Abstract
Accurate HCV knowledge is lacking among high-risk groups, including people with HIV/AIDS (PLWHA). Liver disease primarily due to HCV has emerged as a serious cause of mortality among PLWHA. We used an interrupted time series design to evaluate a social-ecologically based intervention for PLWHA, where an infectious disease clinic serving a six-county intervention area was monitored before (7 months) and after (17 months) intervention onset. The intervention included education of PLWHA and medical providers, HIV/HCV support groups and resource guides, and adaptation of the patient chart top sheet to include HCV test information. Clinic-level outcomes were assessed prospectively every other week for two years by interviewing patients (n=259) with clinic appointments on assessment days. Abrupt, gradual and delayed intervention effects were tested. Weighted regression analyses showed higher average HCV knowledge and a higher prevalence of patients reporting HCV discussion with their medical providers after intervention onset. A delayed effect was found for HCV awareness, and a gradually increasing effect was found for knowing one‘s HCV status. Other communities may consider adopting this intervention. Additional HCV interventions for PLWHA with HIV are needed.
Keywords: HIV, Hepatitis C, Intervention research, Prevention, Interrupted Time Series
INTRODUCTION
The identification and treatment of people with multiple diseases, especially stigmatized diseases, can be difficult. Yet co-morbidities are common, especially when diseases share routes of transmission. Such is the case for HIV and chronic hepatitis C virus (HCV) infection, which represents a serious and difficult public health challenge. HIV/AIDS is estimated to affect 1.1 million people in the United States (US) [1], with approximately 56,000 new HIV infections occurring annually [2]. The virus HIV is found in blood, semen, and vaginal secretions and is primarily transmitted through sexual activity, followed by shared needle use) [3]. HCV infection is estimated to affect 3.2 million people in the US [4]. As a blood-borne virus, HCV is transmitted largely through shared needle use, with 48.4% of HCV-positive persons in the National Health and Nutrition Examination Survey (NHANES) study with a history of injection drug use [4]. Sexual transmission among monogamous heterosexual couples appears to be low [5], but NHANES did show an increased risk of HCV among persons with 10 or more lifetime sexual partners [4]. The impact of HIV on transmission of HCV among heterosexual couples is an area of controversy, but an increased risk of HCV transmission appears to be present for men who have sex with men with no other identifiable risk factor except unsafe sex [6]. Arguably, HCV‘s most serious effect on health is as a major cause of advanced liver disease, including cirrhosis and hepatocellular carcinoma, and HCV is the most common indication for liver transplantation in the United States [7]. HCV infection prevalence among people living with HIV/AIDS (PLWHA) is estimated to be 33% in Europe [8] and 16–35% in the US [9–11], compared to 1.6% in the US general population [4].
Among people living with HIV/AIDS (PLWHA), liver disease, largely caused by HCV, is a leading cause of death [12]. Mortality from opportunistic infections has dramatically decreased due to use of highly active antiretroviral therapy [13]. As a result, PLWHA are living longer, are less likely to die from HIV-related co-morbidities, and are thus more likely to die from HCV-related end-stage liver disease. Mortality rates for PLWHA due to end-stage liver disease and related to HCV-associated cirrhosis are rising [11]. Death rates related to liver disease are higher for persons with HIV-HCV co-infection compared to those with HIV only [8], because HIV and HCV interact such that HIV infection spurs increased HCV-RNA serum levels [14, 15] and faster progression to cirrhosis [16, 17], liver failure [18], and hepatocellular carcinoma [19]. In France in 2003, end-stage liver disease infection was the leading cause of death among PLWHA [20]. In a large sample of HIV-infected patients in North America and Europe, liver disease was the second leading cause of death, behind HIV but ahead of cardiovascular disease [12].
Public health options to decrease liver disease among PLWHA include HCV education focused on HCV prevention and treatment; identifying HIV-HCV co-infection through testing efforts; needle exchange and other harm reduction efforts for persons who use substances; and increasing access to HCV antiviral treatment for co-infected persons. The extent to which people in the US are aware of chronic HCV is unclear. Several non-profit organizations exist that seek to improve HCV awareness in both the general population and at-risk groups. For example, the National Hepatitis C Coalition, Inc. (http://nationalhepatitis-c.org/) is devoted to educating the public on HCV and supporting patients living with HCV. The Hep-C Outreach Project (http://www.hcop.org/) provides education programs for the general population and also targeted to PLWHA and people co-infected with HIV and HCV, and Hepatitis Foundation International (http://www.hepfi.org/) provides peer counseling and doctor referrals to those diagnosed with HCV, along with public education. Largely unknown is the effect of these intervention efforts on HCV awareness and knowledge. To our knowledge, no empirical evaluation of such efforts has been published. Data are also lacking on long-term outcomes of screening for HCV in otherwise healthy asymptomatic adults [21], despite recommendations to use screeners to identify patients at high-risk for HCV and offer them HCV education [22].
Research has shown that accurate HCV knowledge is lacking among groups that are at high risk for HCV. For example, in a sample of methadone maintenance patients, only 50% knew the correct risk factors for HCV [23] and only 34% knew that HCV can be treated [24]. In a study of syringe exchange participants, 50% did not know that HCV is spread through shared, un-sterilized injection equipment [25], and participants gave more accurate answers to HIV than HCV questions [26]. Based on these studies, education on HCV transmission routes and protection behaviors for persons at risk for HCV is needed. Persons at risk for HCV also need to know that HCV treatment is available, to give them a reason to get tested.
This paper describes the first HCV knowledge intervention study of which we are aware. Specifically, our intervention sought to increase understanding of HCV, knowledge of one‘s HCV status, and patient-provider communication about HCV. A particularly promising approach to address public health problems is a multi-level intervention design based on the Socioecological Framework (SEF) [27]. Intervening at multiple levels may be particularly important for people with HIV who are at risk for HCV, because they intersect with multiple arms of the health care system.
