Abstract
Rationale
Anxiety disorders and alcohol use disorders frequently co-occur in humans perhaps because alcohol relieves anxiety. Studies in humans and rats indicate that alcohol may have greater anxiolytic effects in organisms with increased genetic propensity for high alcohol consumption.
Objectives and methods
The purpose of this study was to investigate the effects of moderate doses of alcohol (0.5, 1.0, 1.5 g/kg) on the acquisition and expression of anxiety-related behavior using a fear-potentiated startle (FPS) procedure. Experiments were conducted in two replicate pairs of mouse lines selectively bred for high- (HAP1 and HAP2) and low- (LAP1 and LAP2) alcohol preference; these lines have previously shown a genetic correlation between alcohol preference and FPS (HAP>LAP; Barrenha and Chester, Alcohol Clin Exp Res 31:1081–1088, 2007). In a control experiment, the effect of diazepam (4.0 mg/kg) on the expression of FPS was tested in HAP2 and LAP2 mice.
Results
The 1.5 g/kg alcohol dose moderately decreased the expression of FPS in both HAP lines but not LAP lines. Alcohol had no effect on the acquisition of FPS in any line. Diazepam reduced FPS to a similar extent in both HAP2 and LAP2 mice.
Conclusions
HAP mice may be more sensitive to the anxiolytic effects of alcohol than LAP mice when alcohol is given prior to the expression of FPS. These data collected in two pairs of HAP/LAP mouse lines suggest that the anxiolytic response to alcohol in HAP mice may be genetically correlated with their propensity toward high alcohol preference and robust FPS.
Keywords: Alcohol, Anxiety, Fear, Genetics, Startle, Stress, Behavior
Introduction
Alcohol use disorders (AUDs) co-occur with anxiety disorders in over 35% of the US population (Kessler et al. 1996). One primary hypothesis put forward to explain the link between AUDs and anxiety disorders is the “tension-reduction” or “self-medication” hypothesis, which states that AUDs arise because individuals consume alcohol to alleviate anxiety symptoms (Bowen et al. 1984; Cappell and Herman 1972; Conger 1956; Sher 1987; Sinha et al. 1998; Weiss and Rosenberg 1985). A cyclic pattern of excessive alcohol consumption in response to anxiety symptoms may be exacerbated to the point that individuals develop full-blown symptoms of alcohol dependence. Another hypothesis is that there are common inherited genetic factors that increase the risk for developing cooccurring anxiety disorders and AUDs (Maier et al. 1993; Merikangas et al. 1994; 1998; Munjack and Moss 1981). Evidence suggests that these hypotheses are not necessarily mutually exclusive and each hypothesis may appropriately explain the comorbid relationship between anxiety disorders and AUDs in certain subsets of afflicted people.
The question of whether alcohol actually relieves anxiety symptoms remains open. In humans, some studies indicate that anxiety symptoms are reduced (Abrams et al. 2001; Levenson et al. 1980; Moberg and Curtin 2009; Nesic and Duka 2006; Sayette et al. 1990; Terra et al. 2004;Thomas et al. 2003), increased, (McDougle et al. 1995; Terra et al. 2004) or unaltered (Curtin et al. 1998; Himle et al. 1999; Naftolowitz et al. 1994; Zimmermann et al. 2004) in the presence of alcohol. Discrepant results in the literature may be due to several factors including the nature of the anxiety symptoms/disorder and variations in methods for administering alcohol or for assessing anxiety symptoms. In addition, whether or not alcohol has anxiolytic effects is likely influenced by factors such as personal history of alcohol use, trait or state anxiety levels, and family history of alcohol use and/or anxiety disorders. Indeed, several investigators have found differential sensitivity to alcohol's anxiolytic effects in special populations at higher risk for AUDs and/or anxiety disorders. For example, alcohol has been shown to reduce anxiety symptoms in individuals with a positive but not negative family history of alcoholism (Sher and Levenson 1982; Sinha et al. 1998), in individuals with a positive but not negative family history of anxiety disorders (Sinha et al. 1998), and in “high anxiety sensitive” but not in “low anxiety sensitive” individuals (Zack et al. 2007). Further, although not the direct indices of anxiety symptoms, alcohol reduces stress-related responses, such as muscle tension (Schuckit et al. 1981), heart rate (Finn and Pihl 1987; Stewart et al. 1992), and cortisol (Croissant and Olbrich 2004) and prolactin (Zimmermann et al. 2009) release, in people with a family history of alcoholism compared to controls. Some studies indicate that these effects may be sex dependent; that is, women with a family history of alcoholism or anxiety disorders have shown greater “stress-response dampening” effects of alcohol than men (Levenson et al. 1987; Sinha et al. 1998).
In rodents, alcohol has been shown to reduce anxiety-related behavior in unconditioned anxiety models, such as the elevated plus maze (Abreu-Villaca et al. 2008; Boehm et al. 2002; Correia et al. 2009; Durcan and Lister 1988; LaBuda and Fuchs 2002; Spanagel et al. 1995), the plus maze discriminative learning avoidance task (Gulick and Gould 2009a; 2009b; Kameda et al. 2007), the social interaction test (File et al. 1976), the holeboard test (Durcan and Lister 1988; File 1976), the light/dark box test (Costall et al. 1988), and defensive behavior tasks (Blanchard et al. 1993). There have been a few reports of alcohol effects on anxiety-related behavior in rats that differ in genetic propensity toward alcohol consumption. Stewart et al. (1993) found greater anxiety-related behaviors in rats selectively bred for alcohol preference (P) than in rats selectively bred for nonpreference (NP) and alcohol treatment reduced anxiety in P but not NP rats. P (Pandey et al. 2006) and Sardinian alcohol preferring (sP) rats (Colombo et al. 1995) have shown greater anxiety in the elevated plus maze than their alcohol-nonpreferring counterparts (NP and sNP), and voluntary alcohol consumption in P and sP rats reduced their anxiety-related behavior. These findings in rats support studies in humans suggesting that alcohol may have greater anxiolytic effects in organisms with increased genetic propensity for high alcohol consumption.
Posttraumatic stress disorder (PTSD) is one anxiety disorder that frequently co-occurs with AUDs (Brady et al. 2000; Brown et al. 1998; Kessler et al. 1996; McFarlane 1998), especially among US active military personnel and veterans (Davidson et al. 1990; Hoge et al. 2004; Kang and Hyams 2005; Milliken et al. 2007; Stewart 1996). Fear-conditioning models of anxiety, such as fear-potentiated startle (FPS), are commonly used in rodents to study processes that may contribute to fear-related disorders such as PTSD (Kim and Jung 2006). Similar to that seen with models of unconditioned anxiety-related behavior, selectively bred P rats show greater FPS than NP rats (McKinzie et al. 2000). We have also reported that mouse lines selectively bred for high alcohol preference (HAP) show greater FPS than mouse lines selectively bred for low alcohol preference (LAP; Barrenha and Chester 2007). These data suggest that genes contributing to high or low alcohol preference may also influence propensity to show anxiety- and fear-related behaviors. In this regard, the HAP/LAP mouse lines may be an animal model that represents increased genetic risk to develop AUDs comorbid with PTSD in humans.
