Abstract
Objective
Oxidative stress plays a causative role in diabetic embryopathy. We tested whether mitigating oxidative stress, using superoxide dismutase 1 (SOD1) transgenic mice, would block hyperglycemia-induced specific PKC isoform activation and its downstream cascade.
Study Design
Day 8.5 (E8.5) embryos from non-diabetic WT control (NC), diabetic mellitus WT (DM) and diabetic SOD1-Tg mice (DM-SOD1-Tg) were used for detection of phosphorylated (p-) PKCα/βII and p-PKCδ, and levels of two prominent PKC substrates, p-MARCKS and RACK1, and lipidperoxidation markers, 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA).
Results
Levels of p-PKCα/βII, p-PKCδ, p-MARCKS, 4-HNE and MDA were significantly elevated in the DM group compared with those in the NC group and the DM-SOD1-Tg group. The NC and DM-SOD1-Tg groups had comparable levels of these protein and lipidperoxidation markers. RACK1 levels did not differ among the three groups.
Conclusions
Mitigating oxidative stress by SOD1 overexpression blocks maternal hyperglycemia-induced activation of specific PKC isoforms and downstream cascades.
Keywords: SOD1 transgenic mice, PKC isoforms, MARCKS, lipidperoxidation, diabetic embryopathy
INTRODUCTION
Pregestational diabetes is a significant risk factor for congenital malformations including major structure birth defects, neural tube defects (NTDs)1. Maternal hyperglycemia increases the production of reactive oxygen species and impairs the intracellular antioxidant capability leading to oxidative stress2–9. Multiple evidence8, 10–13 support that hyperglycemia-induced oxidative stress causes malformations. Both in vivo and in vitro treatments with variety of antioxidants8, 10–13 can effectively reduce hyperglycemia-induced NTDs. Overexpression of the antioxidant enzyme, SOD1, in transgenic mice ameliorates maternal diabetes-induced NTDs13. Although oxidative stress appears to be a central mechanism underlying hyperglycemia-induced malformations, it is unclear whether the downstream intracellular signals mediate oxidative stress as well.
Oxidative stress activates multiple kinase signaling pathways. The Protein Kinase C (PKC) family consists twelve isoforms that involve diverse physiological and pathophysiological functions including cell proliferation, differentiation and apoptosis14. In diabetic embryopathy, prolonged PKC activation is associated with maternal diabetes-induced NTDs15. We have further reported that hyperglycemia specifically activates PKCα/βII and PKCδ16. Specific pharmacological inhibitors to PKCα, PKCβII or PKCδ have been shown to significantly reduce hyperglycemia-induced NTDs16, strongly implicating that activation of these specific PKC isoforms plays a causative role in the induction of NTDs by hyperglycemia. PKC activation results in lipidperoxidation which leads to cell membrane damage17. PKC activation has been linked to altered AA metabolism during lipidperoxidation18. In diabetic embryopathy, hyperglycemia-induced lipidperoxidation alters cell membrane lipid metabolism by shifting arachidonic acid (AA) metabolism from prostaglandin E2 to isoprostanes19. The loss of membrane AA destabilizes the cell membrane structure and function. Conversely, AA supplementation has been shown to reduce the incidence of diabetic embryopathy11, 12, 20. In the present study, we will determine if oxidative stress-induced specific PKC isoforms activation triggers lipidperoxidation which, in turns, intensifies the degree of oxidative stress in embryos exposed to hyperglycemia.
Beside the differential activation mechanisms of the twelve PKC isoforms, individual PKC isoforms exerts distinct physiological and pathophysiological functions via substrate specificity. Limited number of PKC substrate has been identified. Among the known PKC substrates, Myristoylated Alanine-Rcih Protein Kinase C Substrate (MARCKS) is a prominent PKC substrate that primarily resides in neural tissues21. Furthermore, it has been reported that MARCKS are specific substrate of PKCβII22 and PKCδ23. Another prominent PKC substrate is the Receptor for Activated C Kinase 1 (RACK1) which is originally discovered through its binding to active PKCβII and other classic PKC isoforms24. RACK1 participates in multiprotein signaling complexes and can enhance PKC-dependent JNK activation25, which plays a causative role in the induction of diabetic embryopathy26. Therefore, in the present study, we will assess the activation and levels of these two PKC substrates with a goal to define the role of PKC substrates in oxidative stress-mediated teratogenicity in diabetic embryopathy.
