Abstract
AIM: To investigate the expression of programmed death (PD)-1, PD ligand 1 (PD-L1) and PD-L2 in liver tissues in the context of chronic hepatitis and hepatocellular carcinoma (HCC).
METHODS: Liver biopsies and HCC specimens from patients were collected and histologically examined. The expression of PD-1, PD-L1, and PD-L2 in biopsy specimens of chronic hepatitis and HCC specimens was evaluated by immunohistochemical staining. The association between the expression level of PD-1, PD-L1, and PD-L2 and clinical and pathological variables was analyzed statistically.
RESULTS: Expression of PD-1 was found in liver-infiltrating lymphocytes. In contrast, PD-L1 and PD-L2 were expressed in non-parenchyma liver cells and tumor cells. The expression of PD-L1 was significantly correlated with hepatitis B virus infection (1.42 ± 1.165 vs 0.50 ± 0.756, P = 0.047) and with the stage of HCC (7.50 ± 2.121 vs 1.75 ± 1.500 vs 3.00 ± 0.001, P = 0.018). PD-1 and PD-Ls were significantly up-regulated in HCC specimens (1.40 ± 1.536 vs 5.71 ± 4.051, P = 0.000; 1.05 ± 1.099 vs 4.29 ± 3.885, P = 0.004; 1.80 ± 1.473 vs 3.81 ± 3.400, P = 0.020).
CONCLUSION: PD-L1 may contribute to negative regulation of the immune response in chronic hepatitis B. PD-1 and PD-Ls may play a role in immune evasion of tumors.
Keywords: Hepatitis B virus, Programmed death-1, Programmed death ligands, Hepatitis, Hepatocellular carcinoma
INTRODUCTION
Though active vaccination against hepatitis B virus (HBV) infection is successful, chronic HBV infection remains an important medical issue. There are approximately 400 million individuals chronically infected with HBV worldwide. Chronic HBV infection is one of the major causes of liver cirrhosis and hepatocellular carcinoma (HCC).
It is generally accepted that an appropriate T-cell response to HBV is crucial for viral elimination[1]. It has been suggested that HBV-specific CD8+ T cells have dual functions: the production of antiviral cytokines to down-regulate HBV replication in hepatocytes and the elimination of residual HBV-infected cells by cytotoxic activities[2]. Chronic HBV infection is characterized by weak or absent specific T-cell responses to HBV in peripheral blood. In the liver, low numbers of HBV-specific CD4 and CD8 T cells have been found. However, those T cells show a low expression of interferon (IFN)-γ and low cytotoxic activity[3,4]. Mechanisms leading to decreased cellular immune responses to HBV are not yet defined. Though there are many approaches suitable for priming specific CTL responses, their ability to break immune tolerance mechanisms in chronically infected HBV patients requires further investigation. In this respect, understanding tolerance mechanisms in chronic HBV infection will further help the design of strategies to overcome the unresponsiveness of T cells.
Programmed death (PD)-1 is a member of the CD28 family and is involved in the regulation of T-cell activation[5]. PD-1 is expressed on T cells, B cells, and myeloid cells. Two ligands for PD-1, PD ligand 1 (PD-L1) and PD-L2 (also known as B7-H1 and B7-DC), have been identified and have features as co-stimulatory molecules. A large number of publications have indicated a role for PD-L1 in the negative regulation of T-cell functions and the maintenance of peripheral tolerance[6]. The exact role of PD-L2 requires further definition. In murine liver tissues, PD-L1 was found to be expressed on Kupffer cells (KCs) and liver sinusoidal epithelial cells (LSECs)[7]. Hepatocytes express constitutively low levels of PD-L1 but show enhanced expression of PD-L1 upon stimulation with interferons[8]. It has also been shown in cell culture that PD-L1 expression on hepatoma cells induces apoptosis in T-cells. PD-L1 deficiency leads to hepatic accumulation and impaired apoptosis of T-cells in a murine model[9], and PD-1 deficiency leads to enhanced proliferation of effector cells in the liver during adenoviral infection[10].
Recent data from other chronic viral infections, such as lymphocytic choriomeningitis virus, human immunodeficiency virus, and hepatitis C virus infections, indicate that up-regulation of the PD-1/PD-L1 system may be responsible for the impairment of T-cell function[11-18]. Blocking of PD-L1 led to the rescue of exhausted CD8 T-cells even in the absence of Th functions[15-18]. Kassel et al[19] examined the expression of PD-1, PD-L1, and PD-L2 in patient liver samples and found that there was a direct link between the degree of inflammation and the expression of PD-1/PD-L1. Gao et al[20] found in patients with HCC that overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence.
