Abstract
Background
Patients who have completed Phase II cardiac rehabilitation have low rates of maintenance of exercise after program completion, despite the importance of sustaining regular exercise to prevent future cardiac events.
Purpose
The efficacy of a home-based intervention to support exercise maintenance among patients who had completed Phase II cardiac rehabilitation versus contact control was evaluated.
Design
An RCT was used to evaluate the intervention. Data were collected in 2005–2010 and analyzed in 2010.
Setting/participants
One hundred and thirty patients (mean age = 63.6 years [SD=9.7], 20.8% female) were randomized to exercise counseling (Maintenance Counseling group, n=64) or contact control (Contact Control group, n=66).
Intervention
Maintenance Counseling group participants received a 6-month program of exercise counseling (based on the Transtheoretical Model and Social–Cognitive Theory) delivered via telephone, as well as print materials and feedback reports.
Main outcome measures
Assessments of physical activity (7-Day PAR), motivational readiness for exercise, lipids and physical functioning were conducted at baseline, 6 and 12 months. Objective accelerometer data were collected at the same time-points. Fitness was assessed via maximal exercise stress tests at baseline and 6 months.
Results
The Maintenance Counseling group reported significantly higher exercise participation than the Contact Control group at 12 months (difference of 80 minutes, 95% CI 22,137). Group differences in exercise at 6 months were nonsignificant. The intervention significantly increased the probability of participants’ exercising at or above physical activity guidelines and attenuated regression in motivational readiness versus the Contact Control Group at 6 and 12 months. Self-reported physical functioning was significantly higher in the Maintenance Counseling group at 12 months. No group differences were seen in fitness at 6 months or lipid measures at 6 and 12 months.
Conclusions
A telephone-based intervention can help maintain exercise, prevent regression in motivational readiness for exercise and improve physical functioning in this patient population.
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability in men and women in the U.S. particularly among older Americans. 1 The survivors of myocardial infarctions, patients with stable angina, and those who had coronary bypass surgery are potential candidates for Phase II (outpatient) cardiac rehabilitation (CR) services. 2 CR programs aim at maintaining physical functioning and preventing second coronary events, cardiac re-hospitalizations, and cardiac disability among those with established coronary artery disease. 3 These are typically multi-factor, medically supervised programs that combine monitored exercise training, education on nutrition and medication use, and risk factor education to modify behaviors associated with cardiac disease. The Agency of Healthcare Policy and Research (AHCPR) 2 and the American Heart Association (AHA) 4 have endorsed the importance of regular, aerobic exercise for primary and secondary prevention of CVD. Despite the emphasis that continued exercise training is required to sustain improved exercise tolerance, 2 by 6 months only 30%–60% of patients report regular exercise. 5,6,7 These data are particularly troubling given that CVD is the nation’s foremost cause of mortality.
Prior studies have evaluated methods to improve maintenance of exercise after CR. These have included assignment to usual care or to five group counseling sessions. 8 Participants in the usual care group were 76% more likely than those in the intervention group to stop exercising 1 year later. In another study, two in-person exercise consultations and two support phone calls led to the maintenance of self-reported exercise but not to differential fitness outcomes (peak oxygen uptake, V02) at 12 months in the intervention versus control condition. 9 Patients enrolled in CR were offered a pedometer-based intervention plus four behavioral counseling telephone calls over 18 weeks in a third trial. 10 At 6 months, minutes of physical activity, number of activity sessions and number of walking sessions increased significantly in the intervention versus control group. However, there were no significant group differences in cardio-respiratory fitness. Finally, researchers tested the effects of using a diary of physical activities and quarterly group exercise sessions versus usual care at 1 year after either in- or out-patient CR. 11 Seventy-three percent of the intervention group reported regular physical activity (defined as at least 3 times/week for at least 30 minutes/day) versus 40% in the usual care group.