The connection between oxidative stress and PKC activation has not been explored. Because both SOD1 overexpression in vivo13 and PKC inhibition in vitro16 reduce hyperglycemia-induced malformations, in the present study we will use SOD1-Tg mice to test whether SOD1 overexpression in vivo, blocks hyperglycemia-induced specific PKC isoforms activation and its downstream cascade.
Material and methods
Animals and reagents
C57BL/6J mice (median body weight 22 g) were purchased from Jackson Laboratory (Bar Harbor, Maine). Streptozotocin (STZ) from Sigma was dissolved in sterile 0.1 M citrate buffer (pH 4.5). Sustained-release insulin pellets were purchased from Linplant (Linshin, Canada). SOD1-Tg mice in C57BL/6J background were revived from frozen embryos by the Jackson Laboratory (Stock number: 002298).
Mouse models of diabetic embryopathy
The procedures for animal use were approved by the Institutional Animal Care and Use Committee of University of Maryland School of Medicine. Eight-week old Wildtype (WT) and SOD1-Tg mice were intravenously (iv) injected daily with 75 mg/kg STZ over two days to induce diabetes. Once a level of hyperglycemia indicative of diabetes (>250 mg/dl) was achieved, insulin pellets were subcutaneously implanted in these diabetic mice to restore euglycemia prior to mating. The mice were then mated with male mice of the same respective genotype. On Day 5 of pregnancy (E5), insulin pellets were removed to permit frank hyperglycemia (>250 mg/dl glucose level), so the developing conceptuses would be exposed to a hyperglycemic environment at E7 onwards. WT non-diabetic female mice with vehicle injections and sham operation of insulin pellet implants served as non-diabetic controls. On E8.5, mice were euthanized, and conceptuses were dissected out of the uteri for analysis. To avoid any redundancy, data of malformation incidences was not collected because it has been published elsewhere9. The typical malformations observed in embryos from diabetic WT mice are NTDs.
Western Blotting
Western Blotting was performed as described by Yang, at el.27. Briefly, embryonic samples were sonicated in 80 μl ice-cold lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 10 mM NaF, 2 mM Na orthovanadate, 1 mM PMSF and 1% Triton 100) containing a protease inhibitor cocktail (Sigma, St. Louis, MO). Equal amounts of protein (50 μg) were resolved by SDS-PAGE and transferred onto an Immunobilon-P ((Millipore). Membranes were incubated for 18 h at 4 °C with the following primary antibodies at 1:1000 to 1:2000 dilutions in 5% nonfat milk: anti-p-PKCα/βII (#9375), anti-p-PKCδ (#9374), anti-p-MARCKS (#2741), anti-RACK1 (#4716) and anti-SOD1 (human specific, #4266), all from Cell Signaling; anti-MDA (EMD, #442730); anti-4-HNE (Chemicon, #AB5605). Membranes were exposed to goat anti-rabbit, anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies. To ensure that equivalent amounts of protein were loaded among samples; membranes were stripped and probed with a mouse antibody against β-actin (Abcam, Cambridge, MA). Signals were detected using an Amersham ECL Advance Detection Kit (GE Healthcare, Piscataway, NJ) and chemiluminescence emitted from the bands was directly captured using a UVP Bioimage EC3 system (UVP, Upland, CA). Densitometric analysis of chemiluminescence signals were performed by VisionWorks LS software (UVP, Upland, CA). Images of representative immunoblots were arranged using Adobe Photoshop and Microsoft PowerPoint software. All experiments were repeated three times with the use of independently prepared tissue lysates.
Statistics
Data are presented as means ± SE (standard error). One way ANOVA were performed using SigmaStat 3.5 software. In ANOVA analysis, Tukey-test was used to estimate the significance of the results. Statistical significance was accepted at p< 0.05.