The SEF describes five levels that influence health: (1) the Intrapersonal level, which consists of an individual‘s beliefs and characteristics; (2) the Interpersonal level, which consists of relationships between the individual and key persons and small social networks, such as one‘s spouse, family, and close friends; (3) the Community level, which consists of shared identities, experiences, and resources for health; (4) the Institutional level, which consists of rules, regulations, policies, and ethos that may promote or endanger health; and (5) the Policy level, which consists of policies, environments, and structures that impact health. The SEF proposes that individual behavior and social influences are inter-related [28], and that each level of the SEF is capable of impacting other SEF levels. Patients with HIV only or HIV-HCV co-infection experience their illnesses in the context of family and other relationships, as well as community structures such as infectious disease clinics, liver clinics, substance use treatment centers, and health departments. Accordingly, the SEF is particularly useful for tailoring a community-based intervention to a particular socio-cultural context for people with HIV and HCV, in addition to allowing for inclusion of individual-level interventions. Thus, the SEF guided our intervention design and provided a holistic framework.
Our multi-level intervention was designed to be community-wide, broadly affecting all individuals with HIV in a six-county geographic area. By targeting a community rather than individuals, we were challenged to find an appropriate evaluation design. We rejected a randomized controlled trial design because the multi-level nature of the intervention did not allow us to randomize individuals within the study, and yet we did not have the resources to intervene with enough communities to randomize communities. We rejected a case-control design using a comparison community because our previous HIV research in the area has taught us that there are substantial differences between HIV populations in neighboring communities. We therefore selected an Interrupted Time Series (ITS) design in which we sampled community-level prevalence rates at regular intervals before introducing the intervention, and then continued data collection throughout the intervention period. By being able to compare trends before and after the start of the intervention rather than simply comparing pre versus post averages, this design eliminates many threats to internal validity. For example, the presence of a pre-existing trend can erroneously lead to the conclusion that an intervention was effective if only pre and post means are compared. In a time series design, pre-existing trends can be included in the model, and thus intervention effects exceeding pre-existing trends can be tested.
Another advantage of the ITS design is that it can address threats to validity that occur when other initiatives within the same community are put into effect during a similar timeframe as the intervention. If such other initiatives exist, comparisons of pre and post means cannot disentangle the effects of the intervention from those of other community influences. A time series design, however, can test the presence of effects starting at different times. Overall, the ITS design is considered to be a very strong quasi-experimental design, with the primary threat to causality being the unplanned introduction of an intervention occurring at exactly the same time as that of the study intervention [29]. Researchers have lamented how rarely the ITS design is used in community research and have called for its increased use [30].
In sum, despite high rates of HIV/HCV co-infection and the fact that HCV is a leading cause of death among persons with HIV, to our knowledge, no hepatitis C interventions for persons with HIV have been designed and evaluated. In this intervention research study, we designed and tested such an intervention, using an evaluation design that is under-utilized. Our research questions were, what impact, if any, did the intervention have on: 1) what HIV-positive patients know about hepatitis C; 2) HIV-positive patients knowing their hepatitis C status; and 3) HIV medical providers talking to HIV-positive patients about hepatitis C.
METHODS
Intervention
Our intervention was composed of components at four SEF levels. The primary target of our intervention was people with HIV who were either already co-infected with HCV or were at risk for HCV. To reach them, we also intervened with medical and social service providers who interact with people in those groups, but with the ultimate aim of reaching people with HIV and HCV, or at risk for HCV.
Intrapersonal
We provided case management to nearly 200 patients co-infected with HIV/HCV, starting in July 2005 and continuing through the end of data collection in February 2007. In addition, from September 2005 through the data collection period, mental health and substance abuse counseling were provided based on need to 80 patients with HIV and either co-infected with HCV or at risk for HCV. Such counseling is important to help people qualify for HCV antiviral therapy, because therapy recommendations include mental health stability and alcohol and substance use abstinence. While substance use abstinence is the ideal, the integrated HIV-substance abuse treatment [31] used by our therapists incorporated harm reduction strategies, including reduced and clean needle use.
Interpersonal
We conducted monthly support groups for co-infected persons at two sites. These groups were regularly attended by 8 participants per month. One group began in April 2005 and the other one month later, with sparse initial attendance building to full group size by September 2005. The two groups continued throughout the data collection period.
Community
Community education was provided through health classes to the general public and to over 800 PLWHA and other people at risk for HCV through a variety of venues. For example, monthly health classes were provided to people in the local county jail from February 2005 throughout the data collection period. Starting in July 2005, HIV peer health educators were regularly trained. Starting in September 2005, health classes were repeatedly offered at several homeless shelters and substance use transition homes. We also launched in September 2005 a website and a public media campaign to impart information on HCV testing, transmission, and treatment.
Institutional
We changed the HIV clinic system through using Information Technology to create a face sheet that was placed in the front of patient charts. This face sheet reported whether or not a patient had been tested for HCV, the test date and results, and whether or not the patient had been vaccinated for hepatitis A and B (these infections can be more severe in the patient with pre-existing HCV). The face sheets were phased in as data entry occurred between August 2005 and February 2006. Through provider interviews, we learned that medical providers routinely used the face sheets. In addition, we sought to enhance clinic and organizational knowledge about HCV through educating HIV medical and non-medical providers. Because universal HCV testing was not in place, we held two testing event contests in the academic medical center infectious diseases clinic to encourage medical providers to test for HCV. Clinical education, which began in July 2005 and continued throughout the data collection period, was provided to 60 medical care providers to extend their knowledge on HIV-HCV co-infection patient care, HCV treatment, and the benefits of HCV testing. Information on HCV testing, transmission, and treatment was provided to over 190 non-medical providers (case managers, social workers, health educators) working in the field of HIV to increase their awareness of HCV and provide knowledge and tools to talk with their clients about it. We also changed institutional policy by providing staff for HCV testing at the Durham County Health Department.
