Set7 associates with the MyoD transcription factor to enhance expression of genes required for muscle differentiation.
Abstract
The molecular events that modulate chromatin structure during skeletal muscle differentiation are still poorly understood. We report in this paper that expression of the H3-K4 histone methyltransferase Set7 is increased when myoblasts differentiate into myotubes and is required for skeletal muscle development, expression of muscle contractile proteins, and myofibril assembly. Knockdown of Set7 or expression of a dominant-negative Set7 mutant impairs skeletal muscle differentiation, accompanied by a decrease in levels of histone monomethylation (H3-K4me1). Set7 directly interacts with MyoD to enhance expression of muscle differentiation genes. Expression of myocyte enhancer factor 2 and genes encoding contractile proteins is decreased in Set7 knockdown myocytes. Furthermore, we demonstrate that Set7 also activates muscle gene expression by precluding Suv39h1-mediated H3-K9 methylation on the promoters of myogenic differentiation genes. Together, our experiments define a biological function for Set7 in muscle differentiation and provide a molecular mechanism by which Set7 modulates myogenic transcription factors during muscle differentiation.
Introduction
Gene expression is tightly controlled, frequently requiring coordinated regulation between chromatin remodeling, chromatin modifications, and the activities of transcription factors. Determination of the myogenic lineage and differentiation of skeletal muscle cells are precisely orchestrated by the MyoD family of basic helix-loop-helix proteins (Weintraub et al., 1991; Molkentin and Olson, 1996; Arnold and Winter, 1998; Tapscott, 2005). The ability of MyoD to convert cells of many different lineages and differentiation states to skeletal muscle suggests that MyoD can both access genes in a repressive chromatin context and actively remodel the appropriate loci independent of cell lineage or differentiation state.
Chromatin modification events, which include histone acetylation, methylation, phosphorylation, and ubiquitination, are central to the regulation of gene expression (Klose and Zhang, 2007; Ruthenburg et al., 2007). Histone acetyltransferases were shown to interact with MyoD and acetylate promoter histones as well as MyoD itself (Sartorelli et al., 1999; Polesskaya et al., 2000; Berkes and Tapscott, 2005). Histone acetyltransferases with the subsequent recruitment of SWI (switch)–SNF (sucrose nonfermentable) complexes positively regulate MyoD activity at the onset of skeletal muscle differentiation (Berkes and Tapscott, 2005; Forcales and Puri, 2005; Sartorelli and Caretti, 2005). In contrast, histone deacetylases condense chromatin and inhibit the accessibility of transcription factors to regulatory elements (promoters and/or enhancers) of their target genes and, thereby, repress gene expression (McKinsey et al., 2001). Similar to the acetylation and deacetylation process, modification of histones by methylation and demethylation also plays an important role in the activation of gene expression (Klose and Zhang, 2007). Globally, the levels of mono- and dimethylation of histone H3 at lysine 4 (H3-K4m1 and H3-K4m2, respectively) correlate with gene transcriptional levels (Barski et al., 2007). Suv39h1, a histone H3 lysine 9 (H3-K9) methyltransferase associated with transcriptional silencing (Kouzarides, 2002; Sims et al., 2003), has been demonstrated to associate with MyoD on the promoters of muscle genes, resulting in transcriptional inhibition in proliferating myoblasts (Mal, 2006).
Set7, also known as Set9, is a SET domain–containing histone 3 lysine 4 (H3-K4) methyltransferase (Wang et al., 2001; Nishioka et al., 2002). Set7 is known to stimulate activator-induced transcription in vivo (Nishioka et al., 2002; Kouskouti et al., 2004), indicating that its activity is likely modulated or associated with other factors. Set7 converts unmodified H3-K4 into monomethylated H3-K4 but is incapable of further methylation using monomethylated H3-K4 as a substrate (Kouzarides, 2002; Xiao et al., 2003; Couture and Trievel, 2006). Intriguingly, the methylation of H3-K4 by Set7 and the methylation of H3-K9 by Suv39h1 are mutually exclusive (Wang et al., 2001; Nishioka et al., 2002). Furthermore, Suv39h1 and the associated methylation at myogenic loci suppress MyoD-mediated myogenic differentiation (Mal, 2006). We hypothesize that Set7 and the associated methylation of H3-K4 promote MyoD-mediated myogenic differentiation by suppressing Suv39h1-mediated transcriptional repression.
Herein, we show that Set7 physically interacts with MyoD on myogenic promoters to activate muscle gene expression. siRNA knockdown of Set7 or overexpression of a dominant-negative Set7 mutant impaired MyoD-mediated muscle differentiation. Consistent with these observations, knockdown the expression of endogenous Set7 in zebrafish embryos dramatically affects skeletal muscle development. Our experiments therefore establish a central role of Set7 in muscle gene expression and skeletal muscle development.
Results
Increased expression of Set7 during skeletal muscle differentiation
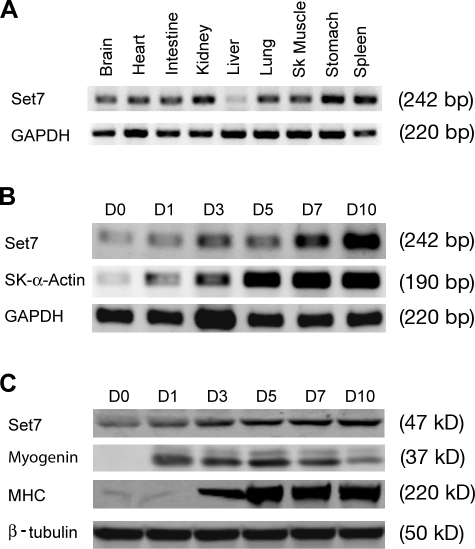
We examined the expression of Set7 in different adult mouse tissues. RT-PCR analysis showed that Set7 is expressed in all tissues tested, including skeletal muscle (Fig. 1 A). We next investigated the potential change of Set7 expression during muscle differentiation. C2C12 myoblasts are a well-established cell line that can faithfully mimic skeletal muscle differentiation in vitro, as indicated by the terminal differentiation and expression of muscle differentiation marker genes, upon serum withdrawal (Blau et al., 1985; Soulez et al., 1996; Lu et al., 2000; Chen et al., 2006). RT-PCR and Western blot analyses demonstrate that the expression level of both Set7 transcript and protein increases during C2C12 differentiation (Fig. 1, B and C), occurring in parallel with increased expression of the muscle differentiation markers myogenin and myosin heavy chain (MHC; Fig. 1, B and C). Together, these data demonstrate that the expression of Set7 is dynamically regulated during normal skeletal muscle differentiation, suggesting a role in skeletal muscle development.
Figure 1.
The expression of Set7 is increased during skeletal muscle differentiation. (A) RT-PCR analyses of Set7 expression using RNAs isolated from adult mouse tissues as indicated. GAPDH was used as a loading control. (B) RT-PCR analyses of the expression of Set7 and skeletal muscle (Sk muscle) α-actin (SK-α-Actin) using total RNA isolated from C2C12 myoblasts cultured in differentiation medium for 0, 1, 3, 5, 7, and 10 d (D0–D10). GAPDH was used as a loading control. (C) C2C12 myoblasts were cultured in differentiation medium for 0, 1, 3, 5, 7, and 10 d. Western blot analyses were performed with cell extracts using antibodies that recognize the indicated proteins. β-Tubulin was used as a loading control.
Set7 is required for the terminal differentiation of skeletal muscle myoblasts
To study the potential function of Set7 in skeletal muscle cell differentiation, we first overexpressed Set7 in C2C12 myoblasts. We cotransfected a pcDNA-Set7 expression plasmid and a control plasmid encoding GFP into C2C12 myoblasts. Cells were then either maintained in growth medium or switched into differentiation medium after transfection (see Materials and methods). Unexpectedly, overexpression of Set7 did not appear to affect muscle differentiation, as there was no observable morphological difference between Set7 and control GFP-transfected cells (Fig. 2 A). Furthermore, there were no detectable changes in the expression of early and late myogenic markers, including MyoD, myogenin, and MHC, in Set7-transfected cells (Fig. 2, B–D; and Table I). In addition, myoblast proliferation was not affected by Set7 overexpression, as indicated by the observation that the expression level of phosphorylated histone H3 (phospho–histone H3), a marker for cell proliferation, was not changed (Fig. 2, C and D; and Table I). When MyoD was used as a positive control in those experiments, it significantly promoted myoblast differentiation and the expression of myogenic differentiation marker genes (unpublished data).
