Abstract
Immunohistochemical profiles of normal mesothelium and histiocytic/mesothelial hyperplasia (HMH) are unknown. A 19-year-old man was treated by thoracoscopic resection of bullae of left lung. Histologically, there were cell proliferative foci composed of round cells without significant atypia (histiocyte, mesothelium and T-lymphocytes). The cell proliferative foci were patch-like, and no invasive features were seen. Because it is composed of histiocytes, mesothelium, and T-lymphocytes, the diagnosis was HMH. Immunohistochemically, cell components of HMH showed the following immunoreactions: calrenitin 3+, D2-40 3+, pancytokeratin AE1/3 3+, pancytokeratin CAM5.2 3+, cy-tokeratin (CK) 34βE12 1+, CK5/6 1+, CK7 1+, CK8 3+, CK 14 1+, CK18 2+, CK19 2+, p53 10%, Ki67 20%, CD68 3+, CD45 2+, CD45 RO 2+, vimentin 3+, Ber-EP4 -, CK20 -, EMA -, desmin -, CEA -, CA19-9 -, TTF-1 -, S100 protein -, αsmooth muscle actin -, CD34 -, CD20 -, chromogranin -, synaptophysin -, NSE -, CDX2 -, CD56 -, HER2 -, MUC1 -, MUC2 -, MUC5AC -, and MUC6 -. The normal mesothelium showed the following immunoprofile: calrenitin 3+, D2-40 3+, pancytokeratin AE1/3 3+, pancytokeratin CAM5.2 3+, CK34βE12 3+, CK5/6 2+, CK7 2+, CK8 3+, CK 14 -, CK18 3+, CK19 2+, vimentin 1+, p53 -, Ki67 1%, CD68 -, CD45 -, CD45 RO -, Ber-EP4 -, CK20 -, EMA -, desmin -, CEA -, CA19-9 -, TTF-1 -, S100 protein -, α-smooth muscle actin -, CD34 -, chromogranin -, synaptophysin -, NSE -, CDX2 -, CD56 -, HER2 -, MUC1 -, MUC2 -, MUC5AC -, and MUC6 -. These findings indicate that the immunoprolfile of mesothelium in HMH was immunohistochemically very similar to that of normal mesothelium except for CD68, p53 protein, Ki-67 labeling, CD45 and CD45 RO. These indicate that the HMH was reactive phenomenon and HMH is composed of hyperplastic mesothelium, histiocytes and T-lymphocytes. The immunoprofile of normal mesothelium provide basic knowledge of mesothelial pathology.
Keywords: Mesothelium, histiocytic methothelial hyperplasia, immunohistochemical profile
Introduction
In the past and recent years, mesothelial pathology was focused on the distinction between malignant mesothelioma and lung cancers and metastatic carcinoma. Recently, benign mesothelial proliferative conditions are increasingly reported [1], because benign mesothelial conditions were occasionally difficult to distinguish from malignant mesothelioma [1-3]. Histiocytic/mesothelial hyperplasia (HMH) is one of such benign mesothelial lesions [4]. Only several cases of HMH have been reported [5, 6]. Immunoprofile of normal mesothelium is unknown, to the best of the author's knowledge. Here reported is a case of HMH with an emphasis on immunohistochemical findings. In addition, the author reports the immunoprofile of the normal mesothelium.
Case Report
A 19-year-old Japanese man consulted to our hospital because of breathing difficulty. Chest XP reveled left pneumothorax, and the patient was treated aspiration of left thoracic air. Imaging modalities including XP and CT revealed bullae in the apex and the left lung. The patient underwent segmental resection of the left upper lobe by video-assisted thoracoscopy. The patient recovered, and is now healthy 6 months after the operation. No tumors are found now.
