Abstract
Objective
We hypothesize that following a sudden decrease in cerebral blood flow velocity (CBFV) in adolescents at faint, rapid hyperemic pulsatile CBFV occurs upon the return to the supine position, and is associated with post-syncopal headache.
Study design
This case-control study involved 16 adolescent subjects with history of fainting and headaches. We induced faint during 70° tilt-table testing and measured mean arterial pressure (MAP), heart rate (HR), end-tidal CO2, and CBFV. Fifteen control subjects were similarly evaluated with a tilt but did not faint, and comparisons with fainters were made at equivalent defined time points.
Results
Baseline values were similar between groups. Upon fainting, MAP decreased 49% in fainters vs. 6% in controls (P<0.001). HR decreased 15% in fainters and increased 35% in controls (P<0.001). In fainters, cerebrovascular critical closing pressure increased markedly resulting in reduced diastolic (-66%) and mean CBFV (-46%) at faint; systolic CBFV was similar to controls. Pulsatile CBFV (systolic – diastolic CBFV) increased 38% in fainters, driving flow-mediated dilation of cerebral vessels. Returning to supine, fainters’ CBFV exhibited increased systolic and decreased diastolic flows compared with controls (P<0.02).
Conclusion
Increased pulsatile CBFV during and following faint may cause post-syncopal cerebral vasodilation and headache.
Keywords: Headache, Syncope, Cerebral Blood Flow, Adolescent, Critical Closing Pressure
Syncope is defined by a transient loss of consciousness and postural tone resulting from cerebral hypoperfusion. Reflex syncope, also known as simple faint, may account for up to 80% of syncopal episodes in the young and tends to be benign (1, 2). Prolonged orthostatic stress (upright posture) is the most common precipitant of reflex syncope in the young, and loss of consciousness often follows decreased arterial blood pressure (AP) and heart rate (HR), resulting in cerebral hypoperfusion as shown by “Faint” in Figure 1. Hyperpnea prior to faint causes decreased end-tidal CO2 (ETCO2), which contributes to cerebral vasoconstriction and further decreases cerebral perfusion (3). The combined results are transient cerebral ischemia with loss of postural tone and loss of consciousness.
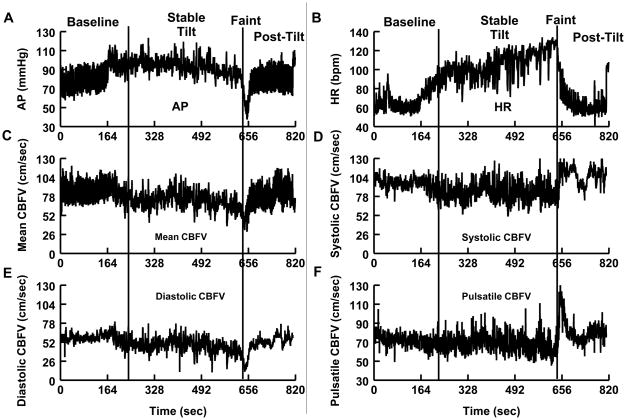
Figure 1.
A) Arterial pressure (AP), B) heart rate (HR), C) mean cerebral blood flow velocity (CBFV), D) systolic CBFV, E) diastolic CBFV, and F) pulsatile CBFV for a representative fainter. The fainter is representative of the typical “vasovagal” pattern seen during fainting in adolescents. During the stable tilt period, AP, HR, and CBFV were steadily maintained. Prior to fainting, AP and HR decrease and then sharply fall at the time of faint. At the time of faint, systolic CBFV is relatively maintained, diastolic CBFV decreases greatly, and pulsatile CBFV increases. During early post-tilt, there is an increase in systolic CBFV; diastolic CBFV remains low and slowly increases; pulsatile CBFV is increased greatly.
Following such reflex syncopal episodes, individuals characteristically complain of headache, a symptom known to be associated with all forms of orthostatic intolerance (1, 4). The causes of these types of headaches are uncertain, but it has been proposed that rapid decreases followed by rapid increases in cerebral blood flow (CBF) may be important (5–10). Data from our laboratory indicate that such unstable CBF can result from diminished cerebral autoregulation which immediately precedes fainting (11). During this period of ineffective autoregulation, we hypothesize that a sudden decrease in CBF will occur at faint, followed by a rapid hyperemic increase in CBF upon return to the supine position, and this leads to a headache that is driven by enhanced pulsatile blood flow.
Methods
We tested 16 subjects with a history of fainting and associated headaches (11 female) and 15 healthy non-fainting control subjects (10 female) aged 13–19 years old. Fainters previously gave a medical history and underwent a physical examination, electrocardiography, and echocardiography to exclude cardiac and other medical causes of their fainting. Fainters were referred to our center for testing after experiencing at least 3 episodes of fainting within the last 6 months. Healthy control subjects reported no clinical illness, no orthostatic intolerance, and had never fainted.
Exclusionary criteria for participation in this study included any infectious or systemic disease (including cardiovascular disease), other forms of orthostatic intolerance, competitive athletic training, recent long-term bed rest, pregnancy within the last 3 months, and the use of all medications. The use of nicotine containing products was also an exclusion. All subjects were instructed to refrain from caffeine and xanthine-containing products for at least 72 hours prior to testing. All subjects were instructed to fast for 12 hours prior to testing. The study was approved by the Institutional Review Board of New York Medical College. All subjects 18 or older signed an informed consent; those younger than 18 assented to participate and their parent or legal guardian signed an informed consent.