Figure 2.
Set7 affects skeletal muscle myoblast proliferation and differentiation. (A) C2C12 myoblasts were transfected with Set7 or a dominant-negative mutant of Set7 (Set7-dn) together with a GFP expression construct. Cells were transferred to differentiation medium for 72 h, and myoblast differentiation was observed by fluorescent microscopy. The quantitative analysis result of the mean myotube numbers from 10 randomly chosen microscopic fields was presented on the right. Data represent the means ± SD from at least three independent experiments. Bar, 30 µm. ***, P < 0.001. (B) C2C12 myoblasts were transfected with Set7 or Set7-dn. Cells were transferred to differentiation medium for the indicated time, and the expression of myogenic differentiation genes was assayed by RT-PCR. GAPDH was used as a loading control. (C) C2C12 myoblasts were transfected with Set7 or Set7-dn. Cells were transferred to differentiation medium for the indicated time, and expression of myogenic differentiation proteins was assayed by Western blot analysis. β-Tubulin was used as a loading control. (D) C2C12 myoblasts were transfected with Set7 or Set7-dn. Cells were transferred to differentiation medium for the indicated time, and myocyte proliferation and differentiation were assayed by immunostaining with the indicated antibodies. Phos H3, phospho–histone H3; MHC, myosin heavy chain. Bar, 20 µm. (E) The MCK promoter luciferase reporter (MCK-Luc) was cotransfected with a MyoD expression plasmid together with an increasing amount of Set7 or the Set7-dn expression plasmid. Luciferase activity was determined 48 h after transfection and was presented as relative luciferase activity in which the control was assigned a value of 1. Data represent the means ± SD from at least three independent experiments in duplicate. SK, skeletal muscle; MCK, muscle creatine kinase; D0, day 0. *, P < 0.05; **, P < 0.01.
Table I.
Effect of Set7 and the Set7-dn mutant on myoblast proliferation and differentiation
| Treatment | Growth medium | Differentiation medium at 24 h | Differentiation medium at 48 h | |||||||
| Phospho-H3–positive cells | Phospho-H3–positive cells | Myogenin-positive cells | Myogenin-positive cells | MHC-positive cells | ||||||
| Total number | Relative to control | Total number | Relative to control | Total number | Relative to control | Total number | Relative to control | Total number | Relative to control | |
| % | % | % | % | % | ||||||
| pcDNA | 94 | 100.0 | 59 | 100.0 | 197 | 100.0 | 363 | 100.0 | 120 | 100.0 |
| Set7 | 92 | 97.9 | 62 | 105.1 | 193 | 98.0 | 370 | 101.9 | 113 | 94.2 |
| Set7-dn | 135 | 143.6 | 95 | 161.0 | 142 | 72.1 | 309 | 85.1 | 81 | 67.5 |
Next, we tested the effect of a Set7 H297G mutant on C2C12 myoblast differentiation. The Set7 H297G mutant contains a point mutation within its SET domain and was previously shown to function as a dominant-negative mutant (Wang et al., 2001; Nishioka et al., 2002). When C2C12 myoblasts were transfected with the Set7 H297G dominant-negative mutant (hereafter referred to as Set7-dn in this study), myogenic differentiation was dramatically inhibited, as there were fewer myotubes formed in Set7-dn–transfected C2C12 cultures (Fig. 2 A). Molecular marker analysis confirmed that the expression of myogenic differentiation marker genes was substantially inhibited in Set7-dn–transfected cells (Fig. 2, B–D; and Table I).
Muscle creatine kinase (MCK) expression is induced during skeletal muscle myoblast differentiation. It has been well documented that the transcription of the MCK gene is MyoD dependent (Lassar et al., 1989). We therefore tested whether Set7 or Set7-dn could affect MyoD-dependent transactivation of the MCK gene. We cotransfected C2C12 myoblasts with a MCK promoter luciferase reporter gene and an expression plasmid encoding MyoD along with (or without) different amounts of Set7 or the Set7-dn mutant. Set7 or Set7-dn alone did not significantly affect the expression of the MCK promoter luciferase reporter. Cotransfection of Set7 had no obvious effect on the expression of MyoD-activated MCK luciferase reporter gene (Fig. 2 E). However, the Set7-dn mutant caused about a twofold suppression of MyoD-activated MCK luciferase reporter gene expression (Fig. 2 E). Together, these data suggest that Set7 is a critical factor necessary for myoblast differentiation.
To confirm the aforementioned observations, we performed additional independent experiments wherein we knocked down endogenous Set7 in C2C12 myoblasts using siRNAs. We transfected Set7 siRNA or control scrambled siRNA into C2C12 myoblasts before differentiation (see Materials and methods). Set7 siRNA, but not the control siRNA, dramatically knocked down the endogenous Set7 protein level (Fig. 3 C). After 3 d of differentiation, myogenic differentiation was impaired in Set7 siRNA-transfected cells but not in control cells (Fig. 3, A and B). Consistent with the results from the Set7-dn overexpression experiments, knockdown of Set7 by siRNA repressed the expression of myogenic differentiation genes: MyoD, myogenin, myocyte enhancer factor 2 (MEF2), and MHC. We therefore conclude that Set7 is necessary for myogenic differentiation of skeletal muscle myoblasts. Conversely, cell proliferation appears slightly enhanced in Set7 siRNA-transfected myoblasts, as indicated by an increase in phospho–histone H3 level (Fig. 3 C).
Figure 3.
Knockdown of Set7 impairs skeletal muscle myocyte differentiation. (A) C2C12 myoblasts were transfected with control siRNA or siRNA against Set7 together with a GFP expression construct. Cells were transferred to differentiation medium for 72 h, and myoblast differentiation was observed under a fluorescent microscope. Bar, 40 µm. (B) The quantitative analysis result of the mean myotube numbers from 10 randomly chosen microscopic fields. Data represent the means ± SD from at least three independent experiments. si, siRNA. ***, P < 0.001. (C) C2C12 myoblasts were transfected with control siRNA or siRNA against Set7. Cells were transferred to differentiation medium for the indicated time, and Western blot analyses were performed using antibodies that recognize the indicated proteins. β-Tubulin was used as a loading control. Phos H3, phospho–histone H3; MHC, myosin heavy chain; Crtl., control. Black lines indicate that intervening lanes have been spliced out.
Set7 is required for MyoD to convert fibroblasts into myoblasts
MyoD is able to convert nonmuscle cells into myoblasts when ectopically overexpressed (Weintraub et al., 1989). We examined whether Set7 could affect MyoD-mediated myogenic conversion in 10T1/2 fibroblasts. When 10T1/2 fibroblasts were transfected with a MyoD expression plasmid, cells were converted into myoblasts after switching into differentiation medium. Set7 by itself did not affect myogenic conversion of 10T1/2 fibroblasts, and neither did it affect cellular proliferation nor differentiation (unpublished data). When both MyoD and Set7 were overexpressed in 10T1/2 fibroblasts, Set7 did not appear to affect MyoD-mediated myogenic conversion. However, when both MyoD and the Set7-dn mutant were overexpressed, MyoD-mediated myogenic conversion was repressed, as indicated by reduced myotube formation (Fig. 4 A). Quantitative analyses of MHC-positive cells further support the view that the Set7-dn mutant impaired MyoD-mediated myogenic conversion (Fig. 4 B). In addition, Western blot analysis demonstrates a significant decrease in the MHC protein expression in Set7-dn–transfected cells (Fig. 4 C). Consistent with our observations using the Set7-dn mutant, MyoD-mediated myogenic differentiation is significantly impaired, as is MHC expression, when endogenous Set7 expression is repressed by siRNA (Fig. 4, D and E). Together, these results suggest that Set7 plays an important role in MyoD-mediated myogenic induction and muscle gene expression.
Figure 4.