To our knowledge, there are only two published studies in which alcohol effects on FPS have been examined in rats; one reported that alcohol decreased FPS (Miller and Barry 1960) while the other reported no effect (Hijzen et al. 1995). The present study examined the effects of various doses of alcohol on the acquisition and expression of FPS in replicate pairs of the HAP/LAP mouse lines. Based on the results from human and rat studies previously described, it was hypothesized that HAP lines would be more sensitive than LAP lines to alcohol's anxiolytic effects during the acquisition and the expression of FPS. We tested male and female mice from both replicate pairs of mouse lines to adequately address the hypothesis that sensitivity to alcohol's anxiolytic effect on FPS is a genetically correlated response to selection for alcohol preference (Crabbe et al. 1990) and to determine whether the correlated response depends on sex.
Materials and methods
Subjects
Subjects were alcohol-naïve adult male and female HAP and LAP mice from replicate lines 1 and 2. The selectively bred HAP1/LAP1 and HAP2/LAP2 mouse lines were derived from a progenitor population of outbred HS/Ibg mice (Institute of Behavioral Genetics, Boulder, CO) at the Indiana Alcohol Research Center in Indianapolis, IN (Grahame et al. 1999). Subjects in the present experiments were derived from 25 different HAP1 families, 33 different LAP1 families, 30 different HAP2 families, and 31 different LAP2 families. Replicate 1 HAP mice were from the 27th, 34th, 37th, and 39th generation of selection. Replicate 1 LAP mice were from multiple generations of offspring from generation 27 breeders maintained with relaxed selection. Replicate 2 HAP and LAP mice were from the 27th, 29th, 31st, 34th (HAP2 only), 35th (LAP2 only), and 37th generation of selection. Multiple replications of the experiments were conducted over a period of 22 months (experiment 1) and 26 months (experiment 2). Experiment 3 was conducted in one replication. Subject representation in each replication was balanced across replicate, line, sex, and litter of origin to the best extent possible. At the start of experimental procedures, mice were between 53 and 100 days old in experiment 1, 67–124 days old in experiment 2, and 72–94 days old in experiment 3. Mice were housed in polycarbonate cages (29.2×19.0×12.7 cm) with aspen wood shavings in groups of two to four per cage. Ambient room temperature was maintained at 21±2°C. Mice had free access to food (Rodent Lab Diet 5001, Purina Mills Inc., St Louis, MO) and water in the home cage at all times, except when testing procedures took place. The experimental procedures were conducted during the light phase of a 12:12 light/dark cycle (lights off at 1900 hours). All experimental procedures were approved by the Purdue Animal Care and Use Committee and were conducted in accordance with the principles of laboratory animal care.
Drugs
Alcohol was diluted from a 95% (v/v) solution to a concentration of 20% (v/v) with physiological saline (0.9%) and was administered as intraperitoneal (IP) injections at doses of 0.5, 1.0, and 1.5 g/kg of body weight and in an injection volume of 3.1, 6.5, and 9.4 ml/kg, respectively. Saline-treated groups received saline in a volume of 6.5 ml/kg. The chosen alcohol doses have been shown to dose-dependently reduce contextual fear-conditioned responses in C57BL/6 mice (Gould 2003) and are below the dose of alcohol shown to produce locomotor stimulation in HAP mice (Grahame et al. 2000). Diazepam (Sigma-Aldrich, St Louis, MO, USA) was dissolved in a 10% 2-hydroxypropyl-β-cyclodextrin solution (Sigma-Aldrich) and administered IP at a dose of 4.0 mg/kg in an injection volume of 10 ml/kg (Smith et al. 2011). Vehicle-treated groups received 10% 2-hydroxypropyl-β-cyclodextrin solution in a volume of 10 ml/kg. The 4.0 mg/kg diazepam dose has been shown to significantly reduce FPS in C57BL/6J mice (Smith et al. 2011).
Fear-potentiated startle apparatus
FPS was assessed using two dark, sound-attenuated Coulbourn Instruments (Allentown, PA, USA) Animal Acoustic Startle System chambers, as previously described (Barrenha and Chester 2007). Startle stimuli consisted of 100 dB, 40 ms white noise bursts (frequency range, 20 Hz–20 kHz). Subjects' startle responses were measured as the amount of force in grams exerted against a weight-sensitive platform during the 200 ms after the onset of each acoustic stimulus. The force measurement does not include the subject's bodyweight. A ventilating fan provided continuous 70–71 dB background noise.
Fear-potentiated startle procedure
FPS procedures consisted of one conditioning and one test session separated by 24 h. During each conditioning trial, fear-conditioned groups received 40 trials of a 30-s, 7 W light stimulus paired with a 0.5-s, 0.8 mA footshock [2-min intertrial interval (ITI)]. The footshock occurred during the last 0.5 s of the light stimulus presentation. Control groups (experiment 1 only) received the same number of light and shock presentations as the fear-conditioned group but these stimuli were explicitly unpaired during each of the 40 2-min intervals (interstimulus range 13–118 s). All mice in the control groups received the same sequence of randomized light and shock presentations. The FPS test session occurred 24 h after the conditioning session and consisted of a 5-min habituation period followed by 36 total trials (2-min ITI) presented on a random schedule (range, 12–108 s) to reduce habituation to any single trial type. Twelve of the trials were blank (no stimuli), 12 were noise alone (100 dB, 40 ms), and 12 were light (7 W, 30 s) + noise (100 dB, 40 ms). On light + noise trials, the noise stimulus was presented immediately after the light stimulus ended. FPS parameters were chosen based on our previous work in HAP/LAP replicate lines (Barrenha and Chester 2007), but the number of conditioning trials was increased from 20 to 40 in this study to avoid a potential floor effect in alcohol-treated LAP groups.
Study procedures
Experiment 1: effects of alcohol on FPS expression
Two-hundred and thirteen HAP1 (120 males, 93 females), 234 LAP1 (115 males, 119 females), 272 HAP2 (118 males, 154 females), and 241 LAP2 (101 males, 140 females) were randomly assigned to either a fear-conditioned or control group and further divided into one of four treatment groups: saline (0 g/kg), 0.5, 1.0, or 1.5 g/kg alcohol. The main purpose of including control groups in this experiment was to examine whether alcohol might have nonspecific effects on the behavior which could compromise the interpretation of any effect of alcohol on the expression of FPS.
