Abstract
The pathogenic mechanisms underlying the occurrence of cerebral malaria (CM) are still incompletely understood but, clearly, cerebral complications may result from concomitant microvessel obstruction and inflammation. The extent to which brain edema contributes to pathology has not been investigated. Using the model of P. berghei ANKA infection, we compared brain microvessel morphology of CM-susceptible and CM-resistant mice. By quantitative planimetry, we provide evidence that CM is characterized by enlarged perivascular spaces (PVS). We show a dramatic aquaporin 4 (AQP4) upregulation, selectively at the level of astrocytic foot processes, in both CM and non-CM disease, but significantly more pronounced in mice with malarial-induced neurological syndrome. This suggests that a threshold of AQP4 expression is needed to lead to neurovascular pathology, a view that is supported by significantly higher levels in mice with clinically overt CM. Numbers of intravascular leukocytes significantly correlated with both PVS enlargement and AQP4 overexpression. Thus, brain edema could be a contributing factor in CM pathogenesis and AQP4, specifically in its astrocytic location, a key molecule in this mechanism. Since experimental CM is associated with substantial brain edema, it models paediatric CM better than the adult syndrome and it is tempting to evaluate AQP4 in the former context. If AQP4 changes are confirmed in human CM, it may represent a novel target for therapeutic intervention.
Keywords: Brain edema, aquaporin 4, astrocyte, endothelium, experimental cerebral malaria
Introduction
Severe malaria remains a major problem of public health [1-5]. This has been studied in various ways, including the use of experimental models. The syndrome of cerebral malaria (CM), the major fatal complication of plasmodium infection, invariably occurs, in susceptible mouse strains, at day 7 after infection with Plasmodium berghei ANKA (PbA) and is not directly related to parasite count in the blood [6]. The fatal outcome is generally attributed to the sequestration of activated blood cells (notably monocytes / macrophages, parasitized erythrocytes, and platelets) in cerebral vessels and to inadequate immune responses in the host [7, 8]. The pathogenic mechanisms underlying the occurrence of cerebral lesions are still incompletely understood but, clearly, cerebral complications may result from concomitant microvessel obstruction and inflammation [9-11].
Blood-brain barrier (BBB) alterations and edema formation are among the major features of severe malaria (reviewed in [12-14]). Post mortem observations of CM patients' brains have revealed parasitized erythrocyte sequestration, edema, BBB breakdown and petechial hemorrhages [15]. The few isolated cases of adults with CM studied by in vivo imaging techniques have shown brain swelling, small hemorrhagic lesions and focal lesions in cerebrum and brainstem [16-20]. Among malaria patients, the pediatric syndrome has been reported to be associated with pronounced degrees of brain edema [21], while adults are suggested to present milder signs of edema [18]. However, brain edema also is substantial in adults and shows prognostic value [19]. In the mouse model, brain edema further worsens ischemia by compressing cerebral arteries, which subsequently leads to a collapse of the blood flow that ultimately may represent a cause of death [22].
The two main types of brain edema are cytotoxic and vasogenic [23]. Vasogenic edema involves accumulation of excess fluid in the extracellular space of the brain parenchyma because of a leaky BBB [24]. Cytotoxic edema consists of intracellular fluid accumulation that occurs during anoxic conditions [25].
Recently, the bidirectional water channel aquaporin 4 (AQP4) has been found to play an important role in brain-water homeostasis [25-30]. Aquaporins are a family of unique trans-membrane molecules acting as bidirectional water channels [31]. Twelve members have been identified but AQP4 is the main water channel expressed in the brain [32]. AQP4 is expressed in astrocyte foot processes, surrounding the brain capillary endothelial cells, but little is known about the molecular mechanisms involved in its regulation [25, 33]. AQP4 protein is expressed strongly in astroglia at the BBB and cerebro-spinal fluid (CSF)-brain interfaces, suggesting involvement in water movement between fluid compartments (blood and CSF) and brain parenchyma [34, 35]. AQP4 appears to facilitate water movement into brain astroglia in cytotoxic edema, and water movement out of the brain in vasogenic edema [30]. Thus the mechanisms by which brain AQPs are regulated will be of the utmost clinical importance, since perturbed water flow via brain AQPs has been implicated in many neurological diseases and, in brain edema, water flow via AQP4 may have a harmful effect [33].