Set7-dn mutant inhibits MyoD-mediated conversion of 10T1/2 fibroblasts into myoblasts. (A) 10T1/2 fibroblasts, which stably expressed Set7 or the Set7-dn mutant, were transiently transfected with a MyoD expression plasmid. Cells were then transferred to differentiation medium for 72 h, and myogenic conversion was scored by positive staining for MHC expression (red). DAPI counter stains the nucleus. (B) The result of quantitative analysis was presented as a percentage of nuclei in myotubes from 10 randomly chosen microscopic fields. Data represent the means ± SD from at least three independent experiments. (C) 10T1/2 fibroblasts, which stably expressed Set7 or the Set7-dn mutant, were transiently transfected with a MyoD expression plasmid. Cells were then transferred to differentiation medium for 72 h, and myogenic conversion was determined by Western blot analysis for the MHC protein. The expression levels of Set7 and Set7-dn proteins are indicated. β-Tubulin was used as a loading control. (D) 10T1/2 fibroblasts were transfected with control siRNA or siRNA against Set7 together with a MyoD expression plasmid. Cells were then transferred to differentiation medium for 72 h, and myogenic conversion was scored by positive staining for MHC expression (red). DAPI counter stains the nucleus. (E) 10T1/2 fibroblasts were transfected with control siRNA or siRNA against Set7 together with a MyoD expression plasmid. Cells were then transferred to differentiation medium for 72 h, and myogenic conversion was analyzed by Western blot analysis using antibodies that recognize the indicated proteins. β-Tubulin was used as a loading control. Crtl., control. Bars, 40 µm.
Set7 directly interacts with MyoD
MyoD has been shown to interact with many transcription factors and other transcriptional modulators to control muscle gene expression and muscle differentiation (Tapscott, 2005). We next tested whether Set7 physically interacts with MyoD. We first applied GST fusion protein pull-down assays to determine the interaction between Set7 and MyoD. Glutathione beads containing bacterially expressed GST-Set7, GST–Set7-dn fusion proteins, or control GST protein alone were incubated with HEK293T cell nuclear extracts in which the Myc-MyoD was overexpressed. Subsequent Western blot analysis using anti-Myc antibodies demonstrated that both Set7 and the Set7-dn mutant proteins directly interact with MyoD (Fig. 5 A). We further confirmed the interaction between Set7 and MyoD by coimmunoprecipitation (IP; co-IP) experiments with ectopically expressed proteins from HEK293T cells (Fig. 5 B).
Figure 5.
Set7 and MyoD interact in vitro and in vivo. (A) MyoD specifically interacted with GST-Set7 and GST–Set7-dn, but not with GST alone, in GST pull-down assays. Coomassie-stained proteins corresponding to relative amounts of GST and GST-Set7 proteins used in the pull-down assay are shown. (B) HEK293T cells were transfected with Flag-tagged Set7 and/or Myc-tagged MyoD as indicated. (top) Set7 proteins were immunoprecipitated by anti-Flag antibodies, and anti-Myc antibodies were used to detect the presence of MyoD proteins in the immunoprecipitates by Western blot analysis. (bottom) One fifteenth of cell extracts were directly immunoblotted to detect the presence of Set7 and MyoD proteins. (C) Cell extracts from C2C12 myocytes at indicated differentiation dates (0, 1, or 3 d [D0, D1, and D3]) were immunoprecipitated using anti-Set7 antibodies (or IgG in controls), and the presence of MyoD proteins in the immunoprecipitates were detected by anti-MyoD antibodies. The expression of Set7 and MyoD was demonstrated in Western blot analysis. β-Tubulin was used as a loading control. (D, top) Schematic diagram of Set7 mutants for MyoD interaction. The arrowhead in indicates the Set7 point mutation that created the Set7-dn. (bottom) Myc-tagged MyoD was detected in Flag-Set7 aa 1–212 and aa 1–343, but not in aa 212–343 immunoprecipitates. (E) Schematic diagram of MyoD mutants for the Set7 interaction. (F) Myc-tagged MyoD mutants were cotransfected with Flag-tagged Set7, and their interaction was detected in Flag-Set7 immunoprecipitates. IB, immunoblot; HLH, helix-loop-helix.
The expression of Set7 and MyoD at the protein level increases when myoblasts are induced to differentiate into myotubes (Figs. 1 C and 5 C). We therefore examined whether endogenous Set7 and MyoD proteins interact in C2C12 myoblasts and myotubes. Co-IP experiments clearly show that Set7 interacts with endogenous MyoD in C2C12 cells. Most importantly, their interaction is enhanced during skeletal muscle differentiation (Fig. 5 C), which may be caused by, at least in part, the increased expression levels of MyoD and Set7. Together, these data demonstrate that Set7 and MyoD physically interact, which further suggests that this interaction contributes to the regulation of skeletal muscle gene expression and myoblast differentiation.
To determine the regions/domains of Set7 and MyoD that mediate their interaction, we performed additional GST protein pull-down and co-IP experiments with multiple Set7 and MyoD truncation mutants. The Set7 protein contains a conserved SET domain in its C terminus (Fig. 5 D). Although both full-length Set7 and the Set7-dn mutant interact with MyoD (Fig. 5 B), the N-terminal regions of Set7 without the SET domain (aa 1–212) appeared to bind to MyoD with higher affinity (Fig. 5 D), suggesting that the SET domain may have a negative effect on the interaction of Set7 and MyoD. Consistent with this notion, we found that the SET domain alone (aa 212–343) failed to bind to MyoD (Fig. 5 D). Our findings are consistent with the view that the N-terminal region of Set7 serves as its protein–protein interaction domain, as has been previously determined structurally (Xiao et al., 2003).
MyoD belongs to the basic helix-loop-helix family of transcription factors, and it contains several domains that mediate the interactions with other proteins (Fig. 5 E; Tapscott, 2005). We constructed MyoD deletion mutants and tested their ability for Set7 binding. Co-IP experiments demonstrate that both the N- and C-terminal regions of MyoD are required for its interaction with Set7 (Fig. 5, E and F). Additional co-IP experiments demonstrate that both the C/H (cysteine-histidine) domain (aa 63–99) and the helix III domain (aa 245–258) of MyoD are required for its interaction with Set7. In contrast, deletion of the basic (aa 102–135) or the helix-loop-helix domain (aa 143–162) was found to have no effect on the interaction with Set7 (Fig. 5, E and F; and not depicted). These results indicate that the C/H and helix III domains of MyoD mediate its interaction with Set7.
Set7 is required for MyoD to associate with the promoters/enhancers of muscle differentiation genes
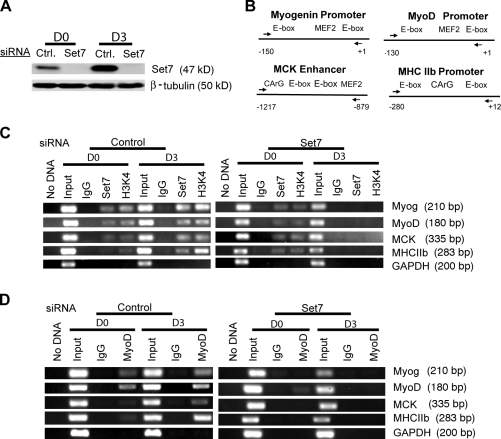
Set7 is a histone methyltransferase suggested to influence gene expression, at least partially, by modifying local chromatin at H3-K4 (Wang et al., 2001; Nishioka et al., 2002; Francis et al., 2005; Callis et al., 2008). We investigated whether Set7 associates with chromatin at the promoters/enhancers of skeletal muscle differentiation genes and, if so, whether such an association alters H3-K4 methylation during myoblast differentiation. We first performed chromatin IP (ChIP) assays using antibodies specific for Set7 and monomethylated H3-K4. After cross-linking, immunoprecipitated DNA fragments bound by proteins were then detected by semiquantitative PCR using specific primers spanning the regulatory regions of myogenic differentiation genes, including MyoD, myogenin, MHC, and MCK (Fig. 6 B). Indeed, Set7 associated with the regulatory regions of myogenic differentiation genes on chromosomes, which were also monomethylated at H3-K4 (Fig. 6 C). Furthermore, both the Set7 association and the H3-K4 monomethylation status on the promoters/enhancers of myogenic differentiation genes substantially increased during myogenic differentiation (Fig. 6 C).
Figure 6.
