Abstract
Hematopoietic stem cell transplantation (HSCT) is a curative treatment for otherwise incurable diseases. Conditioning regimen is an important part of HSCT and consists of chemotherapy with or without irradiation. Conditioning exerts myelosuppressive, immunosuppressive and antitumor effects, but also contributes to HSCT-related complications including graft-versus-host disease (GVHD). Since almost 50% of the transplanted patients are conditioned with cytostatics without irradiation, we developed and characterized a GVHD mouse model following conditioning with busulphan and cyclophosphamide. Recipient Balb/c female mice were treated with busulphan (20 mg/kg/day for 4 days) and cyclophosphamide (100 mg/kg/day for two days). After one day of rest, recipient mice were transplanted with 2×107 bone marrow and 3×107 spleen cells from male C57BL/6 (allogeneic group) or female Balb/c (syngeneic/control group) mice. The allogeneic, but not syngeneic transplanted mice developed GVHD. Histopathology of the major internal organs (liver, pancreas, spleen, lungs, heart and kidney) was examined before conditioning start, after conditioning's end and 5, 7 and 21 days after transplantation using hematoxylin-eosin staining. Decreased spleen cellularity and diminished glycogen content in the liver were observed after conditioning regimen. Histopathological changes such as vasculitis, inflammation and apoptotic cell forms in liver, spleen, pancreas, lungs and heart were observed in allogeneic transplanted mice, however, only hypocellular spleen and extramedullar hematopoiesis were detected in syngeneic transplanted animals. No morphological changes were observed in kidney in either HSCT setting. This is the first study describing early histopathological changes after conditioning regimen with busulphan/cyclophosphamide and dynamics of GVHD development in several major internal organs.
Keywords: Graft-versus-host disease, hematopietic stem cell transplantation, mouse, conditioning regimen, busulphan, cyclophosphamide
Introduction
Hematopoietic stem cell transplantation (HSCT) is the treatment of choice for malignant and non-malignant diseases. Although HSCT has a high curative potential for otherwise incurable diseases, transplantation related morbidity deteriorates clinical outcome and life quality of the patients [1]. Graft-versus-host disease (GVHD) remains one of the most serious transplantation -related complications despite intensive research on this issue. Factors such as disparity in major and/or minor histocompatibility antigens between donor and recipient, sex mismatch, stem cell source and conditioning regimen contribute to GVHD development [2]. Conditioning regimen is an important part of HSCT and exerts an immunosuppressive, myelosuppressive/ myeloablative and anti-tumor effects. Conditioning regimens consist of cytostatics with or without irradiation and with or without antithymocyte globuline (ATG). Type and intensity of the conditioning regimen is determined with regards to several factors such as the diagnosis, patient's age, co-morbidity, type of donor and HLA-d is parity, and risk for transplantation-related complications.
Conditioning regimen is implicated in GVHD pathophysiology through inducing damage in host tissues [3]. Tissue damage results in the secretion of inflammatory molecules, elevation of adhesion molecules and expression of histocompatibility antigens that attract donor T cells to the site of the inflammation. Further, the interaction between the antigen presenting cells (APC) with the donor T cell leads to T cells activation, proliferation and differentiation. Subsequently, activated donor T cells migrate to target tissues through expressing chemokine receptors and other chemotaxes signals [4]. Phagocytic and cytotoxic cells are recruited and worsen the tissue damage [5-6]. GVHD may be divided according to its onset in two types, acute (aGVHD) and chronic (cGVHD). Both types are T cell mediated but differ in symptoms and involved organs.
