Abstract
Impetigo is a highly contagious bacterial skin infection affecting children worldwide that is caused by the Gram-positive bacteria Staphylococcus aureus, Streptococcus pyogenes, or both. Staphylococcus species can quickly develop drug resistance rendering mupirocin, fusidic acid, and erythromycin ineffective. Preclinical and clinical studies demonstrated that NVC-422 (N, N-dichloro-2, 2-dimethyltaurine) rapidly kills pathogens without the development of drug resistance. 129 patients with clinically diagnosed impetigo were randomized to three dose groups (0.1, 0.5, or 1.5% NVC-422 topical gel) in a study conducted at 2 centers; 125 patients (97%) had microbiologically confirmed infection. Treatment was administered three times a day (TID) for 7 days to all randomized subjects. Response was measured at the completion of treatment (Day 8) and 1 week post treatment (Day 15) by the Skin Infection Rating Scale (SIRS) and by microbiological response. A total of 120 subjects (96%) completed all 7 days of treatment and were assessed at end of treatment (EOT). Clinical response rate at EOT in the PPC population was excellent in each of the dose groups (84.6%, 87.2%, and 92.3% in the 0.1%, 0.5% and 1.5% dose groups respectively). The majority of the infections were caused by S. aureus, alone (106/125, 85%) of which approximately 10% were MRSA. There were no clinical recurrences in any treatment groups. Treatment-emergent adverse events were seen in 5.4% of the subjects (7/129) and were mild to moderate and resolved. NVC-422 topical gel administered TID was well tolerated, with high rates of clinical and microbiological responses for treating impetigo.
Keywords: Impetigo, NVC-422, Clinical Phase 2, Staphylococcus, Streptococcus, MRSA
Introduction
Impetigo is a highly contagious bacterial skin infection (pyoderma) affecting children worldwide caused by the Gram-positive bacteria Staphylococcus aureus, Streptococcus pyogenes (a group A beta-hemolytic Streptococcus; GABHS), or both [1, 2]. In the United States and Europe, S. aureus is the usual causative pathogen while in tropical countries the Streptococcus species predominates and the disease is often endemic [3]. In the past 15 years however there has been an etiologic change from Streptococcus to Staphylococcus and with it an increased frequency of methicillin resistant S. aureus (MRSA) impetigo [4-6]. A 2005 report showed that S. aureus was the most prevalent pathogen of both types of impetigo in Europe and the United States; and MRSA impetigo has been reported in hospitals in England [7, 8]. Community-associated methicillin-resistant S. aureus (CA-MRSA) has been reported as a notable public health problem in the United States [9]. In 2007 CA-MRSA accounted for 7-20% of impetigo infections in Houston, Texas [10]. Most impetigo cases occur in children but children and adults can asymptomatically carry Staphylococcus in the anterior nares which can serve as a reservoir for the infection to other people [11].
Clinically, impetigo caused by Staphylococcus, Streptococcus or MRSA appear similar. However, impetigo caused by MRSA can predispose a patient to a deeper and more serious infection. Staphylococcus species quickly develop resistance to drugs and therefore can render agents such as mupirocin, fusidic acid, and erythromycin ineffective [12-17]. Furthermore, a recent in vitro passage study from the United Kingdom investigating XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vanco-mycin against MRSA, suggests that drug resistance to retapamulin could also develop [18, 19]. Resistance reports for mupirocin have come from New Zealand where mupirocin became available over the counter in 1991. By 1999, a mean value of 28% of S. aureus isolates was resistant to mupirocin [20]. In Western Australia in 1993, mupirocin was used frequently to treat infected skin lesions. This resulted in high-level mupirocin resistance among clinical MRSA isolates which reached 15% [21]. Fusidic acid resistance was reported in the United Kingdom where levels of between 11.5% and 18.5% have been seen with especially high rates in children [22]. In Greece, among the CA-MRSA isolates, where fusidic acid is commonly used for the empiric therapy of suspected staphylococcal infections, 88.9% were resistant [23]. Since impetigo is a contagious skin infection, infected children may be required to stay at home from school, often in the care of a parent or guardian, until the lesions are resolved.