Set7 is required for MyoD to bind to the promoters/enhancers of skeletal muscle differentiation genes. (A) C2C12 myoblasts were transfected with control siRNA or siRNA against Set7. Cells were transferred to differentiation medium for the indicated times and Western blot analyses were performed with cell extracts using antibodies that recognized Set7 proteins. β-Tubulin was used as a loading control. (B) Schematic diagrams of promoter or enhancer regions of indicated myogenic differentiation genes. Arrows indicate PCR primers used for the PCR reaction. (C) IPs were performed with anti-Set7 and anti–H3-K4 antibodies as indicated. (D) IPs were performed with anti-MyoD antibodies. (C and D) The amount of DNA in each sample (input) is shown. IPs performed with IgG were used as controls. GAPDH was used as a loading control. Myog, myogenin; Crtl. control; D0, day 0.
Next, we examined whether knockdown of endogenous Set7 in C2C12 myoblasts could impact H3-K4 monomethylation status on the promoters/enhancers of myogenic differentiation genes. We achieved near complete knockdown of the endogenous Set7 protein by siRNA treatments (Fig. 6 A). As expected, the association of Set7 with the regulatory regions of myogenic differentiation genes was completely abolished when Set7 was knocked down in differentiated myotubes (Fig. 6 C). Consistent with the role of Set7 in H3-K4 monomethylation, knockdown of Set7 also dramatically decreased H3-K4 monomethylation on the promoters/enhancers of myogenic differentiation genes (Fig. 6 C). Association of Set7 with the promoter/enhancer regions of myogenic differentiation genes as well as the H3-K4 monomethylation was unchanged in the presence of the scrambled control siRNA, further demonstrating the specificity of the Set7 siRNA (Fig. 6 C).
Our results establish that Set7 is required for proper myoblast differentiation (Figs. 2 and 3). We demonstrate that Set7 and MyoD physically interact (Fig. 5), suggesting that Set7 may achieve its biological function through modulation of MyoD. We therefore investigated whether Set7 is required for MyoD to associate with the regulatory regions of myogenic differentiation genes. Results from ChIP experiments demonstrate that depletion of Set7 by siRNA treatment abolishes the association of MyoD with the promoters/enhancers of myogenic differentiation genes in differentiated myotubes (Fig. 6 D). Our results suggest that Set7 is required for MyoD to bind to the regulatory region of muscle differentiation genes to activate gene expression and to initiate the myogenic differentiation process. Of note, MyoD expression level was decreased in Set7 knockdown cells, which may also contribute to the decreased MyoD binding to the promoters/enhancers.
Set7 is required for skeletal muscle development and myofiber assembly in vivo
The Set7 protein is highly conserved, and homologues for the mouse Set7 gene have been identified in species from zebrafish to human. Using the BLASTp (basic local alignment search tool for proteins) algorithm, we found that the zebrafish Set7 (NCBI Protein database accession no. NP_001002456) and mouse Set7 (NCBI Protein database accession no. NP_542983) share 72% identity at the amino acid level (unpublished data). Therefore, to assess the function of Set7 during skeletal muscle development in vivo, we used the zebrafish model system in which gene expression and function can be specifically inhibited by morpholino oligomers (MOs) during embryonic development. Morpholinos can either bind to the 5′ untranslated region of mRNAs to block translational process or interfere with pre-mRNA–splicing steps. We designed morpholino oligomers to block the splicing of zebrafish Set7 pre-mRNA, as this approach will allow us to conveniently monitor the splice modification events by RT-PCR.
Injection of Set7 MO, but not the control MO, led to a block of pre-mRNA splicing, as indicated by a band shift after gel electrophoresis of RT-PCR products (Fig. 7 A). Interestingly, the gross morphology of Set7 MO-injected embryos (Set7 morphants) appears to be normal when compared with controls (Fig. 7 B). However, all morphant embryos are paralyzed and fail to respond to tactile stimuli (unpublished data), suggesting a defect in musculature function. Immunohistochemistry analysis revealed that Set7 morphants exhibit a dramatic decrease in the expression of the MHC protein (Fig. 7 C). Further analyses using antibodies that specifically label fast and slow MHCs demonstrate that both fast and slow MHC were dramatically decreased in Set7 morphant embryos (Fig. 7, D and E).
Figure 7.
Set7 is required for skeletal muscle development and myofibril assembly. Zebrafish embryos were injected with control (CTRL) or Set7 morpholino (MO) at the single-cell stage, and embryos were collected 24 h after fertilization. (A) RT-PCR analysis of zebrafish Set7 transcripts from control and Set7 morpholino embryos. Mutant (Mut) and wild-type (Wt) transcripts are indicated. (B) Bright-field embryonic images of control and Set7 morpholino. (C) Set7 morphants display defects in myofibril assembly as indicated by MHC immunostaining. Higher magnification is shown on the right. DAPI counter stains the nucleus. Bars, 60 µm. (D) Set7 morphants display defects in fast MHC expression. (E) Set7 morphants display defects in slow MHC expression. (F) MEF2 protein expression level was significantly decreased in Set7 morphants. Bar, 30 µm.
Our in vitro experiments showed that the expression of MEF2 was down-regulated in Set7 knockdown myoblasts. Interestingly, Hinits and Hughes (2007) recently reported that disruption of MEF2 expression in zebrafish leads to defects in muscle development and myofibril assembly, including perturbed MHC expression, a phenotype very similar to what we observed in our Set7 morphants. We therefore examined the expression of MEF2 by immunostaining. Indeed, we found that MEF2 is significantly decreased in Set7 morphants (Fig. 7 F), indicating that the MEF2 family of transcription factors is likely involved in Set7-mediated regulation of muscle development and myofibril assembly.
The aforementioned observations suggest that Set7 is required for the proper expression of myogenic contractile proteins and the assembly of myofibrils. We next examined the structure of skeletal muscles in control and Set7 morphants using transmission electron microscopy. As seen in Fig. 8 A, there are significantly fewer and shorter myofibrils in Set7 morphants as compared with controls. Close examination of myofiber sarcomeres revealed periodic absent z lines (Fig. 8 B, arrow) and noticeably narrower sarcomeres as compared with control (Fig. 8 B). In addition, an increased frequency of A-band branching is observed in Set7 morphants (Fig. 8 B, arrow). Together, these data demonstrate that Set7 is required for normal development of skeletal muscle and the assembly of myofibrils.
Figure 8.
Set7 morpholino-treated zebrafish exhibit impaired sarcomerogenesis and morphological defects in skeletal muscle. Transmission electron microscopy examinations of control or Set7 morphant skeletal muscle. (A) Set7 MO treatment results in morphologically altered myocytes with fewer and shorter myofibrils as compared with control. Bars, 10 µm. (B) Higher magnification reveals periodic absent z lines (arrow) and noticeably narrower sarcomeres as compared with control. An increased frequency of A-band branching (arrow) is observed with morpholino treatment. Bars, 500 nm. Images are representative of dorsal skeletal myocytes from n = 3 fish for both control and Set7 morphants.
Set7 competes with Suv39h1 for the binding of the promoters/enhancers of muscle genes
Set7 activates chromatin at the promoters and enhancers of its regulatory target genes, at least in part, by inhibiting Suv39h1-mediated chromatin repression. Interestingly, the modifications of H3-K4 by Set7 and H3-K9 by Suv39h1, a H3-K9–specific methyltransferase, are mutually exclusive (Wang et al., 2001; Nishioka et al., 2002). Furthermore, it has been reported that Suv39h1 can interact with MyoD to repress myogenic differentiation (Mal, 2006). We therefore tested whether Set7 could modulate the methylation status of H3-K9 and the association of Suv39h1 with the promoters/enhancers of myogenic differentiation genes. We first examined the expression levels of the Suv39h1 protein and the methylation status of H3-K4, H3-K9 in undifferentiated myoblasts, and differentiated myotubes. Western blot analyses indicate that there is no substantial change in Suv39h1 protein expression level during muscle differentiation (Fig. 9 A, compare lanes 1 and 3). Similarly, the levels of H3-K4 monomethylation and H3-K9 trimethylation are not changed during myoblast differentiation (Fig. 9 A, compare lanes 1 and 3). We further examined the expression level of additional methyltransferases in Set7 knockdown C2C12 cells. As shown in Fig. 9 B, we found that the expression levels of Ash11 (ash1 [absent, small, or homeotic]-like) and Prmt5 (protein arginine methyltransferase 5) were not altered in Set7 knockdown C2C12 cells.