Animal models were developed to investigate the mechanism underlying GVHD, since pathophysiological studies in humans are limited due to ethical and practical reasons. The vast majority of the models on GVHD pathophysiology are based on total body irradiation-based conditioning despite the fact that about one half of the transplanted patients are conditioned with cytostatics without irradiation. Alkylating agents busulphan (Bu) and cyclophosphamide (Cy) are used as conditioning regimen before transplantation for acute and chronic myeloid leukemia, inherited metabolic diseases and chronic granulomatous disease since the 1970's [7]. Conditioning regimen with Bu/Cy has an intensive immunosuppressive and myeloablative effect, but it is also involved in several transplantation-related complications such as mucositis, liver toxicity and hemorrhagic cystitis [8]. Tissue damage induced by Bu/Cy has been also implicated in development of GVHD. We have developed and characterized a mouse model of GVHD after conditioning regimen with Bu/Cy [9-10]. Conditioning regimen, together with disparity in the major and minor MHC antigens between recipient Balb/c and donor C57BL/6 mice, results in acute GVHD that is mediated by CD8+ T cells. In this investigation we present the dynamics of the early histopathological changes that occur after conditioning regimen with Bu/Cy and during subsequent development of acute GVHD.
Material and methods
Materials
Materials were purchased as follows: busulphan, cyclophosphamide and ethylenediamine-tetraacetic acid (EDTA) from Sigma-Aldrich, Stockholm, Sweden; phosphate buffer saline (PBS) and fetal bovine serum (FBS) from Invitrogen AB, Stockholm, Sweden; 4% formaldehyde from Solveco AB, Rosersberg, Sweden.
Animals
Female Balb/c mice (H-2Kd) 10 to 12 weeks old and C57BL/6 (H-2Kb) mice 10 to 14 weeks old were purchased from Charles River, Germany. The experiments were approved by Stockholm south ethics committee for animal research in compliance with Swedish animal welfare law. The mice had access to food and water ad libitum and were kept in pathogen free HEPA filtered individually ventilated cages (IVCs) under controlled conditions with 12 hours light/dark cycle, humidity 55% ± 5% and temperature 21°C ± 2°C. Upon delivery, the mice were allowed to acclimatize for one to two weeks before the start of experiment.
Conditioning and HSCT
Conditioning regimen and HSCT were performed as described previously [9]. Briefly, female Balb/c mice were treated with busulphan at dose of 20 mg/kg/day for 4 days (day -7 - day -4) and cyclophosphamide at dose of 100 mg/ kg/day for two days (day -3 and day -2). Then mice were allowed to rest for one day. Day of stem cell injection is assigned as day 0, days before stem cell injection are numbered backwards with “-” sign and days after stem cell injection forward with “+” sign. Conditioned recipient mice were transplanted with 2 × 107 bone marrow and 3 × 107 spleen cells from male C57BL/6 (allogeneic group) or female Balb/c (syngeneic group) on day 0. Cells were administered intravenously through the tail vein. The health status of the mice was regularly monitored and the weight was recorded from the start of treatment until the day of sampling. After transplantation, the animals were assessed for GVHD signs i.e. weight loss, posture, activity, fur texture, and skin integrity [11].
Four to seven animals were examined per time point in each group. Untreated controls were sampled before conditioning start (day -7). The treated animals were sampled at day 0 (before stem cell administration) and at day +5, +7 and +21. Since the intensity of Bu/Cy conditioning does not allow for autorecovery of hematopoiesis and thus, inevitably results in early death, syngeneic transplanted mice served as controls for chemotherapy induced tissue damage.
Tissue processing and staining
At the appropriate time point, mice were given general anesthesia and killed by cervical dislocation. Immediately the whole vascular system was perfused with solution of phosphate buffer saline/ 10 mM ethylenediaminetetraacetic acid (EDTA)/ 2% fetal bovine serum through the left ventricle while the inferior vena cava was cut. Heart, liver, spleen, kidney and pancreas were harvested and placed in a sealed container with 4% para-formaldehyde. After harvesting, lungs were perfused with 4% para-formaldehyde through the trachea, then transferred to the sealed container. The tissues were dehydrated in increasing concentration of ethanol, cleared with xylene and embedded in paraffin. Tissues sections were cut 5 µm thick and deparafinized in Tissue Clear, rehydrated in series of ethanol in decreased concentrations. Then the sections were stained with hematoxylin & eosin and dehydrated in ethanol. The Tisse Tek Prisma (Sakura FinetekInc, Torrance, CA, USA) automated slide stainer was used for staining procedure according to manufacturer's recommendations. Then the slides were cleared using xylene and mounted with coverslips.
