Cognitive dysfunction remains prevalent in the era of effective antiretroviral therapy. A recent study found that HIV-associated neurocognitive disorders (HAND) were present in 64% of HIV-infected individuals who were aviremic and without cognitive complaints.1 Such high rates of cognitive dysfunction are of major public health significance as even mild cognitive impairment may impair an individual’s ability to live independently and is associated with an increased mortality.2,3 The etiologies responsible for this continued cognitive dysfunction are likely multifactorial and may include persistent impairment that developed in the period predating use of effective antiretroviral therapy, on-going failure of current antiretroviral therapy (ARV) to effectively cross the blood brain barrier, toxicity of these medications, or the inability of these medications to fully suppress the effects of HIV per se among cells important to central nervous system (CNS) inflammation such as monocyte/macrophages, microglia or astrocytes.4 One alternative hypothesis is that etiologies responsible for cognitive dysfunction in the general population such as cerebrovascular disease (CVD) may also have an influence on cognition in this population. Multiple CVD risks such as glucose intolerance/diabetes, hypercholesterolemia, and hypertension are elevated in the HIV-population either due to the effect of HIV and/or to the impact of therapy. Several recent studies have demonstrated that poorer cognitive test performance in the HIV population is associated with vascular risk factors.5,6
The cognitive impact of CVD is presumably from the damage sustained from multiple microinfarcts particularly within the white and deep gray matter of the brain. It is now well established that CVD risk added to another degenerative disease process such as Alzheimer’s Disease is capable of accelerating the onset of otherwise subclinical disease to one with overt clinical functional consequences.7 Similar concerns may be valid in the context of HIV-associated cognitive dysfunction.
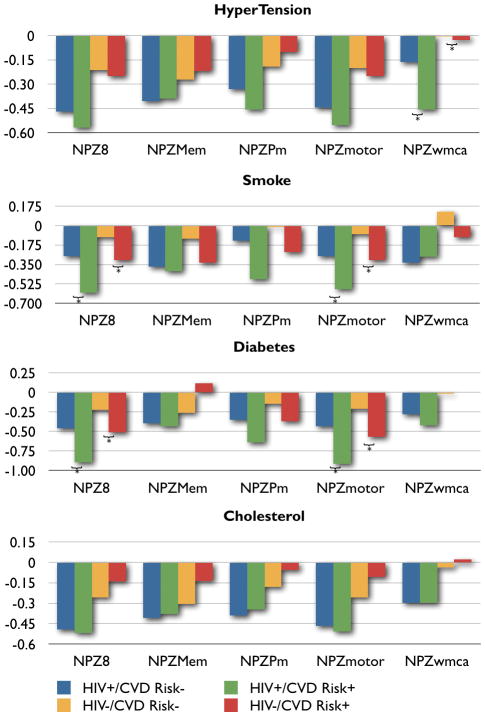
CVD risk data was collected in the Hawaii Aging with HIV Cohort (HAHC) Study, a longitudinal cohort study assessing cognitive and neurologic outcomes in older HIV-infected individuals which has been detailed elsewhere.8 Briefly, 158 older (50 years of age or older) and 128 younger (20–39 years old) HIV-infected individuals were recruited between October 2001 to October 2005 with age, education, ethnicity, and gender-matched controls. Exclusion criteria included major neurologic or psychiatric illness, learning disability, major head injury, brain opportunistic infection, and primary language other than English. Baseline and annual evaluations included demographic data, medical/medication/substance abuse histories, neurologic examination, neuropsychological testing, and HIV laboratory parameters. The IRB at the University of Hawaii approved the study, and all subjects signed informed consent. Based on baseline data from a subset of 158 HIV+ and 123 HIV− age-, education-, and gender-matched HAHC subjects ≥ 50 years of age, we explored the differential effects of several CVD risk factors (diabetes, hypercholesterolemia, smoking and hypertension) on neuropsychological performance (NP) in HIV+ and HIV− subjects. Diabetes was defined by patient report of diagnosis, use of anti-diabetic medication, or fasting glucose ≥ 126 mg/dL. Hypercholesterolemia was defined by patient report of diagnosis, use of lipid-lowering agents, or fasting total cholesterol > 200 mg/dL. Smoking was defined as either current or past cigarette use, and hypertension based on prior diagnosis, use of anti-hypertensive medications, or resting BP ≥ 140/≥ 90 mm Hg. An extensive NP battery was performed, and age- and education-adjusted NP composite scores were calculated as previously described 9 to assess global cognitive function (NPZ8) and NP subdomains including psychomotor speed (NPZpm), motor function (NPZmotor), memory (NPZmem), and working memory/attention and concentration (NPZwmca) where a lower NPZ composite score implies worse cognitive impairment (see Figure legend for NP tests included in composite scores). The five NP composite scores (i.e., NPZ8, NPZpm, NPZmotor, NPZmem, NPZwmca) were used as the outcome. HIV-status and the addition of each CVD risk factor were considered separately (e.g., HIV-status and the presence or absence of diabetes; HIV-status and the presence or absence of hypercholesterolemia) in a multiple regression model to determine the effect of HIV and each individual CVD risk factor on NP.