Microbial resistance is emerging faster than we are replacing our armamentarium of antimicrobial agents. To address this evolving issue of antimicrobial drug resistance, NovaBay Pharmaceuticals, Inc. is developing NVC-422 (N, N-dichloro-2, 2-dimethyltaurine) as a first of a class of synthetic stable chlorotaurines exhibiting a fast-acting, broad-spectrum activity against Gram-positive and Gram-negative pathogens [24]. These agents have been named Aganocide® compounds by NovaBay. Preclinical and clinical studies have demonstrated that NVC-422 has a good safety profile, solution and gel formulation stability, and more importantly, rapidly kills pathogens without the development of drug resistance (manuscripts in preparation). Impetigo is a topical disease readily accessible to the clinician and the infection is localized to the epidermis providing the perfect opportunity for developing NVC-422 as a topical gel. The mechanism by which NVC-422 kills pathogens is inactivating surface proteins through the preferential oxidation of sulfur-containing groups found in the protein's amino acid residues (manuscripts in preparation). This new mechanism of action differentiates Aganocide compounds from traditional antibiotics. By simultaneously attacking multiple targets on the surface of pathogens, NVC-422 kills rapidly through a variety of pathways, thereby making it extremely difficult for pathogens to develop drug resistance.
We herein report the activity and tolerability of NVC-422 in a stable topical gel for the treatment of impetigo in the first proof of concept human clinical evaluation.
Materials and methods
Minimum bactericidal concentration and the testing of bacterial clinical isolates
One hundred and twenty one (121) recent clinical isolates of S. aureus and S. pyogenes were obtained from Eurofins Medinet (Herndon, VA). Activity testing of NVC-422 against antibiotic-sensitive and resistant bacteria strains was conducted using a modified CLSI method as described previously [24]. Briefly, a CLSI protocol for Minimum Bactericidal Concentration (MBC) testing was modified by substituting 0.9% acetate saline buffer, pH5 (ASB) for Cation-Adjusted Mueller Hinton Broth (CAMHB). This change was done specifically to minimize the reaction of NVC-422 with components of the CAMHB culture media. Due to the rapid cidal effect of NVC-422, the MBC assay was shortened from 16–20 hours at 35 °C to 60 min at room temperature. The MBC was defined as the lowest concentration achieving >99.9% microbial kill. Microorganisms were first grown to mid-log phase, centrifuged and suspended in ASB. Next, the organisms were added to serial 2-fold dilutions of the test compound in ASB to a final inoculum of 105–106 CFU/mL and incubated for 60 min at room temperature. Aliquots of the cell suspension were plated on agar with growth media; incubated 24 h at 35 °C and CFUs were quantified.
Topical gel preparation
The active pharmaceutical ingredient (NVC-422) and drug product preparations were manufactured under cGMP conditions. NVC-422 topical gel consists of fully solubilized NVC-422 in water formulated with hydrated Noveon® AA-1 (purchased from Lubrizol Corporation) and sodium chloride. The pH of the drug product was adjusted to 5. Three concentrations of NVC-422 topical gel, 0.1%, 0.5% and 1.5% were manufactured at Dow Pharmaceutical Sciences Inc. (DPSI) and stored in amber glass vials at 2–8°C for stability and clinical studies. The stability of NVC-422 topical gel was monitored using the standard ICH conditions of 5°C, 25°C/60% relative humidity (RH) and 40°C/75% RH. The stability of all concentrations was monitored beyond the duration of the clinical trials with satisfactory data.
Clinical microbiology methods
The microbiology testing of the clinical samples was performed by using culture swabs obtained from the target lesion for each study patient. The patient samples were forwarded to a single microbiology laboratory at the Instituto Dermatológico for processing. All culture swabs were processed the same day that they were collected. Each specimen was plated to a Blood Agar Plate (BAP), MacConkey Agar Plate (MAC) and a Columbia CNA Agar with 5% Sheep Blood. Culture plates were incubated up to 48 h at 35° C, then examined for colony morphology consistent with S. aureus and S. pyogenes. Identification of S. aureus and/or S. pyogenes colonies included the tube coagulase test, slide latex typing and the Vitek® 2 system. The Vitek® 2 system was also used for antibiotic sensitivity, including oxacillin for S. aureus.