Results
Weight loss was observed in both groups starting already during the treatment with cytostatics. Weight loss reached nadir at day +3 in syngeneic mice and at day +7 in allogeneic mice. While the mice in the syngeneic group recovered their weights by 21 days post-transplant, the allogeneic transplanted animals remained underweight. The weight loss was well related to GVHD signs expressed as hunched posture, ruffled fur and hair loss observed only in allogeneic transplanted animals. No signs of GVHD were observed in the syngeneic group.
Pancreas
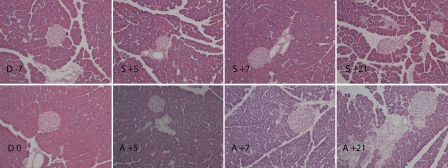
Conditioning regimen did not affect the microscopic morphology of the pancreas (Figure 1). However, in the allogeneic group, smaller size of acinis together with progressive loss of apical secretion granules between day +5 and day +7 was observed. Apoptotic cell morphology in both exocrine and endocrine part of pancreas was observed at day +7. Signs of recovery in pancreatic tissue were observed at day +21, however, high inter-individual variability among animals was also detected. The findings varied from almost normal structure of exocrine pancreas to fragmentation among acini with fibrotic reaction and edema with influx of predominantly granulocytes. At day +21, the allogeneic transplanted animals exhibited vasculitis, in some cases extensively. No apparent structural changes in exocrine or endocrine part of pancreas were observed in the syngeneic group throughout the study period.
Figure 1.
Histology of pancreas from conditioning start until day +21 post HSCT. Conditioning regimen (Bu/Cy) did not affect the microscopic structure of the pancreas (D 0) compared to that observed in untreated controls (D -7). In the allogeneic group, smaller size of acins and progressive loss of apical secretion granules between day +5 (A +5) and day +7 (A +7) was observed. Apoptotic cell morphology in both exocrine and endocrine part of pancreas was observed at day +7 (A +7). Fragmentation among acini with fibrotic reaction and edema with influx of predominantly granulocytes and signs of vasculitis was observed at day +21 (A +21). No apparent structural changes in exocrine or endocrine part of pancreas were observed in syngeneic group at day +5, +7 and +21 (S +5, S +7, S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×20.
Liver
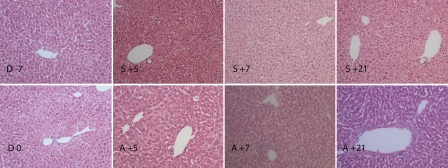
After conditioning regimen, the hepatocytes became smaller in size, rather eosinophilic and showed a homogenously stained cytoplasm lacking the cytoplasmic vacuolization typical of glycogen accumulation (Figure 2). In the allogeneic group, extramedullar hematopoiesis, particularly myelopoiesis, was detected already at day +5. Some accumulation of inflammatory cells adjacent to particularly central veins without apparent changes in the endothelium or vascular integrity was observed. The vascular involvement was more apparent at day +7, when granulocytes adhering to endothelium and apoptotic cell forms probably of endothelial origin were observed. However, 21 days after stem cell transplantation vascular injury was apparently localized in the porta triad in some animals. The microscopic structure of the liver was relatively well preserved in syngeneic group at day +5, +7 and +21. Extramedullar hematopoiesis was presented in the liver of syngeneic mice as in allogeneic group, but no signs of vascular injury were observed.
Figure 2.
Histology of liver from conditioning start until day +21 post HSCT. After conditioning regimen (D 0), the hepatocytes became smaller in size, rather eosinophilic and the glycogen content diminished compared to untreated controls (D -7). In allogeneic transplanted group, some accumulation of inflammatory cells adjacent to particularly central veins without apparent changes in the endothelium or vascular integrity was observed 5 days after transplantation (A +5). The vascular involvement was more apparent at day +7, when granulocytes adhering to endothelium and apoptotic forms probably of endothelial origin were observed (A +7). The vascular injury was apparently localized in porta triad at day +21 (A +21). The microscopic structure of the liver was relatively well preserved and no signs of vascular injury were observed in syngeneic group at day +5, +7 and +21 (S +5, S +7, S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×20.
