Abstract
A diagnosis of lung cancer at its early stages is vital for improving the survival rate of patients. MicroRNAs (miRNAs), a family of 19- to 25-nucleotide non-coding small RNAs, are frequently dysregulated in lung cancer. The objective of this study was to investigate the potential of circulating miRNAs for early detection of lung cancer. We searched the published literature for the miRNA microarray data of primary lung cancer and selected 15 miRNAs that were most frequently up-regulated in lung cancer tissues. Total plasma RNA including miRNAs was isolated, polyade-nylated and reverse-transcribed into cDNAs. The levels of miRNAs were determined by real-time RT-PCR in 74 lung cancer patients and 68 age-matched cancer-free controls. We found that the levels of miR-155, miR-197, and miR-182 in the plasma of lung cancer including stage I patients were significantly elevated compared with controls (P<0.001). The combination of these 3 miRNAs yielded 81.33% sensitivity and 86.76% specificity in discriminating lung cancer patients from controls. The levels of miR-155 and miR-197 were higher in the plasma from lung cancer patients with metastasis than in those without metastasis (P<0.05) and were significantly decreased in responsive patients during chemotherapy (P<0.001). These results indicate that miR-155, miR-197, and miR-182 can be potential non-invasive biomarkers for early detection of lung cancer.
Keywords: Plasma microRNA, lung cancer, real-time RT-PCR, early diagnosis, metastasis, prognosis
Introduction
Lung cancer is the leading cause of cancer-related deaths in both men and women worldwide, as well as in the United States [1]. Since there is no validated population-based screening procedure available, most patients with lung cancer are diagnosed at advanced stages with an overall five-year survival rate of only 15% [1]. To improve the outcome of the lung cancer patients, multiple large scale clinical trials to validate screening procedures including chest X-rays, sputum cytology, chest CT, or a combination have been conducted, but none have shown to significantly improve overall mortality over the past 25 years [2]. Recently a large well-designed National Lung Screening Trial (NLST) showed that the low-dose helical CT screening in an older, high-risk population reduced lung cancer mortality by 20% [3, 4]. However, recent studies also indicated that among 20 to 60% of abnormalities by screening CT scans most of them are not lung cancer [4, 5]. The high rate of a false positive result may cause anxiety for the individuals or even lead to unnecessary biopsies or surgery. Therefore, development of a reliable, noninvasive, and cost-effective confirmatory test would reduce the overdiagnosis and facilitate the implementation of screening CT scan procedure in the near future [5].
MicroRNAs (miRNAs) are a class of small non-coding cellular RNAs that regulate gene expression at the posttranscriptional level [6]. Many genes involving in basic biological functions such as cellular proliferation, differentiation, and apoptosis are targets of miRNAs. Accordingly, numerous studies have identified aberrant miRNA expression profiles in many types of human diseases including cancer [7]. Profiles of miRNA expression differ between normal tissues and tumor tissues and vary among tumor types [8]. Therefore, miRNAs can potentially be used as biomarkers in the diagnosis and classification of human malignancies. Moreover, the high stability of miRNAs constitutes an enormous advantage from a clinical diagnostic point of view. It allows an efficient isolation from clinical specimens including sputum [9], plasma [10-12], serum [13], and even formalin-fixed paraffin-embedded tissue samples stored for 10 years [14]. Investigation of cancer-specific miRNAs in the circulation is an emerging and exciting field of study. In this study, we evaluated the plasma levels of miRNAs in patients with or without lung cancer and the stability of plasma miRNAs in the clinical laboratory setting to validate it as an acceptable biomarker for early diagnosis in patients with lung cancer.
Materials and methods
Patients and samples
Following an Institutional Research Board (IRB) approval, blood samples were collected from 74 patients with lung cancer, and 68 age-matched patients with no current or previous malignancy as controls. The lung cancer patients included 23 squamous cell carcinoma, 18 adenocarcinoma, 17 small cell carcinoma, 7 large cell carcinoma, and 9 others (carcinoid or mixed tumor). These patients were at various clinical stages including 21 stage I, 12 stage II, 11 stage III and 30 stage IV, as determined according to the International Association for the Study of Lung Cancer staging system (Table 1). Fresh whole blood samples in EDTA preservative were centrifuged at 1,600 g for 10 min at room temperature, and the plasma was collected and stored at-80°C until use.
Table 1.
