Abstract
Blood-borne nucleated cells participate not only in inflammation, but in tissue repair and regeneration. Because progenitor and stem cell populations have a low concentration in the blood, the circulation kinetics and tissue distribution of these cells is largely unknown. An important approach to tracking cell lineage is the use of fluorescent tracers and parabiotic models of cross-circulation. Here, we investigated the cross-circulation and cell distribution kinetics of C57/B6 GFP+/wild-type parabionts. Flow cytometry analysis of the peripheral blood after parabiosis demonstrated no evidence for a “parabiotic barrier” based on cell size or surface characterstics; all peripheral blood cell subpopulations in this study reached equilibrium within 14 days. Whole blood fluorescence analysis indicated that the mean exchange flow rate was 16μl/hr or 0.66% of the circulating blood volume per hour. Studies of peripheral lymphoid organs indicated differential cell distribution kinetics. Some subpopulations, such as CD8+ and CD11c+, equilibrated in both lymph nodes and spleen indicating a residence time less than 28 days; in contrast, other lymphocyte subpopulations, such as B220+ and CD4+ cells, had not yet reached equilibrium at 28 days. We conclude that parabiosis can provide important insights into defining tissue distribution, residence times, and recirculating pools using fluorochrome markers of cell lineage.
Introduction
A centrifuged peripheral blood sample has a thin white layer of nucleated cells between the sedimented red blood cells and blood plasma; a layer descriptively referred to as the “buffy coat.” Recent molecular and functional studies of the buffy coat have revealed complex subpopulations of cells that participate in not only inflammation, but tissue repair and regeneration (Korbling and Estrov, 2003). Because of their relatively small representation in the peripheral blood, the circulation kinetics and tissue distribution of these cells is largely unknown.
Attempts to track the fate of blood-borne nucleated cells have primarily relied upon bone marrow transplantation (Voswinckel et al., 2003), ex vivo labeling (West et al., 2001a) and parabiosis (Wright et al., 2001). Parabiosis establishes a common blood circulation by surgical pairing; that is, creating a surgical union of twin animals or parabionts (Bunster and Meyer, 1933). More recently, the development of genetically engineered mice expressing green fluorescent protein (GFP) have provided an opportunity to track blood borne cells with a persistent cytoplasmic marker (Swenson et al., 2007). Parabiosis using a GFP+ parabiont has been used to investigate the migration of dermal fibroblast/myofibroblast progenitors (Barisic-Dujmovic et al., 2010), lung progenitors (Abe et al., 2004), diabetic wound healing (Pietramaggiori et al., 2009), non-bone marrow progenitors (Aicher et al., 2007), endothelial progenitors (Purhonen et al., 2008) and bone marrow stem cells (Goldman et al., 2009). Despite the biologic interest in parabiotic cell populations, the kinetics of blood flow and cell distribution between parabionts remains incompletely described.
In this report, we investigated the development of cross circulation in murine C57/B6 GFP+/wild-type parabionts. Based on the measured blood exchange rate of 16μl/hr, calculated cross-circulation equilibrium was established within 12 days. The distribution of GFP+ cells in the peripheral tissues varied by cell subpopulation. Our findings suggest that parabiosis is a valuable approach to defining the distribution of all blood cells, but particularly cell subpopulations with a low frequency in the peripheral blood.
Methods
Mice
Eight to ten week old wild type C57BL/6 (Jackson Laboratories, Bar Harbor, ME) and GFP+C57BL/6-Tg (UBC-GFP) 30Scha/J (Jackson) with similar weights were selected for parabiosis. The care of the animals was consistent with guidelines of the American Association for Accreditation of Laboratory Animal Care (Bethesda, MD).