Clinical study design
The protocol and informed consent forms were approved by each site's local Ethics Committee (EC) prior to the start of the study. After obtaining written informed consent by their parent or guardian, children aged 2-12 years with micro-biologically documented primary non-bullous impetigo were enrolled and randomized into a dose-ranging parallel group study testing 3 different doses: a low (0.1%), medium (0.5%), and high (1.5%) concentration of NVC-422 topical gel. Subjects were randomized in each dose group across two phases in the study. Treatment was administered topically three times a day (TID) for 7 days to all randomized subjects. Sequential Design: Part 1 was 0.1% & 0.5% NVC -422 (20:40 subjects); Part 2 was 0.1% & 1.5% NVC-422 (20:40). A target total of 120 subjects were to be enrolled and randomized; forty in each treatment group. Four scheduled study visits: Day 1 -Screening/Treatment Begins, Day 4 (±1) -Interim Visit/Safety, Day 8 (+1) End of Treatment (EOT) and Day 15 (±2) Follow-up (F/ U). Clinical and bacteriological assessments and efficacy evaluations were done at EOT and F/U. Response was measured at the completion of treatment (Day 8) and 1 week post treatment (Day 15) by the Skin Infection Rating Scale (SIRS) and by microbiological response. Microbiological samples were taken at Day 1 and Day 8 of the clinical trial. The Day 1 pre-treatment isolates were analyzed on the day of collection to determine the type of bacterial infection by using the Vitek® 2 system. Photographic documentation of clinical trial progress was obtained. Safety (skin reactions and clinical adverse events) was assessed throughout the treatment phase and post-treatment follow up.
Skin infection rating scale (SIRS)
Patients were evaluated by a Skin Infection Rating Scale (SIRS) which evaluated 5 Signs and Symptoms: exudate/pus, crusting, erythema/inflammation, itching, and pain on a scale (0-3): 0 = absent, 1 = mild, 2 = moderate, 3 = severe. Enrollment criteria was to include 2-12 years of age with a clinical diagnosis of non-bullous impetigo with ≤10 impetigo lesions in total and a Gram stain of the target lesion showing Gram-positive cocci, a total SIRS score of ≥4; at least 3 of 5 signs and symptoms present at baseline; and a score of ≥1 for exudate/pus. Subjects were screened, randomized, and began treatment all on the same day. To be eligible for the study, subjects' parent or legal guardian must have signed a written informed consent document and been willing and able to comply with the requirements of the protocol.
Patient demographics
For the demographics, continuous variables (e.g., age) were tabulated using the number of subjects, mean, standard deviation, median, minimum, and maximum, and were analyzed by one-way analysis of variance (ANOVA) model with term treatment group as the factor. Categorical data (e.g., gender, clinical success or failure) were summarized by frequency counts and percentages, and were analyzed using Fisher's exact test (Cochran-Mantel-Haenszel test if appropriate).
Clinical response to treatment
Clinical response was determined as either a clinical success or clinical improvement at Day 8 End of Treatment (EOT) and/or Day 15 Follow-up. Clinical success was determined by sufficient resolution of signs and symptoms of infection of the target lesion such that no additional antimicrobial therapy is required to treat the impetigo, as evidenced by the SIRS score of 0 each for exudate/pus, crusting and pain and 0 or 1 each for erythema/inflammation and itching. Clinical improvement was determined by a SIRS score of 0 for exudate/pus which does not meet all the criteria for clinical success. Clinical failure was determined by a SIRS score of 1 or greater for exudate/pus. Unevaluable was recorded when a valid clinical assessment could not be made.
Clinical efficacy
The evaluation of clinical efficacy involved an evaluation of number of patients, their age, gender, and type of infection. The treatment regimen was a TID application of the topical gel to the target lesion and non-target lesions. The patients were evaluated at each visit to the clinic. A pretreatment and post-treatment SIRS score was determined. Bacteriological Success was determined when the causative pathogen isolated from the target lesion at baseline (Staphylococcus aureus and/or Streptococcus pyogenes) was eliminated on culture, or clinical response was such that no exudate material was available for culture, as evidence of pathogen eradication. Bacteriological failure was determined by the non-eradication of the organism from the target lesion that was isolated at baseline. Finally “Unevaluable” was determined when bacteriological evaluation could not be made due to a reason other than no culture material available. Clinical and bacteriological efficacy analyses were provided for each treatment group, along with the corresponding 95% confidence interval for the difference in proportions, based on the normal approximation to the binomial distribution. Differences between treatment groups were compared using Fisher's exact test. All statistical tests were two-tailed. No adjustments for multiple comparisons were made in this study. Descriptions of the populations evaluated are described in the results section.