From a histopathological perspective, enlargement of perivascular spaces is recognized as a sign of brain edema and has been described using electron microscopy [36] but not appraised quantitatively in CM. In the PbA mouse model of CM, our MRI data demonstrated the coexistence of inflammatory and ischemic lesions and proved the preponderant role of edema in the fatal outcome of experimental CM. We previously have demonstrated that CM involves both cytotoxic and vasogenic edema [22, 37].
Along these lines – and in particular considering the suggestion of a causal relationship between brain edema, AQP4 expression, and the clinical severity of malaria – we set out to compare the CM-susceptible (CM-S) mouse strain, CBA/CaH, and CM-resistant (CM-R) BALB/cA mice in terms of their brain microvessel morphology upon infection with PbA. In this study we provide evidence that CM is characterized by enlarged perivascular spaces (PVS), and markedly increased leukocyte sequestration. We show a dramatic AQP4 upregulation, selectively at the level of astrocytic foot processes, in both CM and non-CM disease, but significantly more pronounced in mice with malarial-induced neurological syndrome. These findings are consistent with a pathogenic role of brain edema and astrocytic AQP4 in the development of cerebral complications.
Materials and methods
Animals and parasites
Animal studies were undertaken in accordance with the guidelines under the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and approved by the University of Sydney Animal Ethics Committee. Fourteen female CBA/CaH and 15 female BALB/c mice (8–10 weeks old) were used for this study, 3 of each as controls and 11 of CBA/CaH and 12 of BALB/c that were infected with P. berghei ANKA by intraperitoneal injection of 106 parasitized erythrocytes, as described [38]. The mice were housed in individual ventilation cages and fed ad libitum in the laboratory animal facility of the Medical Foundation Building, Department of Pathology, University of Sydney. Parasitemia was monitored on days 4 and 7 of infection by examining DiffQuick® (Jorgensen laboratory, J322A3)-stained blood smears. Mice were screened on a daily basis for neurological manifestations.
Specimen processing
Mice were euthanized with over dose inhalation of Isoflurane® on day 7 post inoculation and brains collected and fixed in 10% v/v neutral buffered formalin for 8 h at 4°C. Fixed specimens were dehydrated in graded alcohol and processed using standard procedures. The tissue was embedded in paraffin and sectioned at 7 µm. Serial tissue sections were mounted on SuperFrost plus slides (Menzel GmbH & Co KG, SF41296PL) for immunohistochemistry and normal glass slides for H&E staining.
Immunohistochemistry
Heat-induced antigen retrieval with citrate buffer, pH 6 was used to unmask the antigen. Endogenous peroxidase was quenched with 1% v/v hydrogen peroxide in methanol after sections were cooled. Sections were washed with 0.2% v/v Tween in Tris buffered saline (TBS) and blocked with 10% w/v skim milk for 20 min. Sections were incubated for 30 min at room temperature with 1:40 rabbit anti-GFAP (Biogenex, San Ramon, CA, USA) diluted in TBS with 1% v/v normal goat serum (NGS, Vector, USA, S1000). The sections were washed in TBS and incubated for 30 min with 1:200 biotinylated goat anti-rabbit antibody (Vector, USA, BA1000) in 1% NGS/TBS at room temperature. Slides were washed, incubated with avidin biotin peroxidase complex (ABC Vectastain, Vector, USA, PK4000) in TBS for 30 min at room temperature, and visualized with diaminobenzidine (DAB, DAKO, K3468). Sections were again blocked with 10% skim milk after several washes with 0.2% Tween in TBS and incubated overnight at 4°C with 1:80 polyclonal rabbit anti-rat AQP4 (Chemicon, AB3068). The sections were washed in TBS and incubated for 30 min with 1:200 biotinylated goat anti-rabbit antibody (Vector, USA, BA1000) in 1% NGS/TBS at room temperature. Slides were washed in TBS, incubated for 30 min with avidin biotin alkaline phosphatase complex (ALP; DAKO RealTM Detection System, K5005) and visualized with liquid permanent red (LPR, DAKO, K0604). Slides were counterstained with hematoxylin before permanent mounting with Vectamount (Vector, USA). Image analysis was performed using the AnalySIS Five software (Olympus).