Figure 9.
Set7 antagonizes Suv39h1 to promote MyoD-mediated skeletal muscle myoblast differentiation. (A) C2C12 myoblasts were transfected with control siRNA (Ctrl.) or siRNA against Set7. Cells were transferred to differentiation medium for the indicated times, and Western blotting was performed with cell extracts using antibodies that recognize the indicated proteins. β-Tubulin was used as a loading control. (B) Methyltransferase gene expression during C2C12 differentiation. Quantitative RT-PCR analysis of Ash1l, myogenin (Myog), Set7, and Prmt5 gene expression at day 0 (D0) and day 3 (D3) of differentiation with and without Set7 siRNA treatment (siSet7). Data represent the means + SD from three independent experiments. *, P < 0.05. (C) IPs were performed with anti–H3-K4, anti–H3-K9, and anti-Suv39h1 antibodies as indicated. The amount of DNA in each sample (input) is shown. IPs performed with IgG were used as controls. GAPDH was used as a loading control. (D) MEF cells from wild type (+/+) or Suv39h1/h2 double knockout (−/−) were transiently transfected with an expression plasmid for MyoD. Myogenic conversion was scored by positive staining for MHC expression (red) 6 d after differentiation induction. DAPI counter stains the nucleus (blue). Bar, 40 µm. The result of quantitative analysis, which was presented as the percentage of nuclei in myotubes from 10 randomly chosen microscopic fields, is presented on the right. Data represent the means ± SD from at least three independent experiments. P = 0.002. (E) RT-PCR analyses of the expression of Suv39h1, Suv39h2, and Set7 transcripts using total RNA isolated from wild-type (+/+) or Suv39h1/h2 double knockout (−/−) MEFs. GAPDH was used as a loading control. (F) Western blot analyses using antibodies that recognize Set7 and H3-K4 proteins from cell extracts of wild-type (+/+) or Suv39h1/h2 double knockout (−/−) MEFs. β-Tubulin was used as a loading control. (G) A working model to account for the molecular regulation of MyoD-mediated skeletal muscle differentiation, which is modulated by Set7 and Suv39h1. In undifferentiated myoblasts, MyoD is associated with Suv39h1/2 on the promoters/enhancers of myogenic differentiation genes. Suv39h1-mediated H3-K9 methylation resulted in condensation of chromatin and transcriptional repression. During skeletal muscle differentiation, increased Set7 expression will compete with Suv39h1 for MyoD association and H3-K4 methylation. This will consequently lead to the occupation of MyoD at the promoters/enhancers of myogenic differentiation genes, resulting in myogenic activation.
Consistent with a previous study indicating that Suv39h1 repressed myogenic gene expression (Mal, 2006), our ChIP results revealed that the association of Suv39h1 with the promoters/enhancers of myogenic differentiation genes was significantly decreased in differentiated myotubes (Fig. 9 C). This decrease is accompanied by a decrease in the trimethylation of H3-K9 (H3-K9me3) at the regulatory regions of myogenic differentiation genes (Fig. 9 C). In contrast, H3-K4 monomethylation was substantially increased during muscle differentiation (Fig. 9 C). Knockdown of Set7 by siRNAs did not affect Suv39h1 protein level or H3-K9 trimethylation, as demonstrated by Western blot analyses (Fig. 9 A). However, the level of H3-K4 monomethylation was decreased (Fig. 9 A, lane 4). Interestingly, knockdown of Set7 results in an increase in the association of Suv39h1 with the promoters/enhancers of myogenic differentiation genes and a significant increase in H3-K9 trimethylation accompanied by a decrease in H3-K4 monomethylation (Fig. 9 C). Our results therefore support the view that the modifications of H3-K4 by Set7 and H3-K9 by Suv39h1 are mutually exclusive on the promoters/enhancers of myogenic differentiation genes. Furthermore, these observations are consistent with a previous study demonstrating that Suv39h1-mediated H3-K9 trimethylation represses muscle gene expression and inhibits skeletal muscle differentiation (Mal, 2006).
Genetic deletion of Suv39h1 and Suv39h2 in mice (Suv39h−/−) results in severe impairment of animal development and chromosomal stabilities (Peters et al., 2001). We therefore tested whether loss of Suv39h proteins could affect MyoD-mediated myogenic conversion and myogenic gene expression in fibroblasts. Primary mouse embryonic fibroblasts (MEFs) derived from wild-type or Suv39h−/− animals were transfected with a MyoD expression plasmid and were subsequently switched to differentiation medium. Although MyoD is able to convert wild-type MEFs into MHC-positive myoblasts (albeit with lower efficiency when compared with that of 10T1/2 fibroblasts), there is a substantial increase in MHC-positive myoblasts from MyoD-transfected Suv39h−/− MEFs (Fig. 9 D). These results are consistent with a previous study in which Suv39h1 was shown to repress MyoD-mediated myogenic differentiation (Mal, 2006). Loss of Suv39h proteins in Suv39h−/− MEFs does not appear to affect the expression level of the Set7 transcript or protein (Fig. 9, E and F). H3-K4 protein expression in Suv39h−/− MEFs is indistinguishable from that of wild-type MEFs (Fig. 9 F). Together, these data clearly demonstrate that Set7 controls the H3-K4 monomethylation status at the promoters/enhancers of myogenic differentiation genes. Our results also suggest that Set7 may influence the trimethylation status of H3-K9, at least in part, via inhibiting the function of Suv39h1, thereby modulating MyoD-mediated muscle differentiation and muscle gene expression (Fig. 9 G).
Discussion
In this study, we show that Set7 is necessary for skeletal muscle gene expression and myogenic differentiation. Knockdown of Set7 and the accompanying decrease in H3-K4 monomethylation impairs MyoD-mediated muscle differentiation. Set7-mediated H3-K4 monomethylation results in a decrease in the association of Suv39h1 as well as H3-K9 trimethylation at the promoter/enhancers of myogenic differentiation genes. This evidence is further supported by in vivo experiments demonstrating the role of Set7 in muscle development in zebrafish. Our findings provide insights into the molecular mechanism of how chromatin-remodeling enzymes work in concert with tissue-specific transcription factors to control muscle gene expression and muscle cell development.
MyoD functions as a central piece of a transcriptional network for myogenic gene expression and has been shown to interact with a variety of other transcriptional cofactors, including the MEF2 family of transcription factors (Tapscott, 2005). Furthermore, MyoD transactivity is modulated by the chromatin status of its target genes (Tapscott, 2005). However, it is less clear how chromatin-remodeling enzymes and MyoD work in concert to direct myogenic gene expression.
Previous studies have shown that Set7-mediated histone methylation may function in transcriptional activation by competing with histone deacetylases or by precluding H3-K9 methylation (Wang et al., 2001; Nishioka et al., 2002). In vitro, the methylations of H3-K4 by Set7 and of H3-K9 by Suv39h1 were shown to be mutually exclusive (Wang et al., 2001; Nishioka et al., 2002). A role for Suv39h1 in myogenesis was reported in which Suv39h1 and the associated methylation on myogenic loci suppressed MyoD-mediated myogenic differentiation (Mal, 2006). It was further suggested that MyoD and Suv39h1 physically interact in a manner that the C/H domain, basic domain, and helix-loop-helix domain of MyoD are required.
Our observation that Set7 physically interacts with MyoD at both C/H and helix III domains is intriguing. These two domains have been shown to be necessary for MyoD to initiate chromatin remodeling at myogenic loci, thereby allowing the activation of a subset of specific muscle differentiation genes (Gerber et al., 1997; Berkes et al., 2004). Given that the C/H domain is also required for MyoD to interact with Suv39h1 (Mal, 2006), we speculate that during skeletal muscle differentiation, increased Set7 expression will compete with Suv39h1 for MyoD association. This will consequently lead to the occupation of MyoD at the promoters/enhancers of myogenic differentiation genes, which are normally located in a region of repressed chromatin as marked by the enrichment of H3-K9 methylation of histones in undifferentiated myoblasts (Mal and Harter, 2003). With the help of MyoD, Set7 can access the silencing nucleosome and monomethylates H3-K4 at the myogenic regulatory regions, leading to increased affinity of MyoD to those regions. Conversely, MyoD failed to associate with the promoters/enhancers of myogenic differentiation genes when Set7 was knocked down, resulting in myogenic repression (Fig. 9 G).
