Abstract
Background
Type 2 diabetes is an important risk factor for heart failure and is common among patients with heart failure. The impact of weight on prognosis after hospitalization for acute heart failure among patients with diabetes is unknown. The objective of this study was to examine all-cause mortality in relation to weight status among patients with Type 2 diabetes hospitalized for decompensated heart failure.
Methods
The Worcester Heart Failure Study included adults admitted with acute heart failure to all metropolitan Worcester medical centers in 1995 and 2000. The weight status of 1,644 patients with diabetes (history of Type 2 diabetes in medical record or admission serum glucose ≥200 mg/dl) was categorized using body mass index calculated from height and weight at admission. Survival status was ascertained at 1 and 5 years after hospital admission.
Results
65% of patients were overweight or obese and 3% were underweight. Underweight patients had 50% higher odds of all-cause mortality within 5 years of hospitalization for acute heart failure than normal weight patients. Class I and II obesity were associated with 20% and 40% lower odds of dying. Overweight and Class III obesity were not associated with mortality. Results were similar for mortality within 1 year of hospitalization for acute heart failure.
Conclusions
The mechanisms underlying the association between weight status and mortality are not fully understood. Additional research is needed to explore the effects of body composition, recent weight changes, and prognosis after hospitalization for heart failure among patients with diabetes.
Keywords: heart failure, diabetes, obesity, mortality
INTRODUCTION
The prevalence of Type 2 diabetes has increased in recent years and currently 10% of Americans aged 40 – 59 years and one-fifth of those 60 years and older have diabetes.1 Adults with diabetes are at increased risk for developing heart failure and develop heart failure at a considerably younger age than those without diabetes.2 An estimated 20% to 40% of patients with heart failure have comorbid diabetes.3-6 Among patients with heart failure, diabetes has been associated with higher all-cause mortality.3,5,6
Obesity is a known risk factor for diabetes7-9 and the prevalence of diabetes is higher among obese compared to normal weight patients hospitalized with heart failure.10-12 Patients with comorbid diabetes tend to be heavier than heart failure patients without diabetes.3,5,6 Several studies have shown an inverse relationship between weight and all-cause mortality in patients with heart failure.10-13 However, whether this inverse association is present among patients with comorbid heart failure and diabetes is unknown. Using data from the population-based Worcester Heart Failure Study, we examined 1- and 5-year all-cause mortality following hospitalization for heart failure in relation to weight status among patients with diabetes.
METHODS
The Worcester Heart Failure Study included adult residents from the Worcester, MA metropolitan area (2000 census estimate = 478,000) hospitalized for possible heart failure at all 11 greater Worcester medical centers during 1995 and 2000.14,15 The University of Massachusetts Medical School Institutional Review Board approved this study. The medical records of patients with primary and/or secondary International Classification of Disease (ICD)-9 discharge diagnoses consistent with possible heart failure (ICD-9 code 428) were reviewed in a standardized manner.14,15 Trained study physicians and nurses also reviewed the medical records of patients with discharge diagnoses of rheumatic heart failure, hypertensive heart and renal disease, acute cor pulmonale, cardiomyopathy, pulmonary heart disease and congestion, acute lung edema, edema, dyspnea, and respiratory abnormalities to identify patients with new onset heart failure.14,15 Confirmation of the diagnosis of heart failure included the presence of two major Framingham criteria or presence of one major and two minor criteria.16 An incident event of heart failure was defined as the absence of a prior hospitalization for heart failure, prior physician diagnosis of heart failure, or past treatment for heart failure based on the medical record review. Patients who developed heart failure secondary to admission for another acute illness (e.g., acute myocardial infarction) or after an interventional procedure or surgery were not included. Medical record abstraction included patients’ age, sex, race, prior comorbidities, physical examination findings, laboratory findings, and clinical characteristics. Quality control checks conducted by the principal investigator (RJG) and a physician study coordinator included redundant review of 5 –10% of all completed charts. Patients were considered to have Type 2 diabetes if a history of Type 2 diabetes was indicated in their medical record and/or if their admission blood glucose level was 200 mg/dl or higher. Body mass index (BMI; kg/m2) was calculated from height and weight recorded in patients’ medical records at hospital admission. Patients’ weight status was categorized as either underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), Class I obesity (30 kg/m2 ≤ BMI < 35 kg/m2), Class II obesity (35 kg/m2 ≤ BMI < 40 kg/m2), or Class III obesity (40 kg/m2 ≤ BMI).17
Patients’ long-term survival status was ascertained through the review of hospital medical records at all participating medical centers for subsequent hospitalizations or medical care contacts and through the review of the Social Security Death Index and death certificates at the Massachusetts State Health Department.18 We examined all-cause mortality at 1 and 5 years following hospital admission for decompensated heart failure.