The expression of PD-Ls was further suggested as a mechanism of immune evasion for tumors[21]. Elevated expression of PD-L1 was found in different tumor entities[22-29]. It could be shown that tumor cells expressing PD-L1 were able to induce apoptosis of T-cells. Thus, it is necessary to investigate the expression of PD-L1/2 and PD-1 in the liver in the context of viral hepatitis and HCC.
MATERIALS AND METHODS
Specimens
Specimens of liver tissues were obtained by biopsy or surgery from 20 hepatitis patients (including 12 with HBV infection and 8 with non-viral hepatitis) and 26 HCC patients (including 21 with HBV infection) at Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, China, between 2006 and 2007 (Tables 1, 2 and 3). Surgically resected or biopsy specimens were fixed in formalin and embedded in paraffin for routine histological diagnosis, and then embedded in OCT compound and snap frozen in liquid nitrogen for immunohistochemical analysis. The histological activity index (HAI) was assessed according to the classification of Ishak et al[30], which combines grading and staging scores (Table 2). All patients with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection were excluded. No patient underwent antiviral drug treatment prior to biopsy. Tumors were classified as stage I to III based on the Chengdu conference[31] and as grade I to IV based on the Edmondson-Steiner Guidelines (Table 3). The Chengdu conference stage classification was based on tumor dimension and lobar distribution, vascular thrombosis, lymph node metastasis, distant metastasis and Child-Pugh staging. No patient underwent radiation or chemotherapy prior to surgery.
Table 1.
Patient information
| Patients | Age (mean, yr) | Sex (M/F) | Etiology (viral/non-viral) |
| Hepatitis | 36.6 | 14/6 | 12/8 |
| HCC | 49.3 | 21/5 | 21/5 |
| Total | 43.8 | 35/11 | 33/13 |
HCC: Hepatocellular carcinoma.
Table 2.
Characteristics of patient samples with hepatitis
| No. | Age (yr) | Sex | Disease, etiology | HAI |
| LB10 | 35 | M | Hepatitis, HBV | 2 |
| LB24 | 18 | M | Hepatitis, HBV | 2 |
| LB18 | 43 | M | Hepatitis, HBV | 3 |
| LB23 | 33 | M | Hepatitis, HBV | 3 |
| LB26 | 36 | F | Hepatitis, HBV | 3 |
| LB11 | 72 | M | Hepatitis, HBV | 4 |
| LB21 | 40 | M | Hepatitis, HBV | 4 |
| LB7 | 26 | M | Hepatitis, HBV | 4 |
| LB9 | 40 | F | Hepatitis, HBV | 5 |
| LB25 | 25 | M | Hepatitis, HBV | 6 |
| LB31 | 28 | M | Hepatitis, HBV | 6 |
| LB8 | 22 | M | Hepatitis, HBV | 13 |
| LB4 | 36 | F | Hepatitis | 2 |
| LB19 | 56 | F | Hepatitis | 2 |
| LB22 | 28 | F | Hepatitis | 2 |
| LB16 | 43 | M | Hepatitis | 3 |
| LB20 | 42 | F | Hepatitis | 3 |
| LB27 | 19 | M | Hepatitis | 3 |
| LB30 | 55 | M | Hepatitis | 3 |
| LB17 | 35 | M | Hepatitis | 4 |
HBV: Hepatitis B virus; HAI: Histological activity index.
Table 3.
Characteristics of patient samples with hepatocellular carcinoma
| No. | Sex | Age (yr) | Disease, etiology | Grade | Stage |
| c002 | M | 71 | HCC, HBV | I | I |
| c005 | M | 42 | HCC, HBV | I | I |
| c025 | M | 34 | HCC, HBV | I | I |
| c009 | M | 58 | HCC, HBV | I | IIb |
| c021 | M | 50 | HCC, HBV | I | IIb |
| c042 | M | 48 | HCC, HBV | I | |
| c045 | M | 49 | HCC, HBV | I | |
| c014 | M | 48 | HCC, HBV | II | I |
| c046 | F | 53 | HCC, HBV | II | |
| c023 | F | 34 | HCC, HBV | III | IIa |
| c027 | M | 45 | HCC, HBV | III | IIa |
| c026 | F | 18 | HCC, HBV | III | IIb |
| c029 | M | 43 | HCC, HBV | III | IIb |
| c043 | M | 61 | HCC, HBV | III | |
| c044 | M | 56 | HCC, HBV | III | |
| c020 | M | 49 | HCC, HBV | IV | IIb |
| c040 | M | 52 | HCC, HBV | IV | |
| c004 | M | 64 | HCC, HBV | ||
| c011 | M | 41 | HCC, HBV | ||
| c019 | F | 40 | HCC, HBV | ||
| c028 | M | 50 | HCC, HBV | ||
| c015 | M | 48 | HCC | I | I |
| c001 | M | 37 | HCC | I | IIa |
| c003 | M | 62 | HCC | I | IIa |
| c007 | F | 65 | HCC | III | IIa |
| c022 | M | 64 | HCC | IV | IIa |
HCC: Hepatocellular carcinoma; HBV: Hepatitis B virus.