Characteristics of lung cancer patients
| Lung cancer (n=74) | |
|---|---|
| Age | Ave 64.2 (SD 10.9, range from 43-87) |
| Sex | |
| Male | 40 |
| Female | 34 |
| Tumor types | |
| SCLC | 17 |
| NSCLC | 48 |
| AC | 18 |
| SC | 23 |
| LC | 7 |
| Other | 9 |
| Stages | |
| Stage I | 21 |
| Stage II | 121 |
| Stage III | 11 |
| Stage IV | 30 |
| Metastasis | |
| YES | 30 |
| NO | 44 |
Abbreviations: SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer; AC, adenocarcinoma; SC, squamous cell carcinoma. Ave, average. Controls, n=68 including male 37, female 31. Average age of 61.2 with SD 14.0, ranged from 36 to 93 years old.
Plasma RNA extraction
Total RNA containing small RNA was extracted from 350µl of plasma using miRVana PARIS Kit (Ambion, Austin, TX) according to the manufacturer's protocol. The final elution volume was 100µl in water. The concentration and purity of sample RNA were determined by NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE).
MicroRNA quantification by real-time RT-PCR
A SYBR Green-based quantitative RT-PCR assay was employed for miRNA quantification in extracted plasma samples [15]. In brief, 20 ul of total RNA sample containing miRNA was polyadenylated by poly(A) polymerase (PAP, Ambion) and reversely transcribed to cDNA using SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions with a poly(T) adapter primer (5′GCGAGCACAGAATTAATACGA CTCACTATAGGTTTTTTTTTTTTTTTVN-3′) [15]. Real -time PCR was performed using iQ SYBR Green Supermix (Bio-Rad, Hercuis, CA) with the miRNA specific forward primers (sequences as shown in Table 2) and the sequence complementary to the poly(T) adapter as the reverse primer (5′-GCGAGCACAGAATTAATACGAC-3′) in iQ5 Realtime PCR system (Bio-Rad). The PCR was carried out as follows: initial denaturation at 95°C for 3min, followed by 50 cycles of 95°C for 15 s and 60°C for 40 s and then a dissociation curve analysis was conducted to confirm the specificity. All assays were repeated three times in duplicates.
Table 2.
The sequence of synthetic primers and single-stranded miRNAs
| Primers and miRNAs | Primer Sequence |
|---|---|
| Let-7a primer | TGAGGTAGTAGGTTGTATAGTT |
| Let-7b primer | TGAGGTAGTAGGTTGTGTGGTT |
| miR-17 primer | CAAAGTGCTTACAGTGCAGGTAG |
| miR-21 primer | TAGCTTATCAGACTGATGTTGA |
| miR-24 primer | TGGCTCAGTTCAGCAGGAACAG |
| miR-106a primer | AAAAGTGCTTACAGTGCAGGTAG |
| miR-125b primer | TCCCTGAGACCCTAACTTGTGA |
| miR-128 primer | TCACAGTGAACCGGTCTCTTT |
| miR-155 primer | TTAATGCTAATCGTGATAGGGGT |
| miR-182 primer | TTGGCAATGGTAGAACTCACACT |
| miR-183 primer | TATGGCACTGGTAGAATTCACT |
| miR-197 primer | TTCACCACCTTCTCCACCCAGC |
| miR-199b primer | CCCAGTGTTTAGACTATCTGTTC |
| miR-203 primer | GTGAAATGTTTAGGACCACTAG |
| miR-205 primer | TCCTTCATTCCACCGGAGTCTG |
| miR-210 primer | CTGTGCGTGTGACAGCGGCTGA |
| miR-221 primer | AGCTACATTGTCTGCTGGGTTTC |
| miR-155 RNA | UUAAUGCUAAUCGUGAUAGGGGU |
| miR-182 RNA | UUUGGCAAUGGUAGAACUCACACU |
| miR-197 RNA | UUCACCACCUUCUCCACCCAGC |
Generation of standard curves for absolute quantification of miRNAs
Synthetic single-stranded RNA oligonucleotides corresponding to the mature miRNA sequences (miRBase Release v.17) were purchased from Integrated DNA Technologies (IDT, Coralville, IA) (Table 2). Synthetic miRNAs were used in the PAP and reverse transcription (RT) reactions over an empirically-derived range of copies to generate standard curves for each of the miRNA SYBR PCR assays. The absolute concentration of miRNA into the RT reaction was converted to femtomole of miRNA per liter plasma (fmol/L).
