Abstract
Intraprostatic leukocyte function may vary depending on local inflammatory or malignant cell microenvironment. Interleukin (IL)-17 producing cells play key roles in chronic inflammation and autoimmunity. Little is known about the relevance of IL-17 producing cells at sites of prostate tissue inflammation and/or prostate adenocarcinoma. In this study, we analyzed thirty formalin-fixed paraffin-embedded whole-mount radical prostatectomy specimens of prostate cancer patients. Immunohistochemistry was employed to identify IL-17 producing cells in all sites of mononuclear cell accumulation, noting their relationships to areas of prostate cancer, proliferative inflammatory atrophy (PIA), or hyperplastic benign tissue. Levels of IL-17 producing cells were similar in zones of benign prostate tissue and areas of prostate cancer. Pronounced intraluminal and peri-glandular IL-17 producing cell accumulations were identified in the mononuclear cell infiltrates associated with PIA lesions. Glandular and peri-glandular CD68+ macrophages and neutrophils were the predominant IL-17 producing cells in PIA lesions. The accumulation of IL-17 expressing cells in PIA lesions presents direct evidence of an inflammatory microenvironment that may support the development of prostate cancer.
Keywords: Chronic inflammation, IL-17, proliferative inflammatory atrophy, prostate cancer, macrophages, neutrophils, mononuclear cells
Introduction
Accumulated epidemiologic studies support the notion that chronic inflammatory diseases are frequently associated with increased risk of various human cancers [1, 2]. It is accepted that almost 25% of all human malignancies are connected to chronic inflammatory diseases [3]. Although a causal link between chronic inflammation and carcinogenesis was presumed more than century ago [4], only in the last decade has the causal connection between inflammation and carcinoma of the prostate been extensively investigated. Chronic inflammation is characterized by sustained tissue damage, damage-induced cellular proliferation, and tissue repair [5]. The central causative role in this process is played by growth factors, reactive oxygen species, and especially, pro-inflammatory cytokines.
Our previous findings in a prospective five year follow-up study in needle biopsy specimens demonstrate a strong association between chronic prostatic inflammation, pre-malignant, and malignant changes in the prostatic epithelium [6].
It has been observed that proliferative inflammatory atrophy (PIA) lesions are associated with chronic inflammation of the prostate, and histological transitions have been noted between areas of PIA and high-grade prostate intraepi-thelial neoplasia (HGPIN), and between PIA and prostate cancer [7]. A key feature of the PIA lesion is the presence of mononuclear cells (MNC) and/or polymorphonuclear cells (PMNC) in both epithelial and stromal compartments [7]. One important aspect of PMNC and monocytes/macrophages is their ability to synthesize pro-inflammatory cytokines that modulate the inflammatory response. In this respect, inter-leukin (IL)-8, tumor necrosis factor (TNF)-α, IL-17, and IL-1β are pro-inflammatory cytokines with a central role in inflammatory processes. IL-1β is the most potent inducer of nuclear factor-kappaB (NF-κB)-responsive genes in the prostate gland, promoting aberrant NF-κB activity and thus leading to development of inflammation in the prostate [8]. The transcription factor NF-κB has been identified as a potential molecular bridge between inflammation and cancer [9]. Downstream NF-κB signaling is crucial for initiation of a new wave of pro-inflammatory IL-17 signaling favorable to chronic intraprostatic inflammation [10].
IL-17-expressing CD4+ T helper (TH17) cells were recently proposed to be a third lineage along with TH1 and TH2 cells [11]. In addition to TH17 cells, a wide variety of T cells including CD8+ T cytotoxic (Tc17) cells [12], γδT cells [13], and natural killer T (NKT) cells [14] and innate immune cells, such as neutrophils [15] and monocytes [16] have the ability to produce IL-17 under various pathological conditions. Previous studies have reported that high numbers of tumor associated macrophages in many tumors correlates positively with increased angiogenesis and accelerated cancer progression. Studies have further shown that IL-17 expressing macrophages promote invasiveness in breast cancer cells and increase microvessel density in breast and colon tumors [17]. To date there have been no published investigations concerning the prevalence of IL-17 expressing cells in prostate cancers, and consequently no data is available concerning their phenotype or their sites of localization in prostate adenocarcinoma. Based on our previous findings that IL-1β-induced NF-κB activation in the prostate favors the accumulation of IL-17-positive leukocytes in prostatic stroma [10], we hypothesized that IL-17 producing cells may be targets of specific pro-inflammatory signals that initiate intraprostatic inflammation. We investigated this hypothesis by evaluating a large number of radical prostatectomy specimens from prostate cancer patients for IL-17 expression and characterizing the phenotype of IL-17 producing cells in these specimens. Our studies, for the first time, demonstrate that aggregates of macrophages and neutrophils that lie in close association with PIA lesions are the predominant IL-17 producing cells in prostate adenocarcinoma specimens.
Materials and methods
Patients and specimens
We studied the whole-mounted radical prostatectomy specimens of 30 men who underwent surgery for biopsy-proven adenocarcinoma. Patients ranged from 45 to 72 years of age (mean = 61.0 ± 6.8 [SD]). In selecting our cases, we chose those subjects that demonstrated the presence of adenocarcinoma, high-grade prostatic intraepithelial lesions, and significant components of benign prostatic hyperplasia. A total of seventeen specimens exhibited cancer with a Gleason score of 7, nine specimens exhibited a Gleason score of 6, two specimens exhibited a Gleason score of 9, and one specimen exhibited a Gleason score of 5 and another one a Gleason score of 8. These studies were approved by the Institutional Review Board at the University Hospitals Case Medical Center.