Subjects arrived at our center at 9:30 AM, which is climate-controlled at 70° F and were instructed about the procedures and were instrumented while supine. Beat-to-beat blood pressure was measured with a Finometer (FMS, Amsterdam, The Netherlands) on the left middle finger calibrated to the brachial artery. The Finometer has a built-in height sensor and software to compensate for the distance from the finger to the heart during standing. Additionally, the Finometer uses a “modelflow” algorithm to estimate cardiac output (CO). Transcranial Doppler ultrasound (Multigon, Yonkers, New York) insonated the left middle cerebral artery (MCA), and the signal was optimized for depth and signal strength. A custom headband held the 2 MHz probe in place. Respiratory plethysmography (Respitrace, NIMS Scientific, Miami Beach, FL) and a capnograph (Smith Medical PM, Waukesha, WI) measured changes in respiration and ETCO2. An ECG measured HR from the beat-to-beat cardiac electrical interval.
Following instrumentation, subjects remained awake while supine for 30 minutes to acclimate. Subjects were then tilted head-up to 70°. A vasovagal faint was defined as a loss of consciousness and postural tone with a concomitant fall in systolic blood pressure (SBP) below 60 mmHg and a fall in HR, following presyncopal symptoms such as lightheadedness, nausea, or diaphoresis. All subjects defined as fainters fainted during this protocol, and all fainters remained upright until they fainted. Upon faint, fainters were lowered back to the supine position and monitored. All fainters complained of post-syncopal headaches. Control subjects were tilted upright for a maximum of 10 minutes. We have previously found this time limit to be comparable with fainters because in our laboratory, most individuals faint within 10 minutes of upright tilt, and this duration also avoids false-positive faints (3, 11). No control subjects experienced presyncopal symptoms, a fall in SBP or HR, or a headache. After 10 minutes of tilt, control subjects were returned to the supine position and monitored. Subjects did not receive any pharmaceutical agents to provoke fainting, such as nitroglycerin.
We defined the “baseline” time interval of measurement as the last 5 minutes of the 30 minute supine period. We excluded the first minute of tilt because often adolescent and young adult subjects experience an initial orthostatic hypotension that is not related to fainting. The “stable tilt” time interval was defined in fainters as the period after the first minute of tilt where blood pressure was maintained. In controls, this time period was minutes 1–9.5 of tilt. Ten minutes was chosen as the end of testing because, in our laboratory, control subjects remaining upright for longer than 10 minutes run the risk of fainting, and fainting does not occur during tilts lasting less than or equal to 10 minutes. For fainters, the “faint” time interval was defined as the 30 second period of time where blood pressure and heart rate fell and the subject fainted. The comparison time period in control subjects was called “end tilt” and it consisted of the last 30 seconds of tilt. For fainters and controls, the “early post-tilt” time interval was defined as 0–2 minutes after the return to the supine position and “late post-tilt” was defined as 2–5 minutes after the return to the supine position. Thus, we compared equivalent time periods between groups. We have previously employed similar techniques to define time intervals that aid in quantization of hemodynamic events that accompany faint (3, 11).
Mean arterial pressure (MAP) was calculated from the systolic blood pressure (SBP) and diastolic blood pressure (DBP) as MAP = 1/3 * SBP + 2/3 * DBP. Pulse pressure (PP) was calculated from the difference between SBP and DBP [PP = SBP – DBP]. Pulsatile cerebral blood flow velocity was calculated from the difference between the systolic CBFV and the diastolic CBFV [pulsatile CBFV = systolic CBFV – diastolic CBFV]. The cerebrovascular resistance index (CVRi) was calculated as the MAP at the level of the MCA (MAPMCA) divided by the mean CBFV [CVRi = MAPMCA/mean CBFV]. MAPMCA was calculated as MAP – d * 0.735 * sin 70°, where d is the distance from the transcranial Doppler probe to the 2nd intercostal space (12). Similarly, SBP at the MCA (SBPMCA) and DBP at the MCA (DBPMCA) were calculated using the same formula.
Critical closing pressure (CCP) is the estimated calculated perfusion pressure at which blood flow ceases in an arterial vessel (13). We estimated the critical closing pressure of the cerebral vasculature at the level of the MCA for each heart beat based on the linear regression between the mean CBFV and the MAPMCA using the formula of a straight line y = mx + b, where y is the mean CBFV, m is the slope of the regression line, x is MAPMCA, and b is the y-intercept. The coefficient of determination R2 was used to determine the proportion of the data that the regression line fit for each heart beat. The critical closing pressure was estimated as the x-intercept (the intercept on the MAPMCA axis where mean CBFV = 0) of the regression line and was equal to (−b/m). Typically, CBFV leads MAPMCA in time. Thus, prior to regression, the waveforms of MAPMCA and mean CBFV were aligned for each beat, such that peaks and troughs occurred at the same time for both signals. This resulted in a relationship between MAPMCA and mean CBFV that was linear during each individual cardiac cycle. The slope of the linear relationship represents the cerebrovascular conductance at frequencies exceeding the inverse of the RR interval. The cerebral vasculature functions as a high-pass filter at frequencies exceeding approximately 0.07 Hz. Conductance is therefore not equivalent to the inverse of 0 Hz (D.C.) resistance but relates to hydraulic impedance terms which are beyond the scope of the present investigation. Custom written software calculated critical closing pressure for each cardiac cycle over the entire time series. While upright, the blood pressure was corrected for the difference in height during tilt to allow for an appropriate estimate of critical closing pressure.