Statistical analysis
Nonparametric Kruskal Wallis test, Friedman test, and Mann-Whitney U-tests were performed with GraphPad Prism 4.0 software and p < 0.05 was considered significant. Results are expressed as mean ± SEM.
Results
Quantitation of perivascular space
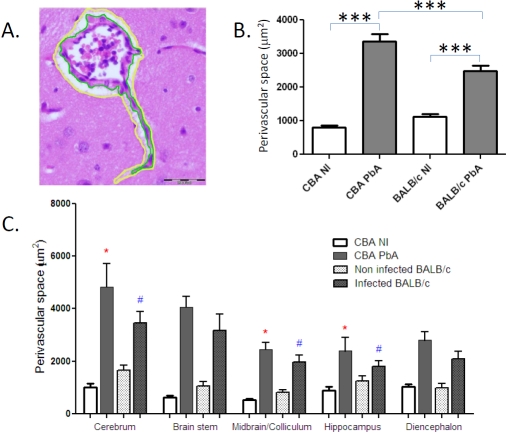
Non-infected and PbA-infected mice of both strains were sacrificed, on day 7 post-inoculation (p.i.), i.e., at the time of CM onset in CBA mice, and brain tissue collected and processed with buffered formalin, which gave the most consistent results in terms of tissue preservation and reproducibility. On all vessels that were visible on each H&E stained section, i.e., without any selection, the outline of the perivascular space (PVS) was drawn manually so as to delineate its outer and inner limits, with a yellow and a green line, respectively, as shown in Figure 1A. The “Polygon” selection of the Analysis Five software was used to draw the lines and the surfaces of these two areas were computed by planimetry for each vessel. The PVS was calculated by subtraction of these two surfaces and expressed in m2. While infection with PbA was associated with a highly significant PVS enlargement both in CM-S CBA and CM-R BALB/ c mice, this increase was significantly greater in CM-S than in CM-R animals (Figure 1B). The infection-induced PVS enlargement was seen in all the CNS areas studied, with differences between infected CBA and infected BALB/c mice being significant only in cerebrum, midbrain and hippocampus (Figure 1C).
Figure 1.
Quantitation of brain edema during PbA infection. A. Delineation of the perivascular space (PVS) with a green line (inner part) and a yellow line (outer part); B. Changes in PVS in CM-S versus CM-R mice, as quantified by planimetry. The numbers of vessels analysed were 302 in non-infected (NI) CBA, 274 in PbA-infected CBA, 227 in NI BALB/c and 247 in PbA-infected BALB/c (total numbers of vessels, in 5 or 6 mice per group). All vessels on each sections were analyzed: this explains the different numbers of vessels in each group. C. Topographical distribution of PVS changes in CM-S and CM-R mice upon PbA infection. *: p<0.05 between infected and non-infected;#: p<0.05 between infected CBA and infected BALB/c; ***: p<0.0001, non-parametric Mann-Whitney U tests.