Parabiotic surgery
The animals were paired based on a modified technique described by Bunster (Bunster and Meyer, 1933). The animals were anesthetized with an intraperitoneal injection of ketamine 100mg/kg (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine 10mg/kg (Phoenix Scientific, Inc., St. Joseph, MO). The right side of the wild type mouse and the left side of the GFP+ mouse was shaved and sterilized. Matching skin incisions from the olecrenon to the knee were made in each mouse. The subcutaneous tissue was dissected to create a 0.5 cm free skin flap. The forelimb and hindlimb were joined by a 0-silk suture (Ethicon, Inc., Somerville, NJ), followed by skin closure using interrupted 4-0 Nylon sutures (Ethicon, Inc., Somerville, NJ). The animal recovered in a heated cage. Postoperatively, each parabiont was given a subcutaneous 1 mL bolus of warmed 0.9% sodium chloride (Abbott Laboratories, North Chicago, IL) twice daily for 48 hours. Analgesia (Buprenorphine, 0.05mg/kg, Webster Generics, Sterling, MA) was given twice daily for 48 hours and titrated to activity. 1% Sulfatrim water (Webster Generics, Sterling, MA) was provided in the cage for 30 days.
Microfluorimetry
The fluorescence intensity of adherent cells was read on a 50μl (“spot”) whole blood data was collected at room temperature and placed in a 96-well flat-bottomed microtiter plate (BD Biosciences, Franklin Lakes, NJ) for analysis by the CytoFluor 4000 Fluorescent Measurement System (Applied Biosystems, Foster City, CA). The CytoFluor 4000 is a computer-controlled multi-well fluorescence scanning device with a tungsten halogen lamp and broadband interference filters: ex 485 ± 20 nm and em 530 ± 25 nm. The blood samples were promptly measured and the data exported to Microsoft Excel (Redmond, WA) spreadsheet for data analysis. Baseline fluorescence measurements of the wild-type (minimum) and GFP+ (maximum) parabiont pereipheral blood, minus background subtraction, were used for subsequent GFP fraction calculations.
Cross-circulation kinetics
The calculations for cross-circulation have been described previously (Marsagli and Thomas, 1971). Applied to parabiosis, these calculations assumed 1) the cells are confined to the vascular space during the experimental interval, and 2) there was near-instantaneous mixing. We also assumed that q1= q2 because the flow rates were presumed to be equal in each direction. Although the blood volume of the two mice may have had slight variations, the use of age- and size-matched mice suggested that V1 = V2. The cell concentration in the respective pairs, x1(t) and x2(t), was obtained by the product of concentration and blood volume.
To calculate cross-circulation kinetics, where
Cell concentration in V1 and V2 was obtained by
| (1) |
| (2) |
These equations can be simplified immediately after parabiosis surgery and before the onset of cross-circulation; that is,
Then the concentration of GFP+ cells was
| (3) |
| (4) |
Monoclonal antibodies
For flow cytometric analyses, fluorescein isothiocynate (FITC), phycoerythrin (PE), allophycocyanin (APC), PE-Cy7 or biotin-conjugated monoclonal antibodies (mAb) were used: anti-CD16/CD32 (Fc block, Clone 2.4G2), anti-B220 (PE, RA3-6B2), anti-CD4 (PE, H129.19), anti-GR-1 (APC, RB-8C5) and biotinylated anti-CD8a (53-6.7) were obtained from BD Biosciences (San Diego, CA). Anti-CD11c (APC, N418) and anti-MHC Class II (APC, M5/114.15.2) were obtained from eBioscience (San Diego, CA). Anti-CD11b (PE-Cy7, M1/70) was obtained from Biolegend (San Diego, CA). Biotinylated anti-F4/80 (BM8) was obtained from Caltag Laboratories (Invitrogen, Carlsbad, CA). Fluorescent-conjugated secondary antibodies to Strepavidin (PE and PE-Cy7) and isotype control anti-Armenian Hamster IgG (HTK888) were obtained from biolegend. Biotinylated isotype control anti-rat IgG2a (eBR2a) was obtained from eBioscience.