Adverse events
Adverse events (AE) were determined by the investigator and assessed to be unrelated to treatment, possibly related or probably related to treatment.
Results
Profile of NVC-422 against S. aureus and S. pyogenes Clinical Isolates
One hundred and twenty one (121) recent clinical isolates of S. aureus and S. pyogenes were obtained from Eurofins Medinet (Herndon, VA). Activity of NVC-422 against MRSA, mupirocin-resistant, fusidic acid resistant, vancomycin-intermediate and resistant, linezolid-resistant, daptomycin non-sensitive S. aureus as well as erythromycin-resistant S. pyogenes is shown (Table 1). NVC-422 was rapidly bactericidal against all isolates with MBC killing 90% of organisms (MBC90) of 4 µg/mL against S. aureus and 2 µg/mL against S. pyogenes regardless of resistance phenotype.
Table 1.
MBC (µg/mL) profile of NVC-422 against S. aureus and S. pyogenes clinical isolates
| Organism | Phenotype | Total | Range | Mode | MBC50 | MBC90 |
|---|---|---|---|---|---|---|
| Staphylococcus aureus | All | 121 | 0.5-8 | 4 | 2 | 4 |
| MRSA | 55 | 1-4 | 2 | 2 | 4 | |
| VISA | 10 | 1-8 | 2 | 2 | 4 | |
| VRSA | 9 | 0.5-4 | 4 | 4 | 4 | |
| DAP-NS | 10 | 2-4 | 4 | 4 | 4 | |
| LZD-R | 11 | 1-4 | 4 | 4 | 4 | |
| FUS-R | 7 | 2 | 2 | 2 | 2 | |
| MUP-R | 5 | 2-4 | 2 | 2 | 4 | |
| Streptococcus pyogenes | All | 31 | 0.5-4 | 2 | 2 | 2 |
| ERY-R | 15 | 0.5-4 | 2 | 2 | 2 |
Clinical and microbiological response
A total of 129 patients with clinically diagnosed impetigo were randomized to three dose groups (0.1, 0.5, or 1.5% NVC-422) in the study conducted at 2 centers. 125 patients (97%) had microbiologically confirmed infection. Treatment was administered three times a day (TID) for 7 days to all randomized subjects. Response was measured at the completion of treatment (Day 8) and 1 week post treatment (Day 15) by the Skin Infection Rating Scale (SIRS) and by microbiological response. A total of 120 subjects (96%) completed all 7 days of treatment and were assessed at end of treatment. Clinical response rate in the per-protocol complete (PPC) population was excellent in each of the dose groups (84.6%, 87.2%, and 92.3% in the 0.1%, 0.5% and 1.5% dose groups respectively). The majority of the infections were caused by S. aureus, alone (106/125, 85%) of which approximately 10% were MRSA. The response rate for MRSA infections was 10/10. A minority of the infections was mixed infections (10%) or S. pyogenes alone (6%), with similar clinical and microbiologic success rates.There were no clinical recurrences in any treatment groups. Treatment-emergent adverse events were seen in 5.4% of the subjects (7/129) and were mild to moderate and resolved. NVC-422 topical gel administered TID was well-tolerated, with high rates of clinical and microbiological responses for treating impetigo. The intent-to-treat (ITT), modified intent-to-treat (mITT) and PPC study populations are summarized in Table 2.
Table 2.