Characterization of the stability of plasma miRNAs and purified miRNAs
To assess the stability of miRNAs in plasma, four plasma specimens were randomly selected, two from patients with lung cancer and two from cancer-free control patients. Each freshly isolated plasma sample was divided into 5 parts. The 350 ul of aliquots from EB011 (lung cancer) and EB049 (control) were maintained at 4° C for 24 h and 48 h, respectively. The other two aliquots from the same individuals were subjected to 3 and 6 cycles of freeze-thawing and the last aliquots were stored at -20°C and served as control. Two aliquots of plasma from EB036 (lung cancer) and EB045 (control) were maintained at 37°C for 24 h and 48 h, respectively. The additional two aliquots were incubated with the addition of 0.01 mg/ml and 1mg/ml RNase A (Qiagen, Valencia, CA) for 24 h and the last aliquots were stored directly at -20°C and served as control.
To further evaluate the stability of miRNAs in isolated form, the purified total RNA from individual plasma were combined and then divided into 3 parts. One part was stored at -20°C as control, and the remaining two parts were incubated at 37 °C for 2 h with or without the addition of 0.01mg/ml RNase A. Two pooled RNA samples were tested, one from five patients with lung cancer and another from five control patients without cancer. After treatment under different conditions, all samples were subjected to RNA extraction, polyadenylation and RT reaction as described. The levels of endogenous miRNAs (miR-155, miR-197, miR-17, and miR-21) were measured by real-time RT-PCR. All experiments were performed in duplicate and repeated three times.
Statistical analysis
The 2-sample t-test was used for all 2 sample comparisons and ANOVA, followed by the Tukey HSD post hoc test, was used to compare the mean response between the levels of the subject factors of interest. Due to the magnitude and range of miRNA levels observed, results were log transformed for analysis when needed. The sensitivity and specificity were calculated according to standard formulas. A multivariate logistic regression model was established and leave-one-out cross-validation was used to find the best logistic model. The receiver operating characteristic curves (ROC curve) were established for discriminating patients with from without lung cancer. The area under the ROC curve (AUC) was calculated in order to better identify microRNAs and a combination of microRNA that classify samples accurately. A random classifier has AUC=0.5, whereas an optimal classifier with perfect sensitivity and 100% specificity has AUC= 1. All p values are two-sided and less than 0.05 was considered statistically significant. All statistical calculations were performed by Graphpad Prism 5 software (GraphPad Software) except the multivariate logistic regression which was performed by the SPSS 17.0 for Windows (SPSS Inc.).
Results
Sensitivity, specificity, and linear range of miRNA quantification by real-time PCR
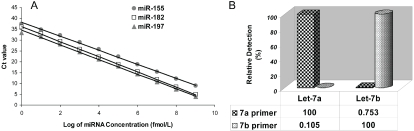
To define the dynamic range and sensitivity of miRNA quantification by real-time PCR, the synthesized miR-155, miR-197, and miR-182 underwent poly (A) addition reaction and a reverse transcription reaction. The cDNA was diluted by ten orders of magnitude and subjected to realtime PCR. As shown in Figure 1A, the real-time RT-PCR assay demonstrated excellent linearity between the log of miRNA concentration and cycle threshold (Ct) value, indicating that the assay had a dynamic range of 9-10 logs and was thus capable of detecting as few as 5 copies of miRNA per PCR reaction, and the correlation coefficient was 0.998 for miR-155, 0.998 for miR-197, and 0.999 for miR-182.
Figure 1.
Sensitivity and specificity of miRNA quantification by a SYBR Green-based real-time RT-PCR (qRT-PCR) method. (A) Standard curves of miR-155, miR-197, and miR-182. The assay showed high sensitivity and a broad dynamic range. (B) Discrimination power (specificity) of the assay on let-7 miRNA family members. Relative detection (%) was calculated based on Ct value difference between perfectly matched and mismatched targets let-7a and let-7b.
To determine the specificity of real-time PCR assay for miRNAs, the synthesized two Let-7 miRNA family members, Let-7a and Let-7b, were detected with primers specific for Let-7a and Let-7b, respectively. Relative detection efficiency was calculated from Ct value differences between perfectly matched and mismatched targets, assuming 100% efficiency for the perfect match. Very low levels of non-specific amplification (less than 1%) were observed (Figure 1B), similar to the method of stem-loop RT-PCR [16]. These results suggested that this SYBR Green-based quantitative RT-PCR assay is highly specific and can discriminate miRNAs that differ by as few as two nucleotides.