Of the 4,536 patients with confirmed acute heart failure in 1995 and 2000, we excluded 65 patients who were missing information on prior diabetes and/or admission blood glucose levels and 2,424 patients without Type 2 diabetes. We additionally excluded patients who were missing height or weight (n = 338), age (n = 2), blood pressure (n = 28), or estimated glomerular filtration rate (GFR, n = 9), and those hospitalized for 30 days or longer (n = 26), resulting in an analytic sample of 1,644 patients with Type 2 diabetes hospitalized with acute heart failure.
Statistical Analyses
We compared demographic and clinical characteristics of the sample in relation to weight status using X2 tests for categorical variables and ANOVA for continuous variables. Lifetable methods were used to estimate cumulative incidence of all-cause mortality at 1-year and 5-years after admission for acute heart failure. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality using Cox proportional hazards models.19 HRs assess the instantaneous probability of death at each time interval, given survival up to that time. We controlled for confounding by multivariable adjustment. Potential confounders (Table 1) were considered based on associations with all-cause mortality. Variables which differed by weight status were included in multivariate-adjusted models and are listed in the footnotes for Table 2 and the Figure. Analyses were conducted in SAS (Version 9.2, SAS Institute, Inc., Cary, NC).
Table 1.
Characteristics of adults with diabetes hospitalized for heart failure, in relation to admission weight status*, M (SD) or %
| Underweight (n = 47) | Normal weight (n = 532) | Overweight (n = 479) | Class I Obesity (n = 297) | Class II obesity (n = 136) | Class III obesity (n = 153) | p-value** | |
|---|---|---|---|---|---|---|---|
| Age, years | 80.6 (8.1) | 78.1 (9.7) | 74.8 (9.8) | 73.6 (10.8) | 68.2 (11.9) | 66.2 (11.4) | < 0.0001 |
| <65 years | 4.3 | 9.0 | 13.4 | 17.8 | 34.6 | 49.2 | |
| 65 – 74 years | 19.2 | 18.6 | 32.4 | 30.3 | 31.6 | 22.9 | |
| 75 – 84 years | 48.9 | 47.6 | 39.0 | 39.4 | 27.2 | 23.5 | |
| >=85 years | 27.7 | 24.8 | 15.2 | 12.5 | 6.2 | 4.6 | |
|
| |||||||
| Male | 31.9 | 44.0 | 49.3 | 46.5 | 36.0 | 24.2 | <0.0001 |
|
| |||||||
| White race | 95.7 | 95.7 | 94.8 | 93.9 | 84.9 | 90.1 | <0.0001 |
|
| |||||||
| Current smoker | 12.8 | 9.4 | 6.5 | 10.4 | 8.1 | 11.8 | 0.22 |
|
| |||||||
| DNR | 55.3 | 30.6 | 21.5 | 21.2 | 16.9 | 20.3 | <0.0001 |
|
| |||||||
| Blood pressure, mmHg | |||||||
| Systolic | 147.8 (33.8) | 148.6 (35.5) | 148.9 (33.1) | 151.6 (30.3) | 149.5 (32.1) | 148.5 (30.4) | 0.87 |
|
| |||||||
| Diastolic | 77.8 (27.4) | 76.7 (20.5) | 75.7 (18.3) | 78.0 (18.9) | 79.4 (18.7) | 77.0 (19.4) | 0.38 |
|
| |||||||
| GFR, ml/min/1.73 m2 | 67.4 (32.3) | 54.2 (27.6) | 57.3 (31.6) | 56.6 (26.4) | 62.9 (31.2) | 67.9 (40.8) | <0.0001 |