Spleen
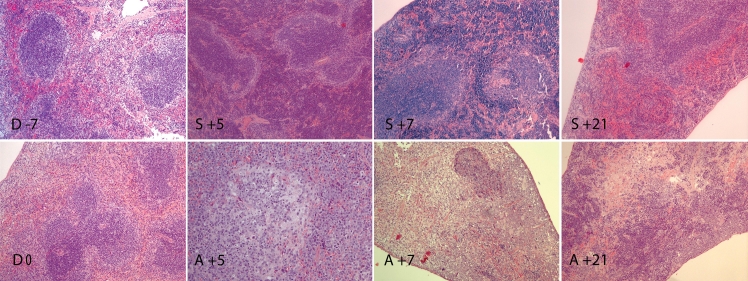
Conditioning regimen resulted in drastically reduced spleen cellularity, both in white and red pulps (Figure 3). In the allogeneic group, the spleen's architecture was altered and progressive hypocellularity was observed within the first week after transplantation. The white pulp became hardly identifiable with sparse areas of lymphocyte accumulation. Only remnants of follicles and marginal zone indicated the original location of the white pulp. Apoptotic forms were observed from day +5. Uneven distribution of hematopoiesis, predominantly myelopoiesis, was observed in red pulp. Three weeks after transplantation, the spleens of allogeneic transplanted animal showed signs of partial recovery in cellularity with varying amounts of lymphocytes. The grade of recovery was highly variable among animals, ranging from missing white pulp with predominance of stromal cells and scattered apoptotic forms to somewhat recovered architecture. Red pulp also showed varying grade of hematopoiesis from almost non-existant to highly active consisting of both myelo -and erythropoiesis.
Figure 3.
Histology of spleen from conditioning start until day +21 post HSCT. Conditioning regimen resulted in decreased spleen cellularity, both in white and red pulps (D 0) compared to untreated controls (D -7). In allogeneic group, the spleen's architecture was altered and progressive hypocellularity was observed at day +5 and +7. White pulp became hardly identifiable with sparse areas of lymphocyte accumulation (A +5, A +7). Apoptotic forms were observed starting from day +5 (A+5, A +7, A +21). Uneven distribution of hematopoiesis, predominantly myelopoiesis, was observed in red pulp (A +5, A +7). Signs of partial recovery in cellularity with varying amount of lymphocytes and variable grade of hematopoiesis in red pulp was observed at day +21 (A +21). In syngeneic group, the white pulp was severely hypocellular, but red pulp showed extensive extramedullar hematopoiesis at day +5 and +7 (S +5, S +7). At day +21, the white pulp was almost re-established, only slightly hypocellular, with moderate hematopoiesis in red pulp (S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×10.
In syngeneic group, the white pulp was severely hypocellular one week post-transplant, but red pulp showed extensive extramedullar hematopoiesis. Three weeks post- transplant, the white pulp was almost re-established, only slightly hypocellular, while moderate hematopoiesis was observed in red pulp.
Lung
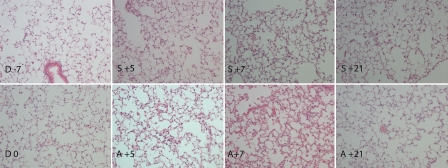
No morphological changes in lungs were observed at the end of the conditioning regimen (Figure 4). In the allogeneic transplanted mice, inflammatory changes with adherence of granulocytic cells in small veins were observed at day +5. These changes were more pronounced at day +7, while variation in inflammation grade from none to granulocytic infiltrates with nuclear dust, foamy macrophages and subpleural fibrotic changes with accumulation of granulocytes were observed at day +21. In the syngeneic mice, alveolar macrophages of foam type were detected at day +5, +7 and +21 without any other abnormity.
Figure 4.
Histology of lung from conditioning start until day +21 post HSCT. No morphological changes in lungs were observed at the end of the conditioning regimen (D 0) compared to untreated mice (D -7). In allogeneic transplanted mice, inflammatory changes with adherence of granulocytic cells in small veins were observed at day +5 (A +5) and these changes were more pronounced at day +7 (A +7). Some granulocytic infiltrates with nuclear dust and foamy macrophages were observed at day +21 (A +21). In syngeneic mice, alveolar macrophages of foam type were detected at day +5, +7 and +21 without any other abnormity (S +5, S +7, S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×20.