Cell Preparation
Retro-orbital blood was collected, mixed in an eppendorf tube with heparinized calcium free phosphate-buffered saline (PBS), and pelleted by centrifugation. The cell pellet was resuspended in red cell lysis buffer (BD Pharmingen, Franklin Lakes, NJ), incubated for 2 minutes, washed in calcium free PBS and stored on ice until staining. The lung was collected in calcium free phosphate-buffered saline (PBS) bath, finely minced using surgical blades and digested in 1mg/mL collagenase (Worthington Biochemical Corp, Lakewood, NJ) at 37°C with continuous agitation for 40 minutes. After filtering with 70μm cell strainer (BD Falcon, Bedford, MA), the sample was pelleted by centrifugation and resuspended in red cell lysis buffer (BD Pharmingen). After a 2 minute incubation, the suspension was washed, resuspended in calcium free PBS at a concentration of 3×107 cells/μl and stored on ice until staining. The spleen was harvested and finely minced using forceps. The tissue was washed in calcium free PBS and passed through a 70 μm cell strainer (BD Falcon, Bedford, MA) and the red cells lysed as above. The suspension was washed, pelleted and resuspended in calcium free PBS to a concentration of 1×107 cells/μl and stored on ice until staining. The lymph node was harvested and finely minced using forceps. The tissue was washed in calcium free PBS and passed through a 70μm cell strainer (BD Falcon, Bedford, MA). The suspension was washed, resuspended in calcium free PBS to a concentration of 1×107 cells/μL and stored on ice until staining.
Flow cytometry
Nonspecific binding was blocked with anti-CD16/32 (Fc-block, Clone 2.4G2) for 5 minutes, and then stained with a 5-fold excess of biotinylated antibody at 4°C for 12 min. The cells were then washed in 25% BSA in calcium free PBS, centrifuged, and incubated in a 5-fold excess of secondary antibody or fluorescently conjugated primary antibody for 12 min. The cells were washed a second time, centrifuged and then processed on a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) with tri excitation laser (405nm, 488nm and 633nm ex). The data were analyzed by FCS Express 4 (De Novo Software, Los Angeles, CA) and described cell marker profiling as dual parameter panels. Debris and dead cells were excluded based on forward and side scatter characteristics and 7AAD staining (Su et al., 2001).