Study Population
In the six-county intervention area, HIV-positive individuals primarily seek health care at two HIV clinics. To assess the impact of our intervention on the broader HIV-positive population, we chose to interview patients at these two clinics; however, 100% of the patients interviewed at the federally-qualified community health center were also seen at the academic medical center. Consequently, this paper focuses on the interviews conducted at the academic medical center, which served approximately 1550 HIV-positive patients in the first year of data collection (2005). Although not all individuals with HIV access HIV medical care, collecting data through HIV medical clinics was determined to be the most efficient way of reaching large numbers of individuals with HIV. The inclusion criteria to be offered participation in the interview were: HIV-positive; age 18 or older; English-speaking; and receiving treatment at one of two Infectious Disease (ID) clinics.
Research Design
As noted above, we used an interrupted time series design. Because the primary threat to validity was simultaneous introduction of another HIV-HCV intervention, we had regular contact with the State HCV Coordinator and the directors of both clinics throughout our intervention period. No other HIV-HCV interventions were introduced.
Procedure
At the medical center infectious disease (ID) clinic, interviews occurred every two weeks from February 2005 – February 2007. The multi-component intervention was phased in with minimal activities starting in February 2005 and all major activities in place around September 2005. This allowed that trends to be observed for 7 months prior to September 2005 and 17 months after September 2005. Since the pre-intervention phase was shorter than the intervention phase, an effort was made to reach a comparable number of patients during this shorter interval to enable tests for changes in the study population. Consequently, interviews occurred two days during every biweekly interval prior to the start of the intervention, and one day during every biweekly interval thereafter. All patients meeting study criteria with a scheduled ID clinic appointment that day were approached, and after completing a consent procedure, were enrolled in the study (n=259). The participant response rate averaged 45.3% of patients meeting study criteria per day (range 27.1–62.9% per day). Questionnaire-based interviews were conducted by trained research staff, and averaged 20 minutes with $10 compensation. In line with our focus on the community-level impact of the intervention, patients could complete interviews multiple times if they met study criteria on multiple interview days, though most participants (n=175, 67.6%) were interviewed only once. In rare instances (n=2) patients were interviewed more than once during a single biweekly interval, but only a single response per patient was included in each biweekly clinic score.
The study period included a total of 54 biweekly intervals, 15 before and 40 after the start of the intervention. Data were collected during 38 (70.4%) biweekly intervals, during 8 (53.3%) of the pre- and 30 (75.0%) of the post-intervention intervals. These differences were not statistically significant (x2(1)=2.4, p=0.12).
Participants
A total of n=259 patients participated in this study. Patients were on average 43.1 (SD=8.8) years old, had known about their HIV status for 9.3 (SD=6.4) years, and were predominantly male (68.3%), and mostly African American (58.3%) or White (30.9%). Eight (3.1%) participants reported being Hispanic. Most participants had completed high school or received their GED (76.1%), and some had received a college degree or higher (23.9%). About 42.1% of the participants reported being employed.
Measures
Patient characteristics
Participants were asked their race, number of years of education, and gender. They were also asked about how long they had known about their HIV status, HCV risk behaviors, and sexual behaviors.
HCV knowledge
HCV knowledge was measured using 11 items developed with a hepatologist and our clinical and community trainers, and pilot-tested with 5 persons with HIV. A full list of items are reported in Proeschold-Bell et al., 2010 [32]. These items fall into the areas of general knowledge (—A person can have both HIV and hepatitis C at the same time ), treatment (—Some people have been cured of hepatitis C, —Many people with both HIV and HCV have benefited from treatment for hepatitis C ), and transmission (—Hepatitis C is passed through blood; —Practicing safer injection techniques is one way to keep from getting hepatitis C ). Participants could respond true,‘ false,‘ or don‘t know.‘ Responses were coded as correct or incorrect, with don‘t know‘ responses coded as incorrect.‘ Community and clinical trainings included content aligned with these 11 items. For example, trainers used the term —cure to align with that specific HCV treatment item.
Knowledge of one’s HCV status
Participants were asked, —Have you ever been tested for Hepatitis C? and if so, —Did the test show that you have Hepatitis C? Response options included —yes, —no, and —don‘t know.
Receipt of education on HCV from providers
Participants were asked, —Has your doctor or other medical provider (nurses, nurse practitioners, physician‘s assistants) talked with you about hepatitis C?
Analytic Strategy
For all outcome variables, weighted regression analyses were conducted, where biweekly averages were the dependent variable, which were weighted by the number of patients who were interviewed during that bi-weekly interval. Dummy-coded variables were used as predictors to model five plausible alternative trends: no changes (i.e., intercept-only model), a pre-existing trend (i.e., linearly increasing dummy code), immediate change (i.e., 0–1 dummy-code for pre and post, respectively), gradual change (i.e., testing for slope and mean differences at the same time), and delayed change (i.e., 0–1 dummy-code dichotomizing 3-months after the start of the intervention versus before). For each outcome, the model with the best fit (i.e., lowest AIC value) was retained. The delay interval was chosen based on how often patients are scheduled for appointments, such that educating a provider might not take effect until the following appointment. At the clinic, patients are routinely scheduled for appointments every 3 months. All analyses were conducted in SAS 9.2. The Durbin-Watson statistic was used to test for the presence of autocorrelations, but was not found to be statistically significant for any of the outcomes, and thus autocorrelations were not modeled. For the primary outcome, performance on the HCV knowledge questionnaire, regular weighted regression could be used, which were performed using the MIXED procedure. The secondary outcomes, HCV awareness, HCV education from medical providers, HCV testing, and knowing status of HCV test, were binary, and their biweekly prevalence had an upper bound at 1. Thus, Tobit models[33], also known as censored regressions, were used for these outcomes, using the QLIM procedure. For all analyses, maximum likelihood estimation was used, which performs very well under missing-at-random data conditions [34].