Pattern and modulation of aquaporin 4 (AQP4) expression
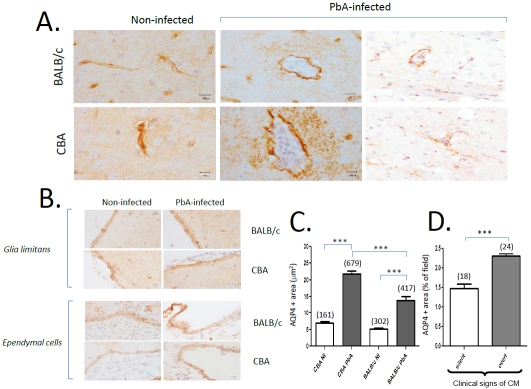
On the same brain samples, we then sought to examine and quantify the expression of AQP4 in relation to malarial infection. In PbA-infected CBA mice, which were exhibiting signs of CM (detailed in 7, 8), we observed an increased AQP4 staining in the PVS, at the level of astrocyte foot processes, when compared to non-infected mice (Figure 2A). No such difference was seen in CM-resistant BALB/c mice. Dual staining for GFAP confirmed the localization of increased AQP4 (Figure 2A, right panels). It is noteworthy that the AQP4 expression at the level of vascular end feet of astrocytes was identical in infected and non-infected mice, irrespective of the mouse strain (Figure 2A, left panels). In contrast to what was seen at the level of PVS, the other two AQP4-rich barriers, namely the glia limitans and the ependyma, did not show any modulation of AQP4 expression with PbA infection in either CM-S or CM-R mice (Figure 2B). When AQP4 expression was quantified using planimetry of the stained surfaces, we found that malarial infection resulted in a significant increase in AQP4 expression in both CM-susceptible and CM-resistant mice (Figure 2C), with a significantly higher expression in the former (p<0.001). Interestingly, the AQP4 expression was found to be significantly higher in CBA mice with clinical signs of neurological involvement i.e., showing palsies, convulsions or ataxia, than in CBA mice also sampled on day 7 but without neurological signs (Figure 2D). In addition, PVS was significantly larger when AQP4 expression was higher (PVS: 367.72 ± 25.83 m2 when AQP4 was 5-10 m2 versus 512.55 ± 27.74 m2, when AQP4 was > 15 m2, p < 0.05).
Figure 2.
Patterns of AQP4 expression in CM-S and CM-R mice upon PbA infection. A. Distinct patterns of AQP4 expression: selectively increased expression in perivascular spaces in CBA mice with CM, on day 7 p.i. The AQP4 expression in astrocytic foot processes is higher in CBA than in BALB/c mice. AQP4 is revealed in brown (DAB) except on the two right panels, where it is revealed in red (LPR) and GFAP in brown (DAB), representative of 5 mice. B. AQP4 patterns in glia limitans and ependymal cells. DAB staining. C. Quantitation of AQP4 expression by planimetry in noninfected (NI) versus PbA-infected mice. The incubation conditions were identical between all sections and all animals by incubating simultaneously for all immunostainings. Numbers in parentheses indicate the numbers of vessels studied. *: p<0.01,***: p < 0.0001. D. AQP4 expression in relation to clinical expression of CM. CBA mice, 7 days p.i., with clinically silent versus clinically overt neurological syndrome of CM. Numbers in parentheses indicate the numbers of vessels studied. Kruskall-Wallis test, non-parametric one-way ANOVA with Bonferroni test.
Quantitation of leukocyte sequestration in brain microvessels during PbA infection
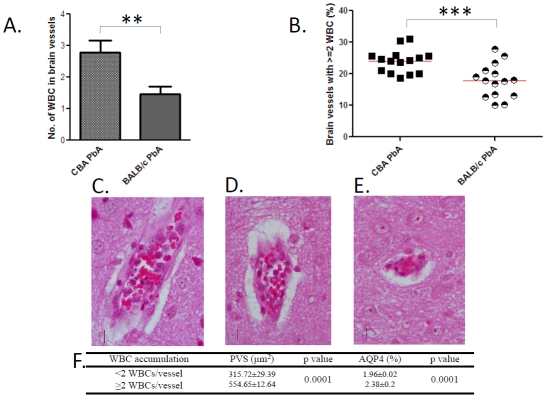
All brain sections analysed for PVS were then examined for intravascular leukocytes. While non-infected mice of the two strains showed no leukocyte in either capillaries or post-capillary venules, PbA-infected mice, on day 7 p.i., presented a substantial accumulation of mononuclear leukocytes. When computed as either the number of white blood cells in brain vessels or as the numbers of brain vessels showing more than 2 white blood cells in their lumen, we found that this phenomenon was significantly more pronounced in CM-S CBA than in CM-R BALB/c mice (Figure 3A and 3B, respectively). Representative illustrations of the leukocyte sequestration are presented in Figure 3C–3E, the intensity of which was higher with large PVS and high APQ4 expression (Figure 3F).
Figure 3.