Statistical analysis
The statistical analysis was based on measurements in at least five different parabionts. The unpaired Student’s t test for samples of unequal variances or repeated measure ANOVA was used to calculate statistical significance. The data was expressed as mean ± one standard deviation. The significance level for the sample distribution was defined as P<.05. Weighted composites of observed blood levels were identified by principal component analysis (Systat 11, Richmond, CA). Prediction bands for a confidence interval of 0.95 were calculated using Origin 8.5 (OriginLab, Northampton, MA).
Results
Parabiotic cross-circulation
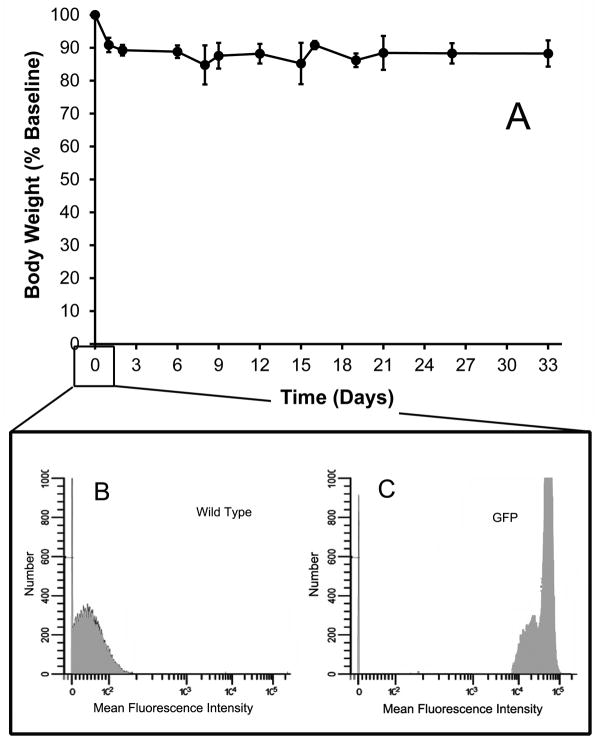
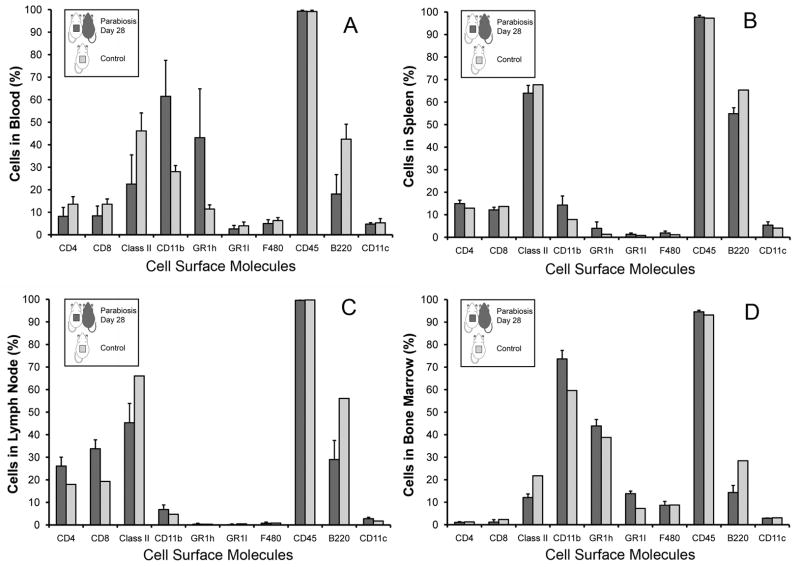
Parabiotic surgery resulted in a modest, but stable, weight loss (Figure 1A) accompanied by rapid behavioral adaptation. At the time of surgery, flow cytometry of nucleated blood cells demonstrated no detectable fluorescent cells in the wild-type parabiont (Figure 1B) and near-100% positive green fluorecence in the GFP parabiont (Figure 1C) To assess any potential systemic differences associated with parabiosis, the difference in peripheral blood cell composition, the systemic composition of single wild-type mice was compared to the composition of wild-type parabionts. In comparing blood, spleen, lymph nodes and bone marrow, there were minor variations in B cell (B220) frequency, but no systematic variation in leukocyte composition (Figure 2).
Figure 1.
Figure 2.
Cross-circulation of GFP+ cells
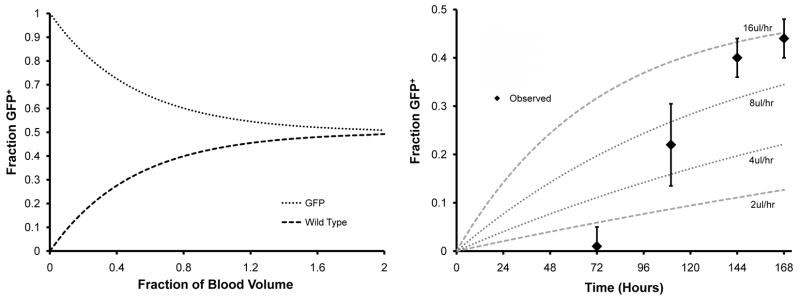
Using theoretical calculations of cross circulation, and assuming equal blood volumes for the two parabiotic mice, the predicted blood exchange would be 43% after one blood volume has flowed in each direction and equilibrium would be almost complete after a cross-circulation flow equal to two blood volumes (Figure 3A). Whole blood “spot” microfluorimetry measurements demonstrated fractional fluorescence greater than 0.4 by post-parabiosis day 7 (Figure 3B). Principal component analysis (N=26) indicated an exchange flow rate of 16μl/hr or 0.66% of the circulating blood volume per hour. Kinetic calculations indicated that a flow rate of 16μl/hr resulted in complete blood exchange in 12 days.
Figure 3.