Immunohistochemical staining
Four micron sections of the specimens were air-dried for 10 min and then fixed in acetone for 10 min. Endogenous peroxidase activity was blocked by treatment with 0.3% hydrogen peroxidase in PBS for 30 min at room temperature. Sections were then washed three times in PBS. After blocking nonspecific binding with normal goat serum for 20 min at room temperature, sections were incubated with primary antibodies in a humidified chamber at 4°C overnight. Anti-PD-1 (J116), anti-PD-L1 (MIH1), anti-PD-L2 (MIH18), anti-FoxP3 (236A/E7) and anti-CD3(UCHT1) antibodies were purchased from eBioscience (San Diego, CA) and used as primary antibodies at the final concentrations of 5, 10, 5, 10 and 5 μg/mL, respectively. The bound primary antibodies were detected with a Dako Envision™ Kit according to the manufacturer’s instructions, and sections were counterstained with hematoxylin. IgG fractions from naïve mice were used instead of the primary antibody as negative controls.
The expression levels of PD-1, PD-L1 and PD-L2 were defined as the quickscore which was calculated according to the Detre S’s method[32]. In brief, the proportion of cells staining positively throughout the section was termed category A and was assigned scores from 1 to 6 (1 = 0%-4%; 2 = 5%-19%; 3 = 20%-39%; 4 = 40%-59%; 5 = 60%-79%; 6 = 80%-100%). The whole section was scanned at low power in order to gauge the general level of intensity throughout. The average intensity, corresponding to the presence of negative, weak, intermediate, and strong staining, was given a score from 0 to 3, respectively, and termed category B. The product (A × B) was recorded as the quickscore.
Statistical analysis
The association between the expression level of PD-1, PD-L1, and PD-L2 and clinical and pathological variables was analyzed statistically by the Student’s t test and Correlation analysis using SPSS 12.0 software. Values of P < 0.05 were considered to indicate statistical significance, and all tests were two-tailed.
RESULTS
Immunostaining of PD-1
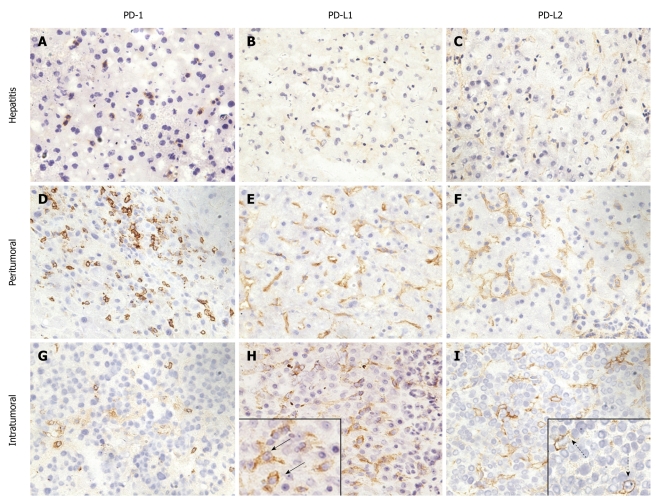
Typically, lymphocyte infiltration occurred in liver tissues of patients with hepatitis (Figure 1A). In these tissues, lymphocytes were recognized as small mononuclear cells and showed positive staining with anti-PD-1. The pattern of staining was consistent with the fact that PD-1 molecules are associated with cell membranes. The PD-1 positive cells showed a scattered pattern in liver tissues. Liver cells were not stained with anti-PD-1, which is consistent with the tissue specificity of PD-1 expression. The expression of PD-1 in liver tissues from patients with hepatitis had no relation to age, gender, alanine aminotransferase (ALT) and total bilirubin (TB) level, HAI or etiology (Table 4).
Figure 1.
Immunohistochemical staining of liver tissues from patients with hepatitis and hepatocellular carcinoma for programmed death-1, programmed death-L1, and programmed death-L2. A-C: Programmed death (PD)-1 (A), PD ligand 1 (PD-L1) (B), and PD-L2 (C) expression in liver tissues with hepatitis; D-I: PD-1 (D and G), PD-L1 (E and H), and PD-L2 (F and I) expression in liver tissues with hepatocellular carcinoma (HCC); D-F: Peritumoral region; G-I: Intratumoral region. A, D and G: PD-1 was expressed on the membrane of infiltrated lymphocytes in liver tissues with hepatitis and HCC; B, E and H: PD-L1 was expressed on the membrane of hepatic cells and/or tumor cells in liver tissues with hepatitis and HCC; C, F and I: PD-L2 was expressed on the membrane of hepatic cells and/or tumor cells in liver tissues with hepatitis and HCC. Solid arrows indicate PD-L1+ tumor cells, and dashed arrows indicate PD-L2+ tumor cells. Magnification 200 ×.