The conditioning session for experiment 1 began with a 5-min habituation period followed by ten startle trials of 100 dB [40 ms; 20 s ITI] noise bursts to acclimate mice and reduce their initial startle reactivity. Two minutes later, the first of the 40 conditioning trials began. Analysis of the ten preconditioning startle trials showed no difference between treatment groups in startle magnitude as a function of replicate, line, or sex. The next day, mice received their respective dose of alcohol or saline 15 min before the start of the FPS test session. A 15-min pretreatment time was chosen based on a study by Gould (2003) in which alcohol disrupted contextual fear-conditioned responses in C57BL/6 mice.
Experiment 2: effects of alcohol on FPS acquisition
Subjects were 115 HAP1 (57 males, 58 females), 126 LAP1 (65 males, 61 females), 114 HAP2 (52 males, 62 females), and 105 LAP2 (47 males, 58 females). Procedures for this experiment differed from experiment 1 in two ways. First, 20–66 h prior to the fear-conditioning session, mice received a baseline startle session to assess initial startle reactivity to ten trials of 100 dB noise bursts (5 min habituation; 40 ms; 20 s ITI). Mice within each replicate/line/sex each were then assigned to one of four treatment groups (0.0, 0.5, 1.0, or 1.5 g/kg alcohol), counterbalanced based on average startle response magnitude. This procedure provided an added safeguard against sampling error by attempting to balance any individual differences in overall startle reactivity across drug treatment groups. Analysis of variance (ANOVA) confirmed that there were no treatment group differences as a function of replicate, line, or sex at the start of the conditioning session. Second, mice received their respective dose of alcohol or saline immediately before the start of the conditioning session, which included a 10-min habituation period, to allow for the absorption and distribution of alcohol by the start of the first conditioning trial.
Experiment 3: effects of diazepam on FPS expression
The purpose of this experiment was to compare the effects of a known anxiolytic drug, diazepam, on the expression of FPS in HAP vs. LAP mice. Forty-three HAP2 (19 males, 24 females) and 41 LAP2 (21 males, 20 females) were randomly assigned to either a vehicle or diazepam (4.0 mg/kg) group. Experimental procedures were the same as that described for experiment 1. Mice received an injection of diazepam or vehicle 25 min before the start of the FPS test session. This pretreatment time was chosen based on a recent study by Smith et al. (2011) in which diazepam significantly reduced FPS in C57BL/6 mice.
Assessment of blood alcohol content
In a separate experiment, blood alcohol content (BAC) was assessed in saline-treated mice from fear-conditioned (experiments 1 and 2) and control (experiment 1) groups. HAP1 male (n=31), HAP1 female (n=25), LAP1 male (n=31), LAP1 female (n=35), HAP2 male (n=31), HAP2 female (n=39), LAP2 male (n=20), and LAP2 female (n=38) mice received an IP injection of alcohol (0.5, 1.0, or 1.5 g/kg; 20% v/v), and blood samples (~30 μl) were obtained from the tip of the tail at 15 and 75 min after injection of alcohol; these two time points corresponded with the beginning and the end of the FPS test session. Tail blood was collected into heparin-coated capillary tubes, immediately centrifuged, and plasma was extracted and frozen at −80°C until analyzed for BAC using an AM1 Analyzer (Analox Instruments, MA, USA).
Statistical analyses
All 12 startle responses on each trial type (noise alone, light + noise) were averaged for each mouse. Mice that did not meet the minimum startle response criterion of 11 g of force and whose data were affected by equipment/experimenter errors were removed from all analyses.
The % FPS measure was obtained using proportional change scores calculated using the following formula: [((startle amplitude on light + noise trials − startle amplitude on noise-alone trials)/startle amplitude on noise-alone trials) × 100]. The % FPS measure adjusts for individual and group differences in startle reactivity. It also adjusts for potential nonspecific drug treatment effects on startle reactivity and thus is indicated to be an accurate and sensitive way to detect selective effects of pharmacological compounds on FPS (Walker and Davis 2002).
Data were analyzed using ANOVA with replicate (1, 2), line (HAP, LAP), sex (male, female), conditioning group (fear-conditioned, control), and treatment group (saline, 0.5, 1.0, and 1.5 g/kg alcohol or vehicle, 4.0 mg/kg diazepam) as between-group factors and time (15 and 75 min post-injection) as a within-group factor, where applicable. Lower-order ANOVAs and Tukey's t test were used to explore interactions and main effects. Pearson product moment correlation coefficients were generated to assess the relationship between body weight and BAC. Probability values less than 0.05 were considered to be significant.
Results
Experiment 1: effects of alcohol on the expression of FPS
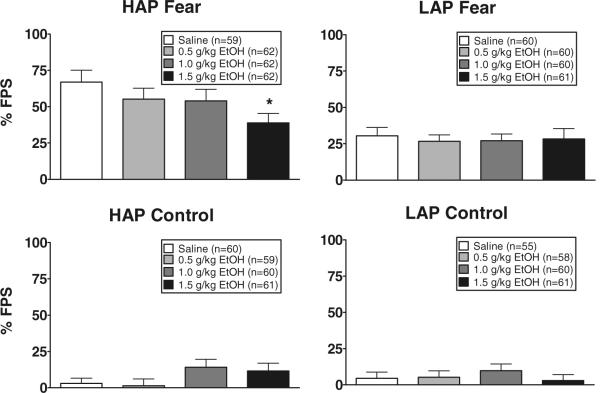
A five-way ANOVA (replicate × line × sex × conditioning group × treatment group) conducted on % FPS scores revealed significant main effects of replicate [F(1,896)=4.8, p<0.05; 1>2], line [F(1,896)=24.3, p<0.01; HAP>LAP], sex [F(1,896)=8.0, p<0.01; male > female], conditioning group [F(1,896)=142.4, p<0.01; fear-conditioned > control], and replicate × sex [F(1,896)=4.6, p<0.05], replicate × conditioning group [F(1,896)=3.9, p=0.05], and line × conditioning group [F(1,896)=15.6, p<0.01] interactions, and a three-way line × conditioning group × treatment group interaction close to significance [F(3,896)=2.4, p<0.07]. To further explore the three-way interaction, conditioning group × treatment group ANOVAs were conducted separately for HAP and LAP mice. A main effect of conditioning group was found for both HAP and LAP mice [F values >38.9 and p values <0.01; fear-conditioned>control], and a significant conditioning group × treatment group interaction was found in HAP but not LAP mice [F(3,477)=3.2, p<0.05]. The source of the interaction was a main effect of treatment group close to significance in the HAP fear-conditioned but not control groups [F(3,241)=2.3, p<0.08], due to reduced % FPS in the 1.5 g/kg alcohol group compared to the saline group (p<0.05 with Tukey's t test). Data are shown in Fig. 1, collapsed across replicate and sex.