Patient characteristics were tested as potential covariates systematically. First, each patient characteristic was tested univariately on the patient level to see if it predicted any of the outcomes, using linear regression for the primary outcome and logistic regression for the four secondary outcomes. Second, if it was statistically significant, a biweekly prevalence level of that characteristic was calculated to be considered for inclusion in the clinic-level biweekly regressions in a stepwise fashion. Only statistically significant covariates were retained in the final models.
RESULTS
A total of 431 interviews were conducted, 201 before and 230 after the start of the intervention. On average, 11.3 (SD=9.6) patient interviews were conducted per interval. The number of interviews conducted per interval varied from 4 to 42, where more interviews per interval were intentionally conducted prior to the start of the intervention (M=25.1, SD=11.5) than afterwards (M=7.7, SD=4.4) to compensate for the shorter pre-intervention assessment phase, with t(7.6)=4.2, p<0.01 using the Satterthwaite approach for unequal variances. Recruitment methods were identical across interviews; recruitment simply occurred on more days per time period prior to the intervention start.
Only 56 (21.6%) participants were interviewed both before and after the start of the intervention. The remaining patients were interviewed either before or after the start of the intervention. To test for differences in the group composition of the study population, chi square and t-tests were conducted and are summarized in Table I. Patients interviewed prior to the start of the intervention did not differ on any of the characteristics with the exception of race, where more American Indian/Alaskan Native patients were interviewed before the start of the intervention than afterwards.1
Table 1.
Patient Characteristics
| Characteristic | Total n=259 | Pre-Intervention n=107 (41.3%) | Intervention n=96 (37.1%) | χ2/t |
|---|---|---|---|---|
| n/M (%/SD) | n/M (%/SD) | n/M (%/SD) | ||
| Demographics | ||||
| Sex (male) | 177 (68.3%) | 71 (66.4%) | 68 (70.8%) | 0.5 |
| Race | 12.8* | |||
| Black/African American | 151 (58.3%) | 59 (55.1%) | 61 (63.5%) | |
| Asian | 6 (2.3%) | 0 (0.0%) | 1 (1.0%) | |
| American Indian/Alaska Native | 15 (5.8%) | 12 (11.2%) | 1 (1.0%) | |
| White | 80 (30.9%) | 33 (30.8%) | 33 (34.4%) | |
| Native Hawaiian/Pacific Islander | 2 (0.8%) | 0 (0.0%) | 0 (0.0%) | |
| Other | 5 (1.9%) | 3 (2.8%) | 0 (0.0%) | |
| Age (at 1st interview, in years) | 43.1 (8.8) | 42.1 (8.4) | 43.7 (9.1) | −1.3 |
| Education | ||||
| Completed high school/GED | 197 (76.1%) | 84 (78.5%) | 76 (79.2%) | 0.0 |
| Completed college | 62 (23.9%) | 29 (27.1%) | 24 (25.0%) | 0.1 |
| Employed (at 1st interview) | 109 (42.1%) | 49 (45.8%) | 43 (44.8%) | 0.0 |
| HIV Status | ||||
| Known about HIV status (in years) | 9.3 (6.4) | 9.0 (6.5) | 9.7 (6.6) | −0.8 |
| Hepatitis C Risk Behaviors | ||||
| Ever injected drugs | 58 (22.4%) | 23 (21.5%) | 23 (24.0%) | 0.2 |
| Used injection drugs in past 6 months | 3 3 (1.2%) | 1 (0.9%) | 1 (1.0%) | 0.0 |
| Ever shared syringe w/o cleaning | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | n/a |
| Sexual Behavior (during 30 days prior to interview on average) | ||||
| Number of different sex partners | 0.5 (0.6) | 0.4 (0.6) | 0.5 (0.7) | −0.9 |
| Had new sex partner(s) | 17 (6.6%) | 4 (3.7%) | 6 (6.3%) | 0.7 |
| Never used condoms | 26 (10.0%) | 9 (8.4%) | 10 (10.4%) | 0.2 |
Note: n=56 (21.6%) were interviewed before and after the start of the intervention, and are excluded from pre-intervention and intervention comparisons;
p<0.05; M=mean, SD=standard deviation
Primary Outcome: HCV Knowledge
The results of the weighted regression analyses for the average HCV knowledge per biweekly interval are summarized in Table II. The best-fitting model was the gradual change‘ model, which tested for both slope and mean differences between the pre-intervention and the intervention phase. The predicted values of this model are displayed in Figure 1 alongside the observed scores. While decreasing trends are visible in both phases, only the mean difference was statistically significant, where HCV knowledge was higher during the biweekly intervals following the start of the intervention.
Table 2.