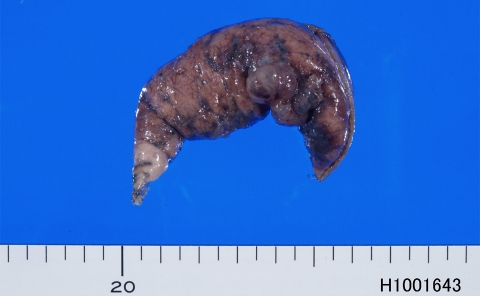
Grossly, the resected part of the lung showed bullae and white and black patches on the visceral pleura (Figure 1). Microscopically, the lung showed bullae and blebs. Abnormal patch-like tissues were seen on the visceral pleura (Figures 2A and 2B). They were composed of round cells with hyperchromatic nuclei (Figure 2C). Collagenization was little or none. No invasion into the lung was seen. No necrosis was seen. No mitotic figures were seen. It was found that the patch-like tissues were composed of histiocytes, mesothelial cells, and T-lymphocytes, as described later.
Figure 1.
Gross features of the resected pulmonary tissue. It shows bullae. In addition, the patches of black and white colors are present on the pleural surface.
Figure 2.
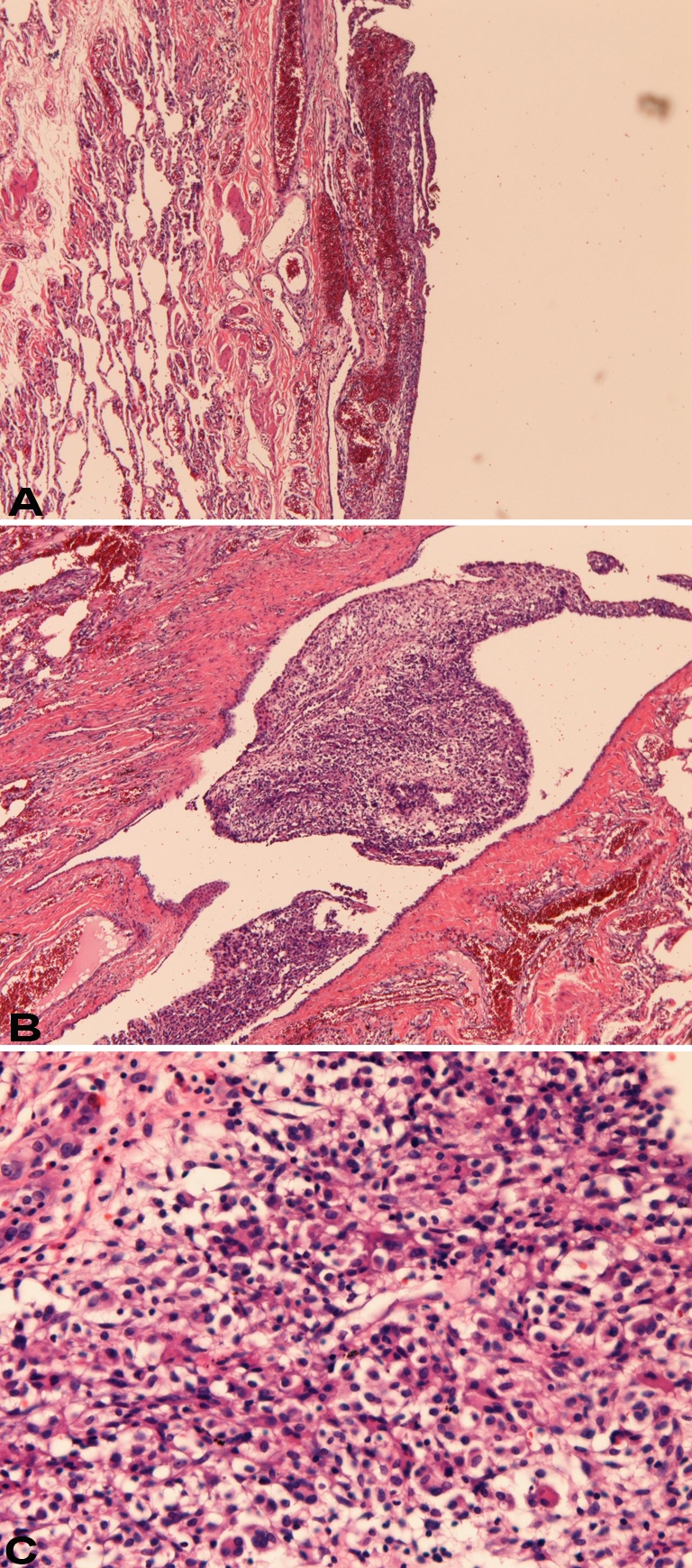
Histology of the pleural patches. A: The patches (left) are located on the visceral pleura. No invasion is seen. HE, × 40. B: This patch is located in the pleural indentation. No invasion is seen. HE, ×40. C: High power view of the patches. They show proliferation of round cells with hyperchromatic nuclei. Nucleoli are absent. Neither mitotic figures nor necrosis is seen. HE, × 200
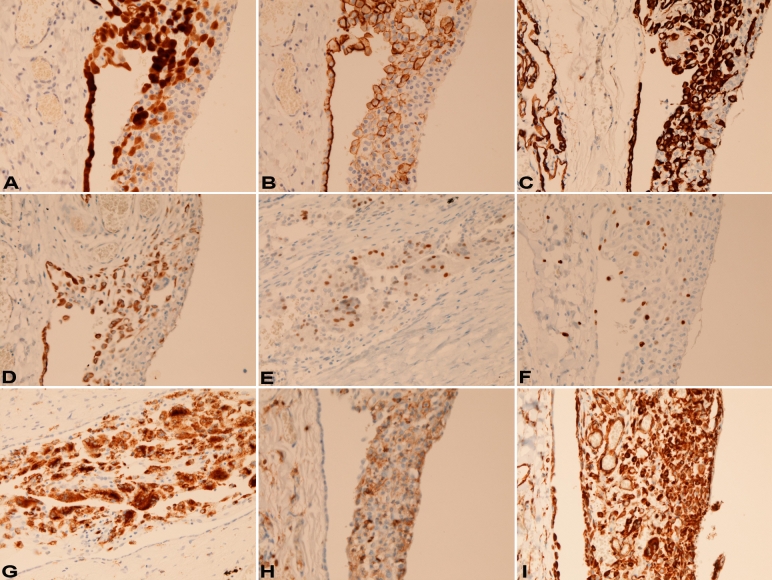