Results
SOD1 overexpression blocks maternal hyperglycemia-induced PKCα/βII and PKCδ activation
To investigate whether mitigating oxidative stress, using SOD1-Tg transgenic mice, blocks hyperglycemia-induced specific PKC isoform activation, E8.5 embryos from non-diabetic WT control (NC), diabetic mellitus WT (DM) and diabetic SOD1-Tg mice (DM-SOD1-Tg) of C57BL/6J background were used for determination of p-PKCα/βII and p-PKCδ levels. SOD1 overexpression did not affect diabetic status because mice in the DM and DM-SOD1-Tg groups had comparable glucose levels which were about 4-fold higher than that in the NC group (Table 1). Levels of p-PKCα/βII and p-PKCδin the DM group were significantly higher than those in the NC group, and levels of p-PKCα/βII and p-PKCδ in the DM-SOD1-Tg group were significantly lower compared to those in the DM group (Fig. 1A and B). The results show that maternal hyperglycemia activates PKCα/βII and PKCδ, and that SOD1 overexpression blocks activation of these PKC isoforms.
Table 1.
Glucose levels in non-diabetic WT Control, diabetic WT and diabetic SOD1-Tg mice
| Group | Average glucose level (mg/dl) |
|---|---|
| Non-diabetic WT Control (NC) | 119.5 ± 5.5* |
| Diabetic Mellitus WT (DM) | 428.5 ± 10.1 |
| Diabetic SOD1-Tg (DM-SOD1-Tg) | 419 ± 8.5 |
Data are presented as mean ± SE (n = 5). Average glucose level is the mean of the glucose levels at E7–E8.
denotes significant differences compared to the DM and the DM-SOD1-Tg groups.
Figure 1.

SOD1 overexpression blocks maternal hyperglycemia-induced p-PKCα/βII and p-PKCδ activation. The upper panels of A and B showed representative images of p-PKCα/β II and p-PKCδ Western blotting, respectively. The lower panels of A and B were the graphic data of densitometric analysis of protein levels of p-PKCα/βII and p-PKCδ, respectively. β-actin served as an equal protein loading control. Data expressed as Mean ± standard errors (SE) (n = 3). NC: non-diabetic WT control. DM: Diabetic WT and DM-SOD1-Tg: diabetic SOD1 Tg. * denotes significant difference (p<0.05) and ** means significant difference (p<0.01) when compared to the diabetic DM group.
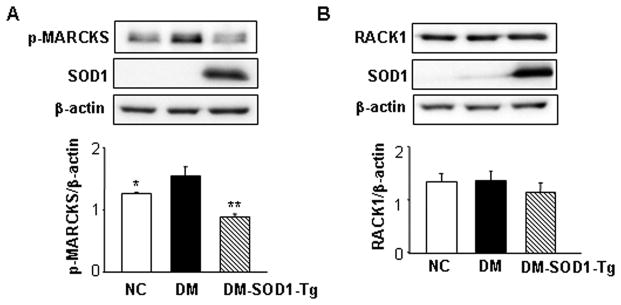
SOD1 overexpression reduces maternal hyperglycemia-induced MARCKS phosphorylation
Because SOD1 overexpression blocks maternal hyperglycemia-induced PKCα/βII and PKCδ activation, we sought to determine the levels and activities of two downstream effectors MARCKS and RACK1 of PKCα/βII and PKCδ. Levels of p-MARCKS in the DM group was significantly higher than that in the NC group, and the level of p-MARCKS in DM-SOD-Tg group was significantly lower than that in the DM group (Fig. 2A). In contrast, RACK1 levels did not differ among the three experimental groups (Fig. 2B). These results provide strong evidence that SOD1 overexpression blocks the maternal diabetes-induced PKC and the cascading sequelae by suppressing the activation of downstream substrates such as MARCKS.
Figure 2.
SOD1 overexpression abolishes maternal hyperglycemia-induced p-MARCKS activations. The upper panels of A and B showed representative images of p-MARCKS and RACK1 Western blotting, respectively. The lower panels of A and B were the graphic data of densitometric. β-actin served as an equal protein loading control. Data expressed as Mean ± standard errors (SE) (n = 3). NC: non-diabetic WT control. DM: Diabetic WT and DM-SOD1-Tg: diabetic SOD1 Tg. * (p<0.05) and ** (p<0.01) denote significant difference when compared to the DM group.