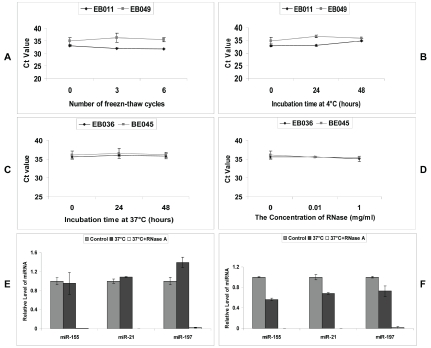
Stability of plasma miRNAs and purified plasma miRNAs
It has been reported that miRNAs were highly stable in sputum, plasma, and serum [9-13] in spite of the fact that these specimens contain RNase. To validate this concept and to understand its mechanisms, we first sought to investigate the stability of endogenous miRNAs in plasma samples. Aliquots of clinical plasma specimens were subjected to 3 or 6 cycles of freeze-thawing or incubated at 4°C or 37°C for 24h and 48h. There was minimal or no effect on the levels of miR-155 (Figure 2A-C), miR-197 and miR-21 (data not shown) at these conditions.
Figure 2.
The stability of miRNAs in plasma. Aliquots of two clinical patient plasma samples were subjected to up to six cycles of freeze-thaw (A) or incubated at 4°C (B) or 37°C (C) for up to 48 h, or digested with addition of different concentrations of RNase A (D). Then miRNAs were extracted and miR-155 was quantified with qRT-PCR. Data was shown in Ct value. E-F, Pools of extracted total RNA from samples of lung cancer patients (E) and normal controls (F) were subjected to incubation in 37 °C for 2 h with or without RNase A digestion. The levels of miR-155, miR-21 and miR-197 were determined and the data was shown as relative level of miRNAs normalized with the original level.
The stability of plasma miR-155 as well as miR-197 and miR-21 (data not shown) was further studied by RNase A digestion. As shown in Figure 2D, plasma miRNAs showed considerable resistance to the enzymatic cleavage of RNase A. However, when purified total RNA was incubated with RNase A, the endogenous miRNAs including miR-155, miR-21, and miR-197 were completely digested, no matter the samples came from the lung cancer patients (Figure 2E) or controls (Figure 2F).
Identification of lung cancer-associated miRNAs in plasma samples
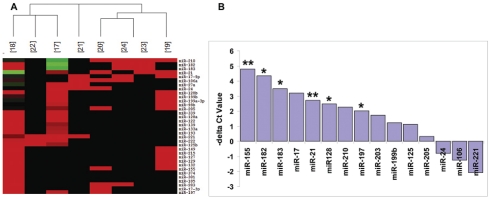
Since plasma miRNAs are remarkably stable and readily detectible by various quantitative methods including real-time RT-PCR, it is reasonable to hypothesize that the levels of tumor-associated miRNAs in the circulation can be used as potential biomarkers for detection of lung cancer. First, we searched for the miRNA microarray data of primary lung cancer published thus far. There are at least eight studies of expression profiles of miRNAs in both NSCLC and SCLC tissues [17-24]. We constructed a heat map of the expression profiles of miRNAs and selected 15 miRNAs (miR-17, 21, 24, 106a, 125b, 128, 155, 182, 183, 197, 199b, 203, 205, 210 and 221) that were reported to be most frequently up-regulated in primary lung cancer tissues (Figure 3A).
Figure 3.
MicroRNA selection and screening by qRT-PCR analysis. (A) Heat map clustering of miRNA microarray data from 8 published references (corresponding reference number in brackets), showing certain degree of consistency of the most up-regulated miRNAs (in red) and fewer down-regulated miRNAs (in green) in primary tumor tissues. (B) A total of 15 miRNAs, which were most frequently up-regulated in lung cancer tissues selected from published data (A), were used for screening in 6 plasma samples of lung cancer patients and 6 of normal individual controls by qRT-PCR. Six out of 15 miRNAs (miR-21, 128, 155, 182, 183, 197) were found significantly elevated in plasma from patients with lung cancer compared with controls. Difference of Ct values between lung cancer and control was shown. Note: **, P<0.01; *, P<0.05.