ITT, mITT and PPC study populations
| Study Population | |
| Intent-to-Treat (ITT) is all subjects who had Gram-positive cocci at screening and received at least one treatment. Only safety analysis was performed on this group. | n=129 |
| Modified-ITT (mITT) is the ITT population who had a positive baseline culture of S. aureus and/or S. pyogenes and at least one post-baseline visit. | n=125 |
| Per-Protocol Complete (PPC) is the mITT population who completed the study per protocol for clinical and bacteriological evaluations. | n=117 (clinical) |
| n=114 (bacteriological) |
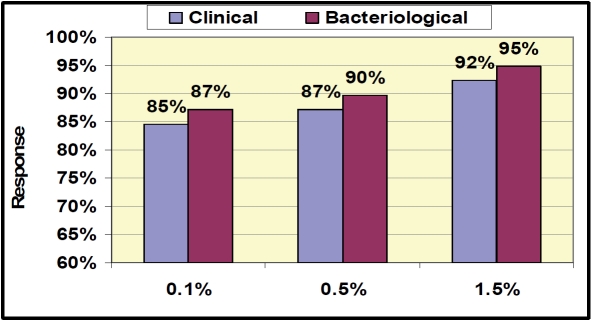
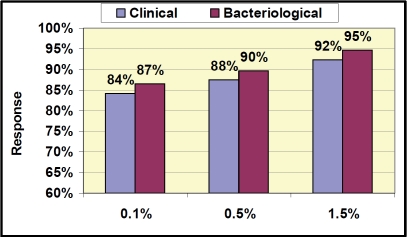
Overall clinical response rate (success and improvement) in the PPC population was equal to or above 85% in each of the dose groups at Day 8 EOT (84.6%, 87.2%, and 92.3% in the 0.1%, 0.5% and 1.5% dose groups respectively; Figure 1). This response rate is substantially higher than the response rate anticipated for historical placebo (30-50%) [25]. Similar responses were seen in the mITT population (83%, 81%, and 90% in the 0.1%, 0.5% and 1.5% dose groups respectively). Response rates for MRSA infections in this study were 100% (10/10) across all treatment groups in the PPC population (Table 3), whether MRSA was the sole organism or in a mixed infection. All subjects that were Clinical Responders at Day 8 (Figure 1) that returned for the follow-up visit at Day 15 (Figure 2) were clinical successes with a SIRS score of 0 (n=103). There were no recurrences of infection at the follow-up visit (Day 15) in any treatment groups. Bacteriological response rates of equal to or above 84% were very similar to clinical response rates at both EOT and follow-up. The clinical and bacteriological response rates across the treatment groups suggest a dose response, although differences were not statistically significant. Adverse events (7/129, 5.4%; Table 4) were mild to moderate in severity and predominantly were local reactions at the application site. All adverse events resolved after the end of treatment. The rate for subject treatment completion was 96%, and 82% of the subjects returned for the Day 15 (follow-up) visit. This proof of concept study demonstrates the activity of NVC-422 topical gel in the treatment of impetigo.
Figure 1.
Clinical and Bacteriological Response Results at End of Treatment (PPC population) Day 8. Clinical Response = Success + improvement, Bacteriological Response = Success (eradication).
Table 3.
Clinical Response (PPC) by Infecting Organism at End of Treatment (Day 8)1
| Infecting Organism(s) | |||||
|---|---|---|---|---|---|
| Treatment Dose | S. aureus (MSSA) (n=89) | S. aureus (MRSA) (n=9) | S. pyogenes (n=7) | S. aureus (MSSA) andS. pyogenes (n=11) | S. aureus (MRSA) andS. pyogenes (n=1) |
| NVC-422 | 22/27 | 4/4 | 4/4 | 3/4 | N/A |
| 0.1% | (81%) | (100%) | (100%) | (75%) | |
| NVC-422 | 29/34 | 2/2 | 3/3 | ||
| 0.5% | (85%) | (100%) | (100%) | N/A | N/A |
| NVC-422 | 26/28 | 3/3 | N/A | 6/7 | 1/1 |
| 1.5% | (93%) | (100%) | (86%) | (100%) | |
N/A denotes organisms were not present in these groups at baseline.
Figure 2.
Clinical and Bacteriological Response Results at End at Follow-up (PPC population) Day 15 Response. Clinical Response = Success, Bacteriological Response = Success (eradication).
Table 4.