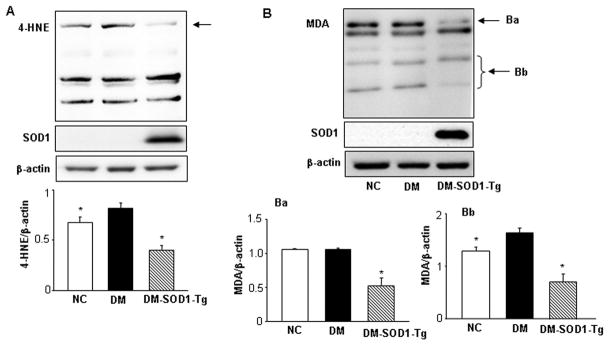
SOD1 overexpression reduces maternal hyperglycemia-induced lipidperoxidation
Because PKC activation directly results in lipidperoxidation, we determined the levels of two major cytotoxic aldehydes, 4-HNE and MDA, which are prominent lipidperoxidation markers in other systems. Levels of 4-HNE were significantly higher in the DM group compared to those in the NC group and the DM-SOD1-Tg group (Fig. 3A). Similarly, levels of MDA in the DM group were significantly increased compared to those in the NC group and the DM-SOD1-Tg group (Fig. 3B). Quantification of specific sizes of protein modified by MDA showed significantly elevated levels in the DM group compared to the NC and DM-SOD1-Tg group (Fig. 3Ba and 3Bb). The NC and DM-SOD1-Tg groups had comparable levels of these two lipidperoxidation markers (Fig. 3A, B). These results demonstrate that maternal diabetes induces lipidperoxidation via PKC activation, and mitigating oxidative stress by SOD1 overexpression blocks lipidperoxidation.
Figure 3.
SOD1 overexpression blocks maternal hyperglycemia-induced lipidperoxidation. The upper panels of A and B showed representative images of 4-HNE and MDA Western blotting, respectively. The lower panel of A was the graphic data of densitometric analysis of the bands which were pointed by the arrow. In panel B, Ba and Bb pointed specific bands which were quantitatively graphed in the underneath bar graphs (Ba and Bb). β-actin served as an equal protein loading control. NC: non-diabetic WT control. DM: Diabetic WT and DM-SOD1-Tg: diabetic SOD1 Tg. Data expressed as Mean ± standard errors (SE) (n = 3). * denotes significant difference (p<0.05) when compared to the DM group.
Comment
In the present study, we have provided for the first time direct in vivo evidence that oxidative stress causes activation of PKCα/βII and PKCδ in diabetic embryopathy. The SOD1-Tg mouse line used in our study is a valid tool in suppressing hyperglycemia-induced oxidative stress. This transgenic line carries the human SOD1 gene and has been demonstrated that the protein products of the transgene expressed in mouse tissue have high enzymatic activities13, 28. Hyperglycemia increases the production of superoxide, and SOD1 effectively reduces oxidative stress by converting superoxide into oxygen and hydrogen peroxide. As previously reported13, we have also found that SOD1-Tg mice do not exhibit any excess embryonic malformations. Our results demonstrate that SOD1 overexpression effectively reduces diabetic embryopathy through blockade of PKCα/βII and PKCδ activation.
Enhanced apoptosis in vulnerable embryonic tissues such as the neural tube in embryos exposed to hyperglycemia leads to malformations6, 16. Oxidative stress activates pro-apoptotic intracellular signaling in diabetic embryopathy6, 8, 9. Because the activation of PKCα/βII and PKCδ mediates the teratogenicity of oxidative stress, we propose that PKCα/βII and PKCδ are pro-apoptotic intracellular signaling intermediates. Indeed, PKCδ induces apoptosis in certain cell types14. We and others have demonstrated that maternal diabetes-induced apoptosis is caspase dependent6, 29. Dietary antioxidant supplementation blocks maternal hyperglycemia-induced caspase activation30. Because activation of PKCα/βII and PKCδ is a downstream event of oxidative stress, inhibition of the activation of PKC isoforms would block caspase-dependent apoptosis. In a separate study (manuscript submitted), we have determined that inhibition of PKCβII blocks hyperglycemia-induced caspase activation and apoptosis. Future studies will aim to delineate the apoptotic mechanism underlying PKCα/βII and PKCδ activation leads to the induction of diabetic embryopathy.