Model fit and parameter estimates for weighted regressions of different intervention effect models
| Model | Parameter | AIC | EST | SE | t | df | p |
|---|---|---|---|---|---|---|---|
| no trend | 93.5 | ||||||
| intercept | 7.71 | 0.11 | 68.52 | 37 | <.0001 | ||
| secular trend | 95.2 | ||||||
| intercept | 7.69 | 0.12 | 66.41 | 36 | <.0001 | ||
| time | 0.00 | 0.01 | 0.58 | 36 | 0.564 | ||
| immediate change | 90.8 | ||||||
| intercept | 7.46 | 0.15 | 48.13 | 36 | <.0001 | ||
| intervention | 0.47 | 0.21 | 2.23 | 36 | 0.032 | ||
| gradual change | 88.1 | ||||||
| intercept | 6.72 | 0.45 | 14.90 | 34 | <.0001 | ||
| intervention | 1.61 | 0.51 | 3.16 | 34 | 0.003 | ||
| time | −0.07 | 0.04 | −1.71 | 34 | 0.096 | ||
| intervention*time | 0.05 | 0.04 | 1.11 | 34 | 0.275 | ||
| delayed change (3-months) | 94.9 | ||||||
| intercept | 7.64 | 0.14 | 52.74 | 36 | <.0001 | ||
| delayed | 0.18 | 0.23 | 0.79 | 36 | 0.432 | ||
Note: Race (being African American), education (having a college degree), and never having injected drugs were statistically significant univariate predictors of performance on the HCV knowledge questionnaire on the patient-level, but were not statistically significant on the biweekly level (coded as prevalence levels), and were thus not included in these models.
Figure 1.
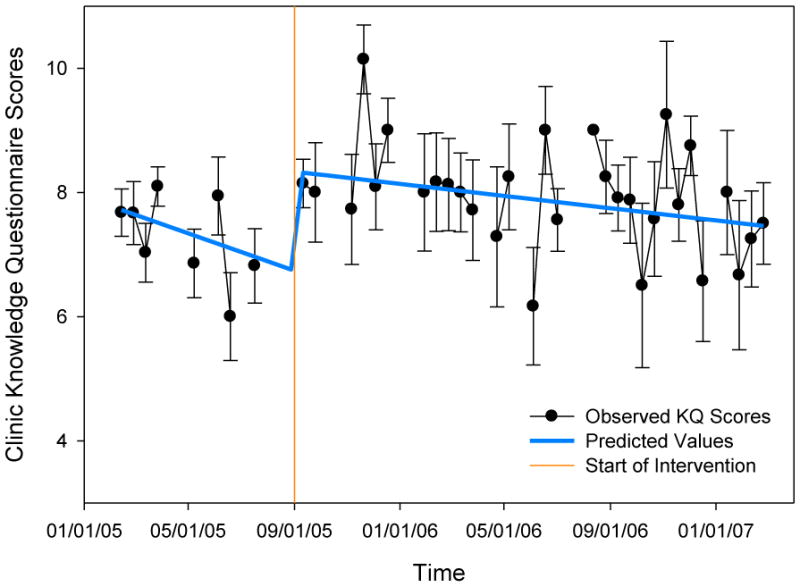
Average scores on the 11-item knowledge questionnaire per biweekly interval are displayed, where standard errors around the mean are represented by error bars. The predicted values of the “gradual change” model, the best fitting model, are displayed in by the thick line.
Secondary Outcomes: Prevalence of HCV Awareness, HCV Education from Medical Providers, HCV Testing, Knowing Status of HCV Test
Model comparisons for the secondary outcomes proceeded in the same way as for the primary outcome. In the interest of space, Table III summarizes the parameter estimates of the best-fitting model per outcome only. For both —HCV education from medical providers and —knowing the status of one‘s HCV test, the gradual change model provided the best model fit. The prevalence of patients having received HCV education from their medical providers was higher on average after the start of the intervention (b=0.28). A slightly decreasing trend prior to the start of the intervention was observed (b=−0.01 per biweekly interval), which was reversed during the intervention (b=0.01). For knowing one‘s HCV status, the average knowledge prevalence before and after the start of the intervention was not statistically significant. Like HCV education, however, an initially downward trend was reversed after the start of the intervention.
Table 3.
Parameter estimates of best fitting model of the intervention effect on clinic prevalence levels using weighted censored regressions
| Outcome | EST | SE | t | df | p | n at upper bound† |
|---|---|---|---|---|---|---|
| Predictor | ||||||
| Before today, ever heard (specifically) about Hepatitis C | 133 | |||||
| intercept | 0.92 | 0.01 | 173.47 | 1 | <.0001 | |
| delayed | 0.09 | 0.01 | 9.25 | 1 | <.0001 | |
| Sigma | 0.08 | 0.00 | 22.72 | 1 | <.0001 | |
| Talked with Medical Provider about Hepatitis C | 63 | |||||
| intercept | 0.39 | 0.04 | 10.59 | 1 | <.0001 | |
| time | −0.01 | 0.00 | −4.52 | 1 | <.0001 | |
| intervention | 0.28 | 0.03 | 8.23 | 1 | <.0001 | |
| intervention*time | 0.01 | 0.00 | 3.91 | 1 | <.0001 | |
| prevalence of IV drug use | 0.84 | 0.07 | 12.77 | 1 | <.0001 | |
| prevalence of having a sex partner | 0.28 | 0.11 | 2.48 | 1 | 0.013 | |
| Sigma | 0.13 | 0.01 | 24.80 | 1 | <.0001 | |
| Got tested for Hepatitis C | 67 | |||||
| intercept | 0.73 | 0.01 | 49.81 | 1 | <.0001 | |
| prevalence of IV drug use | 0.66 | 0.06 | 10.28 | 1 | <.0001 | |
| Sigma | 0.12 | 0.01 | 22.95 | 1 | <.0001 | |
| Knows Hepatitis C Status | 44 | |||||
| intercept | 0.64 | 0.04 | 16.61 | 1 | <.0001 | |
| time | −0.01 | 0.00 | −2.37 | 1 | 0.0176 | |
| intervention | 0.02 | 0.04 | 0.63 | 1 | 0.525 | |
| intervention*time | 0.01 | 0.00 | 2.36 | 1 | 0.018 | |
| prevalence of IV drug use | 0.63 | 0.07 | 9.50 | 1 | <.0001 | |
| Sigma | 0.13 | 0.01 | 23.93 | 1 | <.0001 |
Note:
total number of patients included in biweekly intervals with a prevalence of 100%; covariates only tested for inclusion if significant on the patient-level
For HCV awareness, a significant delayed effect was observed; the prevalence of HCV awareness was on average statistically significantly higher three months after the start of the information than before (b=0.09). Given the delay, however, we cannot attribute the change to the intervention with as much confidence as we could with an immediate effect. For getting tested for HCV, there was no significant intervention effect.
