Abstract
OBJECTIVE
To estimate characteristics and outcomes of pregnant and immediately postpartum women hospitalized with influenza-like illness during the 2009–2010 influenza pandemic, and the factors associated with more severe illness.
METHODS
An observational cohort in 28 hospitals of pregnant and postpartum (within 2 weeks of delivery) women hospitalized with influenza-like illness. Influenza-like illness was defined as clinical suspicion of influenza and either meeting the Centers for Disease Control and Prevention (CDC) definition of influenza-like illness (fever 100.0º F or higher, cough, sore throat) or positive influenza test.
RESULTS
Of 356 women meeting eligibility criteria, 35 (9.8%) were admitted to the ICU and 4 (1.1%) died. Two-hundred and eighteen women (61.2%) were in the third trimester and 10 (2.8%) were postpartum. Over half (55.3%) were admitted in October and 25.0% in November, with rapidly decreasing numbers thereafter. Antiviral therapy was administered to 10.1% of the women before hospitalization and to 88.5% during hospitalization. Factors associated with an increased likelihood of intensive care unit (ICU) admission included cigarette smoking (29.4% vs. 13.4%, OR 2.77, 95% CI 1.19 – 6.45) and chronic hypertension (17.1% vs. 3.1%, OR 6.86, 95% CI 2.19 – 21.51). Antiviral treatment within two days of symptom onset decreased the likelihood of ICU admission (31.4% vs. 56.6%, OR 0.36, 95% CI 0.16 – 0.77).
CONCLUSION
Comorbidities, including chronic hypertension and smoking in pregnancy, increase the likelihood of ICU admission in influenza-like illness hospitalizations, whereas early antiviral treatment may reduce its frequency.
INTRODUCTION
In April 2009, an outbreak of a novel H1N1 influenza A began in Mexico (1); it was a triple recombinant virus including gene segments of human, swine and avian origin. The initial reports described a mortality rate of 7 percent with this infection (1). The virus quickly spread, and on June 11, 2009, the World Health Organization (WHO) raised the pandemic alert to level 6, indicating efficient human to human spread in multiple countries (2). As part of the WHO preparedness statement, individual countries were expected to implement individual, societal, pharmaceutical, and national action plans. Given that the severity of this pandemic was still unknown, and that pregnant women were considered to be at risk of increased morbidity and mortality, it was of clinical and public health importance to capture accurate information on pregnant and immediately postpartum women during the pandemic. The objective of this study was to estimate characteristics and outcomes of pregnant and immediately postpartum women hospitalized with influenza-like illness (ILI) during the 2009–2010 influenza pandemic, and the factors associated with more severe illness.
MATERIALS AND METHODS
This was an observational study of the Maternal-Fetal Medicine Units (MFMU) Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) designed to estimate characteristics and outcomes of pregnant and immediately postpartum women hospitalized with ILI during the 2009–2010 influenza pandemic in the United States. The study was conducted at 28 hospitals in 14 states of the United States and the MFMU Network’s independent data coordinating center. Potential eligible women were identified by daily surveillance from October 1, 2009 through May 31, 2010. Hospital inpatient units, observation units, intensive care units, emergency room departments and mortality logs/mortality committee reviews were screened daily by trained research personnel for women who were pregnant or within 14 days following delivery of a pregnancy of at least 20 0/7 weeks’ gestation by best obstetrical estimate. The two-week postpartum interval was included because women who acquired an ILI would still have pregnancy-associated physiology (3–4). This interval would also have allowed direct comparison with then-current CDC recommendations (5). Hospitalization was defined as any hospital stay lasting 12 or more hours or in-hospital death prior to admission. Influenza-like illness was defined as a clinical suspicion of influenza and either meeting the Centers for Disease Control and Prevention (CDC) definition of ILI (2) (fever 100.0º Fahrenheit or higher and cough and/or sore throat) or having a positive influenza test during a current ILI (including pre-admission, in-hospital, or postmortem).