To explore the potential of these 15 miRNAs as circulating biomarkers for lung cancer, we determined the levels of these miRNAs in 6 plasma samples from patients with lung cancer and 6 plasma samples from healthy cancer-free individuals. Six out of 15 miRNAs (miR-155, 197, 182, 21, 128, and 183) were demonstrated to be significantly elevated in plasma from patients with lung cancer compared with that of controls (Figure 3B, P<0.05).
Clinical validation of miRNA biomarkers in patient plasma samples
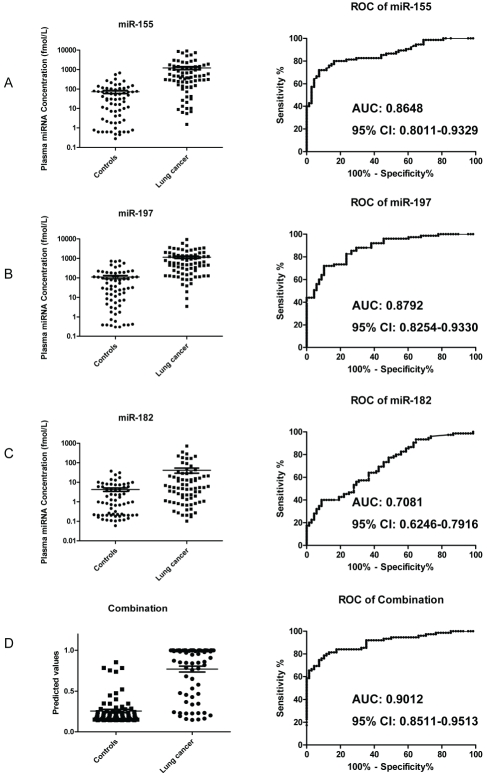
To further evaluate the diagnostic value of these selected miRNAs, the levels of miR-155, 197, 182, 21, 128, and 183 were measured on a total 142 plasma samples, including 74 samples of lung cancer patients at various stages and 68 samples of cancer-free age-matched controls (Table 1). Because there was no consensus on the use of housekeeping miRNA for plasma qRT-PCR quantification [10-13, 20, 25], we decided to measure miRNA expression levels converted to absolute concentration in fmol/L by using a dilution series of known input quantities of synthetic miRNA run simultaneously (on the same plate) as the experimental samples. The levels of the three miRNAs (miR-155, miR-197, and miR-182) were significantly higher, in average, in the lung cancer cohort than in controls (P<0.001), corresponding to an average fold-change of 17.08, 10.46 and 9.74, respectively (Figure 4A-C, left panels). ROC curve analysis showed that all of these three miRNAs could differentiate lung cancer from controls with an AUC of 0.8648 for miR-155 (95% CI: 0.8011-0.9329, P<0.001), 0.8792 for miR-197 (95% CI: 0.8254-0.9330, P<0.001), and 0.7081 for miR-182 (95% CI: 0.6246-0.7916, P<0.001), respectively (Figure 4A-C, right panels). The predicted values of logistic regression showed significant difference between these two groups (Figure 4D, left panel, P<0.001) and combination ROC analysis revealed increased AUC value to 0.9012 (Figure 4D, right panel, 95% CI: 0.8511-0.9513, P<0.001) with 81.33% sensitivity and 86.76 % specificity. However, the other 3 miRNAs, miR-21, miR-128 and miR-183 showed no significant difference although they elevated in lung cancer samples (data not shown).
Figure 4.
Validation of miR-155, miR-197 and miR-182 in plasma samples of 74 lung cancer patients and 68 cancer-free patient controls. A-C, Scatter plots of plasma levels of miR-155 (A), miR-197 (B) and miR-182 (C) on left panels and receiver operating characteristics (ROC) curve analysis of plasma miR-155 (A), miR-197 (B) and miR-182 (C) on right panels for discriminating lung cancer from control. Ct values were converted to absolute number of miRNA molecules in fmol/L by using a dilution series with known input quantities of synthetic miRNA standard run simultaneously (on the same plate). Concentration of miRNAs was shown as log 10 scale on the Y-axis. (D) Scatter plots of predicted value after logistic regression (left panels) and combination ROC curve (right panels) for discriminating lung cancer from controls. The lines represent the mean value and the bars show the SE. The area under the curve (AUC) and 95% Confidence Interval (CI) are shown in the corresponding charts.