Fig. 1.
Mean (± SEM) % FPS in fear-conditioned (top panels) and control (bottom panels) HAP (left panels) and LAP (right panels) mouse lines treated with IP injections of alcohol (0.5, 1.0, and 1.5 g/kg) or saline before the FPS test session.*p< 0.05 saline vs. 1.5 g/kg group
Experiment 2: effects of alcohol on the acquisition of % FPS
A four-way ANOVA (replicate × line × sex × treatment group) on % FPS scores revealed significant main effects of replicate [F(1,428)=5.2, p<0.05; replicate 2 > replicate 1] and line [F(1,428)=16.9, p<0.01; HAP > LAP] and a replicate × treatment group interaction [F(3,428)=2.8, p<0.05], but follow-up analyses of treatment group within each replicate yielded no significant effects. The initial ANOVA also yielded a replicate × line × sex [F(1,428)=6.3, p<0.05] interaction that was followed with line × sex ANOVAs within each replicate. For both replicates, HAP mice showed greater % FPS than LAP mice [F values >6.2 and p values ≤0.01]. A line × sex [F(1,215)=9.6, p<0.01] interaction was also found in replicate 2 mice due to a sex effect in LAP2 [F(1,103)=6.3, p=0.01; female > male] but not HAP2 mice. Table 1 shows these data separately for replicate lines 1 and 2 and male and female mice in fear-conditioned and control groups despite the lack of significant statistical interactions with these factors (Table 2).
Table 1.
Effects of alcohol on the expression of FPS
| Treatment Group | Saline | 0.5 g/kg EtOH | 1.0 g/kg EtOH | 1.5 g/kg EtOH |
|---|---|---|---|---|
| HAP1 Male Fear | 81.5±22.2 | 56.0±15.5 | 69.7±19.4 | 33.3±13.8a |
| HAP1 Male Control | 12.1±6.4 | 7.4±11.2 | 23.7±12.6 | 13.4±15.9 |
| LAP1 Male Fear | 40.4±15.0 | 12.1±8.4 | 27.9±9.7 | 18.8±11.6 |
| LAP1 Male Control | 19.0±9.3 | 9.5±9.2 | 12.7±7.5 | 23.8±9.9 |
| HAP1 Female Fear | 75.7±18.8 | 62.4±25.5 | 60.8±28.4 | 23.9±12.7a |
| HAP1 Female Control | 10.9±11.2 | 18.4±11.1 | 39.7±16.8 | 11.5±10.9 |
| LAP1 Female Fear | 22.1±9.2 | 34.2±8.0 | 29.9±9.0 | 27.1±10.5 |
| LAP1 Female Control | 5.9±9.7 | −1.2±6.8 | 10.6±11.7 | −1.2±9.8 |
| HAP2 Male Fear | 82.6±15.2 | 70.4±11.9 | 32.4±8.6 | 52.7±13.2a |
| HAP2 Male Control | −6.1 ±5.8 | 2.5±8.9 | 3.4±7.3 | 16.7±7.5 |
| LAP2 Male Fear | 44.9±13.9 | 33.9±12.1 | 33.3±10.9 | 68.1±24.8 |
| LAP2 Male Control | 0.2±8.4 | 29.0±8.9 | 1.7±7.4 | −6.1±5.3 |
| HAP2 Female Fear | 39.0±10.2 | 38.3±8.6 | 54.5±8.1 | 42.0±12.1a |
| HAP2 Female Control | −2.5±6.1 | −13.3±5.2 | −0.1±5.7 | 6.2±6.8 |
| LAP2 Female Fear | 19.1±8.4 | 27.2±7.2 | 19.6±8.7 | 7.1±7.3 |
| LAP2 Female Control | −6.5±6.2 | −9.4±8.3 | 12.6±9.6 | −2.8±5.6 |
Mean (± SEM) % FPS data from Fig. 1 separately show the replicate lines 1 and 2 and male and female mice in fear-conditioned and control groups despite the lack of significant statistical interactions with these factors
Statistical comparisons collapsed across replicate and sex (p<0.05 saline vs. 1.5 g/kg group)
Table 2.
Effects of alcohol on the acquisition of FPS
| Treatment Group | Saline | 0.5 g/kg EtOH | 1.0 g/kg EtOH | 1.5 g/kg EtOH |
|---|---|---|---|---|
| HAP1 Male | 87.7±35.2 | 37.8±15.9 | 145.9±61.2 | 28.6±18.9 |
| LAP1 Male | 43.9±18.5 | 40.7±16.5 | 54.7±11.9 | 22.5±11.3 |
| HAP1 Female | 79.1±23.7 | 109.1±45.7 | 86.3±23.7 | 53.6±16.4 |
| LAP1 Female | 21.3±11.7 | 28.2±12.1 | 43.3±14.2 | 35.8±7.8 |
| HAP2 Male | 92.8±19.7 | 78.2±14.1 | 97.5±17.3 | 149.1±35.3 |
| LAP2 Male | 45.5±19.9 | 22.4±10.6 | 43.6±12.3 | 55.1±14.7 |
| HAP2 Female | 68.4±11.9 | 95.9±16.9 | 64.7±20.4 | 78.6±17.3 |
| LAP2 Female | 109.4±31.6 | 92.8±23.3 | 61.2±20.9 | 73.3±37.0 |
Mean (± SEM) % FPS in male and female HAP1/LAP1 and HAP2/LAP2 lines treated with 0 (saline), 0.5, 1.0, and 1.5 g/kg alcohol prior to the fear-conditioning session
Experiment 3: effects of diazepam on the expression of FPS
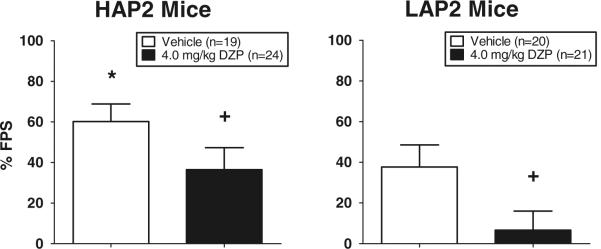
A two-way ANOVA (line × sex × treatment group) conducted on % FPS scores revealed significant main effects of line [F(1,76)=7.3, p<0.01; HAP2 > LAP2] and Treatment Group [F(1,76)=7.3, p<0.01; vehicle > diazepam] but no interaction. Data are shown in Fig. 2, collapsed across sex.