PGE2-like isoprostanes, such as 8-iso-prostagladin F2 (8-iso-PGF2) and 8-iso-PGF2α, have been identified as lipidperoxidation biomarkers in diabetic embryopathy19. In the present study, we have further identified two additional lipidperoxidation biomarkers, MDA and 4-HNE in diabetic embryopathy. The protein adducts resulting from MDA or 4-HNE modifications are induced by oxidative stress. However, SOD1 overexpression abolishes maternal hyperglycemia-increased activities of these two biomarkers. MDA, a low-molecular-weight aldehyde, is a byproduct of oxidized polyunsaturated fatty acid such as AA. The increase in MDA in embryos exposed to diabetes provides further evidence that AA metabolism is altered in diabetic embryopathy. MDA cross-links to protein to form protein adducts which is cytotoxic to the cell31. Plasma MDA levels are robust indicators of oxidative stress in pathophysiological conditions32, 33, suggesting that MDA may be a suitable peripheral biomarker in diabetic embryopathy. 4-HNE is also a cytotoxic aldehyde and is formed during oxidation of omega-6 fatty acids. 4-HNE reacts with several amino acid residues of proteins to form 4-HNE-protein adducts. 4-HNE can directly induce cell apoptosis34, indicating that 4-HNE may directly participate in hyperglycemia-induced apoptosis leading to malformations. 4-HNE can activate c-Jun-N-Terminal kinase (JNK)35, which plays a causative role in the induction of diabetic embryopathy26, suggesting that 4-HNE may act as an intermediate in the link between PKC activation and JNK activation in hyperglycemia-induced malformations.
Our data provide strong evidence that the activity of MARCKS is increased in concert with the activation of PKCα/βII and PKCδ in embryos exposed to maternal hyperglycemia, whereas RACK1 levels are not affected. This is due to the lack of antibody against phosphorylated RACK1 which directly reflects the activity of RACK136. Nevertheless, we have determined that SOD1 overexpression blocks hyperglycemia-enhanced MARCKS activities concurrent with the inhibition of PKCα/βII and PKCδ activation. These findings implicate MARCKS as an important substrate of these PKC isoforms in diabetic embryopathy.
Non-phosphorylated MARCKS is associated with cell membrane via its myristoylation domian37. Membrane-associated MARCKS binds to calmodulin, membrane lipids and F-actin filaments21. PKC phosphorylates the serine residues of MARCKS thus induces MARCKS translocation from the membrane to the cytosol38. It will be of interest to examine if MARCKS membrane disassociation resulted from phosphorylation contributes to hyperglycemia-induced lipidperoxidation and subsequent cell membrane damage. PKC-induced MARCKS phosphorylation may disrupt some of the physiological functions of MARCKS such as cell adhesion, membrane trafficking and cell motility39. In addition, myristoylation and phosphorylation may be two counteractive events in regulation of MARCKS functions under physiological and pathophysiological conditions. Future studies may focus on the differential effects of MARCKS myristoylation and phosphorylation on hyperglycemia-induced oxidative stress, apoptosis and malformations.
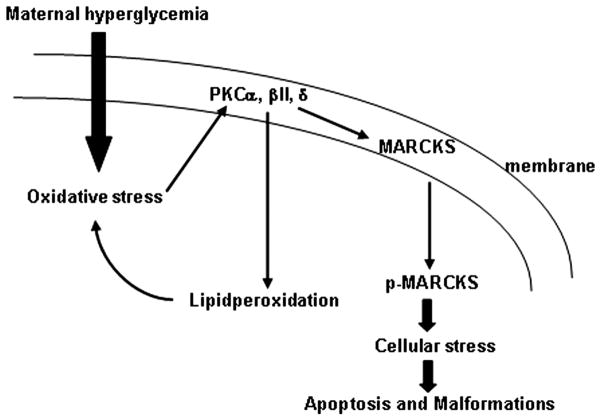
In summary, our study demonstrates that hyperglycemia specifically induces PKCα/βII and PKCδ phosphorylation, and that oxidative stress is responsible for the activation of these specific PKC isoforms (Fig. 4). The activation of these PKC isoforms triggers lipidperoxidation and MARCKS phosphorylation, which may lead to cellular stress and consequently apoptosis and malformations (Fig. 4). SOD1 overexpression abolishes this PKC cascade activation, suggesting that the PKC cascade mediates the teratogenicity of oxidative stress in diabetic embryopathy. Our results provide mechanistic basis for the development of antioxidant and specific PKCα/βII and PKCδ inhibitors to prevent against human NTDs. Antioxidants have been shown effective in animal studies10–12 and we have proposed multi-vitamin clinical trails in diabetic pregnant women. Epidemiological studies1, 40 have shown that pregnant women who take periconceptional multi-vitamin have lower risks to have a baby with birth defects than those who do not. Development of safe and effective PKC isoforms specific inhibitor will be new area with much potential to prevent against maternal-diabetes-induced birth defects.