Relationship between plasma levels of miR-155, miR-197, miR-182 and patient clinical status
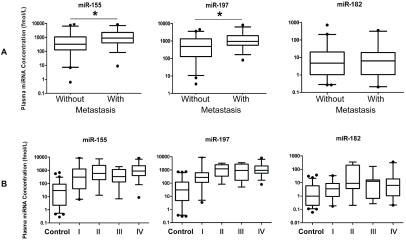
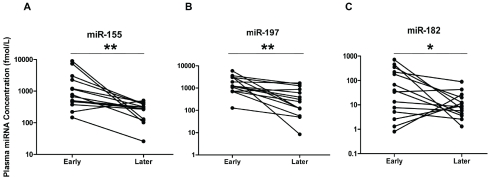
Next, we examined the correlation between the plasma levels of these 3 miRNAs with patient clinical parameters. No significant association was found between the levels of these three miRNAs and sex, age, smoke history, nodal status or histological types of tumor, respectively (P>0.05, data not shown), while the plasma levels of miR-155 and miR-197, but not miR-182, in the patients with metastasis were significantly higher than in those without metastasis (P<0.05, Figure 5A). Patients were further stratified based on the TNM staging of lung cancer. As shown in Figure 5B, the levels of all 3 miRNAs were not significantly different between the stages, however, each of the 4 stages including stage I patients had significantly elevated plasma miR-155, miR-197, and miR-182 levels when compared with the cancer-free controls (Figure 5B, P<0.01). To explore the value of these 3 miRNAs in monitoring of treatment effectiveness by chemotherapy, their plasma levels were measured in an independent set of 14 patients with lung cancer in early and late phases of chemotherapy. It was found that the levels of miRNA-155, miR-197, and miR-182 were significantly reduced after treatment (P<0.01 for miR-155 and miR-197, P<0.05 for miR-182) (Figure 6) in the majority of responsive patients.
Figure 5.
Correlation of plasma levels of miRNAs and patient clinical status. (A) Box plots of plasma levels of miR-155, miR-197 and miR-182 in lung cancer patients without metastasis (n=44) or with metastasis (n=30). Note: *, P<0.05. (B) Box plots showing plasma levels of miR-155 (left panel), miR-197 (middle panel) and miR-182 (right panel) in control subjects (n=68) and lung cancer patients with different TNM stages (I, n=21; II, n=12; III, n=11; IV, n=30). The lines inside the boxes denote the medians. The boxes mark the interval between 25th and 75th percentiles. The whiskers denote the interval between the 5th and 95th percentiles. Filled circles indicate data points outside the 5th and 95th percentiles.
Figure 6.
The changes of plasma levels of miR-155 (A), miR-197 (B) and miR-182 (C) in patients with lung cancer (n=14) at early and late phases of chemotherapy. Concentration of miRNAs was shown as log 10 scale on the Y-axis. Note: **, P<0.01; *, P<0.05.
Discussion
Human cancers commonly exhibit an altered expression profile of miRNAs with oncogenic or tumor-suppressive activity [6-8]. Recent studies have revealed that cancer-associated miRNAs play important roles in tumorigenesis and may serve as diagnostic and prognostic biomarkers in cancer including lung cancer [5, 25-28]. In the present study, we focused on the plasma levels of miR-155, miR-197, and miR-182 in lung cancer patients. Our results demonstrate that these cancer-associated miRNAs can potentially serve as novel noninvasive biomarkers for the early detection and diagnosis of lung cancer. It was found that the levels of miR-155, miR-197, and miR-182 were significantly elevated in the plasma of lung cancer patients in comparison to cancer-free control subjects (17.08-fold, 10.46-fold and 9.73-fold changes, respectively), and these changes could discriminate lung cancer from cancer-free controls with high specificity and sensitivity (Figure 4A, B, C). Combination of these three miRNAs further increased the discrimination power (81.33% sensitivity and 86.76% specificity, Figure 4D). Importantly, all three circulating miRNAs were significantly elevated in patients with stage I lung cancer as well as other stages compared with cancer-free controls (Figure 5B) although there is no difference among the stages, suggesting their potential value for early detection of lung cancer and reduction of false positive rate by screening CT scans [3-5]. In addition, the plasma levels of miR-155 and miR-197 (but not mR-182) were significantly elevated in metastatic cancer patient and all 3 were decreased after chemotherapy in the majority of clinically responsive cancer patients (Figure 5A and 6), showing they may be prognostic factors and for monitoring response to therapy. Most recently, Shen etal [29] reported that the plasma level of 4 miRNAs (miR-21, -126, -210, and 486-5p) can discriminate non-small cell lung cancer (NSCLC) patients from the healthy controls with 86.22% sensitivity and 96.55% specificity, which supports the hypothesis that plasma miRNAs can serve as biomarkers for early detection of lung cancer, although the selection of miRNAs needs further study.