Fig. 2.
Mean (± SEM) % FPS in HAP2 (left panel) and LAP2 (right panel) mouse lines treated with IP injections of 4.0 mg/kg diazepam or vehicle before the FPS test session. *p<0.01 HAP2 vs. LAP2; +p<0.01, vehicle vs. diazepam groups
Blood alcohol content
Pearson product moment correlations between body weight and BAC indicated a significant correlation at the 15-min time point (r=0.2, p<0.01). Thus, statistical analyses of BAC included body weight as a co-factor. A five-way repeated measure analysis of co-variance (replicate × line × sex × treatment group × time with body weight as a co-factor) revealed a significant main effect of treatment group [F(2,225)=299.6, p<0.01] and a replicate × treatment group × time interaction [F(2,225)=4.0, p<0.05] but no line or sex differences in BAC. Data are shown in Table 3.
Table 3.
Blood Alcohol Content in HAP1/LAP1 and HAP2/LAP2 lines
| Treatment Group | 0.5 g/kg |
1.0 g/kg |
1.5 g/kg |
|||
|---|---|---|---|---|---|---|
| Minutes Post-Injection | 15 | 75 | 15 | 75 | 15 | 75 |
| HAP1 Male | 48.0±6.2 | 6.1±1.2 | 102.2±9.3 | 32.7±10.3 | 147.6±18.5 | 84.6±11.4 |
| LAP1 Male | 40.6±5.8 | 5.8±1.1 | 96.3±9.6 | 31.9±5.1 | 160.5±13.6 | 102.5±7.2 |
| HAP1 Female | 39.0±5.6 | 5.3±1.0 | 90.6±12.4 | 18.3±3.3 | 165.0±13.4 | 93.4±4.9 |
| LAP1 Female | 44.7±5.0 | 5.9±2.2 | 94.8±15.3 | 22.0±5.6 | 156.1±13.1 | 103.3±5.9 |
| HAP2 Male | 41.7±4.0 | 3.4±0.8 | 80.3±11.8 | 21.8±4.5 | 114.1±14.6 | 89.8±10.8 |
| LAP2 Male | 36.8±5.2 | 6.0±0.9 | 84.0±16.3 | 27.9±7.4 | 134.1±19.9 | 90.6±20.0 |
| HAP2 Female | 37.2±3.9 | 4.0±0.9 | 78.9±7.7 | 23.5±5.4 | 115.8±15.2 | 98.2±14.5 |
| LAP2 Female | 34.2±2.4 | 4.5±0.8 | 83.9±8.4 | 26.0±4.3 | 108.7±8.3 | 91.1±9.6 |
Mean (± SEM) BAC (mg of alcohol/dl of plasma) at 15 and 75 min in HAP and LAP mouse lines treated with IP injections of 0.5, 1.0, and 1.5 g/kg alcohol
Discussion
The “tension-reduction” and “stress-response dampening” hypotheses of alcohol use (Cappell and Herman 1972; Conger 1956; Sher 1987) set forth the idea that alcohol may reduce anxiety symptoms, which has been suggested as a primary reason for the high rate of comorbidity between AUDs and anxiety disorders. It has also been hypothesized that common inherited genes could be mediating risk factors in the development of these comorbid disorders because some studies indicate people with a positive family history of AUDs and/or anxiety disorders are more sensitive to the effects of alcohol on anxiety symptoms (Sher and Levenson 1982; Sinha et al. 1998; but see Zimmermann et al. 2004) and other stress-related indices (Croissant and Olbrich 2004; Finn and Pihl 1987; Levenson et al. 1987; Schuckit et al. 1981; Stewart et al. 1992; Zimmermann et al. 2009). In the present study, we used the selectively bred HAP/LAP mouse lines to explore the idea that organisms with a genetic propensity toward high alcohol preference may be more sensitive to the anxiolytic effects of alcohol than organisms that do not have this genetic propensity. We have previously suggested the HAP/LAP lines to be an animal model that may represent increased genetic risk to develop AUDs and a comorbid anxiety disorder, specifically PTSD (Barrenha and Chester 2007), in humans because these mouse lines show a positive association between alcohol preference and FPS (HAP > LAP).
One of the primary goals of this study was to examine whether alcohol has anxiolytic effects in the FPS procedure in mice because to our knowledge this has not yet been demonstrated. Although many studies have consistently shown anxiolytic effects of alcohol on unconditioned anxiety-related behaviors in both mice (Abreu-Villaca et al. 2008; Boehm et al. 2002; Correia et al. 2009; Costall et al. 1988; Durcan and Lister 1988; Gulick and Gould 2009a; 2009b; Kameda et al. 2007) and rats (Blanchard et al. 1993; Colombo et al. 1995; File 1976; File et al. 1976; LaBuda and Fuchs 2002; Spanagel et al. 1995; Stewart et al. 1993), there are only two published studies in rats that examined alcohol effects on the expression of FPS. Miller and Barry (1960) reported that alcohol decreased FPS, whereas Hijzen et al. (1995) reported no effect of alcohol on FPS. In the present study, alcohol had no effect on the acquisition of FPS but the highest dose of alcohol (1.5 g/kg) moderately reduced FPS in HAP, but not LAP mice when given during the expression test. This effect was similar in both male and female mice. It should be noted that the magnitude of alcohol's effect on FPS was small and that some statistical terms did not quite reach the p<0.05 significance criterion [the effect size of the line × conditioning group × treatment group interaction term was small (eta squared = 0.03; power = 0.6; p<0.07)].