The prevalence of patients who had ever injected drugs was a statistically significant covariate for three out of the four secondary outcomes: HCV education, HCV testing and knowing one‘s HCV status, where IV drug use was positively associated with each outcome. The only other statistically significant covariate on the clinic-level was the prevalence of patients with past 30-day sex partners, which was positively associated with receiving HCV education from one‘s medical provider. For reference, 137 (53.1%) of the patients did not have a sex partner during the 30 days prior to their interview.
DISCUSSION
This study implemented and evaluated a multi-level intervention to improve PLWHA‘s knowledge of HCV and encourage them to find out their HCV status. The intervention had multiple components across the intrapersonal, interpersonal, community, and institutional levels of the Socioecological Framework, which posits that desired outcomes are most likely to occur when targeted across multiple levels. Study results suggest that our primary outcome, accurate HCV knowledge among patients with HIV, was higher during the intervention phase than pre-intervention phase. Thus, our intervention successfully achieved our primary goal. Interestingly, accurate HCV knowledge was decreasing during both pre-intervention and intervention time periods, though at a much reduced rate during the intervention phase. It is hard to explain why accurate knowledge would decrease prior to the intervention, although it is possible that the act of garnering partners and initiating the study—even pre-intervention—led some providers to share HCV information with their patients early on, and then taper off until the intervention was launched. Post-intervention, it is plausible that patients who had attended a community health class later forgot new HCV learnings. It may be particularly difficult for PLWHA, who know so much about HIV, to retain knowledge about HCV transmission which is similar, but not identical, to that of HIV. Future studies may wish to develop brief review materials that can be posted in the clinic or the community, and also to encourage patients to attend refresher health classes. The deterioration we saw in HCV knowledge may also be related to the fact that we trained the clinic‘s HIV medical providers early in the study but did not offer additional, future trainings. Consequently, we did not reach any new HIV providers, including medical fellows who turnover frequently. Also, it may be that providers decreased their HCV efforts with the elapsing of time since their training. In the future, it may be wise to institute routine prompts, such as prompts in an electronic medical record system, for providers to discuss HCV with at-risk patients. This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC) HCV National Prevention Strategy for Health Professionals [35].
The number of participants who reported ever having been tested for HCV evidenced a significant increase 3 months after implementation of the intervention. In addition, immediately following the start of the intervention, a previously downward trend in the prevalence of patients knowing their HCV test results was reversed, and the prevalence of patients talking with their medical providers about HCV was higher during the intervention phase. We hypothesized that the intervention would lead PLWHA to test for HCV or ask their providers about whether they had ever previously been tested for HCV, enabling them to know their HCV status. We also hypothesized that HIV medical providers would test for HCV more frequently. Based on patient report, the findings indicate an increase in HCV testing and, perhaps more importantly, knowledge of one‘s HCV status. Knowing one‘s HCV status allows people to seek treatment for HCV and to take steps to prevent transmitting HCV.
We also tested intermediate outcomes such as awareness of HCV and patient-provider discussion about HCV. Participant awareness of HCV was significantly higher 3 months after implementation of the intervention. Because our intervention activities were spread out across the 17-month intervention period, it may be that a minimum threshold of intervention implementation had to be reached before awareness measurably increased. Admittedly, it is possible that the research interviews rather than the intervention raised HCV awareness; however, HCV awareness is a precursor to seeking HCV testing and accurate HCV knowledge, and so is important to demonstrate. Participants further reported more HCV discussions with their providers during the intervention phase. This finding lends validity to our primary outcome of more accurate HCV knowledge. Improvements in HCV knowledge may have occurred through our efforts to educate providers who, in turn, communicated with patients. Alternatively, more accurate HCV knowledge may have resulted directly through our community education efforts.
Intervention effects were found for different outcomes at a variety of times (e.g., immediately after the stated intervention date, three months later) and in both abrupt and slope changes. This may be because our intervention began on the date we indicate as the intervention date, but continued over time. The trend in significant findings for multiple outcomes is likely more meaningful than when outcomes were found. We conclude that overall, the intervention met its goals and is worthy of replication. However, it should also be refined in an attempt to improve HCV knowledge and testing further, both of which evidenced room for improvement even during the intervention phase.
We examined a number of covariates, and notably, the intervention was equally effective across race, gender, age, education, employment status, and HIV status. The findings suggest that the intervention was appropriately targeted to participants with a history of injection drug use, who were significantly more likely to report having had an HCV discussion with a medical provider, getting tested for HCV, and knowing their HCV status. Although parenteral transmission through injection drug use more commonly leads to HCV transmission than sexual risk behaviors, rates of current injection drug use in our sample were extremely low. It is therefore unsurprising that our data also suggest that medical providers talked about HCV more often to participants with at least one sexual partner, because sexual risk behavior rather than injection drug use is more common in our population of PLWHA. However, it also appears that medical providers did not necessarily test participants with more sexual partners for HCV. This, too, may be appropriate, because studies indicate that risk for HCV is predicted by many sexual partners, such as more than 20 partners [4], and not just one or two.