Table 4.
Programmed death 1/programmed death ligands expression in liver tissues of patients with hepatitis (mean ± SD)
| n | PD-1 | P | PD-L1 | P | PD-L2 | P | |
| Age (yr) | |||||||
| < 36.6 | 12 | 1.67 ± 1.826 | 0.297 | 1.08 ± 1.240 | 0.873 | 1.83 ± 1.467 | 0.905 |
| ≥ 36.6 | 8 | 1.00 ± 0.926 | 1.00 ± 0.926 | 1.75 ± 1.581 | |||
| Gender | |||||||
| Male | 14 | 1.43 ± 1.604 | 0.903 | 1.07 ± 1.207 | 0.898 | 1.71 ± 1.437 | 0.702 |
| Female | 6 | 1.33 ± 1.506 | 1.00 ± 0.894 | 2.00 ± 1.673 | |||
| ALT level (U/L) | |||||||
| Normal (≤ 40) | 11 | 1.45 ± 1.440 | 0.866 | 1.27 ± 1.191 | 0.33 | 2.00 ± 1.673 | 0.517 |
| Abnormal (> 40) | 9 | 1.33 ± 1.732 | 0.78 ± 0.972 | 1.56 ± 1.236 | |||
| TB level (μmol/L) | |||||||
| Normal (≤ 19) | 13 | 1.46 ± 1.613 | 0.814 | 0.92 ± 0.862 | 0.496 | 1.77 ± 1.691 | 0.903 |
| Abnormal (> 19) | 7 | 1.29 ± 1.496 | 1.29 ± 1.496 | 1.86 ± 1.069 | |||
| HAI score | |||||||
| < 4 | 12 | 1.00 ± 1.477 | 0.159 | 0.67 ± 0.778 | 0.053 | 1.50 ± 1.567 | 0.276 |
| ≥ 4 | 8 | 2.00 ± 1.512 | 1.62 ± 1.302 | 2.25 ± 1.282 | |||
| Etiology | |||||||
| HBV | 12 | 1.83 ± 1.528 | 0.125 | 1.42 ± 1.165 | 0.047 | 2.08 ± 1.311 | 0.304 |
| Unknown | 8 | 0.75 ± 1.389 | 0.50 ± 0.756 | 1.38 ± 1.685 |
PD: Programmed death; PD-L: Programmed death ligand; HBV: Hepatitis B virus; ALT: Alanine aminotransferase; TB: Total bilirubin; HAI: Histological activity index.
In peritumoral regions of HCC and within tumor tissues, PD-1 positive cells accumulated as massive lymphocyte infiltration took place (Figure 1D and G). Therefore, the expression level of PD-1 was determined by lymphocyte infiltration and was not dependent on the parameters of age, gender, tumor stage or grade, or HBV infection (Table 5).
Table 5.
Programmed death 1/programmed death ligands expression in liver tissues of patients with hepatocellular carcinoma (mean ± SD)
| n | PD-1 | P | PD-L1 | P | PD-L2 | P | |
| Age (yr) | |||||||
| < 49.3 | 21 | 6.45 ± 4.510 | 0.397 | 4.56 ± 4.304 | 0.779 | 4.73 ± 3.524 | 0.202 |
| ≥ 49.3 | 5 | 4.90 ± 3.872 | 4.00 ± 3.625 | 2.80 ± 3.120 | |||
| Gender | |||||||
| Male | 21 | 5.60 ± 4.154 | 0.844 | 4.45 ± 4.390 | 0.826 | 3.67 ± 3.619 | 0.769 |
| Female | 5 | 6.00 ± 4.147 | 4.00 ± 3.098 | 4.17 ± 3.061 | |||
| Stage | |||||||
| I | 5 | 4.75 ± 2.500 | 0.252 | 7.50 ± 2.121 | 0.0181 | 2.75 ± 1.893 | 0.624 |
| IIa | 6 | 7.00 ± 5.612 | 1.75 ± 1.500 | 2.60 ± 2.302 | |||
| IIb | 5 | 1.67 ± 1.528 | 3.00 ± 0.001 | 1.33 ± 1.528 | |||
| Grade | |||||||
| I, II | 12 | 5.78 ± 3.632 | 0.958 | 6.80 ± 5.495 | 0.153 | 4.00 ± 3.775 | 0.736 |
| III, IV | 10 | 5.67 ± 4.975 | 2.44 ± 1.878 | 3.44 ± 3.046 | |||
| Etiology | |||||||
| HBV | 21 | 5.60 ± 4.154 | 0.844 | 4.69 ± 4.231 | 0.464 | 3.73 ± 3.615 | 0.876 |
| Unknown | 5 | 6.00 ± 4.147 | 3.00 ± 2.449 | 4.00 ± 3.098 |
The significance among different stages is mainly the difference between stage I and Stage II (including IIa and IIb) according to the SNK analysis. PD: Programmed death; PD-L: Programmed death ligand; HBV: Hepatitis B virus.