Immunohistochemistry
To assess IL-17 expressing leukocytes and analyze their phenotype in formalin-fixed paraffin-embedded whole mount human radical prostatectomy specimens we used primary antibodies as following: IL-17 (R&D System Inc, Minneapolis, MN), CD68 (clone KP1), CD20 (clone L26), CD3 (clone PS1), and CD4 (clone BC/1F6) purchased from Biocare Medical (Concord, CA). For control staining, primary antibodies were replaced with irrelevant isotype-matched antibodies procured from Jackson Immuno-Research (West Grove, PA). Immunohistochemical analysis was performed using a protocol, antibodies, and reagents from Biocare Medical, with slight modifications. Briefly, 5-µm thick sections of whole mounted radical prostatectomy specimens were deparaffinized, rehydrated, and subjected to heated antigen decloaking with the Reveal antigen unmasking solution. The specimens were blocked with PEROXIdazed-1 for endogenous peroxidase activity and BACK-GROUNDsniper to reduce nonspecific background staining, and incubated with the primary Abs against human cell surface markers. Combinations of primary (CD3/CD20, CD3/CD4, IL-17/CD68, and IL-17 only) and secondary (MACH-2 Poly-HRP and MACH-2 Polymer-ALP) antibodies with different staining kits were used for two color staining. The staining was performed using Betazoid DAB kit for MACH-2 Poly-HRP Abs or Vulcan Red Chromogen kit for MACH-2 Polymer-ALP Abs. CTS kit (R&D System Inc, Minneapolis, MN) was applied for detection of IL-17 expressing cells in all specimens. The denaturation of all the antibodies in the first staining protocol after their detection with chromogen was provided by pretreatment with Denaturating Solution. This procedure was used for double staining to make sure that the second staining protocol will not cross react with the first one. After immunostaining, slides were counterstained in Gill's #3 hematoxylin, mounted in crystal mount media, and dried overnight.
Prostate histology
Routine H&E-stained histological sections from the above-noted formalin-fixed paraffin-embedded whole mount radical prostatectomy specimens corresponding to the immuno-stained sections were evaluated for the presence of polymorphonuclear and mononuclear cells, and the spatial relationships of the inflammatory cell infiltrates to areas of PIA, benign prostatic hyperplasia and adenocarcinoma were observed and recorded. Particular attention was given to the presence and distribution of acute and/or chronic infiltrates, and each case was assigned a score on a scale of 0-no, 1-mild, 2-moderate and 3-severe inflammation. The degree of inflammation was assigned according to the approximate percent of stroma and glands in the prostatectomy specimen involved by aggregates of 50 or more lymphocytes designated as “hot spots”, or by granulomatous inflammation on a scale of mild inflammation-1% to 10%, moderate inflammation-11% to 50% and severe inflammation-greater than 50% involvement. The morphological characteristics of adenocarcinoma, HGPIN, and PIA are well described in standard pathology texts.
Assessment of IL-17 expressing cells
Quantification of IL-17+ cells was performed in areas of benign tissue with scattered IL-17 expressing cells (Benign Tissue), in MNC hot spots located within foci of adenocarcinoma (Inside PCa), in MNC hot spots of benign tissue adjacent to foci of adenocarcinoma (Along PCa), in MNC hot spots in benign prostate tissue distant from foci of adenocarcinoma (Hot Spots), and in proliferative atrophy lesions (PIA Lesions) using light microscopy. Sections were examined with an inverted Olympus BH2 microscope (Olympus America Inc., Melville, NY) and images were acquired with Image Proplus software (Media Cybernetics, Carlsbad, CA), digitally scored on a computer. All IL-17+ cells within a visual field with magnification ×125 were counted with the assistance of the software program, noting their relationships to specific pathologic entities. Scattered IL-17+ cells that were outside the chosen visual field (x125) were excluded from analysis. The average number of diffusely distributed IL-17 expressing cells in benign tissue was also calculated in comparison (Benign Tissue) using the MicrosoftVSuite program
Statistical analysis
Statistical analysis was performed using oneway analysis of variance (ANOVA) with 95% confidence limits and Tukey's correction.
Results
Morphologic findings in radical prostatectomy specimens
As stated previously, we chose cases that had substantial amounts of prostatic adenocarcinoma as well as readily recognizable nodules of benign prostatic hyperplasia (BPH). All cases showed areas of chronic inflammation. Some areas of adenocarcinoma were immediately adjacent to areas of BPH, and other areas of cancer were present in a background of benign prostatic tissue that lacked features of BPH. To assess the potential contributions of IL-17 to the pathogenesis of various prostate lesions in patients with prostate cancer, we evaluated the levels of IL-17 expressing cells in hyperplastic and non-hyperplastic prostate tissue, foci of PIA and areas of prostate cancer. Since it has been previously shown that IL-17 positive cells in prostate tissue are mononuclear cells (MNC) rather than neutrophils [10], we focused our attention on sites of MNC accumulation.