Women were excluded if they had infectious conditions in the absence of a positive influenza test: acute pyelonephritis/urosepsis; positive rapid test or culture for group A streptococcal pharyngitis; aspiration pneumonia or pneumonitis from smoke inhalation; pneumocystis pneumonia; Epstein-Barr virus/mononucleosis; cytomegalovirus; and active tuberculosis.
Because of public health concerns and potential selection bias, this study was conducted under a waiver of informed consent and a HIPAA waiver from each of the participating hospitals. The study was approved by the Institutional Review Boards of all participating centers.
Clinical data were collected from available hospital medical records by trained and certified research personnel who abstracted the information onto pre-specified research data forms. Data forms were entered at each clinical center using a web-based data entry system that was managed by the data coordinating center, which was responsible for data analysis.
All registry patients were followed for pregnancy outcomes. The primary outcome was maternal mortality attributed to ILI during the index ILI hospitalization. Secondary maternal outcomes included mortality due to ILI after discharge, cesarean delivery and severe maternal morbidity, defined as intensive care unit (ICU) admission, super-infection with bacterial pneumonia (positive x-ray and positive sputum culture), intubation, renal or liver dysfunction, or bacteremia/sepsis (positive blood culture). The secondary perinatal composite outcome included fetal death, pre-term birth (less than 37 weeks), neonatal influenza infection (positive influenza test), NICU admission, respiratory distress syndrome, grade III/IV intraventricular hemorrhage, necrotizing enterocolitis, neonatal sepsis, neonatal death, or major congenital malformations. These primary and secondary outcomes were determined a priori.
Sample size and power
At the time of study initiation, the estimated general population case fatality rate for 2009 H1N1 influenza A was 0.4% (6), and 0.1% for seasonal influenza (7). Assuming a 50% higher rate for hospitalized patients with ILI (i.e., 0.6%), a sample size of 3490 would be required to detect with 90% power a doubling of the mortality rate for ILI among hospitalized pregnant and immediate postpartum women compared with that expected among the general hospitalized population.
Statistical methods
Continuous variables were compared by type of maternal hospital admission (ICU admission vs. non-ICU admission) with the use of the Wilcoxon rank sum test, and categorical variables with the use of the chi-square test or the Fisher exact test when appropriate. Logistic regression was used to examine which baseline factors were independently associated with ICU admission. Logistic regression was used to examine the association between antiviral treatment and cesarean delivery and between each of the composite secondary outcomes, adjusting for maternal age, smoking, asthma, anemia in current pregnancy, chronic hypertension, diabetes (pregestational or gestational) and medication allergy. The cesarean delivery and perinatal composite outcomes were also adjusted for maternal ICU admission during the index ILI hospitalization. For all secondary outcomes, P values of less than 0.05 were considered to indicate statistical significance and no adjustments were made for multiple comparisons.
Because the characteristics of the impending epidemic were not known, provisions were made for interim analyses and possible early dissemination of results.