Treatment Emergent Adverse Events for the ITT Population (n=129)
| Adverse Event | 0.1% (n=43) | 0.5% (n=45) | 1.5% (n=41) |
|---|---|---|---|
| Allergy/rash | 1 | 0 | 0 |
| Contact dermatitis | 1 | 0 | 0 |
| Dermatitis | 0 | 0 | 1 |
| Dry skin | 0 | 0 | 1 |
| Fever | 0 | 1 | 0 |
| Itching | 0 | 0 | 2 |
| Total | 2 | 1 | 4 |
Photographic examples of successful treatment of impetigo lesions
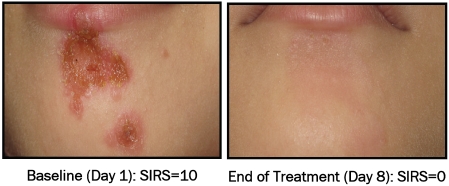
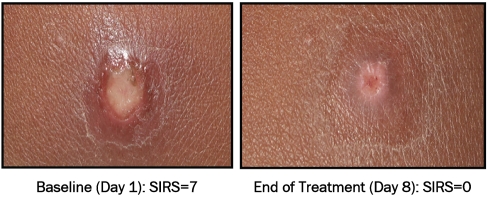
The pictorial examples show the baseline (Day 1) and EOT (Day 8) for the NVC-422 0.1% (Figure 3), 0.5% (Figure 4) and 1.5% (Figure 5) topical gels. In these pictorial examples, the reduction of the SIRS scores is apparent as is the return of skin integrity. It should be noted that, as part of the normal healing process, hyper (hypo) pigmentation should be resolved in a few weeks following this treatment. All three patient examples were clinical successes at end of treatment.
Figure 3.
S. aureus (MRSA) impetigo infection on forearm of 2 yr old female before and after treatment with 0.1% NVC-422 topical gel completed 21 doses (TIDfor7 days).
Figure 4.
S. aureus (MSSA) impetigo infection on lip and chin of 3 yr old female before and after treatment with 0.5% NVC-422 topical gel completed 21 doses (TID for 7 days).
Figure 5.
S. aureus (MRSA) and S. pyogenes mixed impetigo infection on thigh of 2 yr old female before and after treatment with 1.5% NVC-422 topical gel completed 21 doses (TID for 7 days).
Statistical analysis of treatment groups
There was no statistically significant difference between the treatment groups in the baseline demographics other than gender, infecting organism, and SIRS score for exudate/pus (Table 5). More females were enrolled in the 0.1% treatment group than in the other two treatment groups. The 0.5% treatment group had a higher incidence of S. aureus compared to the 0.1% and 1.5% groups. The 1.5% treatment group had a higher incidence of mixed infections compared to the 0.1% and 0.5% groups at baseline. For exudate/pus score, more subjects in the 0.5% treatment group were in the “mild” category than the 0.1% and 1.5% treatment groups.
Table 5.
Summary of subject baseline demographics
| Treatment Groups | |||||
|---|---|---|---|---|---|
| 0.1% NVC-422 n = 41 | 0.5% NVC-422 n = 43 | 1.5% NVC-422 n=41 | P-value | ||
| Age (years) | Mean ± SD | 4.9 ± 2.8 | 4.8 ± 2.4 | 4.7 ±2.7 | 0.9631 |
| Range | 2.0-11.3 | 2.0-11.32.0-1 | 2.0-11.4 | ||
| Gender | Female | 28 (68.3%) | 20 (46.5%) | 17 (41.5%) | 0.0362 |
| Male | 13 (31.7%) | 23 (53.5%) | 24 (58.5%) | ||
| Race | Black/African American | 14 (34.2%) | 20 (46.5%) | 14 (34.2%) | 0.0992 |
| White | 4 (9.8%) | 5 (11.6%) | 0 (0.0%) | ||
| Other | 23 (56.1%) | 20 (46.5%) | 18 (41.9%) | ||
| Infecting Organism | S. aureus | 33 (80.5%) | 40 (93.0%) | 33 (80.5%) | 0.0142 |
| S. pyogenes | 4 (9.8%) | 3 (7.0%) | 0 (0.0%) | ||
| Both | 4 (9.8%) | 0 (0.0%) | 8 (19.5%) | ||
| Exudate/Pus | 0 (Absent) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0042 |
| 1 (Mild) | 23 (56.1%) | 36 (83.7%) | 21 (51.2%) | ||
| 2 (Moderate) | 18 (43.9%) | 7 (16.3%) | 20 (48.8%) | ||
| 3 (Severe) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Total SIRS | Mean ± SD | 7.12 ± 1.49 | 6.88 ± 1.35 | 7.63 ±1.64 | 0.0681 |
| Range | 4-10 | 4-10 | 4-11 | ||
P-values for treatment comparisons from a one-way ANOVA with factor of treatment
P-values for treatment comparisons from CMH tests for general association
Adverse events
As can be seen from the table, the AEs (Table 4) were mild and resolved after treatment was ended.