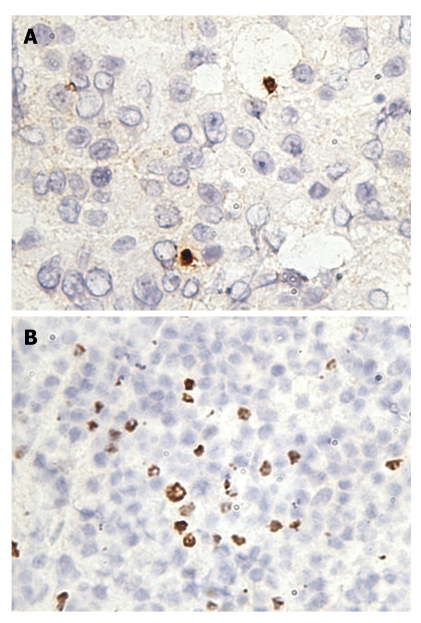
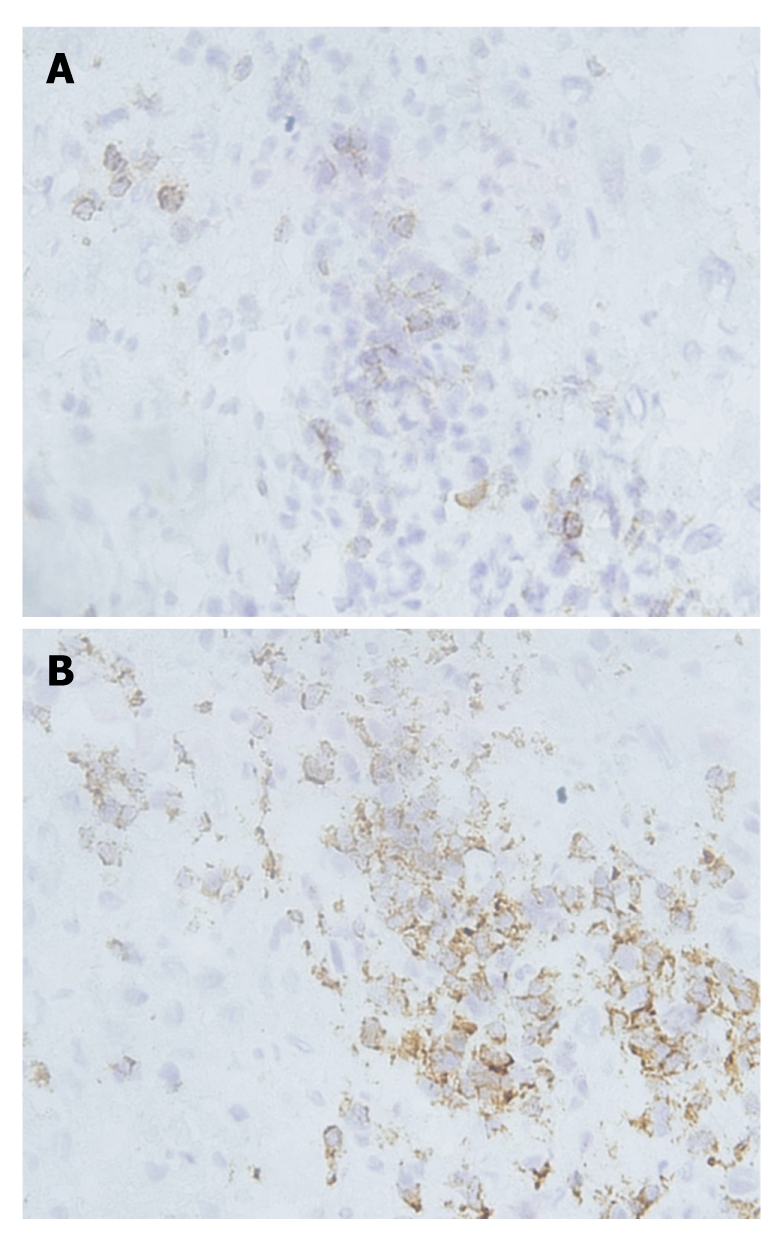
PD-1 was expressed on infiltrating lymphocytes (Figure 2A). These lymphocytes were CD3 positive, as both T-cell types were present in liver tissues and HCC tissues (Figure 2B). In addition, a significant portion of infiltrating lymphocytes were FoxP3 positive, indicating that negative regulation by regulatory T cells is operative in HCC (Figure 3).
Figure 2.