Mean age of HIV+ subjects was 55 years of age (standard deviation, SD = 5.3). A majority of these individuals were white (70%; 110/158), males (70%; 110/158) with at least a high school education (mean years of education = 14.6 years, SD 2.6 years). Seventy five percent of HIV+ subjects were treated with highly active antiretroviral therapy (HAART) and about half of these individuals (54%) had undetectable plasma HIV RNA viral load. Mean log10 HIV viral load was 2.6 copies/mL (SD, 1.3) with a mean CD4 count of 477 cells/uL (SD, 257) and nadir CD4 count of 202 cells/uL (SD, 166). In the HIV+ group, the prevalence of CVD risk factors were as follows: 70% were current or prior smokers, 45% had hypertension, 37% had hypercholesterolemia, and 11% had diabetes. There were no statistically significant differences in frequencies of smoking history and hypertension between HIV+ and HIV− groups. Hypercholesterolemia and diabetes were more prevalent in the HIV+ group. Most groups tended to perform below the mean for age- and education-matched controls from published normative data (Figure). Multiple regression analyses revealed that HIV (p = 0.006), diabetes (p = 0.021), and tobacco use (p = 0.005) were independent predictors of global cognitive function (NPZ8) with an adjusted R2 = 0.05. HIV (p = 0.007), diabetes (p = 0.008), and tobacco use (p = 0.004) were independent predictors of motor function (NPZmotor) with an adjusted R2 = 0.05. HIV (p = 0.001) and hypertension (p = 0.039) were independent predictors of working memory, concentration, and attention (NPZwmca) with an adjusted R2 = 0.06. If HIV-status was fixed, subjects who used tobacco would be expected to score 0.27 standard deviations lower on NPZ8 and 0.27 standard deviations lower on NPZmotor compared to non-smokers. If HIV-status was fixed, subjects with diabetes would score 0.40 standard deviations lower on NPZ8 and 0.45 standard deviations lower on NPZmotor compared to non-diabetics. Subjects with hypertension would score 0.18 standard deviations lower on NPZwmca compared to those without hypertension with HIV-status fixed. There were no significant interactions between HIV and CVD risk factors for any composite NP score. Subjects with multiple CVD risk factors were associated with worse global cognitive performance (p = 0.027).
CVD risk factors were not optimally collected and is a limitation of our study. For example, the components of total cholesterol (i.e., LDL and HDL) were not obtained, and the diagnosis of diabetes was made by self-report. Thus, our results may underestimate the effect of diabetes and cholesterol on NP. Nevertheless, several interesting observations and conclusions are possible. As expected, our data showed that CVD risk factors worsen NP performance both in HIV+ and HIV− subjects. Our limited dataset suggests that CVD have an additive rather than synergistic effect on cognitive performance. Although the modest decreases in NP performance found in our cross-sectional analysis may not result in clinically significant interruptions of activities of daily living or observable deficiencies during a routine clinic visit, this finding is clinically important however since over time, the presence of CVD added to HIV-associated subclinical NP deficits may lead to overt functional cognitive problems. CVD risk factors are treatable with lifestyle modifications and anti-hypertensive, anti-diabetic, and lipid-lowering medications. Increased efforts to control CVD risk factors in the HIV-infected population are warranted.
Acknowledgments
Sources of Support: This manuscript was funded in part by research grants P20RR011091, U54NS43049, and K23-AG032872.
References
- 1.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (London, England) 2010;24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 2.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–31. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 3.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–8. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Annals of neurology. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 5.Wright EJ, Grund B, Robertson K, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75:864–73. doi: 10.1212/WNL.0b013e3181f11bd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker JT, Kingsley L, Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–9. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–74. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–7. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiramizu B, Paul R, Williams A, et al. HIV proviral DNA associated with decreased neuropsychological function. J Neuropsychiatry Clin Neurosci. 2007;19:157–63. doi: 10.1176/appi.neuropsych.19.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]