RESULTS
Eligibility characteristics and timing of the hospitalizations
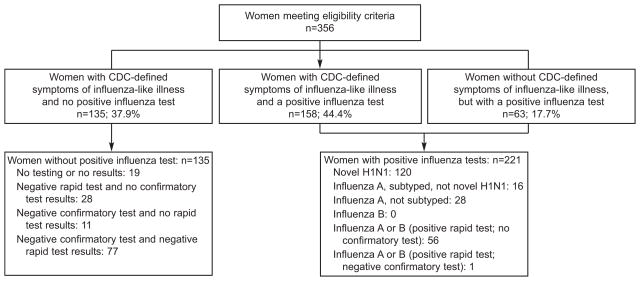
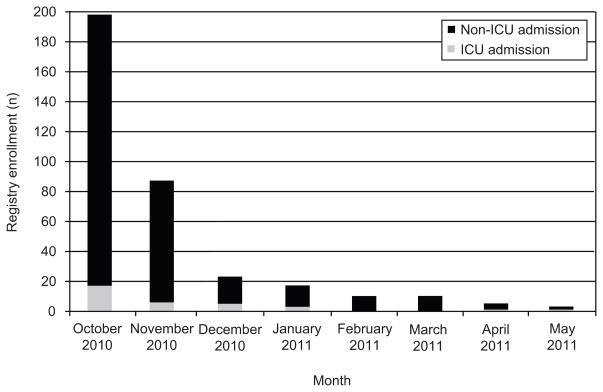
From October 1, 2009 through May 31, 2010, 356 women met eligibility criteria. Figure 1 summarizes eligibility characteristics of the patients. Most of the women (293/356 = 82.3%) had CDC symptom-defined ILI (158 with a positive influenza test plus an additional 135 with no positive influenza test). Sixty-three (17.7%) had a positive influenza test but not CDC symptom-defined ILI, including 56 without a documented fever measured by a health care provider. Of the 221 patients with a positive influenza test, 54.3% were sub-typed novel H1N1, 7.2% were influenza A not novel H1N1 and 38.5% were not sub-typed. No in-hospital death prior to admission was observed. As noted in Figure 2, over half (197/356 = 55.3%) of all women were enrolled in October 2009 and an additional 25.0% (89/356) were in November 2009, with rapidly decreasing numbers thereafter.
Figure 1.
Eligibility criteria.
Figure 2.
Registry enrollment by month and by intensive care unit (ICU) and non-ICU admission.
General characteristics of the patients
The majority of women (61.2%) were admitted in the third trimester, with smaller numbers in the second (28.1%) and first (7.9%) trimesters. Only 2.8% were admitted within the first two weeks after delivery. Of the 346 women who were pregnant when admitted, 26.0% delivered during their ILI hospitalization and 74.0% were discharged undelivered. Thirty-five women (9.8%) required admission to an ICU and three received extracorporeal membrane oxygenation (ECMO). Of the ICU admissions, 42.9% were transferred from another hospital to a MFMU Network study hospital. Only 7.8% of the remaining 321 women were transferred from other hospitals. Excluding maternal deaths, the mean duration of hospital stay was 3.2±3.9 days; 9.6±7.9 days in those admitted to the ICU and 2.6±2.5 days in those not admitted in the ICU. Forty-six women (12.9%) received antiviral medication prior to the current hospital admission and 10.1% received antiviral medication before any hospitalization for the current ILI (before transfer hospitalization if transferred). All but one of these women received oseltamivir; one received zanamavir. Eighty-eight percent of the women received antiviral medications during their hospitalization; 312 received oseltamivir, two received oseltamivir and peramivir and one received zanamavir.
Characteristics by type of hospitalization (ICU admission)
Characteristics of the study population by type of hospitalization are presented in Table 1. Nine women (2.5%) were pregnant with a multiple gestation, with no significant difference between groups. Fifty-three women (14.9%) smoked cigarettes during the current pregnancy, with a significantly higher proportion in the women who required admission to an ICU (29.4% vs 13.4%). Pregnant women with ILI requiring ICU admission were more likely to have chronic hypertension, but were not significantly more likely to have other concurrent medical conditions, including obesity, asthma, anemia, diabetes and medication allergy.
Table 1.