We sampled the data at 200Hz, stored the digital data on a computer hard drive, and analyzed the data offline. NCSS 2007 statistical software was used for analysis. Demographic data was analyzed using an independent Student t-test. All other data were analyzed using repeated-measures ANOVA with a Bonferroni post hoc test. Values are presented as mean ± SEM, and statistical significance was set at P < 0.05.
Results
Comparing fainters with controls, age (16 ± 1 vs. 17 ± 1 years), height (163 ± 1 vs. 164 ± 2 cm), and weight (62 ± 3 vs. 65 ± 2 kg) were not different (P>0.05). Table I shows the baseline supine values for fainters and controls. There were no differences in AP, HR, CO, respiratory rate, ETCO2, or CBFV.
Table 1.
Supine baseline absolute values and percent change baseline during and after tilt for fainters and control subjects.
| Absolute Values Measurement (units) | Baseline | Δ % from Baseline | Stable Tilt | Faint | End Tilt | Early Post-Tilt | Late Post-Tilt | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fainters | Controls | Measurement (Δ%) | Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | |
| MAP(mmHg) | 80±2 | 83±2 | MAP | 7±3 * | 0.3±1 | −49±3 ‡ | −6±3 | −9±6 | −6±2 | −1±3 | 1±2 |
| SBP mmHg) | 116±3 | 122±2 | SBP | 1±2 | −3±1 | −48±3 ‡ | −9±3 | −10±4 | −7±3 | −2±2 | 1±2 |
| DBP(mmHg) | 63±2 | 64±2 | DBP | 13±4 * | 2±2 | −50±3 ‡ | −3±3 | −8±6 | −6±2 | −1±2 | 0±1 |
| PP(mmHg) | 53±3 | 58±2 | PP | −12±3 | −10±1 | −45±2 ‡ | −15±4 | −13±5 | −7±4 | −3±3 | −1±1 |
| HR(bpm) | 68±2 | 65±2 | HR | 46±4 † | 33±2 | −15±5 ‡ | 35±4 | 20±6 | 9±3 | 7±3 | 1±2 |
| CO (L/Min) | 4±0.5 | 5±0.3 | CO | 2±5 | 1±4 | −53±7‡ | −0.1±5 | 14±8 | 6±5 | 1±3 | 1±2 |
| Resp. Rate (breaths*min−1) | 18±1 | 16±1 | Resp. Rate | 9±4 * | −11±7 | 30±15 * | 3±9 | 16±9 | 6±5 | 5±3 | 2±3 |
| ETCO2(mmHg) | 43±1 | 44±1 | ETCO2 | −7±1 | −7±2 | −23±3 ‡ | −8±2 | −10±2 ‡ | 1±2 | 1±2 | 2±1 |
| Mean CBFV(cm/sec) | 78±3 | 78±3 | Mean CBFV | −11±2 | −10±1 | −46±3 ‡ | −12±2 | −13±3 * | −2±1 | −1±2 | −1±2 |
| Systolic CBFV (cm/sec) | 116±4 | 115±4 | Systolic CBFV | −11±2 | −11±1 | −11±8 | −11±2 | 14±3 † | −2±2 | 3±3 | 1±3 |
| Diastolic CBFV(cm/sec) | 54±3 | 55±2 | Diastolic CBFV | −7±2 | −5±2 | −66±3 ‡ | −8±3 | −21±4 † | −8±3 | −6±4 | −3±1 |
| Pulsatile CBFV(cm/sec) | 62±3 | 61±2 | Pulsatile CBFV | −15±3 | −15±2 | 38±13 † | −12±3 | 23±6 * | 4±2 | 3±2 | 1±2 |
| CVRi(mmHg/cm/sec) | 1.1±0.1 | 1.2±0.1 | CVRi | −2±5 | −8±3 | −43±7‡ | −12±4 | 3±6 | −3±2 | 2±5 | 2±4 |
MAP, mean arterial pressure. SBP, systolic blood pressure. DBP, diastolic blood pressure. PP, pulse pressure. HR, heart rate. CO, cardiac output. Resp. Rate, respiratory rate. ETCO2, end-tidal CO2. CBFV, cerebral blood flow velocity. CVRi, cerebrovascular resistance index.
During the stable tilt interval, fainters exhibited an increase in DBP, MAP, HR, and respiratory rate compared with control subjects, but no significant differences in ETCO2 or CO was seen between groups (Table I). During the stable tilt period, there were no differences in any CBFV variables between groups (Figures 1 and 2; available at www.jpeds.com).
Figure 2.