Results from recent studies have revealed the remarkable stability of miRNAs in various clinical samples [9-13, 29]. It was found that endogenous plasma miRNAs exist in a form that is resistant to 4°C or 37°C incubation, freeze-thaw cycles, and even to RNase activity [10, 29]. The results of our study confirmed these important features in the clinical diagnostic setting (Figure 2). The mechanisms by which miRNAs are protected from endogenous RNase activity are still unknown. One tantalizing hypothesis is that they are packaged inside exosomes that are secreted from somatic cells, including cancer cells [30]. Other explanations include protection via association with other molecules or modifications of the miRNAs, or secondary structure of miRNA that make them resistant to RNase activity [27]. Our results showed that plasma miRNAs were resistant to RNase, but the same miRNAs purified with reagents containing detergent that disrupts biomembrane were sensitive to RNase (Figure E, F), which indicated that plasma miRNAs may be protected by the membrane of exosomes.
The first identified over-expressed miRNA in plasma of lung cancer patients was miR-155. As a typical multifunctional miRNA, miR-155 has distinct expression profiles and plays a crucial role in various physiological and pathological processes such as hematopoietic lineage differentiation, immunity, inflammation, cardiovascular diseases, and cancer [31]. In cancer, miR-155 was shown as an oncogenic microRNA in many studies [32, 33]. The results of microRNA profiling studies indicated frequent increase of miR-155 in various types of human malignancy, including lung cancer [23], pancreatic adenocarcinoma [33], and other solid tumors [6, 7, 27]. The studies have also demonstrated an association of elevated miR-155 with late stage and poor overall survival in squamous cell carcinoma of lung [23] and pancreatic adenocarcinoma [33]. MiR-155 can be detected in the exosomes purified from the plasma of lung cancer patients and can not be detected in control samples [30], suggesting it can serve as a blood-based biomarker for cancer detection. Our findings support these observations. Recently, Heegaard etal reported that serum miR-155 in lung cancer patients was decreased compared with non-cancer control group, but increased when European American patients were compared with African American patients [34]. The reason for this discrepancy was not clear, but may be related to aged samples [34].
Although the fold-change of miR-197 was less than that of miR-155 in plasma of lung cancer patients (10.46 vs. 17.08 folds, Figure 4A and B, left panels), it was the most useful biomarker in discriminating lung cancer from cancer-free control subjects (yielding an AUC of 0.894 with 73.3% sensitivity and 83.8% specificity, Figure 4B, right panel). Similar to miR-155, the levels of miR-197 were significantly higher in the plasma from patients with metastasis than those without metastasis (Figure 5A). The miRNA profiling studies showed that miR-197 was over-expressed in the primary lung cancer tissue [17, 19] and could be detected by Solexa sequencing only in the serum of lung cancer patients [13]. This is the first report of the quantitative assessment of plasma miR-197 in lung cancer patients and our results demonstrate that it can be a useful non-invasive biomarker for early lung cancer detection.
MiR-182 has been described to be over-expressed in lung cancer tissue [17, 18]. Ectopic expression of miR-182 in an epithelial ovarian cancer cell line significantly promoted tumor growth in vivo, suggesting a role of miR-182 as a putative oncogene [35]. In this study, we demonstrated that miR-182 was significantly elevated in plasma of patients with lung cancer (Figure 4C, 5B).
In conclusion, differentially altered circulating cancer-related miRNAs in plasma samples of lung cancer patients have been studied in this report. MiR-155, miR-197, and miR-182 have high specificity and sensitivity to discriminate all stages including stage I of lung cancer from cancer-free controls. These markers, once validated in a large-scale clinical trial, may be used as a non-invasive confirmatory screening test complementary to the low-dose helical CT screening procedure in the near future and also used as a clinical test for monitoring and clinical follow-up for patients with lung cancer.