Consistent with the view that Set7 and Suv39h1 antagonize each other to regulate muscle differentiation, we found that MyoD-mediated myoblast conversion from fibroblasts was enhanced in Suv39h1-null MEF cells. We hypothesize that Set7 might be involved in myoblast fusion processes, as we observe substantially fewer multinuclei myotubes in Set7 knockdown or Set7-dn–overexpressed C2C12 myoblasts. Several molecules, including IL4 and myoferlin, have been implicated in myoblast fusion (Horsley et al., 2003; Doherty et al., 2005). However, we did not detect a substantial change in the expression levels of these proteins during myoblast fusion when Set7 is knocked down (unpublished data), implying that other mechanisms are likely involved.
In a recent study, Set7 was deleted from the mouse genome using a gene-targeting approach. A portion of Set7-null animals were viable and fertile. It was reported that p53 failed to activate its target gene expression in response to DNA damage in Set7-null MEFs (Kurash et al., 2008). Interestingly, about half of the Set7-null mice die during embryogenesis, suggesting that Set7 is also required for animal development. However, no embryonic phenotypic analyses were reported. Our in vivo experiments in zebrafish point to an important role of Set7 in muscle development and myofibril assembly. It will be interesting to determine the developmental defects in Set7-null mouse embryos, in particular, to assess skeletal muscle development and muscle gene expression in future studies.
Collectively, our study provides a mechanism for MyoD to activate muscle gene expression and skeletal muscle differentiation, by which MyoD interacts with Set7 to regulate myogenesis program, at least in part, through H3-K4 methylation at the regulatory regions of myogenic differentiation genes. Given that Set7 expression level was increased during muscle differentiation, it is intriguing to speculate that manipulating expression levels of Set7 may be a valuable therapeutic approach of muscle degeneration/regeneration and muscle-related diseases, such as Duchenne muscular dystrophy.
Materials and methods
Plasmids and reporter genes
MyoD expression vectors have been previously described (Lu et al., 2000; Chen et al., 2006). MyoD mutants were generated through PCR-based mutagenesis using the QuickChange kit from Agilent Technologies. All mutations were confirmed by DNA sequencing. The wild-type and mutant Set7 H297G expression vectors were as previously described (Wang et al., 2001). The GST-Set7 mutants were previously described (Francis et al., 2005) and were gifts from R.G. Mirmira (University of Virginia, Charlottesville, VA). The PIG (puro internal ribosome entry site GFP empty vector) Set7 mutants were generated by inserting PCR fragments flanked with EcoRI and XhoI into the PIG-Δ-multiple cloning site vector. The MCK promoter luciferase reporter was as previously reported (Chen et al., 2006).
Cell culture, transfection, and muscle differentiation assays
Transfections of 10T1/2 fibroblasts and C2C12 myoblasts were performed as previously described (Lu et al., 2000; Chen et al., 2006). Transient transfection for luciferase reporter assays, unless otherwise indicated, used 100 ng of reporter plasmid and 100 ng of each activator plasmid. The total amount of DNA per well was kept constant by adding the corresponding amount of the expression vector without a cDNA insert. Cytomegalovirus-lacZ or cytomegalovirus-GFP was included as an internal control for variations in transfection efficiency. All the transfection experiments were repeated at least twice in duplicate.
C2C12 myoblast cells were cultured, and myogenic differentiation was induced as previously described (Lu et al., 2000) with minor modifications. In brief, cells were maintained in DME with 10% FBS. We plated cells at ∼50–60% confluence and then performed the transfection the next day when they reached ∼90–100% confluence. We collected cells on the same day of transfection (∼6 h after transfection) and defined it as day 0. Cells were switched to medium containing 2% horse serum to induce differentiation, and samples were collected at the indicated dates. Myogenesis was monitored by either cotransfecting cells with a pcDNA-GFP construct (Figs. 2 A and 3 A) using a reagent (Lipofectamine LTX; Invitrogen) or staining cells with myogenic markers. Cells that contain two or more nuclei were viewed as myotubes. The Suv39h-null MEFs were provided by T. Jenuwein (Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany).
Generation of stable 10T1/2 cell lines
The PIG vector, PIG Set7, and PIG Set7-dn were transfected into 10T1/2 cells by electroporation with the Nucleofector system according to the manufacturer’s instruction (Lonza). Transfected cells were then plated on 10-cm dishes overnight. On the next day, cells were placed in selection medium that contained 5 µg/ml puromycin for ∼10 d. Cells were passaged, and selection medium was changed every other day.
siRNA knockdown
C2C12 myoblasts cultured in growth medium were transfected with control or Set7 siRNAs by electroporation with the Nucleofector system according to the manufacturer’s instruction. 24 h later, cells were passaged and maintained in growth medium. 16 h later, cells were transfected again for control or Set7 siRNAs using Lipofectamine LTX. Cells were cultured in growth medium for an additional 24 h before switching to differentiation medium.
GST protein-binding assays
Plasmids encoding GST fusion proteins were transformed into the BL21 codon plus cells (Agilent Technologies). The cells were grown at 37°C in 2× YT (yeast extract and tryptone) medium to an optical density of 1.0. 50 µM IPTG was then added to the culture to induce protein expression. After shaking at room temperature for 4–6 h, the cells were harvested, and the GST protein was purified with glutathione beads according to the manufacturer’s procedure (GE Healthcare). For GST protein-binding assays, HEK293T expressing Myc-MyoD lysates were incubated with GST, GST-Set7, or GST–Set7-dn beads. After washing three times with GST-binding buffer, samples were analyzed by SDS-PAGE, and the associated proteins were detected by Western blotting.
Immunoblotting and immunostaining
Western blotting was performed as previously described (Cao et al., 2005) using antibodies against myogenin, MEF2 (Santa Cruz Biotechnology, Inc.), MyoD (BD), β-Tubulin (Sigma-Aldrich), and phospho–histone H3 (Millipore). The MF20 antibody, which recognizes striated muscle-specific MHC, was obtained from the Developmental Studies Hybridoma Bank (University of Iowa). Immunostaining was performed as previously described (Chen et al., 2006, 2010). In brief, cells cultured in plates were fixed in 4% PFA for 10 min, washed with PBS + 0.1% NP-40, blocked with 5% goat serum in PBS + 0.1% NP-40 for 1 h at room temperature, and incubated with primary antibodies overnight at 4°C. After washing, cells were incubated with secondary antibodies for 1 h at room temperature and counterstained with DAPI. Myogenic conversion assays in 10T1/2 cells were performed as previously described (Wang et al., 2004; Cao et al., 2005). In brief, 10T1/2 fibroblasts were maintained in DME with 10% FBS. 1 d before transfection, 10T1/2 cells were plated on 6-well format plates at a density of 105 cells/well. Lipofectamine LTX reagent was used for transfection. 16 h later, cells were switched to differentiation medium (DME with 2% horse serum) for 4 d before immunostaining or Western blot analysis.
All images were acquired at room temperature from cell culture plates by a camera (UFX-DX; Nikon) mounted on an inverted (TE2000; Nikon) or upright fluorescence microscope (Microphot SA; Nikon). Digital fluorescent images were captured at room temperature with a 10× (Plan Fluor, air, and NA 0.30), 20× (Plan Fluor, air, and NA 0.45), or 40× (Plan Fluor, air, and NA 0.60) objective lens using the least possible exposure to minimize bleaching. The images were processed using SPOT (version 3.5.4 for Mac OS [Apple]; Diagnostic Instruments) software and were scaled down and cropped in Photoshop (Adobe) to prepare the final figures.
Co-IP assays
HEK293T cells were transiently transfected with plasmids encoding the epitope-tagged Set7 and/or MyoD proteins as indicated in the figure legends with FuGENE 6 (Roche). Cells were harvested 48 h after transfection in lysis buffer composed of PBS containing 0.5% Triton X-100, 1 mM EDTA, 1 mM PMSF, and complete protease inhibitors (Roche). After a brief sonication and removal of cellular debris by centrifugation, epitope-tagged proteins were precipitated with antibodies as indicated and protein A/G beads (Santa Cruz Biotechnology, Inc.). The bound proteins were washed five times with lysis buffer, resolved by SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Membranes were immunoblotted with antibodies as indicated, and proteins were visualized with a fluorescence detection system (Odyssey; LI-COR Biosciences).