Heart
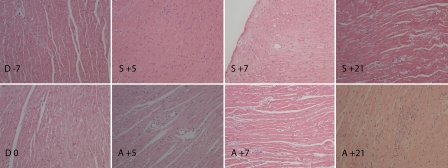
Conditioning regimen did not result in morphological changes in heart histology as assessed at day 0 (Figure 5). At day +5 no changes were observed in the allogeneic group, however, at day +7 occasional apoptotic cells, some loss of cardiomyocytes and subendocardial accumulation of neutrophils were observed. The inflammatory changes, apoptotic cells, vascular wall with loss of endothelial cells and granulocyte accumulation were observed at day +21. No such changes were observed in the syngeneic transplanted mice throughout the study.
Figure 5.
Histology of heart from conditioning start until day +21 post HSCT. Conditioning regimen did not affect microscopic morphology (D 0) compared to untreated animals (D -7). In allogeneic group, no changes were observed at day +5 (A +5), while occasional apoptotic cells, some loss of cardiomyocytes and subendocardial accumulation of neutrophils were observed at day +7 (A +7). The inflammatory changes, apoptotic cells, vascular wall with loss of endothelial cells and granulocytes accumulation were observed at day +21 (A +21). None of these changes were observed in syngeneic transplanted group at day +5, +7 and +21 (S +5, S +7, S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×20.
Kidney
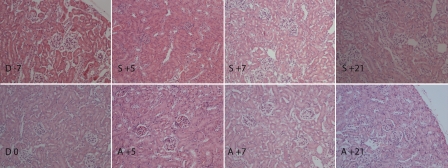
No apparent abnormity in renal structure was observed after conditioning regimen (Figure 6). Neither allogeneic, nor syngeneic transplanted mice developed any apparent changes detectable with histopathological examination until day +21.
Figure 6.
Histology of kidney from conditioning start until day +21 post HSCT. No apparent abnormity in renal structure was observed after conditioning regimen (D 0) compared to untreated animals (D -7). Neither allogeneic, nor syngeneic transplanted mice developed any apparent changes detectable with histological examination at day +5, +7 and +21 (allogenenic group: A +5, A +7, A +21; syngeneic group: S +5, S +7, S +21). Day of stem cell injection is assigned as day 0 (D 0), days before and after stem cell injection are numbered with “-” and “+” sign, respectively. Hematoxylin and eosin staining, magnification ×20.
Discussion
GHVD remains a major complication to HSCT and its pathophysiology is not fully elucidated despite intensive research on this issue. Animal models are used for pathophysiological and histopathological studies on GVHD. In our previous study, we developed a mouse model of GVHD following conditioning regimen with Bu/Cy [9]. Recipient Balb/c and donor C57BL/6 mice are mismatched in MHC class I, which allows the full spectrum of clinically relevant GVHD and magnifies the pathophysiological events. GVHD following Bu/Cy was confirmed by histopathologic changes in skin, intestine and liver and was found to be CD8+ dependent. In the present study, we assessed the dynamics of morphological changes occuring in the major internal organs after conditioning regimen with Bu/Cy and during subsequent development of GVHD.
Pancreatic ductal changes found in autopsy material were related to acute GVHD in humans [12]. In another study on autopsy material, loss of granules and presence of inflammatory cells together with exocrine ducts epithelial cellular atypia were almost pathognomonic for acute GVHD, while no morphological changes were observed in Langerhans islets [13]. In our model, progressive loss of apical granules was observed in the allogeneic group within the first week after transplantation together with apoptotic cell forms in both exocrine and endocrine part of pancreas. Three weeks after transplantation the findings varied from almost complete recovery to extensive inflammation, fibrotic changes and signs of vasculitis. However, no changes in ductal epithelia were found. Since morphological changes were found neither after the end of conditioning nor after transplantation in syngeneic transplanted animals, we suggest that Bu/Cy does not affect the pancreatic endocrine or exocrine tissue and the observed changes in the present investigation are associated mainly with acute GVHD and/or alloreactivity.