Phenotypic subpopulations
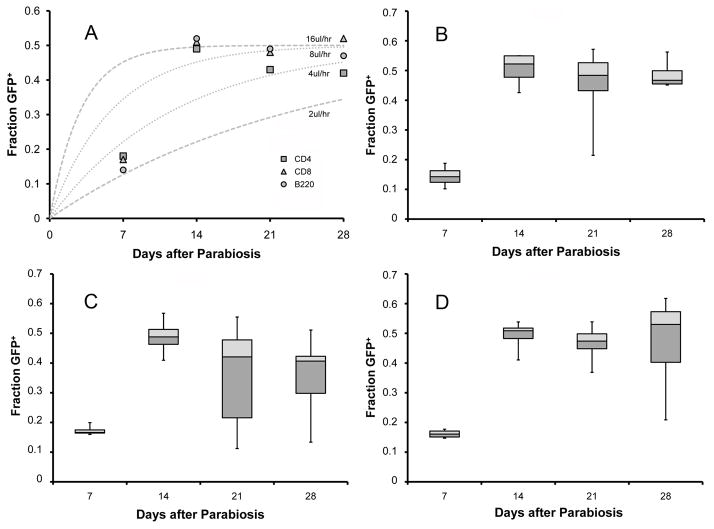
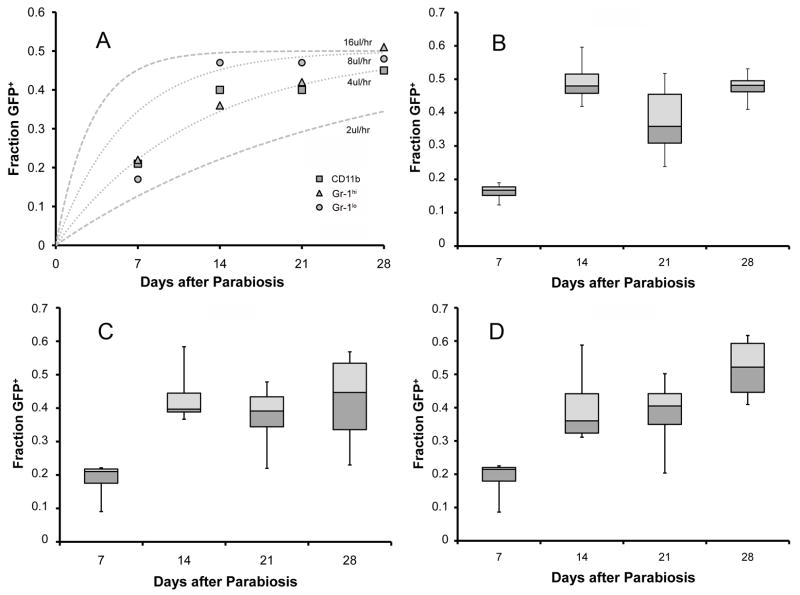
To assess cross-circulation kinetics in peripheral blood cell populations, we analyzed the size and surface marker phenotype of GFP+ cells appearing in the wild-type blood circulation. Flow cytometry analysis of the wild-type parabiont peripheral blood indicated that the appearance of GFP+ cells was independent of cell size; that is, cell size was not correlated with differential appearance of GFP+ cells in the wild-type parabiont peripheral blood (not shown). CD11b, a broadly reactive leukocyte marker (labeling monocytes, neutrophils, macrophages and natural killer cells), approached equilibrium within 2 weeks (Figure 4A). Similarly, neutrophils, (Gr-1hi) and monocytes (Gr-1lo) were at near-equilibrium within 14 days (Figure 4B–D). In contrast, lymphocyte subpopulations demonstrated more variability. There was an initial equilibration of CD4+ cells at 14 days followed by an apparent decline on days 21 and 28 after parabiosis (p=0.34) (Figure 5). There was no significant difference in the calculated rate of exchange between leukocyte populations.
Figure 4.
Figure 5.