An immunohistochemical study was performed with the use of Dako Envision method (Dako, Glostrup, Denmark), as described previously [7,8]. Immunohistochemically, cell components of HMH showed the following immunoreactions: calrenitin 3+ (Figure 3A), D2-40 3+ (Figure 3B), pancytokeratin AE1/3 3+ (Figure 3C), pancytokeratin CAM5.2 3+, cytokeratin (CK) 34βE12 1+, CK5/6 1+ (Figure 3D), CK7 1+, CK8 3+, CK 14 1+, CK18 2+, CK19 2+, p53 10% (Figure 3E), Ki67 20% (Figure 3F), CD68 3+ (Figure 3G), CD45 2+, CD45 RO 2+ (Figure 3H), vimentin 3+ (Figure 3I), Ber-EP4 -, CK20 -, EMA -, desmin -, CEA-, CA19-9 -, TTF-1 -, S100 protein -, αsmooth muscle actin -, CD34 -, CD20 -, chromogranin -, synaptophysin -, NSE -, CDX2 -, CD56 -, HER2 -, MUC1 -, MUC2 -, MUC5AC -, and MUC6 - (Table 1). The normal or reactive mesothelium showed the following immunoprofile: calrenitin 3+, D2-40 3+, pancytokeratin AE1/3 3+, pancytokeratin CAM5.2 3+, CK34βE12 3+, CK5/6 2+, CK7 2+, CK8 3+, CK 14 -, CK18 3+, CK19 2+, vimentin 1+, p53 -, Ki67 1%, CD68 -, CD45 -, CD45 RO -, Ber-EP4 -, CK20 -, EMA -, desmin -, CEA-, CA19-9 -, TTF-1 -, S100 protein -, αsmooth muscle actin -, CD34 -, chromogranin-, synaptophysin -, NSE -, CDX2 -, CD56 -, HER2 -, MUC1 -, MUC2 -, MUC5AC - and MUC6 -. The immunoprofile of alveolar lining epithelium is shown in Table 1.
Figure 3.