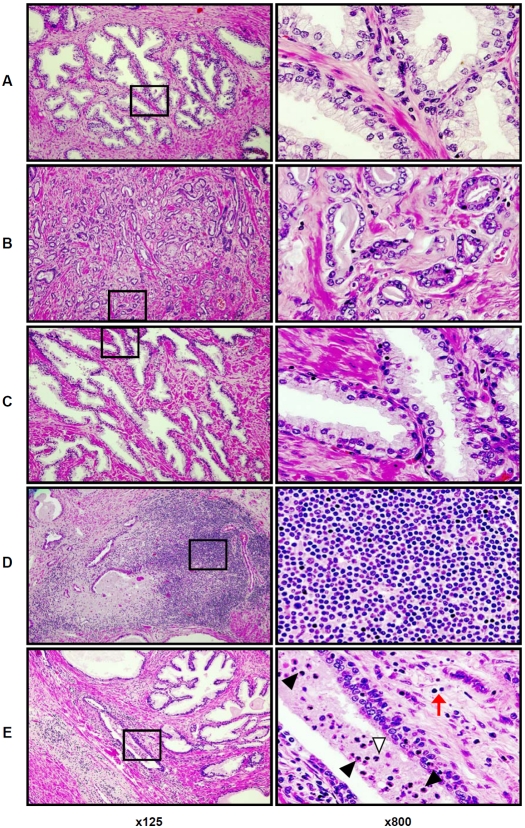
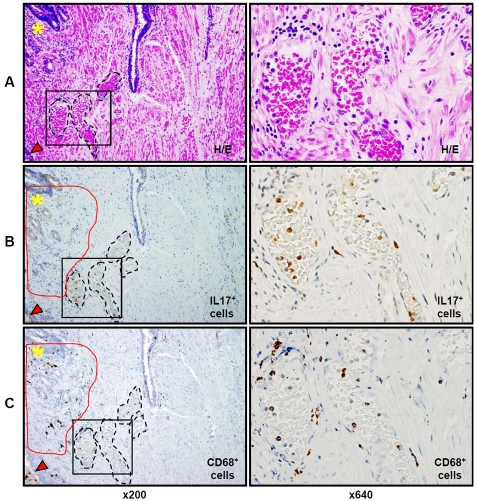
Histological findings in the whole mount prostate glands are shown in Figure 1 at low (x125) and high (x800) magnifications. As shown in Figure 1A, benign prostate tissue distant from areas of adenocarcinoma typically contained scattered intra-stromal MNC. In foci of prostate cancer, scattered MNCs were found in the lumens of malignant acini, and in the stroma surrounding malignant acini (Figure1B). Scattered MNCs were also found in stroma and in benign glandular epithelium at the interface between zones of cancer and adjacent normal prostate tissue (Figure1C). Figure 1D illustrates a “hot spot” of stromal MNC accumulation in benign prostate tissue, composed predominantly of lymphocytes with a few scattered macrophages. PIA lesions were also characterized by significant stromal and intra-acinar accumulations of MNC (Figure1E). However, the MNC infiltrate was different in these sites compared to the inflammatory cell infiltrates in the areas described above: although lymphocytes comprise the majority of MNCs in the stroma around PIA lesions, more abundant cells with the morphologic characteristics of macrophages were present in these sites, and in addition, neutrophils were present in the glandular epithelium and within the lumens of the glands (Figure1E, ×800).
Figure 1.
Representative histological patterns of lesions in the formalin-fixed paraffin-embedded whole mount radical prostatectomy specimens (H&E staining) from patients with prostate cancer. A. area of benign prostate tissue; B – area of prostate adenocarcinoma; C. benign tissue adjacent to an area of adenocarcinoma; D. site of mononuclear cells (MNC) accumulation (“hot spot”) in benign prostate tissue is comprised of cells with the morphologic features of lymphocytes; E. proliferative inflammatory atrophy (PIA) lesion in benign prostate tissue. Black arrowheads (x800) indicate intraluminal cells with monocyte/macrophage morphology and white arrowhead (x800) indicate intraluminal cell with neutrophil morphology. Scattered cells (red arrow, ×800) with lymphocyte morphology (similar to D, ×800) are shown in peri-glandular stroma of PIA lesion. Left column presents prostate histologic zones at magnification ×125. Right column illustrates tissue located inside of the black squares of the left column in the same samples at magnification ×800. Details are described in “Materials and Methods” section.
Sites of MNC accumulation were readily identifiable in all of the radical prostatectomy specimens that we studied. PIA lesions were identified in 21 of 30 cases (70%). Most of the PIA lesions were located in the peripheral zone of the prostate gland. The number of sites of stromal MNC accumulation (“stromal hot spots”) varied considerably between individual specimens. The average number of stromal hot spots was 6-fold higher than the average number of PIA lesions in these specimens (Table 1).
Table 1.
Number of sites of mononuclear cell (MNC) accumulations (“hot spots”) and prolif-erative inflammatory atrophy (PIA) lesions in the whole mount radical prostatectomy specimens of patients with prostate cancer (n=30)
| Hot Spot (mean ± SD) | PIA Lesion (mean ± SD) | |
|---|---|---|
| Average per patient prostate gland | 24 ± 12.9 | 4 ± 4 |
PIA lesions are the sites of accumulation of IL-17 expressing cells in human prostate gland
To assess IL-17-expressing cell distribution in radical prostatectomy specimens, we analyzed consecutive sections stained with H&E and IL-17 respectively, focusing on the morphologic entities specified above, specifically: i) inflammatory cell infiltrates in benign prostate tissue distant from cancer, ii) MNC hot spots in foci of cancer, iii) benign prostate tissue with a width of a visual field at ×125 magnification immediately adjacent to foci of cancer, iv) MNC hot spots in benign tissue distant from cancer, and v) inflammatory cell infiltrates in PIA lesions.