Percent change in systolic, diastolic, pulsatile, and mean cerebral blood flow velocity (CBFV) in fainters (black bars) and controls (gray bars) during stable tilt, faint, and early and late post-tilt time intervals. There were no differences between groups during the stable tilt period. During the faint, mean CBFV and diastolic CBFV decreased markedly in fainters compared with controls, and pulsatile CBFV increased. At early post-tilt, diastolic CBFV remained decreased and pulsatile CBFV remained increased in fainters compared with controls, and systolic CBFV increased above baseline in fainters but not controls. Mean ± SEM. *, P < 0.05; †, P < 0.01; ‡, P < 0.001 compared with controls.
All fainters fainted during the “faint” time interval as previously defined. No control subject fainted or experienced presyncopal symptoms. At the time of faint, fainters had decreases in SBP (60±3 vs. 111±4 mmHg, P<0.001), DBP (31±2 vs. 62±3 mmHg, P <0.001), MAP (41±2 vs. 79±3 mmHg, P<0.001), PP (29±2 vs. 49±2 mmHg, P<0.001), HR (57±4 vs. 88±4 bpm, P<0.001), CO (1.58±0.56 vs. 5.09±0.25 L/min, P<0.001), and ETCO2 (33±1 vs. 41±1 mmHg, P<0.001) compared with controls. Respiratory rate increased compared with controls (23±2 vs. 16±1 breaths/min, P<0.01).
At the time of faint, there was a marked decrease in both mean CBFV and diastolic CBFV (P<0.001 for both) in fainters compared with control subjects, and systolic CBFV was not different between groups (Figure 2). Consequently, pulsatile CBFV increased markedly in fainters and decreased in controls (P<0.01). Figure 1 shows tracings of systolic CBFV, diastolic CBFV, and pulsatile CBFV for a representative fainter during all time intervals.
Compared with controls, fainters continued to exhibit a decrease in the absolute value of ETCO2 at early post tilt (39±1 vs. 44±1 mmHg, P<0.01). Absolute SBP remained lower in fainters than controls at the early post tilt supine period (104±3 vs. 115±3 mmHg, P<0.05), as did absolute CO (3.38±0.41 vs. 5.44±0.33 L/min, P<0.001).
Figure 2 and Table I show that during the early post tilt supine period fainters compared with controls continued to show greater decreases in mean CBFV (P<0.05) and diastolic CBFV (P<0.01). Fainters exhibited an increase in systolic CBFV, and controls exhibited a slight decrease (P<0.01). During the early post-tilt supine period, fainters continued to exhibit a greater increase in pulsatile CBFV than controls (P<0.05). During the early post-tilt supine period, all fainters developed headache following fainting at the time of early post-tilt, whereas control subjects did not.
During the late post-tilt period, no differences were found between groups (Table I). Fainters described easing or relief of headache during the late post-tilt time period.
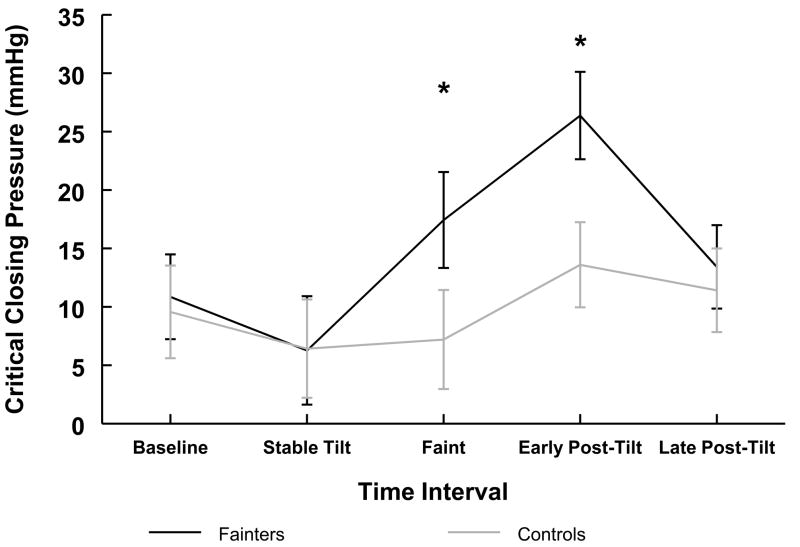
Critical closing pressure was similar between groups during baseline and decreased similarly for both groups during stable tilt (Table II). As shown in Figure 3, during faint, the critical closing pressure rose in fainters compared with controls (P < 0.05). During the early post-tilt period, critical closing pressure remained significantly greater in fainters than controls (P < 0.05) (Figure 3 and Table II).
Table 2.