A. The patch (right) is positive for calretinin. The mesothelium (left) is also positive. Immunostaining, ×200. B. The patch (right) is positive for D2-40. The mesothelium (left) is also positive. Immunostaining, ×200. C. The patch (right) is positive for pancytokeratin AE1/3. The mesothelium (left) is also positive. Immunostaining, ×200. D. The patch (right) is positive for cytokeratin 5/6. The mesothelium (left) is also positive. Immunostaining, ×200. E. The patch is positive for p53. Immunostaining, ×200. F. The patch (right) is positive for Ki-67 antigen (labeling=15%). The reactive mesothelium (left) is also positive only focally. Immunostaining, ×200. G. The patch (right) is positive for CD68. The mesothelium was negative. Immunostaining, ×200. H. The patch (right) is positive for CD45RO. The reactive mesothelium (left) is negative. Immunostaining, ×200. I. The patch (right) is positive for vimentin. The mesothelium (left) is also positive. Immunostaining, ×200.
Table 1.
Immunohistochemical reagents and results
| Antigens | Antibodies (clone) | Sources | Results | ||
|---|---|---|---|---|---|
| HMH | N | ALV | |||
| Pancytokeratin | AE1/3 | Dako Corp. Glostrup, Denmark | 3+ | 3+ | 3+ |
| Pancytokeratin | CAM5.2 | Beckton-Dickinson, CA, USA | 3+ | 3+ | 2+ |
| HMWCK | 34βE12 | Dako | 1+ | 3+ | - |
| Calretinon | DAK-Calret 1 | Dako | 3+ | 3+ | - |
| D2-40 | D2-40 | Nichirei, Tokyo, Japan | 2+ | 3+ | - |
| Epithelial antigen | Ber-EP4 | Dako | - | - | 2+ |
| CK5/6 | D5/16 | Dako | 1+ | 2+ | - |
| CK7 | N1626 | Dako | 1+ | 2+ | 3+ |
| CK8 | DC10 | Dako | 2+ | 3+ | - |
| CK14 | LL002 | Novocastra, Newcastle upon type, UK | 1+ | - | - |
| CK18 | DC10 | Dako | 3+ | 3+ | 3+ |
| CK19 | RCK 108 | Progen, Heidelberg, Germany | 2+ | 2+ | - |
| CK20 | K20.8 | Dako | - | - | - |
| EMA | E29 | Dako | - | - | 3+ |
| Vimentin | Vim 3B4 | Dako | 3+ | 1+ | - |
| Desmin | D33 | Dako | - | - | - |
| TTF-1 | 8G7G3/1 | Dako | - | - | 2+ |
| CEA | polyclonal | Dako | - | - | - |
| CA19-9 | NS19-9 | TBF | - | - | - |
| S100 protein | polyclonal | Dako | - | - | - |
| ASMA | 1A4 | Dako | - | - | - |
| CD34 | NU-4A1 | Nichirei, Tokyo, Japan | - | - | - |
| p53 protein | DO-7 | Dako | 10% | - | - |
| Ki-67 | MIB-I | Dako | 20% | 1% | 1% |
| Chromogranin | DAK-A3 | Dako | - | - | - |
| Synaptophysin | Polyconal | Dako | - | ||
| NSE | BBS/NC/VI-H14 | Dako | - | - | |
| CDX2 | CDX2-88 | Cell Marque, Rocklin CA | - | - | - |
| CD56 | UJ13A | Dako | - | - | - |
| CD68 | KP-1 | Dako | 3+ | - | - |
| CD45 | LCA | Dako | 2+ | - | - |
| CD20 | L26 | Dako | - | - | - |
| CD45RO | UCHL-1 | Dako | 1+ | - | - |
| HER2 | PN2A | Dako | - | - | - |
| MUC-1 | Ma695 | Novocastra | - | - | - |
| MUC-2 | Ccp58 | Novocastra | - | - | - |
| MUC-5AC | CLH-2 | Novocastra | - | - | - |
| MUC-6 | CLH5 | Novocastra | - | - | - |
+++ strongly positive. ++ moderately positive; + weakly positive; -, negative. HMH, histiocytic/masothelial hyperplasia. N, normal mesothelium. ALV, Alveolar lining epithelium. HMWCK, high molecular weight cytokeratin. CK, cytokeratin. TTF-1, thyroid transcriptional factor-1. EMA, epithelial membrane antigen. CEA, carcinoembryonic antigen. CA19-9, carbohydrate antigen 19-9. ASMA, α-smooth muscle antigen. NSE, neuron-specific enolase.