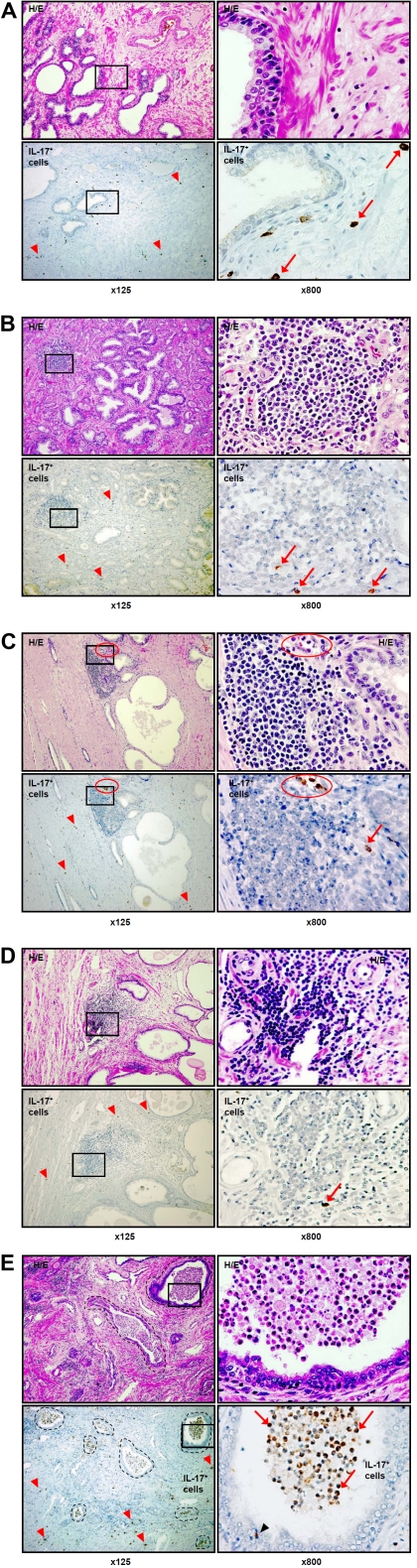
IL-17-immunoreactive cells were simply seen scattered in the areas of benign prostate tissue distant from prostate cancer (Figure2A). IL-17-immunoreactive cells were also randomly scattered in the stroma in zones of prostate cancer, and were also found in stromal hot spots in areas of cancer (Figure2B), in stromal hot spots in tissue at the interface between cancer and benign tissue (Figure2C), and in stromal hot spots in benign tissue distant from prostate cancer (Figure2D). Differences between the densities of IL-17+ cells in these various areas of benign and malignant prostate tissue (Figures2A-D) were undetectable without quantitative statistical analysis.
Figure 2.
H&E and IHC staining for inter-leukin (IL)-17 in histological patterns of formalin-fixed paraffin-embedded whole mount radical prostatectomy from patients with prostate cancer. A. scattered stromal IL-17 expressing cells (brown color, red arrowheads, ×125 and red arrows, ×800) in benign prostate tissue distant from cancer; B. scattered stromal IL-17 expressing cells (red arrowheads, ×125) and scattered IL-17 expressing cells in mononuclear cell (MNC) hot spot (red arrows, ×800) within a focus of ade-nocarcinoma; C. scattered stromal IL-17 expressing cells (red arrowheads, ×125) and IL-17 expressing cells (red arrow, ×800) in a MNC hot spot in benign tissue adjacent to a focus of adenocarcinoma. IL-17 expressing cells with monocyte/ macrophage morphology are present within a capillary lumen (red circle) at the edge of the hot spot; D. scattered stromal IL-17 expressing cells (red arrowheads, ×125) and IL-17 expressing cell (red arrow, ×800) in a MNC hot spot in benign tissue distant from cancer; E. stromal (red arrowheads, ×125), peri-glandular (black arrowhead, ×800) and intra-glandular (black dotted circles, ×125 and red arrows, ×800) accumulation of IL-17 expressing cells (brown color) in a proliferative inflammatory atrophy (PIA) lesion. Left column shows H&E and immunohistochemical staining for IL-17 in various histological patterns at magnification ×125. Right column depicts H&E and immunohistochemical staining for IL-17 in tissues located inside the black squares of the left columns of the same samples with magnification ×800. The images illustrate scant numbers of IL-17 expressing cells scattered in benign prostate tissue (A) and in MNC hot spots (B, C, D) in various his-tologic zones. The most striking accumulations of IL-17 expressing cells appear within the lumens of glands in areas of PIA lesions in the peripheral zone of the prostate (E). Details are described in “Materials and Methods” section. (Figure 2D and E on next page).
It is worth noting that IL-17+ cells with monocyte/macrophage phenotype rather than lymphocytes were observed in micro-vessel lumens but not inside MNC hot spots (Figure2C, H&E and IL-17, ×125, red circles). As discussed later, we propose that the presence of IL-17+ cells with monocyte/macrophage morphology inside prostate microvessels is a unique and previously undescribed finding, and may have some significance in the development of a specific inflammatory microenvironment (Figure2C, H&E and IL-17, ×800, red circles).
In contrast to the findings in the sites described above, numerous IL-17-expressing cells with an unusual distribution were observed in PIA lesions (Figure 2E). Scattered stromal IL-17+ cells were seen throughout the lesions; stromal MNC hot spots in these sites were almost free from IL-17+ cells, as in other zones of the prostate (Figure2E, ×125). However, aggregates of IL-17-immunostaining cells were found within gland lumens (Figure2E, ×125, black dotted circles). Examination at higher magnification confirms that the lymphocyte populations in PIA lesions are confined to the stroma around glands. Abundant inflammatory cells resembling macrophages and neutrophils are present in the gland lumens, and occasional inflammatory cells are within gland epithelium (Figure2E, H&E ×800). These intraluminal and intra-epithelial cells were shown to exhibit intensely positive immunostaining for IL-17 (Figure2E, ×800, red arrows).