Liver GVHD has not been well characterized by histopathological changes. Serum bilirubin levels are used as a clinical correlate to liver GVHD. In human liver material, bile duct damage characterized by cytoplasmic vacuolation and lymphocyte infiltration of ductal epithelium has been associated with acute and chronic GVHD. Lobular hepatitis, inflammation, lymphocyte infiltration and mild endothelial fibrosis as well as portal inflammation have also been reported in chronic GVHD [14]. In a mouse model of acute GVHD following irradiation, the changes in bile ducts were observed together with vasculitis, hepatocellular damage and infiltration of portal tract one month after transplantation [15]. These data are only partially in agreement with our observation. No bile duct damage was observed and the vascular involvement with accumulation of inflammatory cells adjacent to central veins was found within the first week after allogeneic HSCT together with apoptotic cell forms. However, three weeks after transplantation, signs of vasculitis were observed at the portat triads. The difference in mice strains used in these models and/or difference in conditioning regimens might explain differences in alloreactive response.
During foetal development, the liver is a major site of hematopoiesis. Postnatally, hematopoiesis may be activated in liver under increased demand. Extramedular hematopoiesis was detected from day +5 in both syngeneic and allogeneic transplanted animals, however, more extensively in the allogeneic transplated mice. Three weeks after transplantation, extrame-dullar hematopoiesis persisted in both groups, but was higher in allogeneic compared syngeneic transplanted mice. This might be explained by poor recovery of hematopoiesis in bone marrow of mice suffering from GVHD [10].
Conditioning regimen per se had little effect on morphology in the studied organs except for a decrease in liver glycogen accumulation and decreased spleen cellularity. Decrease in intra-cellular glycogen may be explained by weight loss and lower food intake due to high emeto-genic potential of high dose cyclophosphamide. However, we did not determine food intake and thus, we can only speculate on this matter, although the reduced weight is highly suggestive. In the spleen, further decrease in cellularity of white pulp was observed in both syngeneic and allogeneic groups within the first post-transplant week. At three weeks post transplant, the syngeneic transplanted animal showed recovery in spleen architecture and cellularity, while only partial recovery with high inter-individual variability was observed in allogeneic transplanted animals. These findings are in agreement with the findings of Rappaport et al in mouse model of acute GVHD following irradiation conditioning [16].
Inflammatory changes with adherence of granulocytic cells in small lung veins were observed within one week after transplantation in allogeneic group. Three weeks after transplantation the histological findings in the allogeneic group varied from almost normal to inflammation with granulocytic infiltrates, nuclear dust and foamy macrophages, and subpleural fibrotic changes with accumulation of granulocytes. In the mouse model of idiopathic pneumonia syndrome following irradiation conditioning only minor histopathological changes in the form of slight edema in interstitial tissue with little or no infiltration of inflammatory cells were found 3 weeks after transplantation [17]. Twelve weeks after transplantation mice suffering from GVHD displayed prominent perivascular and peribronchiolar inflammation, diffuse infiltrates of mononuclear cells throughout the alveolar inter-stitium and focal organizing inflammatory lesions. Radiation intensity has been shown to play a significant role in the development of lung disease in this model [18]. Busulphan has also been implicated in the development of interstitial pneumonitis (busulphan lung) after long treatment with low doses [19]. Increased risk for idiopathic pneumonia syndrome has also been associated with acute and hyper acute GVHD in children [20]. Our results show early inflammatory changes in the lung following allogeneic transplantation.
The heart is generally accepted as a non-target organ for acute GVHD. In a mouse model of acute GVHD reported by New et al, infiltrating lymphocytes were only rarely detected in the heart [4]. However, in our study, allogeneic transplanted animals displayed varied changes in heart histology. Apoptotic cell forms, some loss of cardiomyocytes and subendocardial accumulation of neutrophils were found at day +7 and progressed until day +21. Conditioning containing cyclophosphamide might be a possible explanation, since cyclophosphamide has been reported to induce cardiotoxicity in humans within 2 – 3 weeks after high-dose treatment [21]. Histological changes related to cyclophosphamide cardiotoxicity include interstitial, myocardial, and vascular changes [22]. In a rat model on cyclophosphamide-induced cardiotoxicity histopathological changes include edema, leukocytic infiltration, muscle necrosis, chronic inflammation, and fibrosis [23]. Acute GVHD has been also implicated in myocardium damage in autopsy material [24].