Expression of CD3 and programmed death-1 on regular liver sections from a patient with hepatocellular carcinoma. Serial sections were prepared from the same liver sample of a patient with hepatocellular carcinoma; CD3 and programmed death (PD)-1 were stained on these serial sections. CD3 was expressed on the membrane of liver-infiltrating lymphocytes, and the expression of PD-1 had a similar pattern to that of CD3, indicating PD-1 was also expressed on liver-infiltrating lymphocytes. A: PD-1; B: CD3.
Figure 3.
Immunohistochemical staining of liver tissues from patients with hepatocellular carcinoma for FoxP3. FoxP3 was expressed in liver-infiltrating lymphocytes in liver tissues with hepatocellular carcinoma. Liver infiltrating lymphocytes were mainly FoxP3 positive. A: Intratumoral region; B: Peritumoral region.
Immunostaining of PD-L1 and PD-L2 in hepatitis
It has been reported that PD-L1 and PD-L2 are abundantly expressed on KCs and LSECs[7]. In liver tissues with hepatitis, the expression of PD-L1 and PD-L2 was weakly detectable (Figure 1B and C), and the percentages of PD-L1 and PD-L2 positive cells were found to be only 0.57% and 1.29% of total cell counts, respectively. Cells with PD-L1 and PD-L2 expression were clearly different from lymphocytes and hepatocytes and represented rather KCs and LSECs.
The PD-L1 expression in liver tissues from patients with hepatitis had no relation to age, gender, or ALT and TB level (Table 4). Enhanced PD-L1 expression seemed to be associated with HBV infection, as compared with non-viral hepatitis (Table 4). In contrast, there was no relationship between PD-L2 expression and age, gender, ALT and TB level, or the etiology of hepatitis (Table 4).
The HAI was assessed to evaluate inflammation in the liver according to the classification of Ishak et al[30]. As shown in Table 4, a weak correlation between PD-L1 expression and HAI was found, which is consistent with previous observations that PD-L1 expression can be stimulated by viral infection and further pulse with IFNs[8]. The correlation between HAI and PD-L1 expression might be explained by the production of proinflammatory cytokines by infiltrating lymphocytes in chronic viral hepatitis. In contrast, there was no relationship between PD-L2 expression and HAI (Table 4).
Immunostaining of PD-L1 and PD-L2 in HCC
It has been reported that PD-L1 is abundantly expressed on cancer cells in various types of tumors. Therefore, 26 HCC specimens and peritumoural tissues prepared from surgery were examined for the expression of PD-L1 and PD-L2. The tumors were classified as stage I (n = 5), stage IIa (n = 6), or stage IIb (n = 5) based on the Chengdu conference, and as grade I (n = 10), grade II (n = 2), grade III (n = 7), or grade IV (n = 3) tumors based on the Edmondson-Steiner Guidelines (Table 3).
In peritumoural tissues, the expression of PD-L1 and PD-L2 was significantly elevated. However, the expression of PD-Ls appeared to still be restricted to cell types like KCs and LESCs (Figure 1E and F). Compared with liver tissues from patients with hepatitis, the expression of PD-Ls was recognizably enhanced in intensity for IHC staining with respective antibodies.
PD-L1 and PD-L2 had focal or scattered expression in 24 (92.6%) and 23 (88.9%) of 26 HCC specimens, respectively (Figure 1H and I). Thus, the enhanced expression of PD-Ls was a general phenomenon in HCC. In tumor tissues, tumor cells expressing PD-L1 and PD-L2 were detected. These cells had a different morphological appearance and were characterized by large nuclei surrounded by thick cytoplasm (Figure 1H and I). Tumor cells were heterogeneous in regards to the expression of PD-L1 and PD-L2 molecules, and less than 50% of cells in tumor tissues expressed these molecules. Thus, the expression of PD-L1 and PD-L2 did not appear to be regulated by an exogenous inducer for cancer cells, but rather was an intrinsic characteristic of these cancer cells.
The expression of PD-L1 was more pronounced at an early stage of HCC, while there was a lower level of PD-L1 expression with increasing stage of HCC (Table 5). Thus, the expression of PD-L1 appeared to be an early event during tumor progression. In contrast, there was no significant correlation between tumor grade and PD-L1 expression level. In addition, HBV infection had no significant influence on PD-L1 or PD-L2 expression in HCC (Table 5).
Compared to liver tissues from patients with chronic HBV infection, PD-1, PD-L1, and PD-L2 expression levels in HCC were greatly enhanced (Table 6).
Table 6.
Comparison of programmed death 1/programmed death-L expression in liver tissues of patients with hepatitis or hepatocellular carcinoma (mean ± SD)
| Diagnosis | n | PD-1 | P | PD-L1 | P | PD-L2 | P |
| Hepatitis | 20 | 1.40 ± 1.536 | 0.000 | 1.05 ± 1.099 | 0.004 | 1.80 ± 1.473 | 0.020 |
| HCC | 26 | 5.71 ± 4.051 | 4.29 ± 3.885 | 3.81 ± 3.400 |
PD: Programmed death; PD-L: Programmed death ligand; HCC: Hepatocellular carcinoma.