Quantification of IL-17 expressing cells in human radical prostatectomy specimens
Since the distribution of inflammatory cells in human prostate glands tends to be patchy rather than diffuse, the numbers of IL-17 immunostained cells were quantitated in all specimens, focusing on the morphologic entities specified above, specifically: i) benign prostate tissue distant from cancer, ii) MNC hot spots in foci of cancer, iii) benign prostate tissue with a width of a visual field at ×125 magnification immediately adjacent to foci of cancer, iv) stromal MNC hot spots in benign tissue distant from cancer, and v) PIA lesions. All IL-17+ cells within a visual field with magnification ×125 were counted, noting their relationships to specific pathologic entities. Scattered IL-17+ cells that were outside the chosen visual field (x125) were excluded from analysis. However, the average number of diffusely distributed IL-17 expressing cells in benign tissue was also calculated in comparison.
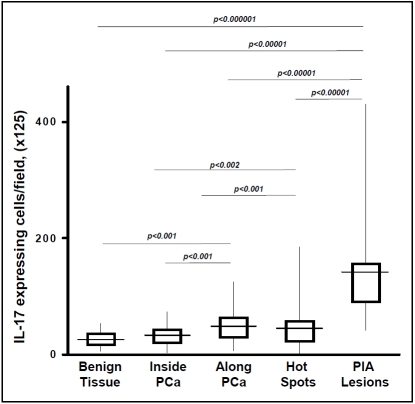
The levels of IL-17+ expressing cells in benign prostate stroma uninvolved by stromal hot spots (27 ± 12 [SD] cells per visual field) and areas infiltrated with prostate adenocarcinoma (32 ± 19 [SD] cells per visual field) were both significantly lower than the number of such cells in the other pathologic areas under study (Figure3). IL-17+ cell accumulation was most pronounced in PIA lesions (141 ± 75 [SD] per visual field). The absolute number of IL-17+ cells in PIA lesions was approximately 4-fold higher than in MNC hot spots within areas of prostate cancer (p<0.000001), approximately 3-fold higher than in MNC hot spots in benign tissue at the periphery of areas of prostate cancer (48 ± 23 [SD] per visual field, p<0.00001), and approximately 3 fold higher than in MNC hot spots in benign tissue distant from prostate cancer (43 ± 26 [SD] cells per visual field, p<0.00001) (Figure3).
Figure 3.
Quantification of interleukin (IL)-17 expressing cells in the formalin-fixed paraffin-embedded whole mount human radical prostatectomy specimens from thirty patients with prostate cancer. Results are expressed as absolute numbers of IL-17 positive cells per visual field with magnification ×125. The average is shown as a horizontal line through the box. The lower and upper margins of the box represent the 25th and 75th percentiles, with the extended arms representing the minimal and maximal number, respectively. The data were analyzed using one-way analysis of variance (ANOVA) with 95% confidence limits and Tukey's correction. Details are described in “Materials and Methods” section.
PIA Lesions are the sites of accumulation of IL-17-producing CD68+ macrophages and neutrophils rather than B or T Lymphocytes
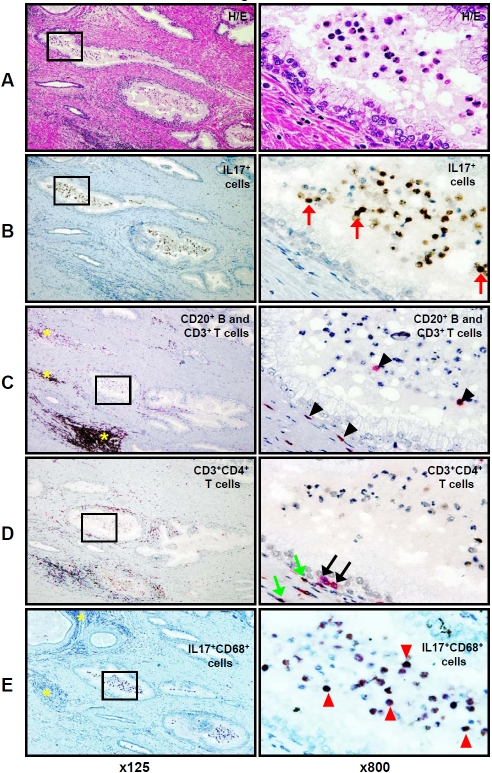
Since recent studies suggest an important role for pro-inflammatory IL-17 producing cells in the development of chronic inflammation, autoimmunity [18], and prostate cancer progression [19], we next focused on phenotyping the IL-17-expressing cells in PIA lesions. Consecutive slide analysis showed abundant cells with the morphologic features of macrophages and neutrophils within gland lumens in PIA lesions (Figure4A H&E, ×800), and these cells exhibited strong IL-17-immunoreactivity (Figure4A and 4B). We found that CD3+ T and CD20+ B lymphocytes were commonly organized as stromal lymphoid MNC follicles (“hot spots”) mostly in the peripheral zone of the prostate (Figure4, yellow asterisks, ×125). Only isolated B and T lymphocytes were detected in the glandular epithelium of PIA lesions and none were found in the gland lumens in PIA lesions (Figure4C, anti-CD20 and anti-CD3, ×800). To investigate the distribution of CD3+CD4+ T helper-inducer lymphocytes in PIA lesions, we used double immunostaining, because of the ability of human monocytes/macrophages co-express CD4 surface marker [20]. As shown in figure 4C, (x125), stromal MNC hot spots are comprised of B and T lymphocytes. Many of these lymphocytes were CD4+CD3+ T helper-inducer cells (Figure4D, ×125). The CD4+ T cells also accumulated along the acinar basement membranes (Figure4D, ×800), but were not identified within gland lumens, (Figure4D, ×125 and ×800), whereas IL-17-expressing leukocytes accounted for the majority of intraluminal inflammatory cells. To confirm the production of IL -17 cytokine by intraluminal cells with the morphologic features of macrophages, we used double immunostaining for consecutive slides with IL-17 and CD68. Numerous IL-17-producing CD68-positive macrophages were demonstrated within gland lumens (Figure4E, ×800), as well as a few of these cells within the stroma of PIA lesions (Figure4E, ×125).