Leukocyte sequestration in brain microvessels and its relation to edema during PbA infection. A. Individual microvessels were analysed in 15 mice per group. 518 and 398 vessels were studied in CBA and BALB/c mice, respectively. Mann-Whitney test. B. Proportion of microvessels containing more than 2 WBC. Each dot represents percentages in individual mice (n = 15). Representative post-capillary venule with 10, 5, or 3 leukocytes, (C, D, E, respectively). PbA-infected CBA mouse, day 7 p.i. Haematoxylin-eosin staining, magnification 400×. F. Intravascular leukocyte numbers correlate with PVS and AQP4 (same vessels studied as in A.). Non parametric Mann-Whitney U test.
Discussion
In this paper we have analysed histopathological parameters in experimental CM by comparing mice that are either susceptible or resistant to the neurological syndrome. First, we analysed the perivascular spaces (PVS), a well recognised change related to brain edema, but which had never been precisely quantified in relation to the neurovascular pathology of CM. We found that malaria causes a marked enlargement of PVS in both strains of mice, compared to non-infected mice. This PVS enlargement was significantly more pronounced in CM-susceptible animals than in their CM-resistant counterparts. In BALB/c mice, the significant degree of PVS enlargement in the absence of neurological signs suggests that that a threshold of edema may be needed to lead to CM.
Since CM involves both cytotoxic and vasogenic edema [22, 37], we focussed our attention on the three brain sites that are rich in AQP4, i.e. where it is known that excess water can be eliminated in the case of either cytotoxic or vasogenic edema: the blood-brain barrier, into the bloodstream; the glia limitans, into the subarachnoid space; and the ependyma, into the ventricles.
In physiological conditions, the pattern of distribution of AQP4 within the perivascular space might be related to the control of the perivascular volume, a function that may be crucial for maintenance of cerebral blood perfusion [39]. The density of AQP4 expression may be related to the size of the extracellular space. The stratum pyramidale, which shows a high degree of AQP4 labeling, exhibits a very low extracellular volume fraction [40]. The presence of AQP4 may also be due to the sensitivity of these regions towards an expansion of the extracellular space [41]. Several studies of pathologic AQP4 expression have been reported. Mice deficient in AQP4 show decreased cerebral edema and improved neurological outcome following anoxia -producing conditions and infectious diseases [25, 26, 35, 42, 43]. AQP4 expression is increased in edematous human brain tumors, traumatic brain injury, ischemia, and various inflammatory lesions [28, 44], notably in rat In our study, we show that it is the AQP4 present in astrocytic foot processes that seems to be relevant to the triggering of CM, while the molecule expressed in glia limitans and ependymal cells is not. In addition, very much like PVS, this AQP4 expression was increased in both CM and non-CM, but was significantly more pronounced in the former. This suggests that a threshold of increased AQP4 expression is needed to lead to neurovascular pathology, a view that is supported by the observation of significantly higher levels in mice presenting with clinically overt signs of CM (Figure 3B).
Leukocyte accumulation in brain microvessels is a central feature of experimental CM histopathology, as shown in several reports [49-53] and we show here that it is quantitatively related to CM. While intravascular leukocytes are not necessarily associated with pathology, as dissociation between these two can be seen upon anti-LFA-1 treatment [54] or in uPA KO mice [55], we demonstrate here that there is a significant correlation between intravascular leukocytes and both PVS enlargement and AQP4 overexpression.
Taken together, our data point toward brain edema as a contributing factor in CM pathogenesis and to AQP4, specifically in its astrocytic location, as a key molecule in this mechanism. Since experimental CM is associated with substantial brain edema, it models paediatric CM [21] better than the adult syndrome [18] and it is tempting to evaluate AQP4 in the former context. If AQP4 changes are confirmed in human CM, it may represent a novel target for therapeutic intervention.
Acknowledgments
This work was supported by grants from the NHMRC (to GEG, NHH and TCL), the Thailand Centre of Excellence for Life Science (TCELS) Programme (to SA), the Rebecca Cooper Foundation and the AL Kerr Bequest, Sydney Medical School (to GEG). the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative (to EP).We also thank Dr Gareth Turner, Dr Urai Chaisri, Dr Apichart Nontprasert and Dr Parn-pen Viriyavejakul (Mahidol University) for critical comments.