Through provider interviews, we discovered several barriers to HCV testing and provider-patient discussions about HCV, despite the fact that guidelines for HIV management include testing for HCV [36]. In particular, many HIV providers indicated that they are more worried about chronic diseases such as diabetes and heart disease in their patients than they are about liver disease. In addition, like other researchers [37], we found that some HIV providers were reluctant to test their patients for HCV, due to the belief that HIV management is more important than HCV management, or that the side effects for antiviral treatment of HCV are too onerous to put patients through. These side effects include fatigue, thyroid abnormalities, bone marrow suppression, and depression [9]. Because of these concerns, we held testing contests in the clinic to bring attention to HCV testing. It is notable that we found trends in HCV testing and patient knowledge despite the reluctance of some providers. We recommend addressing these concerns head-on in future interventions.
It should be noted that this intervention addressed some, but not all, of the principle components of the CDC‘s National HCV Prevention Strategy [35]. In particular, it attempted to educate health care professionals on HCV identification, management, and treatment, and to educate people at risk for HCV about risk factors and testing. The intervention also sought to educate community-based program staff on HCV prevention and to encourage them to identify people who need testing for HCV. The intervention did not address the National HCV Prevention Strategy‘s principle component of surveillance of disease trends, although we did, as recommended, engage in research evaluation of this particular intervention. In addition, HCV prevention initiatives, especially among PLWHA, should include harm reduction strategies in the form of needle and syringe exchange programs, as well as substance abuse treatment, including opioid substitution treatment [38, 39]. Although we offered substance abuse treatment, we did not offer these other harm reduction activities, in part because our particular population had low rates of injection drug use. However, these components are recommended alongside HCV awareness and testing initiatives to create combination approaches for optimal prevention of HCV infection [39].
A rather unique feature of this study is its use of the ITS design. Although researchers have called for ITS to be used in the evaluation of community-level interventions, such use of ITS is still relatively rare [30]. In this study, we devoted at least one full day to data collection every two weeks for two years, a process that was both time-intensive and required significant partnership with the clinics. The result, however, was a stronger research design that provides greater confidence in the causal intervention effects than a case-control design would have offered because it addresses several threats to validity. In this study, for example, we were able to test for any pre-existing trend and rule it out as a rival theory for the observed intervention effect. ITS designs are vulnerable to their own threats to validity, which we controlled for carefully in this study. For example, we rigorously tested for changes in the study population through comparison of participants during the pre-intervention and intervention phases, with only one significant difference (race) resulting. We then conducted sensitivity analyses to ensure that our findings were not a mere byproduct of changes in the study population but rather an intervention effect. As such, this study illustrates how the ITS design can be used effectively in community research given real-life administrative constraints.
This study has several limitations. First, it is limited by its use of patient report rather than medical record abstraction for HCV testing. Also, the intervention itself was extensive and multi-level, requiring ample staff and time resources. Even so, it lacked needle and syringe exchange activities, which future researchers and interventionists would be wise to incorporate. We are unable to tease apart which aspects of the intervention were most impactful on outcomes, even though such information would be useful. In addition, we collected data only before and during intervention activities, such that we are not able to examine whether removal of the intervention led to any decay in outcomes. Finally, despite the strength that ITS offers for internal validity, the ITS design is often limited in its external validity. In this particular study, our conclusions are known only to be generalizable to patients at the ID clinic where the study occurred. This study‘s findings may be generalizable to other clinics and patients, based on the extent to which they are similar to ours. Our sample had a very low self-reported current injection drug use rate, had a mean age of 43, and had known their HIV status on average 9 years, all of which may be different from other clinic populations.
In conclusion, this is the only intervention study to promote HCV knowledge and testing among PLWHA of which we are aware. The high co-morbidity of HIV-HCV combined with the increasing liver-related mortality among PLWHA makes HCV prevention, testing, and treatment a priority for the over 1 million people in the US infected with HIV. Additional interventions paired with rigorous evaluation are needed.
Acknowledgments
This work was funded by a grant from the Healthy Community Access Program (HCAP) of the Bureau of Primary Health Care, of the Health Resources and Services Administration (HRSA), of the U.S. Department of Health and Human Services (G92CSO2237-02-02). We thank F. Lombard, MSW, for his leadership on this study. We thank Elizabeth Goacher, PA, Duke University Department of Medicine, Division of Gastroenterology, for assisting with the development of the HCV knowledge items, Nathan Thielman, MD, MPH, Duke University Department of Medicine, Division of Infectious Diseases, for support in conducting research in the clinic, and Randall Pollard, LCSW, Duke Division of Infectious Diseases, for assisting with participant recruitment and response rate data. The intervention could not have happened without Marc Kolman, Patrick Lee, Donna Tennyson, Teresa Hart, Xavier Purefoy, Betsy Barton, Robin Swift, Milford Evans, Tanya McNeill, and Kimberly Walker. We thank the many study interviewers, including Arit Amana, Christie Raulli, Carolyn Vance, Douglas Thomas, Josette Gbemudu, Sharon Abbruscato, and Brian Flores. Most importantly, we thank all the study participants.
Footnotes
For all subsequent analyses, sensitivity analyses were conducted, where the same analyses were conducted with and without patients of American Indian/Alaskan Native background. The absence or presence of these patients did not alter substantive conclusion of the analyses, and thus the full sample analyses are reported here.