The other primary goal of this study was to determine whether alcohol's effect on FPS is a genetically correlated response to the selection for alcohol consumption. The question of whether the two traits of interest are genetically correlated can be rigorously tested using rodent lines selected in replicate. If both pair of lines show the correlated trait, this outcome provides stronger evidence that the correlated trait is influenced by common genes that also mediate the selection phenotype in the replicate lines (Crabbe et al. 1990). In the current study, we replicated our previous findings of a genetic correlation between alcohol preference and FPS in both pairs of the HAP/LAP mouse lines (Barrenha and Chester 2007). In addition, we found support for the hypothesis that HAP lines would be more sensitive than LAP lines to the anxiolytic effects of alcohol with the finding that the highest dose of alcohol reduced the expression of FPS in HAP but not LAP mice. The similar reduction in % FPS in both HAP1 and HAP2 lines suggests that the anxiolytic response to a moderate dose of alcohol during the expression of FPS may be genetically correlated with predisposition toward high alcohol preference. The analyses of BAC indicate that the effect of alcohol on FPS in HAP mice is not due to line differences in alcohol pharmacokinetics. The anxiolytic effects of alcohol in HAP but not LAP mice is a result consistent with prior reports in selected rat lines where alcohol reduced unconditioned anxiety-related behavior in alcohol preferring but not in alcohol-non-preferring rat lines (Colombo et al. 1995; Pandey et al. 2006; Stewart et al. 1993). Interestingly, Rorick et al. (2003) showed that, under certain conditions, alcohol reversed avoidance learning deficits in rats selectively bred for high alcohol drinking (HAD) but not in their low alcohol drinking (LAD) counterparts, an effect the authors suggested might be due to the greater anxiolytic effects of alcohol in HAD than LAD rats. However, HAP and LAP mice from both replicates 1 and 2 have shown no differences in anxiety-related behavior or in the anxiolytic response to 1.25 and 2.0 g/kg alcohol in the elevated plus maze (Dr. Nicholas J. Grahame, personal communication). Taken together, these data suggest that perhaps line differences in anxiety-related behavior are necessary to observe line-specific anxiolytic effects of alcohol. In addition, whether alcohol produces anxiolytic effects may depend on the type of anxiety-related behavior being measured (conditioned vs. unconditioned) and the method of alcohol exposure (experimenter-administered vs. voluntary self-administration). Further research should address these issues. A primary question we are currently exploring is whether fear conditioning alters subsequent voluntary alcohol consumption and, conversely, whether alcohol consumption reduces the expression of FPS.
Different neural pathways and chemical substrates regulate the acquisition vs. the expression of fear-conditioned behaviors (Davis 2006; Fendt and Fanselow 1999). For example, Davis and colleagues have shown that activation of the basolateral nucleus of the amygdala is particularly important for the acquisition of FPS, whereas the central nucleus of the amygdala (CeA) is critical for the expression of FPS (Davis 2006). It is known that alcohol affects neuronal function in the CeA (see review by McBride 2002) and, specifically, increases inhibitory neurotransmission in the CeA via interaction with gamma-aminobutyric acid (GABAA) receptors (Roberto et al. 2003). Our finding that alcohol affected the expression but not the acquisition of FPS suggests that the CeA in HAP mice may be particularly sensitive to alcohol and that differences in GABAA receptor function could partly account for the observed line difference in alcohol's anxiolytic effect on FPS expression. In order to explore this idea, we examined the effect of diazepam, the prototypical anxiolytic drug and agonist at the GABAA/benzodiazepine receptor binding site (Löw et al. 2000)on the expression of FPS in HAP2 and LAP2 mice. Similar to previous reports in rats (Davis 1979) and mice (Risbrough et al. 2003; Smith et al. 2011), diazepam (4.0 mg/kg) significantly reduced the expression of FPS, but no line difference in the response to diazepam was seen. This small piece of evidence suggests that the line difference in anxiolysis may be specific to alcohol and is perhaps not mediated by the GABAA receptor and/or that the lines do not differ in GABAA receptor function. But, certainly more research is needed to explore the neurochemical basis for the effects reported here. Future studies are planned to investigate line-based differences in amygdala function that may be related to alcohol- and anxiety-related behaviors in HAP and LAP mice.
Data from both experiments are generally inconsistent with a number of reports in which effects of alcohol were examined on contextual fear-conditioned freezing behavior in rodents. Most of these studies indicate that alcohol disrupts the acquisition (Gould 2003; Gulick and Gould 2007; Land and Spear 2004; Melia et al. 1996; Wehner et al. 2004; Weitemier and Ryabinin 2003; but see Stromberg and Hammond 1997), but not the expression of fear-conditioned behavior (Gould 2003; Land and Spear 2004). Lattal (2007) further explored this issue and reported that alcohol (1.5 g/kg) appears to interfere with the acquisition of extinction learning in mice. Findings from these studies were interpreted primarily in the context of alcohol's effect on learning and memory mechanisms, rather than anxiety-related mechanisms per se, and were suggested to indicate that alcohol disrupts neural processes involved in memory encoding but not memory retrieval. It is possible that alcohol's effect on FPS in this study was due to its amnestic effect, but this interpretation seems unlikely given the inconsistencies between the present and prior published data. However, it is important to keep in mind that comparison between the present vs. the prior data may be complicated by the fact that FPS is primarily mediated by the amygdala, whereas contextual fear-conditioned behavior is primarily mediated by the hippocampus (Logue et al. 1997; Phillips and LeDoux 1992), and these two brain areas are differentially sensitive to alcohol (Ryabinin et al. 1997). It would be necessary to conduct additional experiments specifically designed to disentangle alcohol's effects on fear- vs. learning/memory-related mechanisms to adequately address whether alcohol has possible amnestic effects in the FPS procedure. On this point, it is notable that alcohol's effects on anxiety-related behavior seem to be dissociable from its effects on learning/memory (Gulick and Gould 2007; Kameda et al. 2007).
In summary, this is the first study to report the anxiolytic effects of alcohol in mice using an FPS procedure, but the observed effect depended on the genetic background of the mice. Although the overall effect size in this study was small, these data collected in two pairs of male and female HAP/LAP mouse lines suggest that the anxiolytic response to alcohol in HAP mice may be genetically correlated with their propensity toward high alcohol preference and robust FPS. Because FPS is thought to be a particularly relevant model for certain features of PTSD in humans (Grillon 2002; Kolb 1984), the presence of this genetic correlation in selected mouse lines could suggest that the high rate of comorbidity between PTSD and AUDs in humans may be partly due to the influence of common genes on mechanisms related to the development of both psychiatric disorders. The results of this study provide additional evidence that one of the relevant mechanisms of comorbidity could be that organisms with a genetic propensity toward AUDs/high alcohol consumption are more sensitive to the anxiolytic, and therefore, the reinforcing effects of alcohol (Colombo et al. 1995; Pandey et al. 2006; Sher and Levenson 1982; Sinha et al. 1998; Stewart et al. 1993). More studies are encouraged to explore the effects of alcohol on other types of stress- and anxiety-related behaviors in these unique selectively bred mouse lines.
Acknowledgments
This study was supported by AA016843 for JAC. We are grateful to Dr. Nicholas J. Grahame for providing the breeders for the HAP1/LAP1 and HAP2/LAP2 lines.