|
| |||||||
| Glucose, mg/dl | 211.6 (88.2) | 217.6 (99.5) | 214.7 (102.7) | 204.3 (95.2) | 211.2 (99.7) | 196.0 (86.4) | 0.11 |
|
| |||||||
| Length of stay (days) | 7.8 (6.1) | 5.4 (4.6) | 5.6 (4.5) | 5.2 (4.5) | 5.6 (4.3) | 6.8 (5.8) | 0.0003 |
|
| |||||||
| Medical History | |||||||
|
| |||||||
| Hypertension | 53.2 | 71.6 | 74.1 | 74.6 | 76.5 | 68.6 | 0.03 |
|
| |||||||
| COPD | 44.7 | 30.6 | 34.2 | 36.7 | 39.0 | 45.1 | 0.01 |
|
| |||||||
| Liver disease/failure | 4.3 | 1.5 | 3.6 | 3.0 | 0.0 | 2.0 | 0.10 |
|
| |||||||
| Renal disease/failure | 19.2 | 30.3 | 33.0 | 30.6 | 26.5 | 24.2 | 0.16 |
|
| |||||||
| PVD | 10.6 | 22.0 | 28.4 | 21.9 | 19.9 | 18.3 | 0.01 |
|
| |||||||
| PCI | 6.4 | 8.1 | 12.3 | 14.5 | 12.5 | 7.2 | 0.2 |
|
| |||||||
| CABG | 4.3 | 25.9 | 30.1 | 26.6 | 17.7 | 13.1 | <0.0001 |
|
| |||||||
| Atrial fibrillation | 46.8 | 34.0 | 33.0 | 37.7 | 25.7 | 19.6 | 0.0003 |
|
| |||||||
| Heart failure | 85.1 | 82.9 | 80.4 | 83.8 | 80.2 | 81.1 | 0.77 |
Underweight (18.5 kg/m2 < body mass index [BMI]), normal weight (18.5 kg/m2 ≤ BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), Class I obesity (30 kg/m2 ≤ BMI < 35 kg/m2), Class II obesity (35 kg/m2 ≤ BMI < 40 kg/m2), and Class III obesity (40 kg/m2 ≤ BMI).
p-values from X2 tests categorical variables and ANOVA for continuous variables.
Table 2.
All-cause mortality following hospitalization for acute HF in relation to weight status* in patients with diabetes, HR (95% CI)
| Underweight (n = 47) | Normal weight (n = 532) | Overweight (n = 479) | Class I obesity (n = 297) | Class II obesity (n = 136) | Class III obesity (n = 153) | |
|---|---|---|---|---|---|---|
| 1-year mortality | ||||||
| Cumulative incidence (%) | 72.3 | 49.1 | 42.2 | 32.7 | 24.3 | 40.5 |
| Crude | 1.8 (1.3 – 2.6) | (Referent) | 0.8 (0.7 – 1.0) | 0.6 (0.5 – 0.7) | 0.4 (0.3 – 0.6) | 0.8 (0.6 – 1.0) |
| Age-adjusted | 1.8 (1.2 – 2.5) | (Referent) | 0.9 (0.7 – 1.1) | 0.6 (0.5 – 0.8) | 0.5 (0.3 – 0.7) | 1.0 (0.7 – 1.3) |
| Multivariate-adjusted§ | 1.4 (1.0 – 2.0) | (Referent) | 0.9 (0.8 – 1.1) | 0.6 (0.5 – 0.8) | 0.5 (0.4 – 0.8) | 0.9 (0.7 – 1.3) |
| 5-year mortality | ||||||
| Cumulative incidence (%) | 100.0 | 87.2 | 82.5 | 80.1 | 70.6 | 78.4 |
| Crude | 1.9 (1.4 – 2.6) | (Referent) | 0.8 (0.7 – 0.9) | 0.7 (0.6 – 0.8) | 0.5 (0.4 – 0.7) | 0.7 (0.6 – 0.9) |
| Age-adjusted | 1.8 (1.4 – 2.5) | (Referent) | 0.9 (0.8 – 1.0) | 0.8 (0.6 – 0.9) | 0.6 (0.5 – 0.8) | 0.9 (0.7 – 1.1) |
| Multivariate-adjusted§ | 1.5 (1.1 – 2.0) | (Referent) | 0.9 (0.8 – 1.0) | 0.8 (0.6 – 0.9) | 0.6 (0.5 – 0.8) | 0.9 (0.8 – 1.2) |
Underweight (18.5 kg/m2 < body mass index [BMI]), normal weight (18.5 kg/m2 ≤ BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), Class I obesity (30 kg/m2 ≤ BMI < 35 kg/m2), Class II obesity (35 kg/m2 ≤ BMI < 40 kg/m2), and Class III obesity (40 kg/m2 ≤ BMI).