Critical Closing Pressure of the Cerebral Circulation during Tilt
| Measurement | Critical Closing Pressure (mmHg) | Slope (cm/sec/mmHg) | R2 | SBPMCA (mmHg) | DBPMCA (mmHg) | MAPMCA (mmHg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | |
| Baseline | 11±3 | 10±3 | 1.16±0.08 | 1.21±0.07 | 0.78±0.01 | 0.80±0.01 | 116±3 | 122±2 | 63±2 | 64±2 | 80±2 | 83±2 |
| Stable Tilt | 6±4 | 6±2 | 1.19±0.08 | 1.22±0.09 | 0.65±0.01 | 0.69±0.01 | 97±3 | 99±3 | 50±2 | 46±2 | 66±2 | 64±2 |
| Faint | 17±4 * | 7±4 | 0.56±0.08 * | 1.22±0.11 | 0.81±0.02 | 0.75±.0.04 | 40±4 ‡ | 91±4 | 11±3 ‡ | 42±3 | 21±3 ‡ | 59±2 |
| Early Post-Tilt | 26±3 * | 13±4 | 0.86±0.08 * | 1.07±0.06 | 0.87±0.04 | 0.82±0.03 | 104±3 * | 115±3 | 58±5 | 61±3 | 73±4 | 79±3 |
| Late Post-Tilt | 13±3 | 11±3 | 0.94±0.09 | 1.12±0.07 | 0.83±0.01 | 0.84±0.02 | 112±4 | 124±2 | 61±2 | 64±2 | 79±3 | 84±3 |
|
| ||||||||||||
| Mean Δ from Baseline | Critical Closing Pressure (mmHg) | Slope (cm/sec/mmHg) | R2 | SBPMCA (mmHg) | DBPMCA (mmHg) | MAPMCA (mmHg) | ||||||
|
| ||||||||||||
| Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | Fainters | Controls | |
| Stable Tilt | −5±2 | −4±2 | 0.03±0.11 | 0.01±0.06 | −0.13±0.02 | −0.11±0.02 | −22±2 | −23±2 | −15±1 | −18±1 | −18±1 | −20±1 |
| Faint | 6±3 * | −3±1 | −0.41 ±0.20 * | 0.02±0.10 | 0.03±0.03 | 0.05±0.02 | −79±3 ‡ | −30±4 | −54±2 ‡ | −22±2 | −63±2 ‡ | −25±2 |
| Early Post-Tilt | 13±3 * | 5±2 | −0.35±0.11 | −0.14±0.05 | 0.04±0.06 | 0.02±0.06 | −15±8 | −7±3 | −8±4 | −3±2 | −10±4 | −4±2 |
| Late Post-Tilt | 2±2 | 1±2 | 0.22±0.13 | −0.09±0.07 | 0.05±0.04 | 0.04±0.03 | −4±5 | 2±3 | 2±2 | 0±2 | −1±2 | 1±2 |
Systolic (SBPMCA), diastolic (DBPMCA), and mean (MAPMCA) arterial pressure at the level of the middle cerebral artery. Slope represents the slope of the regression line between mean CBFV and MAPMCA. R2 is the coefficient of determination of the regression line.
, P < 0.05.
P < 0.01;
P < 0.001 compared to control.
Figure 3.
Critical closing pressure at the level of the middle cerebral artery for fainters (black) and controls (gray). During baseline, critical closing pressure was similar between groups. Critical closing pressure dropped in both groups during stable tilt. At the time of faint, critical closing pressure rose in fainters compared with controls. At early post-tilt, critical closing pressure was higher in fainters compared with controls. Mean ± SEM. *, P < 0.05 compared with controls.
The slope of the linear regression line for critical closing pressure changed during all time intervals for both groups (Table II). The slope was similar between groups during baseline and stable tilt time intervals. At faint, the slope decreased in fainters compared with controls and remained decreased during the early post-tilt time interval (P < 0.01 for both). The coefficient of determination (R2) for the linear regression was similar during all time intervals for both groups.
Also shown in Table II, SBPMCA, DBPMCA, and MAPMCA were similar between groups during baseline, stable tilt, and post-tilt time intervals. During the faint time interval, SBPMCA, DBPMCA, and MAPMCA all were significantly decreased in fainters compared with controls (P < 0.001 for all). At faint, the critical closing pressure approached the DBPMCA.
Discussion
This study confirms our hypothesis that following a sudden decrease in CBF at faint, rapid hyperemic pulsatile CBF occurs upon return to the supine position, and is associated with post-syncopal headache. Fainting and onset of post-syncopal headaches coincided with an increase in cerebral CCP and a decrease in CBF, potentiated by hyperpnea and hypocapnia (3, 11). The decreased CBF in fainters was confined to diastolic flow, but systolic CBF remained relatively unchanged. Thus, pulsatile CBF increased and was synchronous with a rise in CCP.
Increased pulsatile CBFV and headache may both be mediated through NO; highly pulsatile flow is a potent stimulus for NO release compared with laminar flow (14, 15). Systolic CBFV is thought to be more affected by changes in NO than diastolic CBFV (16). Our data show increased systolic CBFV following faint, that may be due to increased NO and decreased diastolic CBFV that may be limited by increased CCP and hypocapnia (12, 13) resulting in increased pulsatile CBFV.
Pulsatile flow-induced changes in cerebrovascular diameter mediated by NO have been linked to fainting and headaches (17, 18). Migraine and episodic tension-type headaches may also relate to vascular changes of CBF (5, 6, 8, 10). Nowak et al (19) found that children with migraines and chronic-type headaches had increased CBFV during visual stimulation, due to increased cerebrovasodilation. In animals, NO increases in the cerebrovasculature during hypotension (20, 21). Typical vascular headaches have their onset and greatest intensity during amplified vascular pulsations combined with hyperemic CBF. Increased NO may cause release of calcitonin gene-related peptide (CGRP) (14). NO and CGRP are mediators of migraines, either due to increased stretching of the vessels or to stimulation of vascular nociceptors detected as pain via the trigeminal nerve (15, 22, 23). Others have suggested that the trigeminovascular system, through stimulation of C-fibers and release of substance P and CGRP, cause pain and modulate changes in CBF (24–27).