References
- 1.Anstey NM, Price RN. Improving case definitions for severe malaria. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040267. e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejon P, Berkley JA, Mwangi T, Ogadmla E, Mwangi I, Maitland K, Williams T, Scott JA, English M, Lowe BS, Peshu N, Newton CR, Marsh K. Defining childhood severe falcipa-rum malaria for intervention studies. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040251. e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley J, Crawley J, Fegan G, Bauni E, Peshu N, Marsh K, Neville B, Newton C. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297:2232–2240. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO, editor. 2009. World Malaria Report 2009; < http://www.who.int/malaria/ publications/ atoz/9789241563901/ en/ index. html>.
- 6.Lou J, Lucas R, Grau GE. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin Microbiol Rev. 2001;14:810–820. doi: 10.1128/CMR.14.4.810-820.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 8.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 9.Medana IM, Hien TT, Day NP, Phu NH, Mai NT, Chu'ong LV, Chau TT, Taylor A, Salahifar H, Stocker R, Smythe G, Turner GD, Farrar J, White NJ, Hunt NH. The clinical significance of cerebrospinal fluid levels of kynurenine pathway metabolites and lactate in severe malaria. J Infect Dis. 2002;185:650–656. doi: 10.1086/339009. [DOI] [PubMed] [Google Scholar]
- 10.Ball HJ, MacDougall HG, McGregor IS, Hunt NH. Cyclooxygenase-2 in the pathogenesis of murine cerebral malaria. J Infect Dis. 2004;189:751–758. doi: 10.1086/381503. [DOI] [PubMed] [Google Scholar]
- 11.Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Cerebral malaria: role of microparti- cles and platelets in alterations of the blood- brain barrier. Int J Parasitol. 2006;36:541–546. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Brown H, Hien TT, Day N, Mai NT, Chuong LV, Chau TT, Loc PP, Phu NH, Bethell D, Farrar J, Gatter K, White N, Turner G. Evidence of blood-brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999;25:331–340. doi: 10.1046/j.1365-2990.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- 13.Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, Turner G. Blood-brain barrier function in cerebral ma laria in Malawian children. Am J Trop Med Hyg. 2001;64:207–213. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- 14.Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 15.Adams S, Brown H, Turner G. Breaking down the blood-brain barrier: signaling a path to cerebral malaria? Trends Parasitol. 2002;18:360–366. doi: 10.1016/s1471-4922(02)02353-x. [DOI] [PubMed] [Google Scholar]
- 16.Millan JM, San Millan JM, Munoz M, Navas E, Lopez-Velez R. CNS complications in acute malaria: MR findings. AJNR Am J Neuroradiol. 1993;14:493–494. [PMC free article] [PubMed] [Google Scholar]
- 17.Cordoliani YS, Sarrazin JL, Felten D, Caumes E, Leveque C, Fisch A. MR of cerebral malaria. AJNR Am J Neuroradiol. 1998;19:871–874. [PMC free article] [PubMed] [Google Scholar]
- 18.Looareesuwan S, Wilairatana P, Krishna S, Kendall B, Vannaphan S, Viravan C, White NJ. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis. 1995;21:300–309. doi: 10.1093/clinids/21.2.300. [DOI] [PubMed] [Google Scholar]
- 19.Patankar TF, Karnad DR, Shetty PG, Desai AP, Prasad SR. Adult cerebral malaria: prog nostic importance of imaging findings and correlation with postmortem findings. Radiology. 2002;224:811–816. doi: 10.1148/radiol.2243010588. [DOI] [PubMed] [Google Scholar]
- 20.Kampfl AW, Birbamer GG, Pfausler BE, Haring HP, Schmutzhard E. Isolated pontine lesion in algid cerebral malaria: clinical features, management, and magnetic resonance imag ing findings. Am J Trop Med Hyg. 1993;48:818–822. doi: 10.4269/ajtmh.1993.48.818. [DOI] [PubMed] [Google Scholar]