All models adjusted for study cohort (1995 versus 2000).
Adjusted for study cohort (1995 versus 2000), age (categorical), sex, DNR, GFR (continuous), length of stay (continuous), history of hypertension, history of COPD, history of PVD, history of PCI, history of CABG, and history of atrial fibrillation.
Figure.
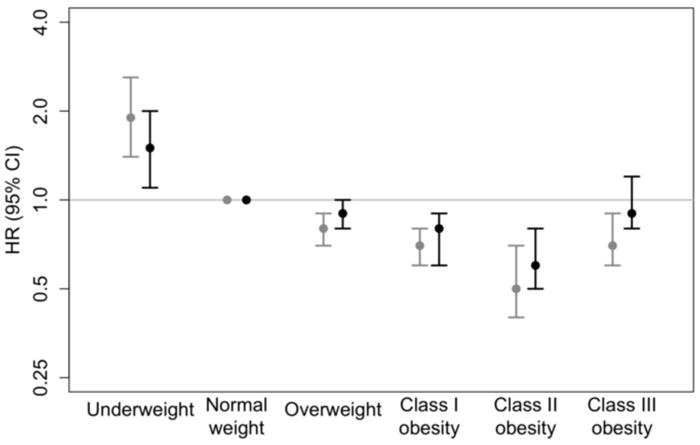
5-year mortality in relation to admission weight status*, among patients with diabetes hospitalized for heart failure
* Underweight (18.5 kg/m2 < body mass index [BMI]), normal weight (18.5 kg/m2 ≤ BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), Class I obesity (30 kg/m2 ≤ BMI < 35 kg/m2), Class II obesity (35 kg/m2 ≤ BMI < 40 kg/m2), and Class III obesity (40 kg/m2 ≤ BMI).
Crude HRs (black) adjusted for study cohort. Multivariate-adjusted HRs (grey) adjusted for study cohort (1995 versus 2000), age (categorical), sex, DNR, GFR (continuous), length of stay (continuous), history of hypertension, history of COPD, history of PVD, history of PCI, history of CABG, and history of atrial fibrillation.
RESULTS
Baseline characteristics
The average age of our study population was 74 years, 57% were female, and 82% had been previously diagnosed with heart failure. Approximately one-third of patients were of normal weight (32.4%), 29.1% were overweight, and 35.6% were obese, including 18.1% with Class I obesity, 8.3% with Class II obesity, and 9.3% with Class III obesity. Only 47 patients (2.9%) were underweight. The majority of patients (n = 1,444; 87.8%) had been previously diagnosed with diabetes, of which 43.6% had serum glucose levels of 200 mg/dl or higher at admission. The proportion of patients with high serum glucose did not differ by weight status (42.9% of underweight patients, 43.7% of normal weight, 44.5% overweight, and 43.2%, 48.0%, and 37.9% of patients with, respectively, Class I, II, and III obesity; p = 0.69).