References
- 1.UNAIDS. Report on the global AIDS epidemic. Geneva: UNAIDS; 2008. [Google Scholar]
- 2.Hall HI, Song R, Rhodes P. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the US. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 5.Vandelli C, Renzo F, Romano L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: Results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99(5):855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 6.van de Laar TJ, van der Bij AK, Prins M, et al. Increase in HCV Incidence among Men Who Have Sex with Men in Amsterdam Most Likely Caused by Sexual Transmission. J Infect Dis. 2007;196(2):230–238. doi: 10.1086/518796. [DOI] [PubMed] [Google Scholar]
- 7.Verna EC, Brown RS. Hepatitis C virus and liver transplantation. Clinical Liver Disease. 2006;10(4):919–940. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192(6):992(11). doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- 9.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345(1):41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 10.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with Human Immunodeficiency Virus: A cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;334:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 11.Verucchi G, Calza L, Manfredi R, Chiodo F. Human immunodeficiency virus and hepatitis C virus coinfection: epidemiology, natural history, therapeutic options and clinical management. Infection. 2004;32(1):33–46. doi: 10.1007/s15010-004-3063-7. [DOI] [PubMed] [Google Scholar]
- 12.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the Human Immunodeficiency Virus: The D:A:D Study. Ann Intern Med. 2006;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 13.Lima VD, Harrigan R, Bangsberg DR. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Def. 2009;50(5):529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cribier B, Rey D, Schmitt C, Lang JM, Kirn A, Stoll-Keller F. High hepatitis C viraemia and impaired antibody response in patients coinfected with HIV. AIDS. 1995;9(10):1131–6. doi: 10.1097/00002030-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Soriano V, Sulkowski M, Bergin C, et al. Care of patients with chronic hepatitis C and HIV co-infection: recommendations from the HIV-HCV International Panel. AIDS. 2002;16(6):813–828. doi: 10.1097/00002030-200204120-00001. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Quijano A, Andreu J, Gavilán F, et al. Influence of HIV type 1 infection on the natural course of chronic parenterally acquired hepatitis C. Eur J Clin Microbiol Infect Dis. 1995;14(11):949–953. doi: 10.1007/BF01691375. [DOI] [PubMed] [Google Scholar]
- 17.Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modified the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 18.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with Human Immunodeficiency Virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 19.García-Samaniego J, Rodríguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96(1):179–183. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenthal E, Poiree M, Pradier C, et al. Mortality due to hepatitis C-related liver disease in HIV-infected patients in France (Mortavic 2001 study) AIDS. 2003;17(12):1803–1809. doi: 10.1097/00002030-200308150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Chou R, Clark M, Helfand M. Screening for hepatitis C virus infection: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(6):465–479. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 22.McGinn T, O‘Connor-Monroe N, Alfandre D, Gardenier D, Wisnivesky J. Validation of a hepatitis C screening tool in primary care. AMA Arch Intern Med. 2008;168(18):2009–2013. doi: 10.1001/archinte.168.18.2009. [DOI] [PubMed] [Google Scholar]
- 23.Weaver MF, Cropsey KL, Fox SA. HCV prevalence in methadone maintenance: Self-report versus serum test. Am J Health Behav. 2005;29(5):387–394. doi: 10.5555/ajhb.2005.29.5.387. [DOI] [PubMed] [Google Scholar]
- 24.Walley AY, White MC, Kushel MB, Song YS, Tulsky JP. Knowledge of and interest in hepatitis C treatment at a methadone clinic. J Subst Abuse Treat. 2005;28:181–187. doi: 10.1016/j.jsat.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Carey J, Perlman DC, Friedmann P, et al. Knowledge of hepatitis among active drug injectors at a syringe exchange program. J Subst Abuse Treat. 2005;29:47–53. doi: 10.1016/j.jsat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Heimer R, Clair S, Grau LE, Blumenthal RN, Marshall PA, Singer M. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97:1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- 27.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Edu Q. 1988;15(4):351–77. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 28.Stokols D. The social ecological paradigm of wellness promotion. In: Jamner MS, Stokols D, editors. Promoting human wellness: New frontiers for research, practice, and policy. Berkeley, CA: University of California Press; 2000. pp. 21–37. [Google Scholar]
- 29.Shadish W, Cook TD, Campbell D. Experimental & quasi-experimental designs for generalized causal inference. New York: Houghton Mifflin Company; 2002. [Google Scholar]
- 30.Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci. 2000;1(1):31–49. doi: 10.1023/a:1010024016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proeschold-Bell RJ, Heine ABP, McAdam K, Quinlivan EB. A cross-site, comparative effectiveness study of an integrated HIV and substance use treatment program. AIDS Patient Care STDs. 2010;24(10):1–8. doi: 10.1089/apc.2010.0073. [DOI] [PubMed] [Google Scholar]
- 32.Proescholdbell RJ, Blouin R, Reif S, et al. Hepatitis C transmission, prevention, and treatment knowledge among patients With HIV. South Med J. 2010;103(7):365–341. doi: 10.1097/SMJ.0b013e3181e1dde1. [DOI] [PubMed] [Google Scholar]
- 33.Amemiya T. Tobit models: A survey. J Econom. 1984;24:3–61. [Google Scholar]
- 34.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. National Hepatitis C Prevention Strategy: A Comprehensive Strategy for the Prevention and Control of Hepatitis C Virus Infection and its Consequences. Division of Viral Hepatitis, National Center for Infectious Diseases, Centers for Disease Control and Prevention; 2001. [accessed October 6, 2010]. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHepCPrevStrategy.htm#summary. [Google Scholar]
- 36.National Institutes of Health. Management of Hepatitis C: 2002. National Institutes of Health Consensus Conference Statement; 2002. [accessed October 27, 2010]. http://consensus.nih.gov/2002/2002HepatitisC2002116html.htm. [Google Scholar]
- 37.Scott JD, Wald A, Kitahata M, et al. Hepatitis C Virus is infrequently evaluated and treated in an urban HIV clinic population. AIDS Patient Care STDs. 2009;23(11):925–929. doi: 10.1089/apc.2009.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee SA. Time to act: a call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376:551–563. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