Discussion
Benign mesothelial proliferation is important because the distinction between malignant mesothelioma and benign mesothelial proliferation is occasionally difficult, particularly in small biopsy specimens [1-3]. HMH belongs to the spectrum of such benign mesothelial proliferations [4-6]. Benign mesothelial proliferations are often seen in places of pleural effusion, ascites, pleural inflammatory diseases, hernia sacs, hydrocele, and pneumothorax [1]. Histopathologically, stromal invasion is the most important [1,2]; the presence of stromal invasion is seen mostly in mesothelioma and not seen in reactive mesothelial proliferation. Other aspects of differential diagnosis are necrosis, cellularity, inflammatory conditions, atypia, location, and mitosis; however, atypia, high celularity and mitotic figures are recognized in benign mesothelial proliferations [1,2]. In the present case, the mesothelial proliferation was seen as patch-like tissues composed of cellular round cell aggregates on the visceral pleura. No invasive features were seen. The cellularity was high, but no mitotic figures were recognized. No necrotic areas were seen. Therefore, the present case belongs to the spectrum of benign mesothelial proliferation.
In the present case, the lesions were composed of round cells with hyperchromatic nuclei. Immunohistochemitry revealed that the cellular elements were composed of CD68-positive histiocytes, CD45-positive and CD45RO positive T-lymphocytes, and mesothelial cells positive for calretinin, D2-40, and various cytokeratins. Because the lesions were composed of histiocytes and mesothelial cells, the diagnosis was HMH. HMH has been reported only several times [5,6]. Thus, the present HMH is very rare.
Several immunohistochemical studies have been performed to differentiate between benign mesothelial proliferations and malignant mesothelioma. Of these, the most important are EMA and desmin immunoreactivity [2]. Other markers includes p53, GLUT-1, K-i67 labeling, bcl-2, p-glycoprotein. Attanoos et al [9] described that immunoreactivities of desmin, EMA and p53 were 34/80 (85%), 8/40 (20%) and 0/40 (0%), respectively, in benign mesothelial proliferations. In contrast, immunoreactivities of desmin, EMA and p53 were 6/60 (10%), 48/60 (80%) and 27/60 (45%), respectively, in mesothelioma. [9]. That is, high expression of desmin, low expression of EMA, and very low expression of p53 are in favor of benign mesothelial proliferations. Other indicators for benign mesothelial proliferations are low expressions of GLUT-1 [10], AgNOR [11], repp86 [12], and telomerase [13]. In the present case, the expressions of desmin and EMA were negative. P53 expression was positive (labeling=10%). The Ki-67 labeling was 20%. Thus, the present case is immunohistochemically not determined with regard to benign or malignant nature.
The present study examined a wide range of immunoreactivities of various antigens in HMH. Such a study has not been performed. It was found that HMM showed wide range of CK reactivity, mesothelioma markers (calretinin, D2-40, CK5/6), CD68, and T-cell markers. It is interesting that the CK profile in HMH was very similar to that of the normal mesothelium, suggesting that CK profile does not alter after mesothelial proliferation. The positive mesothelioma markers (calretinin, D2-40, CK5/6) in HMH indicate that these antigens are not useful in the distinction between reactive mesothelial proliferation and malignant mesothelioma. The positive CD68 indicated that histiocytes or macrophages predominantly accumulated in HMH. The presence of T-cell markers shows that the lymphocytes in HMH were predominantly of T-lymphocytes.
The pathogenesis of HMH in the present case seems to be chronic irritation and chronic inflammation of the mesothelium by bullae and bulla rupture. The following scenario is probable. First, the bullae irritate the mesothelial cells. Rupture of the bullae facilitates this irritation. The irritation cause acute and chronic inflammation, which cause mesothelial proliferation. Due to the inflammation, inflammatory cells of histiocytes and T-lymphocytes accumulate in the lesion, thus creating HMH. The absence of neutrophils in the present HMH may imply that recent irritation is absent or that neutrophilic infiltration is not a phenomenon on serosal inflammation. The absence of fibrosis may denote that the inflammation is not strong or the inflammatory period is not long. The absence of B-lymphocytes in the lesions suggests that T-cell immunity is the major immune response in the serosal membrane.