No inflammatory infiltrates were found in kidneys from of allogeneic transplated animals suffering from GVHD until the end of the study. This is opposite to finding in mouse model of acute GVHD with disparity in MHC class I [25]. In that model, focal mononuclear interstitial infiltrates were usually found in the perivascular areas but occasionally in the peritubular and periglomerular areas accompanied by increased expression of Ia antigens. In another model with disparity in MHC class I the renal changes were of mild character at two weeks after transplantation, but progressed until 3 and 6 months [26]. However, in this model, no conditioning regimen has been used and thus, tissue damage and cytokine storm triggering immunological cascade resulting in acute GVHD has been omitted. This certainly affects the kinetics of GVHD. Moreover, the major changes presented at 3-6 months would be defined as chronic GVHD.
In summary, we report histopathological changes in several major internal organs during the early stages of GVHD development. Vasculitis and inflammatory infiltrates were found in pancreas, liver, lung and heart, i.e. target and non-target organs for GVHD. Surprisingly, no morphologically detectable changes were found in the kidney. Although many animal models of GVHD have been reported, histopathological criteria defining acute GVHD in specific organs are not completely defined. GVHD models differ in mouse strains used, intensity of radiation, amount and source of transplanted cells. These different conditions may affect the subtype of T cells mediating alloreactivity and dynamics of changes. Moreover, intensity of radiation in conditioning regimen affects the occurrence of idiopathic pneumonia syndrome [18] confirming the role of conditioning regimen in induction of tissue damage. Our mouse model is the first one reporting dynamics of histopathological changes during development of GVHD following conditioning regimen with Bu/Cy.
Acknowledgments
The authors would like to express their gratitude to Carin Lundmark for technical assistance with slides preparation. The study was supported by grants from Swedish Cancer Foundation (Cancerfonden) and The Swedish Childhood Cancer Foundation (Barncancerfonden).
References
- 1.Miano M, Faraci M, Dini G, Bordigoni P. Early complications following haematopoietic SCT in children. Bone Marrow Transplant. 2008;(41 Suppl 2):S39–42. doi: 10.1038/bmt.2008.53. [DOI] [PubMed] [Google Scholar]
- 2.Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75–101. doi: 10.1016/j.iac.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mapara MY, Leng C, Kim YM, Bronson R, Lokshin A, Luster A, Sykes M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–634. doi: 10.1016/j.bbmt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.New JY, Li B, Koh WP, Ng HK, Tan SY, Yap EH, Chan SH, Hu HZ. T cell infiltration and chemokine expression: relevance to the disease localization in murine graft-versus-host disease. Bone Marrow Transplant. 2002;29:979–986. doi: 10.1038/sj.bmt.1703563. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. 2003;78:181–187. doi: 10.1007/BF02983793. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Santos GW. Preparative regimens: chemotherapy versus chemoradiotherapy. A historical perspective. Ann N Y Acad Sci. 1995;770:1–7. doi: 10.1111/j.1749-6632.1995.tb31039.x. [DOI] [PubMed] [Google Scholar]
- 8.Petersen FB, Buckner CD, Appelbaum FR, Clift RA, Sanders JE, Bensinger WI, Storb R, Witherspoon RP, Sullivan KM, Bearman SI, et al. Busulfan, cyclophosphamide and fractionated total body irradiation as a preparatory regimen for marrow transplantation in patients with advanced hematological malignancies: a phase I study. Bone Marrow Transplant. 1989;4:617–623. [PubMed] [Google Scholar]