References
- Abrams K, Kushner M, Medina KL, Voight A. The pharmacologic and expectancy effects of alcohol on social anxiety in individuals with social phobia. Drug Alcohol Depend. 2001;64:219–231. doi: 10.1016/s0376-8716(01)00125-9. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Nunes F, Queiroz-Gomes F, Manhaes AC, Filgueiras CC. Combined exposure to nicotine and ethanol in adolescent mice differentially affects anxiety levels during exposure, short-term, and long-term withdrawal. Neuropsycho-pharmacology. 2008;33:599–610. doi: 10.1038/sj.npp.1301429. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res. 2007;31:1081–1088. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Magee L, Veniegas R, Blanchard DC. Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:171–182. doi: 10.1016/0278-5846(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Boehm SL, Reed CL, McKinnon CS, Phillips TJ. Shared genes influence sensitivity to the effects of ethanol on locomotor and anxiety-like behaviors, and the stress axis. Psychopharmacology (Berl) 2002;161:54–63. doi: 10.1007/s00213-002-1000-y. [DOI] [PubMed] [Google Scholar]
- Bowen RC, Cipywnyk D, D'Arcy C, Keegan D. Alcoholism, anxiety disorders, and agoraphobia. Alcohol Clin Exp Res. 1984;8:48–50. doi: 10.1111/j.1530-0277.1984.tb05031.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 7):22–32. [PubMed] [Google Scholar]
- Brown PJ, Sout RL, Gannon-Rowley J. Substance use disorder-PTSD comorbidity. Patients' perceptions of symptom interplay and treatment issues. J Subst Abuse Treat. 1998;15:445–448. doi: 10.1016/s0740-5472(97)00286-9. [DOI] [PubMed] [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction: a review. Q J Stud Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, Gessa GL. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Correia D, Ribeiro AF, Brunialti Godard AL, Boerngen-Lacerda R. Trait anxiety and ethanol: anxiolysis in high-anxiety mice and no relation to intake behavior in an addiction model. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:880–888. doi: 10.1016/j.pnpbp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ. The anxiolytic and anxiogenic actions of ethanol in a mouse model. J Pharm Pharmacol. 1988;40:197–202. doi: 10.1111/j.2042-7158.1988.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Croissant B, Olbrich R. Stress response dampening indexed by cortisol in subjects at risk for alcoholism. J Stud Alcohol. 2004;65:701–707. doi: 10.15288/jsa.2004.65.701. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WG. Alcohol and fear-potentiated startle: the role of competing cognitive demands in the stress-reducing effects of intoxication. J Abnorm Psychol. 1998;107:547–557. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Kudler HS, Saunders WB, Smith RD. Symptom and comorbidity patterns in World War II and Vietnam veterans with posttraumatic stress disorder. Compr Psychiatry. 1990;31:162–170. doi: 10.1016/0010-440x(90)90020-s. [DOI] [PubMed] [Google Scholar]
- Davis M. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology. 1979;62:1–7. doi: 10.1007/BF00426027. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Lister RG. Time course of ethanol's effects on locomotor activity, exploration and anxiety in mice. Psychopharmacology (Berl) 1988;96:67–72. doi: 10.1007/BF02431535. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- File SE. Are central cholinergic paths involved in habituation of exploration and distraction? Pharmacol Biochem Behav. 1976;4:695–702. doi: 10.1016/0091-3057(76)90222-7. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde J, Pool M. Effects of ethanol and chlordiazepoxide on social interaction in rats. Br J Pharmacol. 1976;58:465. proceedings. [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Pihl RO. Men at high risk for alcoholism: the effect of alcohol on cardiovascular response to unavoidable shock. J Abnorm Psychol. 1987;93:230–236. doi: 10.1037//0021-843x.96.3.230. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Li TK, Lumeng L. Limited access alcohol drinking in high- and low-alcohol preferring selected lines of mice. Alcohol Clin Exp Res. 1999;23:1015–1022. [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Acute ethanol has biphasic effects on short- and long- term memory in both foreground and background contextual fear conditioning in C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1528–1537. doi: 10.1111/j.1530-0277.2007.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Effects of ethanol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009a;123:1271–1278. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning, anxiety, and locomotion in C57BL/6 mice in the plus-maze discriminative avoidance task. Neuropsychopharmacology. 2009b;57:302–310. doi: 10.1016/j.neuropharm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijzen TH, Houtzager SW, Joordens RJ, Olivier B, Slangen JL. Predictive validity of the potentiated startle response as a behavioral model for anxiolytic drugs. Psychopharmacology (Berl) 1995;118:150–154. doi: 10.1007/BF02245833. [DOI] [PubMed] [Google Scholar]
- Himle JA, Abelson JL, Haghightgou H, Hill EM, Nesse RM, Curtis GC. Effect of alcohol on social phobic anxiety. Am J Psychiatry. 1999;156:1237–1243. doi: 10.1176/ajp.156.8.1237. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Kameda SR, Frussa-Filho R, Carvalho RC, Takatsu-Coleman AL, Ricardo VP, Patti CL, et al. Dissociation of the effects of ethanol on memory, anxiety, and motor behavior in mice tested in the plus-maze discriminative avoidance task. Psychopharmacology (Berl) 2007;192:39–48. doi: 10.1007/s00213-006-0684-9. [DOI] [PubMed] [Google Scholar]
- Kang HK, Hyams KC. Mental health care needs among recent war veterans. N Engl J Med. 2005;352:1289. doi: 10.1056/NEJMp058024. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry. 1996;66:17–31. doi: 10.1037/h0080151. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb LC. The post-traumatic stress disorders of combat: a subgroup with a conditioned emotional response. Mil Med. 1984;149:237–243. [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Catecholamine depletion by reserpine blocks the anxiolytic actions of ethanol in the rat. Alcohol. 2002;26:55–59. doi: 10.1016/s0741-8329(01)00193-8. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Fear conditioning is impaired in adult rats by ethanol doses that do not affect periadolescents. Int J Dev Neurosci. 2004;22:355–362. doi: 10.1016/j.ijdevneu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Lattal KM. Effects of ethanol on the encoding, consolidation, and expression of extinction following contextual fear conditioning. Behav Neurosci. 2007;121:1280–1292. doi: 10.1037/0735-7044.121.6.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Sher KJ, Grossman LM, Newman J, Newlin DB. Alcohol and stress response dampening: pharmacological effects, expectancy, and tension-reduction. J Abnorm Psychol. 1980;89:528–538. doi: 10.1037//0021-843x.89.4.528. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Oyama ON, Meek PS. Greater reinforcement from alcohol for those at risk: parental risk, personality risk, and sex. J Abnorm Psychol. 1987;96:242–253. doi: 10.1037//0021-843x.96.3.242. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Maier W, Minges J, Lichtermann D. Alcoholism and panic disorder: co-occurrence and co-transmission in families. Eur Arch Psychiatry Clin Neurosci. 1993;243:205–211. doi: 10.1007/BF02190729. [DOI] [PubMed] [Google Scholar]
- McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71:509–515. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Krystal JH, Price LH, Heninger GR, Charney DS. Noradrenergic response to acute ethanol administration in healthy subjects: comparison with intravenous yohimbine. Psychopharmacology (Berl) 1995;118:127–135. doi: 10.1007/BF02245830. [DOI] [PubMed] [Google Scholar]
- McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: the nature of the association. Addict Behav. 1998;23:813–825. doi: 10.1016/s0306-4603(98)00098-7. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Sajdyk TJ, McBride WJ, Murphy JM, Lumeng L, Li TK, Shekhar A. Acoustic startle and fear-potentiated startle in alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 2000;65:691–696. doi: 10.1016/s0091-3057(99)00252-x. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Risch NJ, Weissman MM. Comorbidity and co-transmission of alcoholism, anxiety and depression. Psychol Med. 1994;24:69–80. doi: 10.1017/s0033291700026842. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stevens DE, Fenton B, Stolar M, O'Malley S, Woods SW, Risch N. Co-morbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med. 1998;28:773–788. doi: 10.1017/s0033291798006941. [DOI] [PubMed] [Google Scholar]
- Miller NE, Barry H., 3rd Motivational effects of drugs: methods which illustrate some general problems in psychopharmacology. Psychopharmacologia. 1960;1:169–199. doi: 10.1007/BF00402740. [DOI] [PubMed] [Google Scholar]
- Milliken CS, Auchterlonie JL, Hoge CW. Longitudinal assessment of mental health problems among active and reserve component soldiers returning from the Iraq war. JAMA. 2007;298:2141–2148. doi: 10.1001/jama.298.18.2141. [DOI] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjack DJ, Moss HB. Affective disorder and alcoholism in families of agoraphobics. Arch Gen Psychiatry. 1981;38:869–871. doi: 10.1001/archpsyc.1981.01780330027002. [DOI] [PubMed] [Google Scholar]
- Naftolowitz DF, Vaughn BV, Ranc J, Tancer ME. Response to alcohol in social phobia. Anxiety. 1994;1:96–99. doi: 10.1002/anxi.3070010209. [DOI] [PubMed] [Google Scholar]
- Nesic J, Duka T. Gender specific effects of a mild stressor on alcohol cue reactivity in heavy social drinkers. Pharmacol Biochem Behav. 2006;83:239–248. doi: 10.1016/j.pbb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. Erratum in J Clin Invest (2006) 116:3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Brodkin JD, Geyer MA. GABA-A and 5HT1A receptor agonists block expression of fear-potentiated startle in mice. Neuropsychopharmacology. 2003;25:654–663. doi: 10.1038/sj.npp.1300079. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorick LM, Finn PR, Steinmetz JE. Moderate doses of ethanol partially reverse avoidance learning deficits in high-alcohol-drinking rats. Pharmacol Biochem Behav. 2003;75:89–102. doi: 10.1016/s0091-3057(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Criado JR, Henriksen SJ, Bloom FE, Wilson MC. Differential sensitivity of c-Fos expression in hippocampus and other brain regions to moderate and low doses of alcohol. Mol Psychiatry. 1997;2:32–43. doi: 10.1038/sj.mp.4000206. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Contrada RJ, Wilson GT. Alcohol and correspondence between self-report and physiological measures of anxiety. Behav Res Ther. 1990;28:351–354. doi: 10.1016/0005-7967(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Engstrom D, Alpert R, Duby J. Differences in muscle-tension response to ethanol in young men with and without family histories of alcoholism. J Stud Alcohol. 1981;42:918–924. doi: 10.15288/jsa.1981.42.918. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Stress response dampening. In: Blane HT, Leonard KE, editors. Psychological Theories of Drinking and Alcoholism. Guilford Press; New York: 1987. [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. J Abnorm Psychol. 1982;91:350–367. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S. Stress response dampening: effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology (Berl) 1998;137:311–320. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Smith KS, Meloni EG, Myers KM, Van't Veer A, Carlezon WA, Jr, Rudolph U. Reduction of fear-potentiated startle by benzodiazepines in C57BL/6J mice. Psychopharmacology (Berl) 2011;213:697–706. doi: 10.1007/s00213-010-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, et al. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self administration in rats. Psychopharmacology. 1995;122:373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stewart SH. Alcohol abuse in individuals exposed to trauma: a critical review. Psychol Bull. 1996;120:83–112. doi: 10.1037/0033-2909.120.1.83. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, Pihl RO. The effects of alcohol on the cardiovascular stress response in men at high risk for alcoholism: a dose response study. J Stud Alcohol. 1992;53:499–506. doi: 10.15288/jsa.1992.53.499. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Hammond LJ. The effects of ethanol on a measure of conditioned fear in mice. Alcohol. 1997;14:403–407. doi: 10.1016/s0741-8329(97)87952-9. [DOI] [PubMed] [Google Scholar]
- Terra MB, Figueira I, Barros HM. Impact of alcohol intoxication and withdrawal syndrome on social phobia and panic disorder in alcoholic inpatients. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:187–192. doi: 10.1590/s0041-87812004000400006. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Randall CL, Carrigan MH. Drinking to cope in socially anxious individuals: a controlled study. Alcohol Clin Exp Res. 2003;27:1937–1943. doi: 10.1097/01.ALC.0000100942.30743.8C. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl) 2002;164:318–328. doi: 10.1007/s00213-002-1213-0. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Weiss KJ, Rosenberg DJ. Prevalence of anxiety disorder among alcoholics. J Clin Psychiatry. 1985;46:3–5. [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX, Aramakis VB, Khamba BK, MacLeod CM. Effects of drink-stress sequence and gender on alcohol stress response dampening in high and low anxiety sensitive drinkers. Alcohol Clin Exp Res. 2007;31:411–422. doi: 10.1111/j.1530-0277.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Wittchen HU, Holsboer F. Effects of ethanol administration and induction of anxiety-related affective states on the acoustic startle reflex in sons of alcohol-dependent fathers. Alcoholism Clin Exp Res. 2004;28:424–432. doi: 10.1097/01.alc.0000117835.49673.cf. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Buchmann AF, Spring C, Uhr M, Holsboer F, Wittchen HU. Ethanol administration dampens the prolactin response to psychosocial stress exposure in sons of alcohol-dependent fathers. Psychoneuroendocrinology. 2009;34:996–1003. doi: 10.1016/j.psyneuen.2009.01.015. [DOI] [PubMed] [Google Scholar]