- 21.Saavedra-Lozano J, Booth TN, Weprin BE, Ramilo O. Isolated cerebellar edema and obstructive hydrocephalus in a child with cerebral malaria. Pediatr Infect Dis J. 2001;20:908–911. doi: 10.1097/00006454-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Penet MF, Viola A, Confort-Gouny S, Le Fur Y, Duhamel G, Kober F, Ibarrola D, Izquierdo M, Coltel N, Gharib B, Grau GE, Cozzone PJ. Imaging experimental cerebral malaria in vivo: significant role of ischemic brain edema. J Neurosci. 2005;25:7352–7358. doi: 10.1523/JNEUROSCI.1002-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klatzo I. Evolution of brain edema concepts. Acta NeurochirSuppl (Wien) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- 24.Paul R, Angele B, Popp B, Klein M, Riedel E, Pfister HW, Koedel U. Differential regulation of blood-brain barrier permeability in brain trauma and pneumococcal meningitis-role of Src kinases. Exp Neurol. 2007;203:158–167. doi: 10.1016/j.expneurol.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Papadopoulos G, Sgouropoulou S, Arnaoutoglou E, Petrou A. Postoperative hypoxaemia in a patient with patent foramen ovale. Eur J Anaesthesiol. 2002;19:152–154. doi: 10.1017/s0265021502250267. [DOI] [PubMed] [Google Scholar]
- 26.Manley GT, Hemphill JC, Morabito D, Derugin N, Erickson V, Pitts LH, Knudson MM. Cerebral oxygenation during hemorrhagic shock: perils of hyperventilation and the therapeutic potential of hypoventilation. J Trauma. 2000;48:1025–1032. doi: 10.1097/00005373-200006000-00005. discussion 1032-1023. [DOI] [PubMed] [Google Scholar]
- 27.Vajda Z, Pedersen M, Fuchtbauer EM, Wertz K, Stodkilde-Jorgensen H, Sulyok E, Doczi T, Neely JD, Agre P, Frokiaer J, Nielsen S. Delayed onset of brain edema and mislocalization of aquaporin-4 in dystrophin-null transgenic mice. Proc Natl Acad Sci U S A. 2002;99:13131–13136. doi: 10.1073/pnas.192457099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saadoun S, Papadopoulos M, Bell B, Krishna S, Davies D. The aquaporin-4 water channel and brain tumour oedema. J Anat. 2002;200:528. [Google Scholar]
- 29.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos MC, Saadoun S, Binder DK, Manley GT, Krishna S, Verkman AS. Molecular mechanisms of brain tumor edema. Neuroscience. 2004;129:1011–1020. doi: 10.1016/j.neuroscience.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Zador Z, Bloch O, Yao X, Manley GT. Aquaporins: role in cerebral edema and brain water balance. Prog Brain Res. 2007;161:185–194. doi: 10.1016/S0079-6123(06)61012-1. [DOI] [PubMed] [Google Scholar]
- 32.Bloch O, Manley GT. The role of aquaporin-4 in cerebral water transport and edema. Neurosurg Focus. 2007;22 doi: 10.3171/foc.2007.22.5.4. E3. [DOI] [PubMed] [Google Scholar]
- 33.Gunnarson E, Zelenina M, Aperia A. Regulation of brain aquaporins. Neuroscience. 2004;129:947–955. doi: 10.1016/j.neuroscience.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Verkman AS. Aquaporin water channels and endothelial cell function. J Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verkman AS. Physiological importance of aquaporin water channels. Ann Med. 2002;34:192–200. [PubMed] [Google Scholar]
- 36.Castejon OJ, Castejon HV, Zavala M, Sanchez ME, Diaz M. A light and electron microscopic study of oedematous human cerebral cortex in two patients with post-traumatic seizures. Brain Inj. 2002;16:331–346. doi: 10.1080/02699050110088209. [DOI] [PubMed] [Google Scholar]
- 37.Penet MF, Kober F, Confort-Gouny S, Le Fur Y, Dalmasso C, Coltel N, Liprandi A, Gulian JM, Grau GE, Cozzone PJ, Viola A. Magnetic resonance spectroscopy reveals an impaired brain metabolic profile in mice resistant to cerebral malaria infected with Plasmodium berghei ANKA. J Biol Chem. 2007;282:14505–14514. doi: 10.1074/jbc.M608035200. [DOI] [PubMed] [Google Scholar]
- 38.Grau GE, Piguet PF, Engers HD, Louis JA, Vassalli P, Lambert PH. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J. Immunol. 1986;137:2348–2354. [PubMed] [Google Scholar]