Baseline Characteristics
| Characteristic | ILI-related ICU admissions (n = 35) | ILI-related non-ICU admissions (n = 321) | Significance |
|---|---|---|---|
| Demographic Characteristics | |||
|
| |||
| Age (mean ± SD) | 26.7 ± 6.0 | 26.1 ± 6.3 | 0.53 |
|
| |||
| Race/ethnicity | 0.21 | ||
| Caucasian | 15 (42.9) | 91 (28.4) | |
| African-American | 7 (20.0) | 66 (20.6) | |
| Hispanic | 9 (25.7) | 135 (42.1) | |
| Other/Unknown | 4 (11.4) | 29 (9.0) | |
|
| |||
| Insurance* | 0.92 | ||
| Self pay/uninsured | 5 (14.3) | 47 (14.7) | |
| Government assisted | 20 (57.1) | 192 (60.0) | |
| Private | 10 (28.6) | 81 (25.3) | |
|
| |||
| Cigarette smoking at any time during the current pregnancy* | 10 (29.4) | 43 (13.4) | 0.01 |
|
| |||
| Pregnancy-related characteristics | |||
|
| |||
| Nulliparous | 15 (42.9) | 120 (37.4) | 0.53 |
|
| |||
| Multiple gestation | 1 (2.9) | 8 (2.5) | 1.00 |
|
| |||
| Pregnancy trimester at admission | 0.22 | ||
| First | 4 (11.4) | 24 (7.5) | |
| Second | 6 (17.1) | 94 (29.3) | |
| Third | 23 (65.7) | 195 (60.8) | |
| Postpartum | 2 (5.7) | 8 (2.5) | |
|
| |||
| Gestational hypertension or preeclampsia at admission in those pregnant at the time of admission‡ | 4 (12.1) | 19 (6.1) | 0.26 |
|
| |||
| Concurrent medical conditions | |||
|
| |||
| Obesity (BMI ≥ 30 kg/m2) at admission†‡ | 17 (51.5%) | 132 (47.8%) | 0.69 |
|
| |||
| Asthma | 8 (22.9) | 62 (19.3) | 0.62 |
|
| |||
| Pneumonia in last two weeks | 5 (14.3) | 1 (0.3) | <0.001 |
|
| |||
| Blood clotting disorder | 0 (0.0) | 9 (2.8) | 0.61 |
|
| |||
| Anemia | 7 (20.0) | 42 (13.1) | 0.30 |
|
| |||
| Chronic hypertension | 6 (17.1) | 10 (3.1) | 0.002 |
|
| |||
| Diabetes | 0.18 | ||
| Pre-gestational diabetes | 2 (5.7) | 4 (1.3) | |
| Gestational diabetes at admission‡ | 1 (2.9) | 16 (5.0) | |
|
| |||
| History of cancer | 2 (5.7) | 3 (0.9) | 0.08 |
|
| |||
| Neurocognitive disorder | 3 (8.6) | 6 (1.9) | 0.05 |
|
| |||
| Medication allergy | 10 (28.6) | 68 (21.2) | 0.32 |
Data are n (%) or mean ± standard deviation, unless otherwise specified.
Information missing on 1 woman.
Information missing on 47 women.
Diagnosis of obesity, gestational hypertension, preeclampsia and gestational diabetes are at the time of admission.
Influenza vaccination status was not documented in a large proportion of the patients (26.4% for the 2009/2010 seasonal influenza vaccine and 34.3% for the H1N1 swine flu vaccine). Although women requiring ICU admission appeared less likely to have received the H1N1 vaccine (5.7% vs 13.4%), the high proportion of missing data (57.1% vs 31.8%) precludes any definite conclusion.
Women requiring ICU admission were less likely to have received antiviral treatment within two days of symptom onset than women not admitted to the ICU (31.4% vs. 56.6%). Women requiring ICU admission were more likely to report difficulty breathing (79.4% vs 34.6%; p < 0.001) and to require oxygen support (48.6% vs 2.5%; p < 0.001), but were less likely to self-report a headache (14.7% vs 35.8%; p = 0.01) or fever (64.7% vs 81.0%; p = 0.03) at the time of admission. There were no differences at admission between groups in the frequency of cough, sore throat, nasal stuffiness, body aches/myalgia, nausea, vomiting or diarrhea. All of the women who were intubated at the time of admission were admitted directly to an ICU.