Discussion
Impetigo is a topical disease readily accessible to treatment by the clinician; the infection is localized to the epidermis and easy in terms of evaluating clinical and antimicrobial response. Intact skin is typically resistant to the colonization and subsequent infection of S. aureus or GABHS transiently introduced to the surface of the skin via the environment. The impetigo pathogenesis begins by the colonization of skin surface where humidity, high ambient temperatures, and microscopic trauma to the epidermis provide a convenient entry point for pathogens. Pathogen detection by the body and tissue damage triggers neutrophil migration resulting in pus, tissue inflammation, and desquamation. Morphologic tissue changes caused by the pathogens results in the formation of multiple characteristic yellowish to honey-colored pruritic erythematous crusted lesions on exposed surfaces of the body such as the face, arms and legs observed in impetigo cases.
The non-bullous impetigo, impetigo contagiosa, begins as a red macule or papule that forms as an easily rupturable vesicle with superficial skin erosion. Impetigo lesions are itchy and slightly painful which leads to scratching, particularly in children, which serves to spread the infection by autoinoculation to the face and extremities and by touch to other children. Bullous impetigo can vary from a pea-sized and larger fragile blister that can last from days to weeks without treatment. In the bullous situation, Staphylococcus bacteria produce a toxin that results in a separation between the epidermis and dermis forming a blister (bulla). These bullae can be observed on various skin areas such as the buttocks. Because impetigo lesions are superficial they do not generally leave scars, however the affected skin can appear red after the crusts have gone away and the redness fades in a matter of days to weeks during the healing process.
This report summarizes the in vitro activity of NVC-422 against clinically relevant Gram-positive organisms (Table 1) and the clinical activity and tolerability of NVC-422 topical gel in the treatment of impetigo in a first proof of concept human clinical evaluation. In vitro studies using recent clinical isolates of S. aureus and S. pyogenes demonstrated that NVC-422 is rapidly bactericidal against all isolates tested including many that were resistant to a range of antibiotics. The results of the clinical study showed that NVC-422 topical gel was active and well tolerated as a TID regimen, demonstrating high clinical and microbiological response rates in the treatment of impetigo in children under 12 years of age. These results provide a basis for the continued development of NVC-422 topical gel for the treatment of impetigo and other AB-SSSI [26]. The goal is to make available a global treatment for impetigo and other superficial infections that has a low potential for the development of resistance.
The Aganocide class of compounds may be useful in other topical applications such as eye, bladder, wound, or burn infection and will be the topic of additional manuscripts that are in preparation.
Acknowledgments
The authors wish to thank the Instituto Dermatológico, Santo Domingo, and the Robert Reid Cabral Children's Hospital, Santo Domingo of the Dominican Republic for their participation in this clinical study. The authors also wish to thank Dr. Philippe Andres (Galderma SA) for invaluable suggestions during the development of the clinical protocol.
References
- 1.Koning S, Verhagen AP, van Suijlekom-Smit LWA, Morris AD, Butler C, van der Wouden JC. Interventions for impetigo. Cochrane Database of Systematic Reviews. 2004 doi: 10.1002/14651858.CD003261.pub2. Issue 2. Art. No. : C D 0 0 3 2 6 1 . DOI : 10.1002/14651858.CD003261.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Rørtveit S, Rørtveit G. Impetigo in epidemic and non-epidemic phases: An incidence study over years in general population. Br J Dermatol. 2007;157:100–105. doi: 10.1111/j.1365-2133.2007.07969.x. [DOI] [PubMed] [Google Scholar]
- 3.Steer AC, Jenney AWJ, Kado J, Batzloff MR, La Vincente S. High Burden of Impetigo and Scabies in a Tropical Country. PLoS Negl Trop Dis. 2009;3 doi: 10.1371/journal.pntd.0000467. e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrieri P, Dajani AS, Wannamaker LW. Natural History of Impetigo. I. Site sequence of acquisition and familial patterns of spread of coetaneous streptococci. J Clin Invest. 1972;51:2851–2862. doi: 10.1172/JCI107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 6.Epstein S. Staphylococcic Impetigo Contagiosa. Arch Derm Syphilol. 1940;42:840–855. [Google Scholar]