Figure 4.
H&E and IHC staining for IL-17, CD3, CD20, CD4, and CD68, in the PIA lesion of formalin-fixed paraffin-embedded whole mount radical prostatectomy specimens from patients with prostate cancer. Consecutive prostate tissue slide staining for: A - H&E, B - IL-17 (brown color), C - two color CD3 (T lymphocytes - pink color) and CD20 (B lymphocytes - brown color), D - CD3 (pink color) and CD4 (brown color) with co-localization of CD3 and CD4 (dark brown or black colors) on helper-inducer T lymphocytes (dark brown or black colors), and E - IL-17 (brown color) and CD68 (pink color) with co-expression of IL-17 and CD68 proteins in tissue macrophages (dark brown or black colors). Red arrows (x800) indicate intra-glandular IL-17 expressing cells. Yellow asterisks (x125) indicate sites of B and T cell accumulation in MNC hot spots in the prostate stroma. Black arrowheads (x800) indicate peri-glandular and glandular T cells. Green arrowheads (x800) indicate peri-glandular CD4+CD3+ T helper cells. Black arrows (x800) indicate peri-glandular CD3 T cells without co-localization of CD4 antigen. Red arrowheads (x800) indicate intra- glandular IL-17 producing CD68 positive macrophages. Left column presents H&E staining and leukocyte immunophenotypingof the same PIA lesion with magnification ×125. Right column depicts H&E staining and leukocyte immunophenotyping of tissue of the same PIA lesion located inside of the black squares of the left columns with magnification ×800. The figure shows that IL-17 positive cells (B) are located predominantly intraluminally in PIA lesions and that they are not T or B lymphocytes (C), or classical IL-17 expressing CD4+CD3+ T helper (TH17) cells (D), but rather CD68+ tissue macrophages (E) expressing IL-17 protein. Details are described in “Materials and Methods” section.
Prostate microvessels might deliver IL-17 producing monocytes/macrophages to PIA lesions
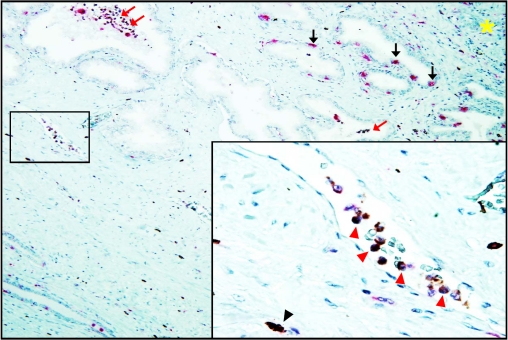
Since inflammatory cell extravasation followed by the appearance of IL-17 positive MNC in prostate stroma is one of the last events of the intraprostatic pro-inflammatory wave following acute systemic IL-1β administration in vivo [10, we next assessed whether resident leukocytes in PIA lesions acquire the ability to express IL-17, or whether PIA lesions represent sites of accumulation of IL-17 expressing cells. The phenotype of IL-17 expressing cells inside stromal capillaries was evaluated in consecutive slides using immunostaining for the CD68 monocyte/macrophage antigen. It is apparent that the majority of nucleated cells within capillaries (Figure5A, ×800) were IL-17 and CD68+, (Figure5B and 5C) indicating a high probability of co-localization for these markers. Additionally, Figure 6 shows direct evidence of a co-expression of IL-17 and CD68 on nucleated cells inside microvessels adjacent to a PIA lesion, indicating that they are IL-17 producing monocytes.
Figure 5.
H&E and IHC staining for IL-17 and CD68 in lumens of capillaries in formalin-fixed paraffin-embedded whole mount radical prostatectomy specimens from patients with prostate cancer. A - H&E staining, B - staining for IL-17 (light brown color) and C - staining for CD68 (dark brown color) expressing cells in lumens of microvessels (black dotted circles, ×200) located in benign tissue between the areas of prostate adenocarcinoma (not visible at right side of the slide), proliferative inflammatory atrophy (PIA) lesion (red arrowheads, ×200) and MNC hot spot (yellow asterisks, ×200). Stromal CD68+ tissue macrophages (dark brown color in red circles, ×200) adjacent to the MNC hot spot show no co-expression of IL-17 protein. Left column presents H&E staining and immunostainingfor IL-17 and CD68 in prostate tissue with magnification ×200. Right column depicts H&E staining and immunostainingfor IL-17 and CD68 in prostate tissue located inside of black squares of the left columns with magnification ×640. Details are described in “Materials and Methods” section.
Figure 6.
IHC staining for IL-17 and CD68 inside microvessel at the PIA lesion of formalin-fixed paraffin-embedded whole mount radical prostatectomy specimens from patients with prostate cancer (magnification ×125). IL-17 (brown color) and CD68 (pink color) proteins are co-expressed (dark brown or black colors) in monocyte/macrophages inside capillaries located in stroma adjacent to PIA lesion. Red arrows indicate intra-glandular IL-17 expressing CD68+ macrophages. Black arrows indicate intra-epithelial IL-17-non-producing CD68+ macrophages. Yellow asterisk indicates site of accumulation of mononuclear cells (“hot spot”) in the prostate stroma. Insertion represents microvessel located inside of the black square: red arrowheads indicate IL-17 producing CD68+ monocyte/macrophages inside of the capillary space; black arrowhead indicates stromal IL-17 expressing CD68+ macrophage (magnification ×800). The figure shows that IL-17 expressing cells can be readily detected inside lumens of capillaries located in the peripheral zone of the prostate, and that CD68 monocytes/macrophages inside the stromal capillaries near PIA lesion express IL-17 protein. Details are described in “Materials and Methods” section.