Cell distribution in peripheral organs
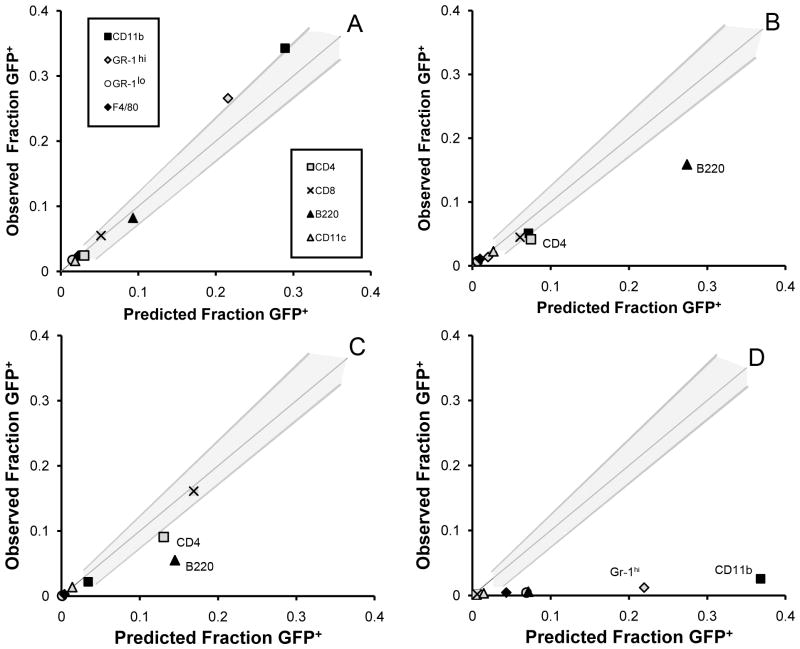
The decline in circulating GFP+ T cells suggested the possibility of a redistribution of lymphocytes into the secondary lymphoid organs. At 28 days after parabiosis, statistical prediction bands were calculated for the peripheral blood and used to assess cell distribution in the lymph nodes, spleen and bone marrow (Figure 6). Cell populations near equilibrium were predicted to fall within these prediction bands. In lymph nodes, the observed fraction of GFP+ B220+ and GFP+ CD4+ cells was significantly less than predicted (Figure 6B). Similarly, these populations were also less than predicted in the spleen (Figure 6C). In contrast, GFP+CD8+ and GFP+CD11c+ cells were in equilibrium in both the lymph node and spleen. None of the 8 cell populations studied neared equilibrium in the bone marrow (Figure 6D).
Figure 6.
Discussion
In this report, we investigated the cross-circulation and cell distribution kinetics of C57/B6 GFP+/wild-type parabionts. We found no evidence for a “parabiotic barrier” based on cell size or surface characterstics; all peripheral blood cell populations in this study reached equilibrium within 14 days. Whole blood fluorescence analysis indicated that the mean exchange flow rate was 16μl/hr or 0.66% of the circulating blood volume per hour. Studies of lymph nodes and the spleen demonstrated variable distribution kinetics; that is, some populations, such as B220+ and CD4+ cells, had not yet reached equilibrium at 28 days. We conclude that parabiosis can provide important insights into defining tissue distribution, residence times, and recirculating pools using cytoplasmic fluorochrome markers of cell lineage.
Although initially assumed to require coelio-anastomosis (Hering, 1829; Sauerbruck and Heyde, 1908), cross-circulation in the rodent parabiosis model was shown to require only surgical union of the chest and abdominal walls between two compatible animals (Bunster and Meyer, 1933). Many authors have investigated the completeness of cross-circulation, examining the surgical site for crossing blood vessels (Duschl and Niekau, 1924) and demonstrating the transmission of substances between pairs (Furth et al., 1940; Grollman and Rule, 1943). Although most authors have agreed that cross-circulation is complete, it is not instantaneous. Huff et al. noted that a “parabiotic barrier” can exist if the clearance rate of the substance is rapid relative to the circulatory flux between animals (Huff et al., 1950); for example, rapidly acting anesthetics may need to be administered separately to the parabiotic twins. Measuring Fe59 tagged erythrocytes, Huff et al. estimated blood flow between parabiotic rats at 0.66% of the blood volume per minute (Huff et al., 1950). Using whole blood microfluorimetry, our estimated blood flow between parabiotic twins was 60-fold lower; that is, 0.66% of the blood volume per hour in mice.