Figure 4.
A schematic shows the oxidative stress-induced PKC cascade in diabetic embryopathy. Maternal hyperglycemia induced-oxidative stress activates PKCα/βII and PKCδ, which trigger lipidperoxidation and MARCKS phosphorylation. Lipidperoxidation and translocation of p-MARCKS from the cell membrane result in cell membrane damage and cellular stress leading to apoptosis.
Acknowledgments
This research is supported by NIH R01 DK083243 (Peixin Yang) and DK083770 (E. Albert Reece). We thank Ms. Hua Li for her technical assistance.
Footnotes
Reprint Requests: Not Available
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Correa A, Gilboa SM, Besser LM, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237, e1–9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Borg LA, Eriksson UJ. Altered metabolism and superoxide generation in neural tissue of rat embryos exposed to high glucose. Am J Physiol. 1997;272:E173–80. doi: 10.1152/ajpendo.1997.272.1.E173. [DOI] [PubMed] [Google Scholar]
- 3.Yang X, Borg LA, Siman CM, Eriksson UJ. Maternal antioxidant treatments prevent diabetes-induced alterations of mitochondrial morphology in rat embryos. Anat Rec. 1998;251:303–15. doi: 10.1002/(SICI)1097-0185(199807)251:3<303::AID-AR5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Sakamaki H, Akazawa S, Ishibashi M, et al. Significance of glutathione-dependent antioxidant system in diabetes-induced embryonic malformations. Diabetes. 1999;48:1138–44. doi: 10.2337/diabetes.48.5.1138. [DOI] [PubMed] [Google Scholar]
- 5.Sivan E, Lee YC, Wu YK, Reece EA. Free radical scavenging enzymes in fetal dysmorphogenesis among offspring of diabetic rats. Teratology. 1997;56:343–9. doi: 10.1002/(SICI)1096-9926(199712)56:6<343::AID-TERA1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198:130, e1–7. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 7.Yang P, Zhao Z, Reece EA. Blockade of c-Jun N-terminal kinase activation abrogates hyperglycemia-induced yolk sac vasculopathy in vitro. Am J Obstet Gynecol. 2008;198:321, e1–7. doi: 10.1016/j.ajog.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am J Obstet Gynecol. 203:75, e1–6. doi: 10.1016/j.ajog.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Cao Y, Li H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am J Obstet Gynecol. 203:185, e5–11. doi: 10.1016/j.ajog.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reece EA, Wu YK. Prevention of diabetic embryopathy in offspring of diabetic rats with use of a cocktail of deficient substrates and an antioxidant. Am J Obstet Gynecol. 1997;176:790–7. doi: 10.1016/s0002-9378(97)70602-1. discussion 797–8. [DOI] [PubMed] [Google Scholar]
- 11.Reece EA, Wu YK, Wiznitzer A, et al. Dietary polyunsaturated fatty acid prevents malformations in offspring of diabetic rats. Am J Obstet Gynecol. 1996;175:818–23. doi: 10.1016/s0002-9378(96)80005-6. [DOI] [PubMed] [Google Scholar]
- 12.Reece EA, Wu YK, Zhao Z, Dhanasekaran D. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. Am J Obstet Gynecol. 2006;194:580–5. doi: 10.1016/j.ajog.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 13.Hagay ZJ, Weiss Y, Zusman I, et al. Prevention of diabetes-associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am J Obstet Gynecol. 1995;173:1036–41. doi: 10.1016/0002-9378(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–38. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu Y, Sekiguchi N, Hayashi M, et al. Diacylglycerol production and protein kinase C activity are increased in a mouse model of diabetic embryopathy. Diabetes. 2002;51:2804–10. doi: 10.2337/diabetes.51.9.2804. [DOI] [PubMed] [Google Scholar]
- 16.Zhiyong Z, Wu YK, Reece EA. Demonstration of the essential role of protein kinase C isoforms in hyperglycemia-induced embryonic malformations. Reprod Sci. 2008;15:349–56. doi: 10.1177/1933719108316986. [DOI] [PubMed] [Google Scholar]
- 17.von Ruecker AA, Han-Jeon BG, Wild M, Bidlingmaier F. Protein kinase C involvement in lipid peroxidation and cell membrane damage induced by oxygen-based radicals in hepatocytes. Biochem Biophys Res Commun. 1989;163:836–42. doi: 10.1016/0006-291x(89)92298-5. [DOI] [PubMed] [Google Scholar]