- 9.Sadeghi B, Aghdami N, Hassan Z, Forouzanfar M, Rozell B, Abedi-Valugerdi M, Hassan M. GVHD after chemotherapy conditioning in allogeneic transplanted mice. Bone Marrow Transplant. 2008;42:807–818. doi: 10.1038/bmt.2008.261. [DOI] [PubMed] [Google Scholar]
- 10.Sadeghi B, Al-Hashmi S, Hassan Z, Rozell B, Concha H, Lundmark C, Gronvik KO, Abedi-Valugerdi M, Hassan M. Expansion and activation kinetics of immune cells during early phase of GVHD in mouse model based on chemotherapy conditioning. Clin Dev Immunol. 2010;2010 doi: 10.1155/2010/142943. 142943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 12.Washington K, Gossage DL, Gottfried MR. Pathology of the pancreas in severe combined immunodeficiency and DiGeorge syndrome: acute graft-versus-host disease and unusual viral infections. Hum Pathol. 1994;25:908–914. doi: 10.1016/0046-8177(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 13.Foulis AK, Farquharson MA, Sale GE. The pancreas in acute graft versus host disease in man. Histopathology. 1989;14:121–128. doi: 10.1111/j.1365-2559.1989.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 14.Quaglia A, Duarte R, Patch D, Ngianga-Bakwin K, Dhillon AP. Histopathology of graft versus host disease of the liver. Histopathology. 2007;50:727–738. doi: 10.1111/j.1365-2559.2007.02679.x. [DOI] [PubMed] [Google Scholar]
- 15.Blatter DD, Crawford JM, Ferrara JL. Nuclear magnetic resonance of hepatic graft-versus-host disease in mice. Transplantation. 1990;50:1011–1018. doi: 10.1097/00007890-199012000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Rappaport H, Khalil A, Halle-Pannenko O, Pritchard L, Dantchev D, Mathe G. Histopathologic sequence of events in adult mice undergoing lethal graft-versus-host reaction developed across H-2 and/or non-H-2 histo-compatibility barriers. Am J Pathol. 1979;96:121–142. [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar G, Bryson JS, Jennings CD, Morris PE, Cohen DA. Idiopathic pneumonia syndrome in mice after allogeneic bone marrow transplantation. Am J Respir Cell Mol Biol. 1998;18:235–242. doi: 10.1165/ajrcmb.18.2.2988. [DOI] [PubMed] [Google Scholar]
- 18.Shankar G, Scott Bryson J, Darrell Jennings C, Kaplan AM, Cohen DA. Idiopathic pneumonia syndrome after allogeneic bone marrow transplantation in mice. Role of pretransplant radiation conditioning. Am J Respir Cell Mol Biol. 1999;20:1116–1124. doi: 10.1165/ajrcmb.20.6.3455. [DOI] [PubMed] [Google Scholar]
- 19.Hasleton PS. Busulphan lung: histopathology. Thorax. 1970;25:257. doi: 10.1136/thx.25.2.257-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant. 2006;38:285–289. doi: 10.1038/sj.bmt.1705436. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth A, Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–763. [PubMed] [Google Scholar]
- 22.Slavin RE, Millan JC, Mullins GM. Pathology of high dose intermittent cyclophosphamide therapy. Hum Pathol. 1975;6:693–709. doi: 10.1016/s0046-8177(75)80078-5. [DOI] [PubMed] [Google Scholar]
- 23.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, Klimberg VS. Oral glutamine protects against cyclophos-phamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–817. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Roberts SS, Leeborg N, Loriaux M, Johnson FL, Huang ML, Stenzel P, Thiede C, Godder KT. Acute graft-versus-host disease of the heart. Pediatr Blood Cancer. 2006;47:624–628. doi: 10.1002/pbc.20621. [DOI] [PubMed] [Google Scholar]
- 25.Wadgymar A, Urmson J, Baumal R, Halloran PF. Changes in Ia expression in mouse kidney during acute graft-vs-host disease. J Immunol. 1984;132:1826–1832. [PubMed] [Google Scholar]
- 26.Niculescu F, Niculescu T, Nguyen P, Puliaev R, Papadimitriou JC, Gaspari A, Rus H, Via CS. Both apoptosis and complement membrane attack complex deposition are major features of murine acute graft-vs.-host disease. Exp Mol Pathol. 2005;79:136–145. doi: 10.1016/j.yexmp.2005.03.007. [DOI] [PubMed] [Google Scholar]