- 7.Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis. 2005;11:928–930. doi: 10.3201/eid1106.040641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayward A, Knott F, Petersen I, Livermore DM, Duckworth G, Islam A, Johnson AM. Increasing Hospitalizations and General Practice Prescriptions for Community-onset Staphylococcal Disease, England. Emerg Infect Dis. 2008;14:720–726. doi: 10.3201/eid1505.070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelsberg J, Taneja C, Zervos M, Haque N, Moore C, Reyes K, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009 doi: 10.3201/eid1509.081228. Sep Available from http://www.cdc.gov/EID/content/15/9/1516.htm. DOI: 10.3201/eid1509.081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen PR. Community-acquired methicillinresistant Staphylococcus aureus skin infections: a review of epidemiology, clinical features, management, and prevention. Int J Dermatol. 2007;46:1–11. doi: 10.1111/j.1365-4632.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- 11.Durupt F, Mayor L, Bes M. Prevalence of Staphylococcus aureus toxins and nasal carriage in furuncles and impetigo. Br J Dermatol. 2007;157:1161–1167. doi: 10.1111/j.1365-2133.2007.08197.x. [DOI] [PubMed] [Google Scholar]
- 12.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin Resistance. Clin Infect Dis. 2009;49(6):935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 13.Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11–18. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Dobie D, Gray J. Fusidic acid resistance in Staphylococcus aureus. Arch Dis Child. 2004;89:74–77. doi: 10.1136/adc.2003.019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rørtveit S, Skutlaberg DH, Langeland N, Rortveit G. Impetigo in a population over 8.5 years: incidence, fusidic acid resistance and molecular characteristics. J Antimicrob Chemother. 2011;66:1360–1364. doi: 10.1093/jac/dkr102. [DOI] [PubMed] [Google Scholar]
- 16.Alsterholm M, Flytström I, Bergbrant IM, Faergemann J. Fusidic acid-resistant Staphylococcus aureus in impetigo contagiosa and secondarily infected atopic dermatitis. Acta Derm Venereol. 2010;90:52–57. doi: 10.2340/00015555-0771. [DOI] [PubMed] [Google Scholar]
- 17.Dagan R, Bar-David Y. Double-blind study comparing erythromycin and mupirocin for treatment of impetigo in children: implications of a high prevalence of erythromycin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 1992;36:287–290. doi: 10.1128/aac.36.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang LP, Keam SJ. Retapamula review of its use in the management of impetigo and other uncomplicated superficial skin infections. Drugs. 2008;68:855–873. doi: 10.2165/00003495-200868060-00008. [DOI] [PubMed] [Google Scholar]
- 19.Farrell DJ, Robbins M, Rhys-Williams W, Love WG. Investigation of the potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus isolates during a 55-passage study. Antimicrob Agents Chemother. 2011;55:1177–1181. doi: 10.1128/AAC.01285-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51:613–617. doi: 10.1093/jac/dkg127. [DOI] [PubMed] [Google Scholar]
- 21.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin Resistance. Clin Infect Dis. 2009;49:935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 22.Dobie D, Gray J. Fusidic acid resistance in Staphylococcus aureus. Arch Dis Child. 2004;89:74–77. doi: 10.1136/adc.2003.019695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katopodis GD, Grivea IN, Tsantsaridou AJ, Pournaras S, Petinaki E, Syrogiannopoulos GA. Fusidic acid and clindamycin resistance in community-associated, methicillin-resistant Staphylococcus aureus infections in children of Central Greece. BMC Infect Dis. 2010;10:351. doi: 10.1186/1471-2334-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Belisle B, Bassiri M, Xu P, Debabov D, Celeri C, Alvarez N, Robson MC, Payne WG, Najafi R, Khosrovi B. Chemical Characterization and Biological Properties of NVC-422, a Novel, Stable N-Chlorotaurine Analog. Antimicrob Agents Chemother. 2011;55:2688–2692. doi: 10.1128/AAC.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting, November 18, 2008 Justification for the Non-Inferiority Margin for the Treatment of complicated Skin and Skin Structure Infections.
- 26.The Division of Anti-Infective and Ophthalmology Products and the Division of Special Pathogen and Transplant Products in the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration; Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment; U.S, editor. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), August 2010.