A limitation of the cross-circulation kinetic analysis was the uncertain rate of parabiotic wound angiogenesis. In our studies, injection with vital dyes, and visual inspection of the wound, demonstrated dye crossing the anastomosis within 7 days. Attempts at intravital microscopy demonstrated a few vessels larger than 25um; most of the observed vessels were capillaries. Consistent with an anastomosis composed of predominantly capillaries, attempts at resin casting of the microcirculation failed--presumably, because the capillaries were too fragile for tissue corrosion. Despite the small vessels involved, the amount of angiogenesis was striking. Based on an estimated average mouse capillary flow rate of 0.012μl/hr (Mayrovitz, 1992), the parabiotic cross-circulation exchange rate of 16μl/hr predicts a capillary density of 1,274 capillaries/anastomosis with a total cross-sectional area of 22,914μm2. Assuming an average capillary length of 160.6μm, the rate of capillary growth in our study approached 7.3mm/day.
Parabiosis of GFP+ and wild-type animals provided several advantages for cell distribution studies. First, GFP was brightly expressed in all blood cells except erythrocytes (Swenson et al., 2007). The stability and brightness of the cytoplasmic GFP fluorochrome was a significant advance over previous approaches to ex vivo labeling (West et al., 2001b). Because we do not suspect a differential rate of migration between GFP+ and wild-type cells, the balanced cross-circulation also created an internal reciprocal control; that is, each subpopulation of blood-borne cells was composed of GFP+ cells and unlabeled cells. At cross-circulation equilibrium, any given subpopulation would be composed of 50% GFP+ and 50% unlabeled cells. This comparison provided insights into the apparent volume of distribution as well as residence time of the cell population within a particular organ. For example, the CD8+ subpopulation in the lymph node at 28 days had an equal percentage of GFP+ and GFP− cells. Because the GFP− cells were a control for production/loss, distribution volume and recirculation behavior of this cell population, the equal concentration of GFP+ and GFP− cells provided convincing evidence that the residence time of CD8+ cells in the lymph node was ≤28 days.
Two theoretical disadvantages of parabiosis are 1) the development of parabiotic-related chronic inflammation, and 2) long-term instability of the cross-circulation. Although subtle differences in antigenic or immunologic composition could lead to ongoing immune activation, evidence for chronic inflammation was limited. The only consistent phenotypic difference between single wild-type mice and wild-type parabionts was a slight decrease in the percentage of B220 (B cells) in the lymph node and spleen. Based on our phenotypic analysis and clinical observations, immunologic incompatibility is rare. A second concern is the stability of the parabiotic cross-circulation. Our systematic evaluation of cross-circulation was limited to 28 days, but random sampling of parabiotic mice several months after parabiotic surgery have occasionally demonstrated mice with a substantially lower percentage of GFP+ cells in the wild-type parabiont. The interpretation of this observation is uncertain, but the decline in GFP+ cells may reflect a maturation of the parabiotic anastomosis and diminished exchange flow rates. Long-term parabiotic studies will be necessary to clarify the biologic mechanism and experimental relevance of these observations.
The results of these studies underscore the utility of parabiosis for the study of cell populations present in the peripheral blood. Parabiosis is uniquely suited to tracking small blood cell supopulations that might be undetectable by conventional studies. For example, the use of fluorescent reporter genes will contribute to the study of low frequency progenitor or stem cell traffic. In contrast to the neutrophil and monocytes populations, lymphocytes are composed of subpopulations with distinct distribution kinetics (e.g. recirculating and nonrecirculating pools) in the blood (Ford, 1975). In future studies, parabiosis using selective GFP expression can play an important role in defining tissue distribution and residence times of these recirculating and nonrecirculating cell populations.