Discussion
Identification and phenotyping of intraprostatic IL-17 producing cells were performed in this study and their distribution in various zones in radical prostatectomy specimens was analyzed. Our findings highlight the long-recognized relationship between chronic inflammation and cancer [21]. Previous studies suggest that the degree of inflammation within areas of prostatic adenocarcinoma may be an independent predictor for prostate cancer recurrence. Patients with high-grade inflammation in the malignant areas of their radical prostatectomy specimens have a significantly higher biochemical recurrence rate than patients with low-grade inflammation within their prostate cancers [22]. More recently, a five year follow-up study has demonstrated that chronic inflammation itself may be a significant risk factor for the development of prostatic adenocarcinoma [6].
Infiltrates of inflammatory cells are commonly associated with malignant neoplasms. Analysis of two major prostate tumor-infiltrating lymphocyte subsets showed that capsular and perineural invasion as well as biochemical progression was related to strong expression of T and B cells [23]. More specifically, elevated densities of CD4+ T helper-inducer lymphocytes [24] and mast cells [25] located in areas of prostate ade-nocarcinoma are associated with poor survival and higher Gleason scores. In contrast, other studies report that a higher tumor-infiltrating lymphocyte density, evaluated by H&E staining, is protective against disease progression [26]. An inverse relationship has been reported between the degree of primary tumor infiltration by CD68+ macrophages and the likelihood of prostate cancer progression [27]. Another recent study demonstrate that patients treated with androgen deprivation therapy exhibit a high density of CD68+ macrophages which was related to and seemed to favor the development of advanced prostate cancer [28].
Tumor-associated macrophages are a significant component of the inflammatory cell infiltrates in most, if not all, tumors. These cells are derived from circulating monocytic precursors, and are attracted to the tumor by chemokines. Appropriate activation of tumor-infiltrating macrophages can destroy prostate tumor cells by cathepsin E-specific growth arrest and induction of apoptosis [29], or by inhibition of angiogenesis [30]. In the current study we found scattered IL-17 positive MNC within foci of prostatic adenocarcinoma. However, our findings indicate that accumulation of IL-17-expressing cells is characteristic only of PIA lesions. In general, PIA may represent injury to the prostate epithelium, regardless of the source of the damage, which as a consequence elicits a stereotypical stress and regenerative response [31]. PIA lesions are by definition associated with chronic prostatic inflammation, and histological transitions between areas of PIA and HGPIN, and between PIA and prostate cancer, have been observed and reported [32, 33].
Previous studies demonstrate that 79% of BPH and 58% of prostate cancer specimens exhibit higher levels of IL-17 mRNA and protein expression [34]. In contrast, normal prostate tissue exhibits weak IL-17 expression restricted to lymphocytes, whereas the hyperplastic glandular epithelium, smooth muscle cells and infiltrating lymphocytes in BPH specimens exhibited reactivity for IL-17. In the present study, although we used anti-human IL-17 antibody procured from the same vender as reported in an earlier study, we did not observe any reactivity to this antibody in the stromal or epithelial cells in the prostatectomy specimens studied. Furthermore, in our study, phenotyping of IL-17-positive cells in prostatectomy specimens indicated that these cells are predominantly CD68+ macrophages and neutrophils, particularly in the PIA lesions. Accumulation of IL-17 expressing macrophages and neutrophils was 3-5 folds higher in PIA lesions as compared the densities of these cells within prostate adenocarcinoma or in benign prostate tissue uninvolved by PIA. These findings suggest that areas of prostate adenocarcinoma, PIA lesions, and areas of benign prostate tissue uninvolved by PIA may represent different microenvironments. Previous studies support the significance of intra-luminal macrophages in PIA lesions in patients with prostate cancer and have addressed the ability of these macrophages either to express COX-2 independently [35] or following induction of COX -2 expression in prostate epithelium in human BPH specimens [36].
It has been shown that the levels of TH17 cells gradually increase in the microenvironments of many mouse and human tumors during tumor development [37] and that IL-17 up-regulates the production of a variety of pro-inflammatory cytokines [38] and proangiogenic factors [39] to promote tumor development [40]. A recent study has shown that prostate-infiltrating lymphocytes seem to be skewed towards the TH17 phenotype in patients with prostate cancer, however, the preponderance of TH17-mediated inflammation was associated with a lower pathologic Gleason score [41]. In the present study we observed that CD68+ macrophages and polymorphonuclear cells, rather that CD4+ TH17 cells, are by far the predominant IL-17 expressing cells in PIA lesions in cancerous prostate glands. This finding suggests that intraprostatic macrophages play a much more significant role than previously recognized in promoting prostate inflammation and possibly additionally promoting the development of prostate cancer.
Documentation of the presence of significant numbers of IL-17-expressing CD68+ monocytes inside stromal capillaries and of the predominance of intraluminal and intraepithelial IL-17 expressing neutrophils and CD68+ monocytes/ macrophages in PIA lesion of patients with prostate cancer are the most significant findings in our present study. These data are in concordance with the findings in a recent study reporting increased numbers of infiltrating monocytes in the early and advanced stages of tumor growth of rat prostate cancer induced by N-methyl-N-nitrosourea plus testosterone [42]. It has been demonstrated that CC chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1) may be one of the tumor microenvironment-derived signals that recruit tumor-associated macrophages, originating from circulating monocytes in tumor-related vasculature [43]. More recently, CCL2 has been highlighted as a factor that increases prostate tumor growth and bone metastasis through recruitment of macrophages and osteoclasts to the tumor site [44]. Our results are in concordance with many of these observations, and suggest that a currently unidentified but powerful PIA-specific chemoattractant for IL-17 expressing blood derived monocytes/ macrophages may significantly contribute to the establishment of chronic prostatic inflammation and the development of prostate cancer.