We recently reported that myogenic cerebral autoregulation may become ineffective prior to, during, and after fainting in young adults (11), and thus CBF is not maintained. During the early recovery phase of faint, as blood pressure recovers, the more distal cerebral vasculature is transiently exposed to increased pressure, which may contribute to evoked pain. Autoregulation loss or decreased effectiveness increases the instability of CBF that may propagate cerebrovascular blood pressure increments, contributing to vascular-related headaches. Our determination of CCP and the linear relationship between mean CBFV and MAPMCA suggests that autoregulation has failed at the time of faint, and that autoregulatory mechanisms continues to be ineffective during the early post-faint. During hypotension, a linear relationship occurs between CBF and MAP that may not denote loss of autoregulation; pulsatile flow may maintain CBF during falling MAP (28). The sympathetic nervous system may modulate cerebrovascular tone during hypotension. Zhang et al (29) and Ogoh et al (30) suggested that following acute hypotension, sympathetically-mediated cerebrovasoregulation may occur, however neither studied vasovagal syncope. Also, sympathetic activity may be associated with headaches as chronic tension-type headaches may be related to decreased Mayer wave activity in the cerebrovasculature (31).
We calculated CCP of the MCA using a linear regression model; our values are similar to that reported by others (12, 32). We found that CCP decreased in fainters and controls during stable tilt. CCP remained relatively unchanged in controls throughout tilt, and fainters exhibited an increase CCP at faint. During early post-tilt, CCP returned to near baseline in controls, but in fainters increased above baseline. A rise in CCP at faint and early post-tilt could interfere with cerebral perfusion, especially during diastole (12, 13).
During upright jugular collapse (33), the pressure difference across the vasculature can be represented by the difference in cerebral perfusion pressure and CCP, which approximates intracranial pressure. This difference is large for healthy controls because perfusion pressure is maintained and CCP is low. This difference is much smaller during faint because perfusion pressure is lower and CCP is relatively increased. Diastolic CBFV may therefore be critically limited during tilt because the values of the DBPMCA and CCP become similar. Thus, pulsatile CBFV increases throughout syncope due to the difference in systolic and diastolic CBFV. We showed that pulsatile flow is most marked during faint but persists into early post-tilt, similar to the increased pulsatile CBFV in healthy subjects during pharmacologically-induced hypotension (28).
Decreased diastolic CFBV occurs when CCP increases, and diastolic CBFV may be influenced by hypocapnia (12, 13, 34). At faint, our fainters were significantly hypocapnic compared with controls. The increases in CCP may be due directly to hypocapnic cerebral vasoconstriction (13). Increased CCP was observed during faint which approached DBPMCA (12). When DBPMCA approaches CCP, diastolic flow may be hindered. Thus, decreased diastolic pressure at the MCA, increased CCP, hypocapnia, ineffective cerebral autoregulation, and inappropriate cerebrovascular regulation may cause decreased diastolic CBFV.
Hypocapnia causes cerebral vasoconstriction which precedes faint. Assuming a 2% change in CBFV per 1 mmHg change in ETCO2, we estimated that the change in ETCO2 could account for ~6% decrease in CBFV for both groups during stable tilt, a 20% decrease for fainters at faint with a 7% decrease for controls at end tilt, a 9% decrease for fainters during the early post-tilt period with a 1% increase in CBFV for controls, and a 1% increase in CBFV for both groups during the late post-tilt period. These changes in CO2 therefore likely contribute to alterations in pulsatile blood flow during faint. Changes in CO2 may also affect headaches as migraineurs, who were headache-free at testing, exhibited greater CBFV and cerebrovascular reactivity to CO2 than healthy control subjects (5,7).
Our current data supports the finding that at faint, fainters’ systolic CBFV remains relatively unchanged and similar to controls due to cerebrovascular dilation (12). Following fainting, we found a relative increased systolic CBFV at early post-tilt that may be caused by a reactive hyperemia following abrupt relief of cerebral ischemia, and by NO release (18, 21). Relief from ischemia and hyperemia occur with the return to the supine position, during which AP and ETCO2 rise in a setting of fully dilated cerebral vasculature. Other changes may account for increased systolic flow as CO and SV also increase on return to the supine position. Ogoh et al (38) found that CO directly influenced CBFV at rest. Also, Verheyden et al (39) found that SV and CO fell steeply at faint and then rose to baseline during recovery, and these changes were major factors leading to hypotension and faint. However, Deegan et al (40) failed to find an influence of CO on cerebrovascular regulation at rest. Studies of changes in CO or SV to relative CBFV during fainting have not yet been done.
The current study was of adolescents; however, we speculate that similar findings would be detected in adults who experience classical vasovagal syncope. We also used control subjects who did not faint; an alternative design may be to use fainters as their own controls in a crossover study. We did not have any objective measure of headache intensity in our subjects. This limits any comparison that could be made between the extent of the increase in pulsatile CBFV and the corresponding headache pain associated with it. Also, CBFV measured by Doppler is always equal to or less than the actual flow velocity. Additionally, we assume the diameter of the MCA remains relatively constant during orthostatic stress.