DISCUSSION
It has been demonstrated that the PD-1/PD-L1/PD-L2 system can deliver a negative signal to T cells and lead to T-cell exhaustion or apoptosis[9,10,33,34]. To assess the role of the PD-1/PD-L1/PD-L2 system in chronic HBV infection and HCC, we analyzed the expression of these molecules in liver tissues from patients with these conditions. From the results of this study, we conclude the following: (1) the expression of PD-L1 and PD-L2 is detectable on KCs and LSECs in liver tissues with viral and non-viral hepatitis; (2) PD-L1 expression in liver tissues is enhanced in patients with chronic hepatitis B; and (3) PD-1 and PD-Ls expression are significantly enhanced in peritumoral and tumor tissues. The first two findings are in agreement with previous findings[19,20]. It will be interesting to investigate the role of PD-L1 expression in chronic HBV infection. While KCs and LSECs are the cell types expressing PD-L1 and PD-L2 in peritumoral tissues, a portion of tumor cells in HCC gained the ability to express PD-L1 and PD-L2. PD-L1 and PD-L2 expression in tumor tissues occurred in the early stage and may represent an important contribution to immune evasion during tumor progression. Thus, HCC may evade immune control by different mechanisms including negative regulation of T-cells.
PD-1/PD-L1 expression may play a role in viral hepatitis[13,14,17,18]. Upon activation, T-cells express PD-1 and are therefore susceptible to negative signaling by its ligands[33,34]. In our work, it is evident that liver-infiltrating lymphocytes were positive for PD-1. The activated T-cells may enter liver tissues due to attraction by chemokines. Due to the expression of PD-L1 and PD-L2 on KCs and LSECs in liver tissues and on cancer cells in HCC, liver-infiltrating cells would frequently encounter negative signals and become “exhausted” T-cells.
In chronic viral infection, virus-specific T cells are dysfunctional either because of interaction with regulatory T cells[35] or interaction between PD-1 and its ligand PD-L1, which results in the down-regulation of T-cell functions[11-14]. Recently, blockage of PD-L1 emerged as an effective measure to promote the proliferation and functions of T-cells in lymphocytic choriomeningitis virus infection of mice[15]. Barber et al[15] demonstrated that virus-specific, exhausted cytotoxic T-lymphocytes regained their ability to proliferate and perform functions such as IFN-γ production and killing of target cells. In HIV- and HCV-positive patients, virus-specific cells also have high expression levels of PD-1, which may explain their dysfunction[12-14,16-18]. Therefore, the blockage of PD-L1 and PD-L2 may restore impaired T-cell functions in individuals with persistent HBV infection and may lead to effective immunological control of viral infection. It is evident that T-cell functions are impaired in patients chronically infected with HBV. In our work, PD-L1 is up-regulated in chronic HBV, suggesting that PD-L1 may play an important role in chronic HBV infection due to the negative control of proliferation and functions of liver-infiltrating T-cells. A recent study demonstrated that a blockage of PD-L1 could restore the functions of peripheral and intrahepatic T-cells from patients with chronic hepatitis B[36].
Our findings have important implications for immunotherapeutic approaches to chronic hepatitis and HCC. Until now, the focus of experimental approaches has been to improve the ability of vaccines to induce a broad, strong T-cell response. However, these efforts could be compromised by the fact that specific T cells may be unable to exert their functions in the liver due to negative regulation, despite effective priming in the peripheral and local lymphoid organs. Thus, future research on immunotherapeutic approaches should consider the blockage of negative T-cell regulation in combination with immunostimulation.
Taken together, PD-L1 and PD-L2 were expressed by KCs and LSECs independent of viral and non-viral hepatitis and were up-regulated by chronic HBV infection and in HCC. The presence of PD-L1 and PD-L2 may lead to the suppression of immune responses and therefore facilitate viral persistence and carcinogenesis. Furthermore, the expression of PD-1, PD-L1, and PD-L2 in HCC was significantly higher than in hepatitis and correlated with the stage of HCC and the number of infiltrating lymphocytes, indicating the importance of the PD-1/PD-Ls system in tumor development.
COMMENTS
Background
Chronic hepatitis B and hepatocellular carcinoma (HCC) remain the big problem in China and around the world. Immune tolerance characterized with weak or absent specific T-cell responses to hepatitis B virus (HBV) or tumor is responsible for the pathogenesis of hepatitis and tumor. In this content, understanding tolerance mechanisms in chronic hepatitis B and HCC will further help the design of strategies to overcome the unresponsiveness of T cells.