Acknowledgments
We thank Sandra Mramor and Linda Hennessey for their assistance in sample collection, and D. Marie Schultz for her editorial assistance. This study was mainly supported by the University of Missouri Intellectual Property Fast Track Initiative Grant (A8881, M.X.W and C.E.F) and was partially supported by NSF 0546165 and NIH GM079613 (L.Q.G).
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics 2011, The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team, editor. The National Lung Screening Trial overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The National Lung Screening Trial Research Team, editor. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011 doi: 10.1056/NEJMoa1102873. Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barba M, Felsani A, Rinaldi M, Giunta S, Malorni W, Paggi MG. Reducing the risk of over-diagnosis in lung cancer: A support from molecular biology. J Cell Physiol. 2011;226:2213–2214. doi: 10.1002/jcp.22558. [DOI] [PubMed] [Google Scholar]
- 6.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 7.Davalos V, Esteller M. MicroRNAs and cancer epigenetics: a macroevolution. Curr Opin Oncol. 2010;22:35–45. doi: 10.1097/CCO.0b013e328333dcbb. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 9.Xie Y, Todd NW, Liu Z, Zhan M, Fang H. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. BrJ Cancer. 2010;102:1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118–126. doi: 10.1002/ijc.25007. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 14.Xi Y, Nakajima G, Gavin E, Morris CG, et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotech-niques. 2005;39:519–525. doi: 10.2144/000112010. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Ridzon DA, Broomer AJ, Zhou Z, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gni178. e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu SL, Chen HY, Chang GC, Chen CY, et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Volinia S, Calin GA, Liu CG, Ambs S, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaihara N, Caplen N, Bowman E, Seike M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garofalo M, Quintavalle C, Di Leva G, Zanca C, et al. MicroRNA signatures of TRAIL resistance in human non-small cell lung cancer. Oncogene. 2008;27:3845, 3855. doi: 10.1038/onc.2008.6. [DOI] [PubMed] [Google Scholar]
- 22.Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer. 2009;45:2197–2206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 23.Raponi M, Dossey L, Jatkoe T, Wu X, et al. MicroRNA classifiers for predicting prognosis of squamous cell lung cancer. Cancer Res. 2009;69:5776–5783. doi: 10.1158/0008-5472.CAN-09-0587. [DOI] [PubMed] [Google Scholar]
- 24.Miko E, Czimmerer Z, Csánky E, Boros G, et al. Differentially expressed microRNAs in small cell lung cancer. Exp Lung Res. 2009;35:646–664. doi: 10.3109/01902140902822312. [DOI] [PubMed] [Google Scholar]
- 25.Hu Z, Chen X, Zhao Y, Tian T, et al. Serum microRNA signatures identified in a genome-wide serum microRNA expression profiling predict survival of non-small-cell lung cancer. J Clin Oncol. 2010;28:1721–1726. doi: 10.1200/JCO.2009.24.9342. [DOI] [PubMed] [Google Scholar]
- 26.Ferracin M, Veronese A, Negrini M. Micromarkers miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10:297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- 27.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 28.Wang QZ, Xu W, Habib N, Xu R. Potential uses of microRNA in lung cancer diagnosis, prognosis, and therapy. Curr Cancer Drug Targets. 2009;9:572–594. doi: 10.2174/156800909788486731. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Todd NW, Zhang H, Yu L, Lingxiao X, Mei Y, et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab Invest. 2011;91:579–587. doi: 10.1038/labinvest.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 31.Tili E, Croce CM, Michaille JJ. miR-155 on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28:264–284. doi: 10.1080/08830180903093796. [DOI] [PubMed] [Google Scholar]
- 32.Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Greither T, Grochola LF, Udelnow A, Lautenschläger C, Würl P, Taubert H. Elevated expression of microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated with poorer survival. Int J Cancer. 2010;126:73–80. doi: 10.1002/ijc.24687. [DOI] [PubMed] [Google Scholar]
- 34.Heegaard NH, Schetter AJ, Welsh JA, Yoneda M, Bowman ED, Harris CC. Circulating microRNA expression profiles in early stage non-small cell lung cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26153. May 4. doi: 10.1002/ijc.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Volinia S, Bonome T, Calin GA, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA. 2008;105:7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]