Acknowledgments
Supported in part by NIH Grants HL94567, HL75426 and HL007734
Abbreviations
- APC
allophycocyanin
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- mAb
monoclonal antibody
- PBS
phosphate buffered saline
References
- Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, Wang X, Hashimoto M, Sharp JG, Rennard SI. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170(11):1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100(4):581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/Myofibroblasts that Participate in Cutaneous Wound Healing are not Derived from Circulating Progenitor Cells. J Cell Physiol. 2010;222(3):703–712. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- Bunster E, Meyer RK. An improved method of parabiosis. Anat Rec. 1933;57:339–343. [Google Scholar]
- Duschl L, Niekau B. Untersuchungen über die Gefäßskommunikation zwischen parabiosierten Ratten Langenbeck’s. Arch Surg. 1924;186:76–97. [Google Scholar]
- Ford WL. Lymphocyte migration and immune responses. Prog Allergy. 1975;19:1–59. doi: 10.1159/000313381. [DOI] [PubMed] [Google Scholar]
- Furth OB, Barnes WA, Brower AB. Studies on resistance to transmissible leukemia in mice by means of parabiosis. Arch Pathol. 1940;29(2):163–174. [Google Scholar]
- Goldman DC, Bailey AS, Pfaffle DL, Al Masri A, Christian JL, Fleming WH. BMP4 regulates the hematopoietic stem cell niche. Blood. 2009;114(20):4393–4401. doi: 10.1182/blood-2009-02-206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman A, Rule C. Experimentally induced hypertension in parabiotic rats. Am J Physiol. 1943;138(4):0587–0592. [Google Scholar]
- Hering E. Versuche, die Schnelligkeit des Blutlaufes und der Absonderung zu bestimmen. Z Phys. 1829;3:85–126. [Google Scholar]
- Huff RL, Trautman R, Vandyke DC. Nature of exchange in parabiotic rats. Am J Physiol. 1950;161(1):56–74. doi: 10.1152/ajplegacy.1950.161.1.56. [DOI] [PubMed] [Google Scholar]
- Korbling M, Estrov Z. Adult stem cells for tissue repair - A new therapeutic concept? N Engl J Med. 2003;349(6):570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- Marsagli G, Thomas ED. Mathematical consideration of cross circulation and exchange transfusion. Transfusion. 1971;11(4):216–219. doi: 10.1111/j.1537-2995.1971.tb04404.x. [DOI] [PubMed] [Google Scholar]
- Mayrovitz HN. Skin capillary metrics and hemodynamics in the hairless mouse. Microvasc Res. 1992;43(1):46–59. doi: 10.1016/0026-2862(92)90005-a. [DOI] [PubMed] [Google Scholar]
- Pietramaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved Cutaneous Healing in Diabetic Mice Exposed to Healthy Peripheral Circulation. J Investig Dermatol. 2009;129(9):2265–2274. doi: 10.1038/jid.2009.60. [DOI] [PubMed] [Google Scholar]
- Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105(18):6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbruck P, Heyde M. Uber parabiose kunstlichvereinigter warmbluter. Munchener Med Wochenschrift. 1908;1:153. [Google Scholar]
- Su M, He C, West CA, Mentzer SJ. Cytolytic peptides induce biphasic permeability changes in mammalian cell membranes. JImmunol Methods. 2001;252(1–2):63–71. doi: 10.1016/s0022-1759(01)00334-9. [DOI] [PubMed] [Google Scholar]
- Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25(10):2593–2600. doi: 10.1634/stemcells.2007-0241. [DOI] [PubMed] [Google Scholar]
- Voswinckel R, Ziegelhoeffer T, Heil M, Kostin S, Breier G, Mehling T, Haberberger R, Clauss M, Gaumann A, Schaper W, Seeger W. Circulating vascular progenitor cells do not contribute to compensatory lung growth. Circ Res. 2003;93(4):372–379. doi: 10.1161/01.RES.0000087643.60150.C2. [DOI] [PubMed] [Google Scholar]
- West CA, He C, Su M, Rawn J, Swanson S, Hay JB, Mentzer SJ. Stochastic regulation of cell migration from the efferent lymph to oxazolone-stimulated skin. J Immunol. 2001a;166(3):1517–1523. doi: 10.4049/jimmunol.166.3.1517. [DOI] [PubMed] [Google Scholar]
- West CA, He C, Su M, Swanson SJ, Mentzer SJ. Aldehyde fixation of thiol-reactive fluorescent cytoplasmic probes for tracking cell migration. J Histochem Cytochem. 2001b;49:511–518. doi: 10.1177/002215540104900411. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]