ChIP assays
ChIP was performed as previously described (Cao and Zhang, 2004) using 2 µg of either normal mouse or rabbit IgG or antibodies against MyoD (sc304 and sc760; Santa Cruz Biotechnology, Inc.), Set7 (ab14820; Abcam), Suv39h1 (07–550; Millipore), monomethylated H3-K4 (07–436; Millipore), and trimethylated H3-K9 (07–442; Millipore). The oligonucleotides used in ChIP PCR were MHCIIb promoter forward, 5′-CACCCAAGCCGGGAGAAACAGCC-3′, and reverse, 5′-GAGGAAGGACAGGACAGAGGCACC-3′; MCK enhancer forward, 5′-AGGGATGAGAGCAGCCACTA-3′, and reverse, 5′-CAGCCACATGTCTGGGTTAAT-3′; myogenin promoter forward, 5′-CCCTGCCCCACAGGGGCTGTG-3′, and reverse, 5′-ACGCCACAGAAACCTGAGCCC-3′ (Caretti et al., 2004); MyoD promoter forward, 5′-GCACTGCCACCGATTCATTTG-3′, and reverse, 5′-CAGGAGGTTTGGAGAGAGACTCAAG-3′ (Francis et al., 2005); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) promoter forward, 5′-AAGCCAAACTAGCAGCTAGG-3′, and reverse, 5′-GGGCTAGTCTATCATTGCAG-3′ (Mal and Harter, 2003).
Quantitative RT-PCR
Total RNA was isolated with TRIZOL reagent (Invitrogen). After extraction and purification, 1 µg RNA was used as a template for reverse transcription with random hexamer primers. All PCR products span the intron region of the genes.
Zebrafish
Zebrafish (Danio rerio) embryos were obtained from the University of North Carolina, Chapel Hill Zebrafish Aquaculture Core Facility. Embryos were injected at cell stage 1–2 with 7–15 pmol zebrafish Set7 (available from GenBank/EMBL/DDBJ under accession no. BC076014) morpholino (5′-GATCACAGCTTGCACCAACCGTTGA-3′; Gene Tools, LLC) conjugated with FITC, or control morpholino (5′-CCTCTTACCTCAGTTACAATTTATA-3′). In six independent experiments, >350 embryos were injected with control MO, and 800 embryos were injected with Set7 MO. In addition, we set 150–200 noninjected fish aside to serve as additional controls in each experiment. Embryos were collected at 18 or 24 h after fertilization for further analysis. RT-PCR assays were used to verify the genotyping of Set7 morphants. In brief, injected fish were collected at 24 h after fertilization, and total RNA was extracted using TRIZOL reagent. For RT-PCR, 1 µg RNA samples were reverse transcribed to cDNA by using random hexamers and Moloney murine leukemia virus reverse transcription (Invitrogen) in a 20-µl reaction system. 1 µl cDNA pool was used per PCR reaction. The sequences of the PCR primers are zebrafish Set7 forward, 5′-TCGCTGGTCATAAACTGCTG-3′, and reverse, 5′-CTCGTCCTTCTCCACAGCTC-3′. PCR detected a 386-bp band for the zebrafish Set7 wild-type transcript, and a 590-bp mutant band in which the splicing of the pre-mRNA of zebrafish Set7 was blocked by MOs.
For immunohistochemistry, embryos were fixed in 4% PFA for 30 min at room temperature or cold methanol for 3–5 min and then washed with PBS + 0.1% NP-40, blocked with 5% goat serum in PBS + 0.1% NP-40 for 1 h at room temperature, incubated with primary antibodies for MEF2 (1:100; c-21; Hinits and Hughes, 2007), MHC (1:10; A4.1025; Developmental Studies Hybridoma Bank; Blagden et al., 1997), slow MHC (1:10; F59 and S58; Developmental Studies Hybridoma Bank; Devoto et al., 1996), and fast MHC (1:10; EB165; Developmental Studies Hybridoma Bank; Blagden et al., 1997), and diluted in blocking buffer overnight at 4°C. After washing, embryos were incubated with an FITC-conjugated secondary antibody. Processed samples were mounted in Vectashield (Vector Laboratories), and the images were acquired with a confocal microscope (FV500 [Olympus]; LSM 5 Pascal [Carl Zeiss]) with a 63× objective lens (Plan Apochromat, oil, and NA 1.40; Pathology Microscopy Facility at University of North Carolina, Chapel Hill). The images were processed using SPOT (version 3.5.4 for Mac OS) software and were scaled down and cropped in Photoshop to prepare the final figures.
For transmission electronic microscopy, control and Set7 MO embryos were fixed in 2% glutaraldehyde plus 6% sucrose in 0.075 M Na-cacodylate buffer, pH 7.4, supplemented with 0.1% tannic acid followed by 2% osmium tetroxide. Samples were stained with uranyl acetate (en bloc). Sections were cut in the Microscopy Services Laboratory in the Department of Pathology and Laboratory Medicine at the University of North Carolina at Chapel Hill. Images were acquired on a transmission electron microscope (Tecnai G2 Spirit BioTWIN; FEI) in the Department of Cell Biology at Harvard Medical School.
Acknowledgments
We thank members of the Wang laboratory and Xiaoyun Hu for excellent technical assistance. Daniel Summer participated the early stage of this work. We thank Edward Flynn and Se-Hee Kim for assistance with fish work, James Minchin for support, and Dr. Mark Majesky for careful reading of the manuscript and comments. We thank Dr. Thomas Jenuwein for providing the Suv39h-null MEFs and Dr. Raghavendra G. Mirmira for GST-Set7 constructs.
This work was supported by the March of Dimes Birth Defect Foundation, National Institutes of Health, and Muscular Dystrophy Association. Y. Zhang is an investigator of Howard Hughes Medical Institute. Z.-P. Huang is a postdoctoral fellow, and D.-Z. Wang is an Established Investigator of the American Heart Association.
Author contributions: Y. Tao and D.-Z. Wang conceived and designed the experiments. Y. Tao, R.L. Neppl, Z.-P. Huang, J. Chen, and R.-H. Tang performed the experiments. Y. Tao, Y. Zhang, S.-W. Jin, and D.-Z. Wang analyzed the data. R. Cao contributed reagents, materials, and analysis tools. D.-Z. Wang supervised all research. Y. Tao, R.L. Neppl, and D.-Z. Wang wrote the paper.