On average, patients with Class III obesity were twelve years younger than patients of normal weight (Table 1). A higher proportion of normal weight, overweight, and patients with Class I obesity were men. Underweight and obese patients were more likely to be current smokers. Utilization of DNR orders was inversely associated with weight status. On average, patients with diabetes had admission blood pressures in the hypertensive range and average readings were similar according to weight status. Underweight patients and patients with Class III obesity had, on average, higher estimated GFR levels and longer hospital stays. Underweight patients were less likely to have a history of hypertension, peripheral vascular disease , or had previously undergone CABG surgery (Table 1). Fewer underweight patients and those with Class III obesity had previously undergone a percutaneous coronary intervention (PCI). Underweight patients were more likely to have a previous diagnosis of atrial fibrillation (Table 1).
Mortality following hospitalization for acute heart failure
Almost one half of normal weight patients (49.1%) died within one year after hospital admission for acute heart failure (Table 2). Class I obesity was associated with 40% lower odds of dying (adjusted HR = 0.6; 95% CI: 0.5 – 0.8) and Class II obesity was associated with 50% lower odds of mortality (adjusted HR = 0.5; 95% CI: 0.4 – 0.8). Underweight patients had 40% higher odds of mortality (adjusted HR = 1.4; 95% CI: 1.0 – 2.0). Overweight and Class III obesity were not associated with 1-year all-cause mortality (Table 2).
By five years after an admission for decompensated heart failure, all 47 underweight patients had died as well as the majority of normal weight (87.2%), overweight (82.5%), and obese (70.6 – 80.1%) patients (Table 2). Class I obesity was associated with 20% lower odds of mortality (adjusted HR = 0.8; 95% CI: 0.6 – 0.9) and Class II obesity was associated with 40% lower odds of mortality (adjusted HR = 0.6; 95% CI: 0.5 – 0.8; Table 2 and Figure). Underweight was associated with 50% higher odds of mortality (adjusted HR = 1.5; 95% CI: 1.1 – 2.0; Table 2). Overweight and Class III obesity were not associated with increased mortality within 5 years following hospitalization for acute heart failure (Table 2).
DISCUSSION
In this population-based study of residents of central Massachusetts, underweight patients had 50% higher odds of mortality by 5 years after hospital admission for decompensated heart failure than normal weight patients, and Class I and II obesity were associated with 20% and 40% lower odds of mortality. These results support previous studies that found an inverse or U-shaped relationship between weight and mortality in patients with heart failure.10-12,20 To our knowledge, this study is the first to examine the impact of weight status on survival following hospitalization for decompensated heart failure among patients with diabetes.
Several mechanisms have been proposed to explain the paradoxical association between increased body mass and reduced mortality in heart failure. Obese patients with heart failure tend to be considerably younger than patients who are normal weight or underweight,12,13 which may confer a survival advantage. In the current study, age was inversely associated with weight status, and adjustment for weight status attenuated the estimated association between weight and all-cause mortality, especially among patients with Class III obesity, lending support to this hypothesis. The observed association may not reflect a benefit of increased adiposity, but rather the adverse influence of cardiac cachexia on mortality in patients with heart failure.21 Cardiac cachexia is characterized by loss of fat, muscle, and bone.22 BMI may not accurately differentiate adults with elevated body fat among the general population23 and specifically in patients with heart failure.24 We did not have data available on body fat or waist circumference to further refine our classification of obesity, which is a common limitation of clinical research studies. Since BMI is correlated with both body fat and lean mass,23,24 the survival advantage of overweight and Class I or II obesity may reflect higher lean mass. This potential misclassification of obesity in terms of body composition may partially explain the observed association between weight status and mortality following hospitalization for decompensated heart failure.
Increased adiposity might also reflect enhanced metabolic reserve which might partially protect patients with heart failure from the negative effects of cachexia. Adipose tissue has important endocrine and paracrine effects that not only regulate metabolism, but also influence cardiac structure and function, natriuretic responsiveness, and metabolism of circulating inflammatory cytokines.25-27 Several inflammatory and adipocytokines are known to be associated with the development of, and poorer prognosis after, heart failure.28,29 Differential expression of these cytokines at different body mass indices may also explain our findings.
Underweight among patients with comorbid diabetes and heart failure is uncommon.6 While only 3% of our sample were underweight, all 47 underweight patients died within five years of hospitalization for heart failure. Underweight may be a sign of occult non-cardiac disease, poorly-controlled diabetes, or may indicate the presence of cardiac cachexia.22 Given the strong link between increased body mass and incident diabetes,7,8 underweight in a patient with diabetes and heart failure may indicate the presence of more severe cardiac cachexia.