Table 2 describes the factors independently associated with ICU admission. After multivariable adjustment, cigarette smoking and chronic hypertension remained significantly associated with an increased frequency of ICU admission, and early antiviral treatment remained significantly associated with a decreased likelihood of ICU admission.
Table 2.
Factors Associated with ICU Admission
| Unadjusted Odds Ratio (95% CI) for ICU admission | P-value | Adjusted* Odds Ratio (95% CI) for ICU admission | P-value | |
|---|---|---|---|---|
| Cigarette smoking | 2.69 (1.21–6.02) | 0.02 | 2.77 (1.19–6.45) | 0.02 |
| Chronic hypertension | 6.44 (2.18–18.97) | <0.001 | 6.86 (2.19–21.51) | 0.001 |
| Antiviral treatment within two days of symptom onset | 0.35 (0.17–0.74) | 0.006 | 0.36 (0.16–0.77) | 0.009 |
CI = confidence interval.
Adjusted for all variables in the table.
Maternal mortality
Four women in this registry died during their index ILI hospitalization, for a maternal mortality rate of 1.1% (95% CI 0.3–2.9). All of these deaths were the result of respiratory failure. No maternal deaths were identified after the index ILI hospitalization discharge through two weeks postpartum.
Maternal morbidity
Table 3 describes the frequency of maternal morbidity and its components by use and timing of antiviral treatment. Overall, antiviral therapy was not associated with maternal morbidity. However, maternal morbidity was significantly less frequent in those receiving antiviral therapy within 2 days of symptom onset. Renal or liver dysfunction could not be assessed because the majority of patients (58.1%) did not have relevant laboratory testing. Superimposed bacterial infections from urine or throat cultures were uncommon: four women had culture proven pyelonephritis/urosepsis and three had Group A streptococcal pharyngitis; all were in women who had a positive influenza test and therefore included in this registry.
Table 3.
Frequency of Maternal Morbidity During the Index ILI Hospitalization by Maternal Antiviral Treatment
| Characteristic | Antiviral treatment within 2 days of symptom onset (n = 192) | Antiviral treatment after 2 days of symptom onset (n = 124) | No antiviral treatment (n = 39) | Antiviral treatment (vs. none) | Antiviral treatment within 2 days of symptom onset (vs. none or not within 2 days) | ||
|---|---|---|---|---|---|---|---|
| Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | ||||
| Maternal morbidity composite | 13 (6.8) | 25 (20.2) | 4 (10.3) | 1.19 (0.40–3.54) p=0.75 |
1.21 (0.38–3.85) p=0.75 |
0.34 (0.17–0.67) p=0.002 |
0.33 (0.16–0.69) p=0.003 |
| ICU admission | 11 (5.7) | 20 (16.1) | 4 (10.3) | ||||
| Super-infection with bacterial pneumonia† | 2 (1.0) | 5 (4.0) | 0 (0.0) | ||||
| Intubation | 2 (1.0) | 13 (10.5) | 2 (5.1) | ||||
| Bacteremia/sepsis‡ | 0 (0.0) | 5 (4.0) | 1 (2.6) | ||||
Data are n (%), unless otherwise specified.
Adjusted for baseline maternal age, smoking, asthma, anemia, chronic hypertension, diabetes, and medication allergy.
Positive x-ray and a positive sputum culture.
Positive blood culture.
Delivery outcomes
Delivery outcomes were obtained in 315 (88.5%) of the patients. Among the patients discharged undelivered, the patients lost to follow-up were more likely to have been transferred from another hospital and uninsured/self-pay (data not shown).
Table 4 describes the frequency of delivery outcomes by use and timing of antiviral treatment. Mode of delivery and the perinatal composite outcome did not vary by antiviral status. The overall preterm birth rate (less than 37 weeks) was 18.7%, including 15.9% in those treated with antivirals within 2 days of symptom onset, 19.6% in those treated after 2 days and 29.7% in those not treated with antivirals; these differences were not statistically significant. No neonate had grade III/IV intraventricular hemorrhage and neonatal influenza infection could not be assessed because only 2.5% had an influenza test.