To the best of the author's knowledge, immunoprofile of normal or reactive mesothelial proliferations has not been studied. The present study shows that normal and reactive mesothelial cells express mesothelioma markers (calretinin, D2-40, CK5/6), various CKs, vimentin, Ki-67 antigen (Table 1). These data may provide basic knowledge in mesothelial pathobiology. For example, the present case showed that the mesothelium was negative for MUC1, MUC2, MUC5AC, and MUC6 gene product. As is well known, the mesothelium produces acidic mucin, suggesting that the mucin production is not associated with genes of MUC1, MUC2, MUC5AC, and MUC6.
In summary, the author reported a case of HMH with an emphasis of immunohistochemical findings. The immunoprofile of normal mesothelium provide basic knowledge of mesothelial pathology.
References
- 1.Churg A, Cagle PT, Roggli VL. AFIP Atlas of tumor pathology. Series 4. Tumors of the serosal membrane. Maryland: ARP Press, Silver Spring; 2006. Separation of benign and malignant mesothelial proliferations; pp. 83–101. [Google Scholar]
- 2.Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathological diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 3.Chung A, Colby TV, Cagle P, Corson J, Gibbs AR, Grimes M, Hammar S, Roggli V, Travis WD. The separation of benign and malignany mesothelial proliferations. Am J Surg Pathol. 2000;24:1183–1200. doi: 10.1097/00000478-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Churg A, Cagle PT, Roggli VL. Hystiocytic/mesothelial hyperplasia. AFIP Atlas of tumor pathology. Series 4. Tumors of the serosal membrane. Maryland: ARP Press, Silver Spring; 2006. Separation of benign and malignant mesothelial proliferations; pp. 136–137. [Google Scholar]
- 5.Rosai J, Dehner LP. Nodular mesothelial hyperplasia in hernia sacs: a benign reactive condition simulating a neoplastic process. Cancer. 1975;35:165–175. doi: 10.1002/1097-0142(197501)35:1<165::aid-cncr2820350122>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Ordonez NG, Ro JY, Ayala AG. Lesions described as nodular mesothelial hyperplasia are primary composed of histiocytes. Am J Surg Pathol. 1998;22:285–292. doi: 10.1097/00000478-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 8.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 9.Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguish reactive from neoplastic mesothelium: a novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and bcl-2. Histopathology. 2003;43:231–238. doi: 10.1046/j.1365-2559.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- 10.Kato Y, Tsuta K, Seki K, Maeshima AM, Watanabe S, Suzuki K, Asamura H, Tsuchiya R, Matsuno Y. Immunohistochemical detection of GLUT-1 can discriminate between reactive mesothelium and malignant mesothelioma. Mod Pathol. 2007;20:215–220. doi: 10.1038/modpathol.3800732. [DOI] [PubMed] [Google Scholar]
- 11.SoosayGN, Griffiths M, Papadaki L, Happerfield L, Bobrow L. The differential diagnosis of epithelial-type mesothelioma from adenocarcinoma and reactive mesothelial proliferation. J Pathol. 1991;163:299–305. doi: 10.1002/path.1711630406. [DOI] [PubMed] [Google Scholar]
- 12.Taheri ZM, Mehrafza M, Mohammadi F, Khoddami M, Bahadori M, Masjedi MR. The diagnostic value of Ki-67 and repp86 in distinguishing between benign and malignant mesothelial proliferation. Arch Pathol Lab Med. 2008;132:694–7. doi: 10.5858/2008-132-694-TDVOKA. [DOI] [PubMed] [Google Scholar]
- 13.Cakir C, Gulluoglu MG, Yilmazbayhan D. Cell proliferation rare and telomerase activity in the differential diagnosis between benign and malignant mesothelial proliferations. Pathology. 2006;38:10–15. doi: 10.1080/00313020500456017. [DOI] [PubMed] [Google Scholar]