Footnotes
Abbreviations used in this paper:
- ChIP
- chromatin IP
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- IP
- immunoprecipitation
- MCK
- muscle creatine kinase
- MEF
- mouse embryonic fibroblast
- MEF2
- myocyte enhancer factor 2
- MHC
- myosin heavy chain
- MO
- morpholino oligomer
References
- Arnold H.H., Winter B.. 1998. Muscle differentiation: more complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 8:539–544. 10.1016/S0959-437X(98)80008-7 [DOI] [PubMed] [Google Scholar]
- Barski A., Cuddapah S., Cui K., Roh T.Y., Schones D.E., Wang Z., Wei G., Chepelev I., Zhao K.. 2007. High-resolution profiling of histone methylations in the human genome. Cell. 129:823–837. 10.1016/j.cell.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Berkes C.A., Tapscott S.J.. 2005. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16:585–595. 10.1016/j.semcdb.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Berkes C.A., Bergstrom D.A., Penn B.H., Seaver K.J., Knoepfler P.S., Tapscott S.J.. 2004. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell. 14:465–477. 10.1016/S1097-2765(04)00260-6 [DOI] [PubMed] [Google Scholar]
- Blagden C.S., Currie P.D., Ingham P.W., Hughes S.M.. 1997. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 11:2163–2175. 10.1101/gad.11.17.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H.M., Pavlath G.K., Hardeman E.C., Chiu C.P., Silberstein L., Webster S.G., Miller S.C., Webster C.. 1985. Plasticity of the differentiated state. Science. 230:758–766. 10.1126/science.2414846 [DOI] [PubMed] [Google Scholar]
- Callis T.E., Deng Z., Chen J.F., Wang D.Z.. 2008. Muscling through the microRNA world. Exp. Biol. Med. (Maywood). 233:131–138. 10.3181/0709-MR-237 [DOI] [PubMed] [Google Scholar]
- Cao D., Wang Z., Zhang C.L., Oh J., Xing W., Li S., Richardson J.A., Wang D.Z., Olson E.N.. 2005. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell. Biol. 25:364–376. 10.1128/MCB.25.1.364-376.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R., Zhang Y.. 2004. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol. Cell. 15:57–67. 10.1016/j.molcel.2004.06.020 [DOI] [PubMed] [Google Scholar]
- Caretti G., Di Padova M., Micales B., Lyons G.E., Sartorelli V.. 2004. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 18:2627–2638. 10.1101/gad.1241904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D.Z.. 2006. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38:228–233. 10.1038/ng1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.F., Tao Y., Li J., Deng Z., Yan Z., Xiao X., Wang D.Z.. 2010. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J. Cell Biol. 190:867–879. 10.1083/jcb.200911036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture J.F., Trievel R.C.. 2006. Histone-modifying enzymes: encrypting an enigmatic epigenetic code. Curr. Opin. Struct. Biol. 16:753–760. 10.1016/j.sbi.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Devoto S.H., Melançon E., Eisen J.S., Westerfield M.. 1996. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 122:3371–3380. [DOI] [PubMed] [Google Scholar]
- Doherty K.R., Cave A., Davis D.B., Delmonte A.J., Posey A., Earley J.U., Hadhazy M., McNally E.M.. 2005. Normal myoblast fusion requires myoferlin. Development. 132:5565–5575. 10.1242/dev.02155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcales S.V., Puri P.L.. 2005. Signaling to the chromatin during skeletal myogenesis: novel targets for pharmacological modulation of gene expression. Semin. Cell Dev. Biol. 16:596–611. 10.1016/j.semcdb.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Francis J., Chakrabarti S.K., Garmey J.C., Mirmira R.G.. 2005. Pdx-1 links histone H3-Lys-4 methylation to RNA polymerase II elongation during activation of insulin transcription. J. Biol. Chem. 280:36244–36253. 10.1074/jbc.M505741200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A.N., Klesert T.R., Bergstrom D.A., Tapscott S.J.. 1997. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 11:436–450. 10.1101/gad.11.4.436 [DOI] [PubMed] [Google Scholar]
- Hinits Y., Hughes S.M.. 2007. Mef2s are required for thick filament formation in nascent muscle fibres. Development. 134:2511–2519. 10.1242/dev.007088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V., Jansen K.M., Mills S.T., Pavlath G.K.. 2003. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 113:483–494. 10.1016/S0092-8674(03)00319-2 [DOI] [PubMed] [Google Scholar]
- Klose R.J., Zhang Y.. 2007. Regulation of histone methylation by demethylimination and demethylation. Nat. Rev. Mol. Cell Biol. 8:307–318. 10.1038/nrm2143 [DOI] [PubMed] [Google Scholar]
- Kouskouti A., Scheer E., Staub A., Tora L., Talianidis I.. 2004. Gene-specific modulation of TAF10 function by SET9-mediated methylation. Mol. Cell. 14:175–182. 10.1016/S1097-2765(04)00182-0 [DOI] [PubMed] [Google Scholar]
- Kouzarides T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198–209. 10.1016/S0959-437X(02)00287-3 [DOI] [PubMed] [Google Scholar]
- Kurash J.K., Lei H., Shen Q., Marston W.L., Granda B.W., Fan H., Wall D., Li E., Gaudet F.. 2008. Methylation of p53 by Set7/9 mediates p53 acetylation and activity in vivo. Mol. Cell. 29:392–400. 10.1016/j.molcel.2007.12.025 [DOI] [PubMed] [Google Scholar]
- Lassar A.B., Buskin J.N., Lockshon D., Davis R.L., Apone S., Hauschka S.D., Weintraub H.. 1989. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 58:823–831. 10.1016/0092-8674(89)90935-5 [DOI] [PubMed] [Google Scholar]
- Lu J., McKinsey T.A., Zhang C.L., Olson E.N.. 2000. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell. 6:233–244. 10.1016/S1097-2765(00)00025-3 [DOI] [PubMed] [Google Scholar]
- Mal A.K. 2006. Histone methyltransferase Suv39h1 represses MyoD-stimulated myogenic differentiation. EMBO J. 25:3323–3334. 10.1038/sj.emboj.7601229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal A., Harter M.L.. 2003. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. USA. 100:1735–1739. 10.1073/pnas.0437843100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey T.A., Zhang C.L., Olson E.N.. 2001. Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet. Dev. 11:497–504. 10.1016/S0959-437X(00)00224-0 [DOI] [PubMed] [Google Scholar]
- Molkentin J.D., Olson E.N.. 1996. Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6:445–453. 10.1016/S0959-437X(96)80066-9 [DOI] [PubMed] [Google Scholar]
- Nishioka K., Chuikov S., Sarma K., Erdjument-Bromage H., Allis C.D., Tempst P., Reinberg D.. 2002. Set9, a novel histone H3 methyltransferase that facilitates transcription by precluding histone tail modifications required for heterochromatin formation. Genes Dev. 16:479–489. 10.1101/gad.967202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A.H., O’Carroll D., Scherthan H., Mechtler K., Sauer S., Schöfer C., Weipoltshammer K., Pagani M., Lachner M., Kohlmaier A., et al. 2001. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 107:323–337. 10.1016/S0092-8674(01)00542-6 [DOI] [PubMed] [Google Scholar]
- Polesskaya A., Duquet A., Naguibneva I., Weise C., Vervisch A., Bengal E., Hucho F., Robin P., Harel-Bellan A.. 2000. CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem. 275:34359–34364. 10.1074/jbc.M003815200 [DOI] [PubMed] [Google Scholar]
- Ruthenburg A.J., Li H., Patel D.J., Allis C.D.. 2007. Multivalent engagement of chromatin modifications by linked binding modules. Nat. Rev. Mol. Cell Biol. 8:983–994. 10.1038/nrm2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Caretti G.. 2005. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr. Opin. Genet. Dev. 15:528–535. 10.1016/j.gde.2005.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Puri P.L., Hamamori Y., Ogryzko V., Chung G., Nakatani Y., Wang J.Y., Kedes L.. 1999. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell. 4:725–734. 10.1016/S1097-2765(00)80383-4 [DOI] [PubMed] [Google Scholar]
- Sims R.J., III, Nishioka K., Reinberg D.. 2003. Histone lysine methylation: a signature for chromatin function. Trends Genet. 19:629–639. 10.1016/j.tig.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Soulez M., Rouviere C.G., Chafey P., Hentzen D., Vandromme M., Lautredou N., Lamb N., Kahn A., Tuil D.. 1996. Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol. Cell. Biol. 16:6065–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S.J. 2005. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 132:2685–2695. 10.1242/dev.01874 [DOI] [PubMed] [Google Scholar]
- Wang H., Cao R., Xia L., Erdjument-Bromage H., Borchers C., Tempst P., Zhang Y.. 2001. Purification and functional characterization of a histone H3-lysine 4-specific methyltransferase. Mol. Cell. 8:1207–1217. 10.1016/S1097-2765(01)00405-1 [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang D.Z., Hockemeyer D., McAnally J., Nordheim A., Olson E.N.. 2004. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature. 428:185–189. 10.1038/nature02382 [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S.J., Davis R.L., Thayer M.J., Adam M.A., Lassar A.B., Miller A.D.. 1989. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl. Acad. Sci. USA. 86:5434–5438. 10.1073/pnas.86.14.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T.K., Turner D., Rupp R., Hollenberg S., et al. 1991. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 251:761–766. 10.1126/science.1846704 [DOI] [PubMed] [Google Scholar]
- Xiao B., Jing C., Wilson J.R., Walker P.A., Vasisht N., Kelly G., Howell S., Taylor I.A., Blackburn G.M., Gamblin S.J.. 2003. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 421:652–656. 10.1038/nature01378 [DOI] [PubMed] [Google Scholar]