Taken together, our present data demonstrate that release of inflammatory IL-17 from monocytes/macrophages and neutrophils may be a characteristic of PIA lesions in prostate adenocarcinoma. The accumulation of IL-17 producing cells in PIA lesions presents direct evidence of an inflammatory microenvironment that may support the development and progression of prostate cancer. The ability of intra-prostatic monocytes/macrophages to actively express pro-inflammatory IL-17 and accumulate in PIA lesions may help to bridge the gaps in our understanding of the relationships between chronic prostatic inflammation and the development of prostate cancer.
Acknowledgments
The studies are supported by the USPHS grants NIH RO1CA10851, RO1AT002709 and the Sullivan Foundation for the study of prostatitis.
Abbreviations
- BPH
Benign prostatic hyperplasia
- CCL2
CC chemokine ligand 2
- IL-17
Interleukin-17
- TH17 cells
Interleukin (IL)-17 expressing CD4+ T helper cells
- MNC
Mononuclear cell
- PIA
Proliferative inflammatory atrophy
- HGPIN
High-grade prostate intraepithelial neoplasia
- IHC
Immunohostochemistry
Disclosure/Conflict of Interest
The authors have no potential conflict of interest to declare.
References
- 1.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Cordon-Cardo C, Prives C. Commentary: at the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190:1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLennan GT, Eisenberg R, Fleshman RL, Taylor JM, Fu P, Resnick MI, Gupta S. The influence of chronic inflammation in prostatic carcinogenesis: A 5-year followup study. J Urol. 2006;176:1012–1016. doi: 10.1016/j.juro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 7.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vykhovanets EV, Shukla S, Maclennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1beta. Prostate. 2008;68:34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 10.Vykhovanets EV, Shukla S, MacLennan GT, Vykhovanets OV, Bodner DR, Gupta S. IL-1beta-induced post-transition effect of NFkappaB provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate. 2009;69:633–643. doi: 10.1002/pros.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 12.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. [DOI] [PubMed] [Google Scholar]
- 13.Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40:1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 14.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. Identification of an IL- 17-producing NK1.1neg iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 16.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 17.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Kazazi F, Mathijs JM, Foley P, Cunningham AL. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J Gen Virol. 1989;70:2661–2672. doi: 10.1099/0022-1317-70-10-2661. [DOI] [PubMed] [Google Scholar]
- 20.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 21.Irani J, Goujon JM, Ragni E, Peyrat L, Hubert J, Saint F, Mottet N. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology. 1999;54:467–472. doi: 10.1016/s0090-4295(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 22.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–4438. [PubMed] [Google Scholar]
- 23.McArdle PA, Canna K, McMillan DC, McNicol AM, Campbell R, Underwood MA. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br J Cancer. 2004;91:541–543. doi: 10.1038/sj.bjc.6601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sari A, Serel TA, Candir O, Ozturk A, Kosar A. Mast cell variations in tumour tissue and with histopathological grading in specimens of prostatic adenocarcinoma. BJU Int. 1999;84:851–853. doi: 10.1046/j.1464-410x.1999.00245.x. [DOI] [PubMed] [Google Scholar]
- 25.Vesalainen S, Lipponen P, Talja M, Syrjanen K. Histological grade, perineural infiltration, tumour- infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- 26.Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000;60:5857–5861. [PubMed] [Google Scholar]
- 27.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009;348:9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, Nakayama KI, Kadowaki T, Tsukuba T, Yamamoto K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007;67:10869–10878. doi: 10.1158/0008-5472.CAN-07-2048. [DOI] [PubMed] [Google Scholar]
- 29.Shin M, Kadowaki T, Iwata J, Kawakubo T, Yamaguchi N, Takii R, Tsukuba T, Yamamoto K. Association of cathepsin E with tumor growth arrest through angiogenesis inhibition and enhanced immune responses. Biol Chem. 2007;388:1173–1181. doi: 10.1515/BC.2007.154. [DOI] [PubMed] [Google Scholar]
- 30.Bardia A, Platz EA, Yegnasubramanian S, De Marzo AM, Nelson WG. Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol. 2009;9:419–426. doi: 10.1016/j.coph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Bergh A, Damber JE. Increased expression of CCAAT/enhancer-binding protein beta in proliferative inflammatory atrophy of the prostate: relation with the expression of COX-2, the androgen receptor, and presence of focal chronic inflammation. Prostate. 2007;67:1238–1246. doi: 10.1002/pros.20595. [DOI] [PubMed] [Google Scholar]
- 32.Nakai Y, Nelson WG, De Marzo AM. The dietary charred meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine acts as both a tumor initiator and promoter in the rat ventral prostate. Cancer Res. 2007:67, 1378–1384. doi: 10.1158/0008-5472.CAN-06-1336. [DOI] [PubMed] [Google Scholar]
- 33.Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003;56:171–182. doi: 10.1002/pros.10238. [DOI] [PubMed] [Google Scholar]
- 34.Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Faith DA, Nelson WG, De Marzo AM, Isaacs WB. Cyclooxygenase-2 is upregulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- 35.Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61:60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- 36.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 37.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol. Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 40.Sfanos KS, Bruno TC, Maris CH, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–6132. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayanan NK, Nargi D, Horton L, Reddy BS, Bosland MC, Narayanan BA. Inflammatory processes of prostate tissue microenvironment drive rat prostate carcinogenesis: preventive effects of celecoxib. Prostate. 2009;69:133–141. doi: 10.1002/pros.20862. [DOI] [PubMed] [Google Scholar]
- 42.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117:2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia. 2007;9:556–562. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]