In addition to the 100% 5-year mortality among underweight patients, 87% of normal weight patients, 83% of overweight patients, and 70 to 80% of obese patients died within 5 years of hospitalization for acute heart failure. These 5-year mortality rates are similar to rates of 63 – 88% observed in two population-based studies of heart failure among adults with diabetes.3,30
Current major guidelines have not reached a consensus regarding recommendations for weight management in obese patients with heart failure. The American Heart Association recommends weight loss for patients with Class III obesity with the goal of reducing their BMI t less than 40 kg/m2,31 the Heart Failure Society of America recommends weight loss in patients with Class II or III obesity,32 and the European Society of Cardiology recommends weight loss for all obese patients.33 Therefore, it is difficult for clinicians to incorporate evidence-based weight loss recommendations into heart failure self-care. A dearth of evidence regarding intentional weight changes among patients with heart failure likely contributes to this lack of consistency in guidelines. Weight loss is common among patients with heart failure; one study found that more than 40% of patients lost at least 5% of their baseline weight and such weight loss during follow-up was associated with higher subsequent mortality.34 Another study found a gradient of elevated mortality with increasing degree of weight loss.35 Among adults with diabetes, intentional weight loss has been linked to lower mortality but unintentional weight loss was associated with higher mortality.36 It is possible that categorization as underweight and normal weight at hospital admission in many patients with diabetes and heart failure is a result of recent unintentional weight loss. Unintentional weight loss could offer an alternative explanation for the higher mortality observed in normal weight patients compared to overweight and obese patients with heart failure. Additional research is needed to better understand the impact of weight changes and weight history on mortality in patients with diabetes and heart failure.
Study strengths and limitations
The strengths of the present study include the population-based sample of patients hospitalized with acute heart failure at all medical centers in Central Massachusetts and the detailed, high-quality clinical data collected using standardized procedures. Our study also has limitations. Since the vast majority (94%) of our sample was non-Hispanic white, we cannot extrapolate our findings to other racial/ethnic groups. Ejection fraction findings during hospitalization were available for only 35% of participants; thus, we could not further classify the study population into those with systolic versus diastolic heart failure. While reduced ejection fraction findings were less often observed among overweight and obese patients with acute heart failure 13 and patients with preserved ejection fraction have lower mortality,12 the relationship between weight status and subsequent mortality was similar in patients with preserved and reduced left ventricular dysfunction.12,13
We considered a plasma glucose level of at least 200 mg/dL (11.1 mmol/L) indicative of Type 2 diabetes mellitus in the absence of a recorded medical history of Type 2 diabetes. It is likely that many of these glucose measurements were obtained while the patient was not fasting and our definition of diabetes may be less sensitive than criteria recommended by the American Diabetes Association37 and may be more likely to identify more severe cases of diabetes. However, the vast majority of our sample had previously-diagnosed diabetes (88%), and the proportion of these patients whose admission glucose levels > 200 mg/dl did not differ by weight status. Additional information on indices of diabetes control, such as HbA1c, was not available. The Worcester Heart Failure Study only collected information about cardiac medications and thus information about medication used to manage diabetes, such as insulin or oral agents, was not available. Therefore, confounding by different diabetes treatment across weight status is possible.
Patients’ BMI was calculated from weights and heights abstracted from their medical records. We did not collect information on recent changes in patients’ weight prior to hospitalization, nor did we have information on patients’ discharge weight following diuresis, which may have resulted in partial misclassification of their weight status. Among patients who did not report symptoms suggestive of recent weight changes and thus potentially misclassified dry weight (reported symptoms of weight gain, weight loss, and/or edema; n = 418), crude and age-adjusted HRs for 1-year and 5-year mortality in relation to weight status were similar to our main results (data not shown). A previous study observed a similar association between weight and mortality in their overall sample and subset of patients without edema,20 suggesting that the use of admission weight status is unlikely to explain our results.