It is not feasible to get direct values for CCP in healthy subjects. Thus, modeling of CCP based on the linear regression of CBFV and MAPMCA is an over-simplified expedient. Another possible source of concern is our use of the calculated CVRi, which uses absolute values for MAPMCA and mean CBFV based on Finometer and transcranial Doppler recordings. The ability of these tools to provide a totally accurate value for either measurement is highly limited.
We describe a group of adolescent fainters who developed headaches following faint. These headaches may be related to pulsatile changes in CBFV. Following faint, fainters exhibited increased systolic CBFV and pulsatile CBFV and decreased diastolic CBFV compared with control subjects. At faint, fainter’s critical closing pressure increased and remained elevated at the early post-tilt period following fainting.
Acknowledgments
We offer much gratitude toward the members of the Division of Pediatric Cardiology. Additional thanks goes to the authors’ mentors, who have inspired their scientific interests.
Supported by the National Heart, Lung, and Blood Institute (grants 1-F30-HL-097380, 1-RO1-HL-074873, 1-RO1-HL-087803, 1-R21-HL091948) and a grant from the CFIDS Association of America.
Abbreviations
- CBF
cerebral blood flow
- MAP
mean arterial pressure
- HR
heart rate
- ETCO2
end-tidal CO2
- CBFV
cerebral blood flow velocity
- AP
arterial pressure
- CO
cardiac output
- MCA
middle cerebral artery
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- PP
pulse pressure
- CVRi
cerebrovascular resistance index
- NO
nitric oxide
- CGRP
calcitonin gene-related peptide
- SV
stroke volume
- Resp. Rate
respiratory rate
- CCP
critical closing pressure
Footnotes
Preliminary data was presented in abstract form at the American Autonomic Society meeting in November 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Driscoll DJ, Jacobsen SJ, Porter CJ, Wollan PC. Syncope in children and adolescents. J Am Coll Cardiol. 1997 Apr;29:1039–45. doi: 10.1016/s0735-1097(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 2.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009): the Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Eur Heart J. 2009 Nov;30:2631–71. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol. 2008 Jul;295:H372–H381. doi: 10.1152/ajpheart.00101.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokri B, Low PA. Orthostatic headaches without CSF leak in postural tachycardia syndrome. Neurology. 2003 Oct 14;61:980–2. doi: 10.1212/01.wnl.0000085868.37963.7d. [DOI] [PubMed] [Google Scholar]
- 5.Abernathy M, Donnelly G, Kay G, Wieneke J, Morris S, Bergeson S, et al. Transcranial Doppler sonography in headache-free migraineurs. Headache. 1994 Apr;34:198–203. doi: 10.1111/j.1526-4610.1994.hed3404198.x. [DOI] [PubMed] [Google Scholar]
- 6.Arjona A, de Torres LA, Serrano-Castro PJ, Guardado-Santervas PL, Olivares J, Rubi-Callejon J. A transcranial doppler study in interictal migraine and tension-type headache. J Clin Ultrasound. 2007 Sep;35:372–5. doi: 10.1002/jcu.20350. [DOI] [PubMed] [Google Scholar]
- 7.Kastrup A, Thomas C, Hartmann C, Schabet M. Cerebral blood flow and CO2 reactivity in interictal migraineurs: a transcranial Doppler study. Headache. 1998 Sep;38:608–13. doi: 10.1046/j.1526-4610.1998.3808608.x. [DOI] [PubMed] [Google Scholar]
- 8.Thie A, Fuhlendorf A, Spitzer K, Kunze K. Transcranial Doppler evaluation of common and classic migraine. Part I. Ultrasonic features during the headache-free period. Headache. 1990 Mar;30:201–8. doi: 10.1111/j.1526-4610.1990.hed3004201.x. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen LL, Iversen HK, Olesen J. Cerebral blood flow velocities are reduced during attacks of unilateral migraine without aura. Cephalalgia. 1995 Apr;15:109–16. doi: 10.1046/j.1468-2982.1995.015002109.x. [DOI] [PubMed] [Google Scholar]
- 10.Wallasch TM. Transcranial Doppler ultrasonic features in episodic tension-type headache. Cephalalgia. 1992 Oct;12:293–6. doi: 10.1046/j.1468-2982.1992.1205293.x. [DOI] [PubMed] [Google Scholar]
- 11.Ocon AJ, Kulesa J, Clarke D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am J Physiol Heart Circ Physiol. 2009 Dec;297:H2084–H2095. doi: 10.1152/ajpheart.00705.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey BJ, Eames PJ, Panerai RB, Potter JF. Carbon dioxide, critical closing pressure and cerebral haemodynamics prior to vasovagal syncope in humans. Clin Sci (Lond) 2001 Oct;101:351–8. [PubMed] [Google Scholar]
- 13.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys. 2003 Oct;25:621–32. doi: 10.1016/s1350-4533(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 14.Strecker T, Dux M, Messlinger K. Nitric oxide releases calcitonin-gene-related peptide from rat dura mater encephali promoting increases in meningeal blood flow. J Vasc Res. 2002 Nov;39:489–96. doi: 10.1159/000067206. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen LL, Olesen J. Nitric oxide in primary headaches. Curr Opin Neurol. 2001 Jun;14:315–21. doi: 10.1097/00019052-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Richards HK, Kozniewska E, Czosnyka M, Pickard JD. Changes in transcranial Doppler flow velocity waveform following inhibition of nitric oxide synthesis. Experimental study in anaesthetised rabbits. Acta Neurochir (Wien) 1997;139:63–9. doi: 10.1007/BF01850870. [DOI] [PubMed] [Google Scholar]