Table 4.
Frequency of Delivery Outcomes by Maternal Antiviral Treatment
| Characteristic | Antiviral treatment within 2 days of symptom onset (n=170) | Antiviral treatment after 2 days of symptom onset (n=107) | No antiviral treatment (n=37) | Antiviral treatment (vs. none) | Antiviral treatment within 2 days of symptom onset (vs. none or not within 2 days) | ||
|---|---|---|---|---|---|---|---|
| Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio* (95% CI) | ||||
| Cesarean delivery | 53 (31.2) | 43 (40.2) | 13 (35.1) | 0.97 (0.48–2.00) p=0.94 |
0.99 (0.46–2.14) p=0.98 |
0.71 (0.45–1.14) p=0.15 |
0.77 (0.47–1.26) p=0.29 |
| Neonatal morbidity/mortality composite | 51 (30.0) | 34 (31.8) | 14 (37.8) | 0.74 (0.36–1.50) p=0.40 |
0.61 (0.27–1.35) p=0.22 |
0.86 (0.53–1.38) p=0.53 |
0.92 (0.54–1.56) p=0.76 |
| Fetal death | 2 (1.2) | 3 (2.8) | 0 (0.0) | ||||
| Neonatal death | 0 (0.0) | 2 (1.9) | 0 (0.0) | ||||
| Pre-term birth | 27 (15.9) | 21 (19.6) | 11 (29.7) | ||||
| Spontaneous†‡ | 11 (6.5) | 7 (6.7) | 3 (8.1) | ||||
| Indicated‡ | 15 (8.9) | 12 (11.4) | 8 (21.6) | ||||
| NICU admission | 32 (18.8) | 21 (19.6) | 10 (27.0) | ||||
| RDS | 11 (6.5) | 4 (3.7) | 3 (8.1) | ||||
| NEC | 0 (0.0) | 1 (0.9) | 0 (0.0) | ||||
| Neonatal sepsis | 0 (0.0) | 1 (0.9) | 1 (2.7) | ||||
| Major congenital malformations | 0 (0.0) | 2 (1.9) | 2 (5.4) | ||||
Data are n (%), unless otherwise specified.
NICU = neonatal intensive care unit; RDS = respiratory distress syndrome; NEC = necrotizing enterocolitis.
Adjusted for baseline maternal age, smoking, asthma, anemia, chronic hypertension, diabetes, medication allergy and maternal ICU admission during the index ILI hospitalization.
Spontaneous pre-term birth defined as delivery before 37 weeks with spontaneous or spontaneous augmented labor or preterm premature rupture of membranes (pPROM).
Three pre-term birth cases were not classified as spontaneous or indicted due to missing data regarding type of labor and/or PROM.
Ninety (25.3%) patients delivered during their index ILI hospitalization with a mean duration of hospital stay of 5.3 ± 5.4 days. Among these women, the percent experiencing preterm labor, preterm rupture of the membranes and a preterm delivery was 5.6%, 7.8% and 26.7%, respectively. Forty (44.4%) delivered via cesarean, 40% of which were due to non-reassuring fetal status. Fourteen women (15.6%) had an emergency cesarean, 8 of which were in intubated women.
DISCUSSION
In this large prospective observational cohort of hospitalized pregnant and recently postpartum women with ILI, 9.8% were admitted to the ICU and 1.1% died. Admission to the ICU was more common if women smoked or had chronic hypertension, and was less common if women received antivirals within two days of the onset of symptoms.
Our data are derived from an active surveillance protocol using detailed real-time review of medical records. This allows for more detailed and precise information than can be acquired from passive reporting and surveillance (8,9). However, this study was conducted under a waiver of consent to minimize selection bias, which precluded interview of hospitalized women or their providers to obtain additional medical history or pregnancy follow-up. As a result, pregnancy outcomes for 41 of 256 (16.0%) women discharged undelivered were not available.