Research frontiers
It has been demonstrated that the programmed death (PD)-1/PD ligand 1 (PD-L1)/PD-L2 system can deliver a negative signal to T cells and lead to T-cell exhaustion or apoptosis. Recent data from other chronic viral infections, such as lymphocytic choriomeningitis virus, human immunodeficiency virus, and hepatitis C virus, and other tumors, such as breast cancer, pancreatic cancer, and non-small cell lung cancer, indicated that up-regulation of the PD-1/PD-L1 system may be responsible for the impairment of T-cell function. In this study, the authors investigated the expression of PD-L1/2 and PD-1 in the liver in the context of chronic hepatitis B and HCC.
Innovations and breakthroughs
Recent reports have highlighted the importance of PD-1/PD-Ls system as a negative immune regulator in pathogenesis of chronic viral infection and tumors. In this study, the authors found: (1) the expression of PD-L1 was significantly correlated with HBV infection and with the stage of HCC; (2) PD-1 and PD-Ls were significantly up-regulated in HCC specimens, which indicated for the first time that PD-L1 may contribute to negative regulation of the immune response in chronic hepatitis B; and (3) PD-1 and PD-Ls may play a role in immune evasion of HCC.
Applications
By identifying the fact that expression of PD-1/PD-Ls is highly up-regulated in hepatitis B and HCC, our study indicated the PD-1/PD-Ls system plays a role in immune tolerance of hepatitis B and HCC, and thus may represent a future strategy for therapeutic intervention in the treatment of patients with hepatitis B or HCC.
Terminology
PD-1 is a member of the CD28 family and is involved in the regulation of T-cell activation. PD-1 is expressed on T cells, B cells, and myeloid cells. Two ligands for PD-1, PD-L1 and PD-L2 (also known as B7-H1 and B7-DC), have been identified and have features as co-stimulatory molecules. A large number of publications have indicated a role for PD-L1 in the negative regulation of T-cell functions and the maintenance of peripheral tolerance. The exact role of PD-L2 requires further definition.
Peer review
This manuscript is impressive in that it investigated the expression of PD-1 and PD-Ls in liver tissue from patients with hepatitis and HCC using immunostaining in detail.
Footnotes
Supported by Grants from the National Mega Research Program of China, No. 2008ZX10002-011; the National Key Basic Research Program of China, No. 2001CB510008, 2005CB522901, 2007CB512804 and 2009CB522500; the Deutsche Forschungsgemeinschaft (SFB/Transregio 60)
Peer reviewer: Atsushi Nakajima, MD, PhD, Professor, Division of Gastroenterology, Yokohama City University Hospital 3-9 Fukuura, Kanazawaku, Yokohama 236-0004 Kanagawa, Japan
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
References
- 1.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari C, Penna A, Sansoni P, Giuberti T, Fiaccadori F. Clonal analysis of intrahepatic T lymphocytes in chronic active hepatitis. Isolation of a T-cell line specific for hepatitis B core antigen from a patient with serological evidence of exposure to HBV. J Hepatol. 1986;3:384–392. doi: 10.1016/s0168-8278(86)80493-7. [DOI] [PubMed] [Google Scholar]
- 4.Kakumu S, Ishikawa T, Wakita T, Yoshioka K, Takayanagi M, Tahara H, Kusakabe A. Interferon-gamma production specific for hepatitis B virus antigen by intrahepatic T lymphocytes in patients with acute and chronic hepatitis B. Am J Gastroenterol. 1994;89:92–96. [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subudhi SK, Alegre ML, Fu YX. The balance of immune responses: costimulation verse coinhibition. J Mol Med. 2005;83:193–202. doi: 10.1007/s00109-004-0617-1. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 8.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 10.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Elrefaei M, Baker CA, Jones NG, Bangsberg DR, Cao H. Presence of suppressor HIV-specific CD8+ T cells is associated with increased PD-1 expression on effector CD8+ T cells. J Immunol. 2008;180:7757–7763. doi: 10.4049/jimmunol.180.11.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 16.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 18.Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, Orlandini A, Missale G, Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625–1637. doi: 10.1002/hep.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 21.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 24.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 29.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 30.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 31.Sheng JM, Zhao WH, Wu FS, Ma ZM, Feng YZ, Zhou XR, Teng LS. The Chinese classification system compared with TNM staging in prognosis of patients with primary hepatic carcinoma after resection. Hepatobiliary Pancreat Dis Int. 2005;4:561–564. [PubMed] [Google Scholar]
- 32.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leibson PJ. The regulation of lymphocyte activation by inhibitory receptors. Curr Opin Immunol. 2004;16:328–336. doi: 10.1016/j.coi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682–693, 693.e1-693.e4. doi: 10.1053/j.gastro.2009.09.052. [DOI] [PubMed] [Google Scholar]