- 17.Busse R, Fleming I. Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium-derived relaxing factors. J Vasc Res. 1998 Mar;35:73–84. doi: 10.1159/000025568. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Ogasawara K, Suga Y, Chida K, Yoshida K, Otawara Y, et al. Early post-ischemic hyperemia on transcranial cerebral oxygen saturation monitoring in carotid endarterectomy is associated with severity of cerebral ischemic insult during carotid artery clamping. Neurol Res. 2009 Sep;31:728–33. doi: 10.1179/174313209X382269. [DOI] [PubMed] [Google Scholar]
- 19.Nowak A, Gergont A, Steczkowska M. Assessment of cerebral blood flow after visual stimulation in children with a migraine and chronic tension-type headache--preliminary reports. Przegl Lek. 2008;65:777–82. [PubMed] [Google Scholar]
- 20.Kobari M, Fukuuchi Y, Tomita M, Tanahashi N, Takeda H. Role of nitric oxide in regulation of cerebral microvascular tone and autoregulation of cerebral blood flow in cats. Brain Res. 1994 Dec 26;667:255–62. doi: 10.1016/0006-8993(94)91503-2. [DOI] [PubMed] [Google Scholar]
- 21.Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol. 2007 Oct;293:H2193–H2201. doi: 10.1152/ajpheart.00190.2007. [DOI] [PubMed] [Google Scholar]
- 22.Arulmozhi DK, Veeranjaneyulu A, Bodhankar SL. Migraine: current concepts and emerging therapies. Vascul Pharmacol. 2005 Sep;43:176–87. doi: 10.1016/j.vph.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 23.May A, Buchel C, Turner R, Goadsby PJ. Magnetic resonance angiography in facial and other pain: neurovascular mechanisms of trigeminal sensation. J Cereb Blood Flow Metab. 2001 Oct;21:1171–6. doi: 10.1097/00004647-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003 Aug;126(Pt 8):1801–13. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 25.May A. The trigeminovascular system in the human. Cerebral blood flow, functional imaging and primary headache. Nervenarzt. 2003 Dec;74:1067–77. doi: 10.1007/s00115-003-1578-2. [DOI] [PubMed] [Google Scholar]
- 26.Tran Dinh YR, Thurel C, Cunin G, Serrie A, Seylaz J. Cerebral vasodilation after the thermocoagulation of the trigeminal ganglion in humans. Neurosurgery. 1992 Oct;31:658–62. doi: 10.1227/00006123-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988 Feb;23:193–6. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 28.Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010 Mar;55:698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002 Oct 1;106:1814–20. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]
- 30.Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008 Jul;39:1979–87. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- 31.Sliwka U, Harscher S, Diehl RR, van SR, Niesen WD, Weiller C. Spontaneous oscillations in cerebral blood flow velocity give evidence of different autonomic dysfunctions in various types of headache. Headache. 2001 Feb;41:157–63. [PubMed] [Google Scholar]
- 32.Sato J, Tachibana M, Numata T, Nishino T, Konno A. Differences in the dynamic cerebrovascular response between stepwise up tilt and down tilt in humans. Am J Physiol Heart Circ Physiol. 2001 Aug;281:H774–H783. doi: 10.1152/ajpheart.2001.281.2.H774. [DOI] [PubMed] [Google Scholar]
- 33.Gisolf J, van Lieshout JJ, van HK, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol. 2004 Oct 1;560(Pt 1):317–27. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schondorf R, Benoit J, Wein T. Cerebrovascular and cardiovascular measurements during neurally mediated syncope induced by head-up tilt. Stroke. 1997 Aug;28:1564–8. doi: 10.1161/01.str.28.8.1564. [DOI] [PubMed] [Google Scholar]
- 35.Diehl RR, Linden D, Chalkiadaki A, Diehl A. Cerebrovascular mechanisms in neurocardiogenic syncope with and without postural tachycardia syndrome. J Auton Nerv Syst. 1999 May 28;76:159–66. doi: 10.1016/s0165-1838(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 36.Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation. 2001 Nov 27;104:2694–8. doi: 10.1161/hc6172.099397. [DOI] [PubMed] [Google Scholar]
- 37.Dan D, Hoag JB, Ellenbogen KA, Wood MA, Eckberg DL, Gilligan DM. Cerebral blood flow velocity declines before arterial pressure in patients with orthostatic vasovagal presyncope. J Am Coll Cardiol. 2002 Mar 20;39:1039–45. doi: 10.1016/s0735-1097(02)01719-9. [DOI] [PubMed] [Google Scholar]
- 38.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, et al. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005 Dec 1;569(Pt 2):697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verheyden B, Liu J, van DN, Westerhof BE, Reybrouck T, Aubert AE, et al. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart Rhythm. 2008 Dec;5:1695–701. doi: 10.1016/j.hrthm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Deegan BM, Devine ER, Geraghty MC, Jones E, Olaighin G, Serrador JM. The relationship between cardiac output and dynamic cerebral autoregulation in humans. J Appl Physiol. 2010 Aug 5; doi: 10.1152/japplphysiol.01262.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]