The majority was admitted in the third trimester (61.2%), a trend noted by other investigators (9–11). This presumably reflects the increased vulnerability imposed by the physiologic changes of late pregnancy (3–4) and likely also reflects increased concerns for fetal well-being. While the increase in cardiac output and extracellular fluid seen in the latter half of pregnancy is more pronounced in women with multifetal pregnancies, we did not see an increased rate of ILI hospitalizations in women with multifetal pregnancies.
This registry is limited to pregnant and recently postpartum women with ILI whose illness was sufficiently severe to warrant hospitalization and our findings should not be extrapolated to the general obstetric population. Nonetheless, women with severe disease requiring admission to an ICU were more likely to smoke cigarettes or to have chronic hypertension.
Twenty percent of our cohort had a diagnosis of asthma. This finding is similar to an early report from Australia in which 21.4% of pregnant women hospitalized with influenza also had underlying asthma (12). Three women required extracorporeal membrane oxygenation, similar to other studies that have described the use of this intervention in pregnant women who were critically ill with influenza pneumonia (12–14).
Likewise, the association of increased influenza severity in individuals with vascular disease has been described (15). The association of chronic hypertension with increased severity of influenza illness during pregnancy, however, has not been commonly reported.
Hospitalized pregnant women with ILI and either asthma or diabetes were not significantly more likely to require ICU admission, although the frequency of influenza in women with asthma or diabetes that did not require hospital admission is not available.
The chronologic distribution of hospital admissions parallels other concurrent real-time reporting systems. Over half occurred in October 2009 and an additional quarter in November 2009, with rapidly decreasing numbers thereafter (Figure 2). It should be noted that the 2009–2010 United States ILI pandemic began in the summer of 2009 and we are unable to comment on the frequency or severity of illness or the response of health care providers prior to October 2009. The absence of an additional large outbreak during the usual United States peak influenza season is at least in part a testament to effective public education and immunization campaigns nationwide during the 2009–2010 influenza pandemic.
Increased rates of preterm labor and/or delivery have been reported during previous H1N1 influenza A pandemics (16–17). Considering the association between infection and preterm birth, this association is not surprising. Early reports from the current pandemic have also suggested an increased rate of preterm birth (12). Similarly, we observed a preterm birth rate of 18.7% overall and 26.7% in those who delivered during their index hospitalization.
The majority of our cohort (88.5%) received antiviral medication during their hospitalization. Women in our study admitted to ICU’s were less likely to have received antiviral treatment within two days of symptom onset, a finding reported by others (18). Consistent with other reports (19), we did not observe any obvious fetal or immediate neonatal adverse effects from in-utero exposure to anti-viral medications (almost entirely oseltamivir). However, our sample size and relatively high lost-to-follow-up rates for delivery outcomes preclude any definitive statement. In addition, the majority of our participating hospitals serve as referral centers for their surrounding communities. As such, it is likely that our reported rates of admissions and complications are higher than those found in a non-referral population.
These data also re-emphasize the importance of smoking cessation efforts for all pregnant women. In addition, 17.7% (Figure 1) of patients had a positive influenza test and symptoms but did not fulfill the CDC ILI diagnostic criteria at time of admission, emphasizing the importance of a high index of suspicion of influenza in symptomatic pregnant women.
Our data can not comprehensively assess the impact of vaccination upon the morbidity associated with 2009/2010 H1N1 pandemic. Although we were relieved that our original sample size estimates were not reached, it remains disappointing to have seen such serious outcomes associated with ILI during a time period when vaccination of the pregnant population was a public health priority.
In summary, chronic hypertension and smoking in pregnancy, but not asthma or diabetes, were associated with an increased likelihood of ICU admission in ILI hospitalizations in this large prospective cohort. In addition, early treatment with appropriate antivirals was associated with with a decreased frequency of ICU admissions.