- 18.Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 19.Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered prostaglandin E2 levels in rat embryos exposed to a diabetic environment. Diabetes. 1999;48:813–20. doi: 10.2337/diabetes.48.4.813. [DOI] [PubMed] [Google Scholar]
- 20.Pinter E, Reece EA, Leranth CZ, et al. Arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy. Am J Obstet Gynecol. 1986;155:691–702. doi: 10.1016/s0002-9378(86)80001-1. [DOI] [PubMed] [Google Scholar]
- 21.Blackshear PJ. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993;268:1501–4. [PubMed] [Google Scholar]
- 22.Chappell DS, Patel NA, Jiang K, et al. Functional involvement of protein kinase C-betaII and its substrate, myristoylated alanine-rich C-kinase substrate (MARCKS), in insulin-stimulated glucose transport in L6 rat skeletal muscle cells. Diabetologia. 2009;52:901–11. doi: 10.1007/s00125-009-1298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, O'Connor KL, Greeley GH, Jr, Blackshear PJ, Townsend CM, Jr, Evers BM. Myristoylated alanine-rich C kinase substrate-mediated neurotensin release via protein kinase C-delta downstream of the Rho/ROK pathway. J Biol Chem. 2005;280:8351–7. doi: 10.1074/jbc.M409431200. [DOI] [PubMed] [Google Scholar]
- 24.Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci U S A. 1994;91:839–43. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–73. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- 26.Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357:749–54. doi: 10.1016/j.bbrc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, Kriatchko A, Roy SK. Expression of ER-alpha and ER-beta in the hamster ovary: differential regulation by gonadotropins and ovarian steroid hormones. Endocrinology. 2002;143:2385–98. doi: 10.1210/endo.143.6.8858. [DOI] [PubMed] [Google Scholar]
- 28.Gurney ME, Pu H, Chiu AY, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 29.Gareskog M, Cederberg J, Eriksson UJ, Wentzel P. Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol. 2007;23:63–74. doi: 10.1016/j.reprotox.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. Am J Obstet Gynecol. 2005;192:967–72. doi: 10.1016/j.ajog.2004.10.592. [DOI] [PubMed] [Google Scholar]
- 31.Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Thorpe SR. Lipoxidation products as biomarkers of oxidative damage to proteins during lipid peroxidation reactions. Nephrol Dial Transplant. 1996;11 (Suppl 5):48–53. doi: 10.1093/ndt/11.supp5.48. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, GRANDJEAN P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–14. [PubMed] [Google Scholar]
- 33.Papalambros E, Sigala F, Georgopoulos S, et al. Malondialdehyde as an indicator of oxidative stress during abdominal aortic aneurysm repair. Angiology. 2007;58:477–82. doi: 10.1177/0003319707305246. [DOI] [PubMed] [Google Scholar]
- 34.Vaillancourt F, Fahmi H, Shi Q, et al. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinna A, Forman HJ. SHP-1 inhibition by 4-hydroxynonenal activates Jun N-terminal kinase and glutamate cysteine ligase. Am J Respir Cell Mol Biol. 2008;39:97–104. doi: 10.1165/rcmb.2007-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang BY, Harte RA, Cartwright CA. RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene. 2002;21:7619–29. doi: 10.1038/sj.onc.1206002. [DOI] [PubMed] [Google Scholar]
- 37.Seykora JT, Myat MM, Allen LA, Ravetch JV, Aderem A. Molecular determinants of the myristoyl-electrostatic switch of MARCKS. J Biol Chem. 1996;271:18797–802. doi: 10.1074/jbc.271.31.18797. [DOI] [PubMed] [Google Scholar]
- 38.Thelen M, Rosen A, Nairn AC, Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991;351:320–2. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- 39.Ramsden JJ. MARCKS: a case of molecular exaptation? Int J Biochem Cell Biol. 2000;32:475–9. doi: 10.1016/s1357-2725(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 40.Kitzmiller JL, Wallerstein R, Correa A, Kwan S. Preconception care for women with diabetes and prevention of major congenital malformations. Birth Defects Res A Clin Mol Teratol. 88:791–803. doi: 10.1002/bdra.20734. [DOI] [PubMed] [Google Scholar]