- 39.Badaut J, Verbavatz JM, Freund-Mercier MJ, Lasbennes F. Presence of aquaporin-4 and muscarinic receptors in astrocytes and ependymal cells in rat braa clue to a common function? Neurosci Lett. 2000;292:75–78. doi: 10.1016/s0304-3940(00)01364-1. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Pinzon MA, Tao L, Nicholson C. Extracellular potassium, volume fraction, and tortuosity in rat hippocampal CA1, CA3, and cortical slices during ischemia. J Neurophysiol. 1995;74:565–573. doi: 10.1152/jn.1995.74.2.565. [DOI] [PubMed] [Google Scholar]
- 41.Traynelis SF, Dingledine R. Role of extracellular space in hyperosmotic suppression of potassium-induced electrographic seizures. J Neurophysiol. 1989;61:927–938. doi: 10.1152/jn.1989.61.5.927. [DOI] [PubMed] [Google Scholar]
- 42.Binder DK, Papadopoulos MC, Haggie PM, Verkman AS. In vivo measurement of brain extracellular space diffusion by cortical surface photobleaching. J Neurosci. 2004;24:8049–8056. doi: 10.1523/JNEUROSCI.2294-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005;95:254–262. doi: 10.1111/j.1471-4159.2005.03362.x. [DOI] [PubMed] [Google Scholar]
- 44.Aoki-Yoshino K, Uchihara T, Duyckaerts C, Nakamura A, Hauw JJ, Wakayama Y. Enhanced expression of aquaporin 4 in human brain with inflammatory diseases. Acta Neuropathol. 2005;110:281–288. doi: 10.1007/s00401-005-1052-2. [DOI] [PubMed] [Google Scholar]
- 45.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 46.Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neu-ropathol. 2004;107:311–318. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 47.Warth A, Simon P, Capper D, Goeppert B, Ta-batabai G, Herzog H, Dietz K, Stubenvoll F, Ajaaj R, Becker R, Weller M, Meyermann R, Wolburg H, Mittelbronn M. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res. 2007;85:1336–1346. doi: 10.1002/jnr.21224. [DOI] [PubMed] [Google Scholar]
- 48.Gu YT, Zhang H, Xue YX. Dexamethasone treatment modulates aquaporin-4 expression after intracerebral hemorrhage in rats. Neurosci Lett. 2007;413:126–131. doi: 10.1016/j.neulet.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 49.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 50.Ball HJ, McParland B, Driussi C, Hunt NH. Isolating vessels from the mouse brain for gene expression analysis using laser capture microdissection. Brain Res Brain Res Protoc. 2002;9:206–213. doi: 10.1016/s1385-299x(02)00147-2. [DOI] [PubMed] [Google Scholar]
- 51.Chan-Ling T, Neill AL, Hunt NH. Early mi-crovascular changes in murine cerebral malaria detected in retinal wholemounts. Am J Pathol. 1992;140:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 52.Amante FH, Haque A, Stanley AC, Rivera Fde L, Randall LM, Wilson YA, Yeo G, Pieper C, Crabb BS, de Koning-Ward TF, Lundie RJ, Good MF, Pinzon-Charry A, Pearson MS, Duke MG, McManus DP, Loukas A, Hill GR, Eng-werda CR. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol. 2010;185:3632–3642. doi: 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 53.Hunt NH, Grau GE, Engwerda C, Barnum SR, van der Heyde H, Hansen DS, Schofield L, Golenser J. Murine cerebral malaria: the whole story. Trends Parasitol. 2010;26:272–274. doi: 10.1016/j.pt.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Grau GE, Pointaire P, Piguet PF, Vesin C, Rosen H, Stamenkovic I, Takei F, Vassalli P. Late administration of monoclonal antibody to leukocyte function-antigen 1 abrogates incipient murine cerebral malaria. EurJ Immunol. 1991;21:2265–2267. doi: 10.1002/eji.1830210939. [DOI] [PubMed] [Google Scholar]
- 55.Piguet PF, Da Laperrousaz C, Vesin C, Tacchini-Cottier F, Senaldi G, Grau GE. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase-and urokinase receptor-deficient mice. Infect Immun. 2000;68:3822–3829. doi: 10.1128/iai.68.7.3822-3829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]