Acknowledgments
The authors thank Peggy Reed, RN, and Jo-Ann Tillinghast, RN, MSN, for protocol development and coordination between clinical research centers, and Elizabeth Thom, PhD, for protocol and data management, and statistical analysis.
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD40544, HD40500, HD53118, HD53097, HD40512, HD40560, HD34208, HD27917, HD40545, HD27915, HD34116, HD40485, HD21410, HD36801, HD27869 and 5UL1RR025777] and its contents do not necessarily represent the official view of NICHD or NIH.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented in part at the 2011 Society for Maternal-Fetal Medicine, San Francisco, CA, February, 10–12, 2011.
Dr. Spong, Associate Editor of Obstetrics & Gynecology,was not involved in the review or decision to publish this article.
References
- 1.WHO Global Alert and Response. http://www.who.int/csr/don/2009_04_30_a/en/index.html.
- 2.CDC Definition of Influenza-Like Illness. http://www.cdc.gov/h1n1flu/casedef.htm.
- 3.Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994;170:849–56. doi: 10.1016/s0002-9378(94)70297-7. [DOI] [PubMed] [Google Scholar]
- 4.Robson SC, Hunter S, Moore M, Dunlop W. Haemodynamic changes during the puerperium: a Doppler and M-mode echocardiographic study. Br J Obstet Gynaecol. 1987;94:1028–39. doi: 10.1111/j.1471-0528.1987.tb02286.x. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed Sept 27, 2009]; http://www.cdc.gov/H1N1flu/pregnancy/
- 6.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): Early findings. Science. 2009;324:1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li FCK, Choi BCK, Sly T, Pak AWP. Finding the real case-fatality rate of H5N1 avian influenza. J Epidemiol Community Health. 2008;62:555–9. doi: 10.1136/jech.2007.064030. [DOI] [PubMed] [Google Scholar]
- 8.Creanga AA, Johnson TF, Graitcer SB, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–26. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 9.Louie JK, Acosta M, Jamieson DJ, Honein MA for the California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 10.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 11.Creanga AA, Kamimoto L, Newsome K, et al. Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. Am J Obstet Gynecol. 2011 Apr 18; doi: 10.1016/j.ajog.2011.02.037. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 influenza A and pregnancy outcomes in Victoria, Australia. CID. 2010;50:686–90. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 13.Welch SA, Snowden LN, Buscher H. Pandemic (H1N1) 2009 influenza, pregnancy and extracorporeal membrane oxygenation. Med J Aust. 2010;192:668. doi: 10.5694/j.1326-5377.2010.tb03675.x. [DOI] [PubMed] [Google Scholar]
- 14.Oluyomi-Obi T, Avery L, Schneider C, et al. Perinatal and maternal outcomes in critically ill obstetrics patients with pandemic H1N1 influenza A. J Obstet Gynaecol Can. 2010;32:443–52. doi: 10.1016/S1701-2163(16)34497-8. [DOI] [PubMed] [Google Scholar]
- 15.Ugarte S, Arancibia F, Soto R. Influenza A pandemics: clinical and organizational aspects: the experience in Chile. Crit Care Med. 2010;38:e133–7. doi: 10.1097/CCM.0b013e3181c87716. [DOI] [PubMed] [Google Scholar]
- 16.Harris JW. Influenza occurring in pregnant women. JAMA. 1919;72:978–80. [Google Scholar]
- 17.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–5. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 18.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greer LG, Sheffield JS, Rogers VL, Roberts SW, McIntire DD, Wendel GD., Jr Maternal and neonatal outcomes after antepartum treatment of influenza with antiviral medications. Obstet Gynecol. 2010;115:711–6. doi: 10.1097/AOG.0b013e3181d44752. [DOI] [PubMed] [Google Scholar]