Conclusions
The results of this population-based investigation provide insights into the role of the weight on 1- and 5-year mortality following hospitalization for decompensated heart failure among patients with diabetes. Underweight patients had 50% higher odds of mortality by 5 years than normal weight patients, and Class I and II obesity were associated with 20% and 40% lower odds of dying. The mechanisms underlying the observed association between weight and all-cause mortality are not fully understood and additional research is needed to explore the relationship between body composition, recent weight changes, and prognosis following hospitalization for heart failure among patients with diabetes.
Acknowledgments
Grant support for this project was provided by the National Heart, Lung, and Blood Institute (R37 HL69874). Partial salary support for Drs. Waring, Saczynski, McManus, Gore, and Goldberg is provided by the National Institutes of Health grants 1U01HL105268-01. This study was made possible by the cooperation of the administration, medical records, and cardiology departments of participating Worcester metropolitan area hospitals.
Footnotes
None of the authors have conflicts of interest to disclose. All authors had access to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999 - 2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 2.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in Type 2 Diabetes. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 3.From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;154(2):277.e1–277.e8. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar D, Deswal A, Ramasubbu K, Mann DL, Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol. 2010;105:373–377. doi: 10.1016/j.amjcard.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005;59:134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol. 2010;171(5):550–556. doi: 10.1093/aje/kwp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgibbons TP, Hardy OT, Lessard D, Gore JM, Yarzebski J, Goldberg RJ. Body mass index, treatment practices, and mortality in patients with acute heart failure. Coron Atery Dis. 2009;20(8):536–543. doi: 10.1097/MCA.0b013e3283324920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M for the ADHERE Scientific Advisory Committee and Investigators. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108927 patients in the acute decompensated heart failure national registry. Am Heart J. 2007;153(1):74–81. doi: 10.1016/j.ahj.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. AJM. 2005;118(7):728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg RJ, Spencer FA, Farmer C, Lessard D, Pezzella SM, Meyer TE. Use of disease-modifying therapies in patients hospitalized with heart failure: a population-based perspective. AJM. 2007;120(1):98.e1–98.e8. doi: 10.1016/j.amjmed.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 16.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. Obes Res. 1998;6(Suppl 2):51. [PubMed] [Google Scholar]
- 18.Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167(5):490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 19.Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4. Cornwall, UK: Blackwell Publishing; 2002. Chapter 17: Survival Analysis; pp. 568–590. [Google Scholar]
- 20.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with heart failure: insights from the Candesartin in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116(6):627–636. doi: 10.1161/CIRCULATIONAHA.106.679779. [DOI] [PubMed] [Google Scholar]
- 21.Anker SD, Ponikowski P, Varney S, et al. Wasting as an independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(1050):1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 22.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36:518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. International Journal of Obesity. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85(7):609–617. doi: 10.4065/mcp.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alpert MA, Terry BE, Mulekar M, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80:736–740. doi: 10.1016/s0002-9149(97)00505-5. [DOI] [PubMed] [Google Scholar]
- 26.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43(9):1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed-Ali V, Goodrick S, Bulmer K, Holley JMP, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol Endocrinol Metab. 1999;277(40):E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 28.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the Cytokine Database from the Vesnarinone Trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 29.Feldman Arthur M, Combes Alain, Wagner Daniel, Kadakomi Toshiaki, Kubota Toru, Li Yun You, McTiernan Charles. J Am Coll Cardiol. 2000;35(3):537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 30.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DCJ. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 31.Riegel B, Moser DK, Anker SD, et al. State of the Science: Promoting Self-Care in Persons with Heart Failure: A Scientific Statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 32.Heart Failure Society of America. Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12(1):10–38. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Dickstein K, Cohen SA, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 34.Anker SD, Negassa A, Coats AJS, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9. [DOI] [PubMed] [Google Scholar]
- 35.Pocock SJ, McMurray JJV, Dobson J, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 36.Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Trying to lose weight, losing weight, and 9-year mortality in overweight U.S. adults with diabetes. Diabetes Care. 2004;27(3):657–662. doi: 10.2337/diacare.27.3.657. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–S48. [PubMed] [Google Scholar]