The goals of this study were to assess the effects of a theory-based 6-month exercise counseling intervention on maintenance of exercise behavior after completion of Phase II CR. The primary hypothesis was that the group receiving the exercise counseling intervention (Maintenance Counseling, MC) would report greater participation in exercise (weekly minutes of at least moderate-intensity exercise) at 6 months versus the Contact Control group (CC). The secondary aims were to assess the effects of the intervention on: (1) exercise behavior at 12 months; (2) meeting guidelines of at least 150 minutes/week of moderate-intensity physical activity 12, 13 at 6 months and 12 months; (3) stage of motivational readiness for exercise at 6 and 12 months; (4) fitness at 6 months, and e) lipid outcomes, c-reactive protein and self-reported physical functioning at 6 months and 12 months.
Methods
Design
In this RCT, 130 patients who had completed a Phase II CR program received brief advice from the CR case managers on the importance of maintaining exercise participation and were then randomized to either the MC or CC group. Assessments were conducted at baseline, 6 months and 12 months. IRBs at the Miriam Hospital (RI) and St. Anne’s Hospital (MA) approved the study. Data were collected in 2005–2010 and were analyzed in 2010.
Recruitment
CR patients who were scheduled to complete Phase II programs received an invitation to participate in the study from the case managers. A Research Assistant (RA) conducted a telephone screen among interested patients for eligibility, and if eligible and interested, obtained the patients’ written informed consent.
Patient eligibility criteria
Men and women aged ≥40 years (1) participating in supervised Phase II CR (generally a 12-week program that includes exercise training 3 times/week for about 90 minutes/session), (2) scheduled to complete Phase II CR in the next 4 weeks, (3) able to read and speak English, (4) providing consent for medical chart review to extract disease and treatment variables, (5) able to walk unassisted and (6) having access to a telephone.
The study was designed to have 80% power to detect a change of at least 75 minutes in favor of MC at 6 months (45-minute increase in MC vs 30-minute decrease in CC) based on a 2-tailed test at the 5% significance level with a common SD of 160 minutes and 72 participants per group after 20% attrition (n=144). Due to recruitment difficulties, the original study goal of 180 randomized participants could not be met.
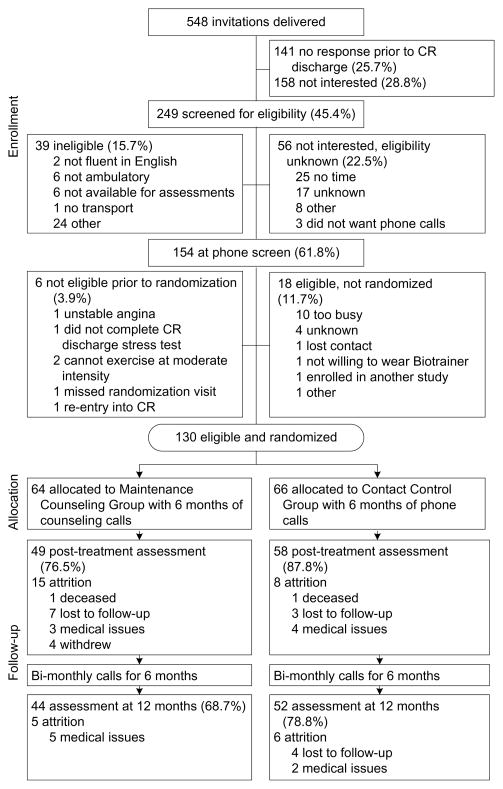
Five hundred and forty-eight patients were invited to participate: 249 were screened for eligibility, 158 were not interested, and 141 did not respond prior to CR discharge (see Figure 1). Of the 249 screened: 54 were not interested/eligibility unknown, 39 were not eligible, and 154 were eligible. Of the 154 eligible participants, 130 (84.4%) were randomized using a stratified scheme that ensure balance across strata defined by age (<65 years vs ≥65 years), gender, and cardiovascular risk (low, intermediate or high per AACVPR guidelines). 2
Figure 1.
Flow diagram of participant recruitment and randomization
CR, cardiac rehabilitation
Procedure
After baseline assessments were completed, the CR case managers were cued by a prompt placed on patients’ charts to deliver brief advice on the importance of adhering to the exercise prescription (based on a maximal stress test) received at CR discharge. Case managers attended a training session (up to 1 hour) on a brief motivational counseling protocol (based on the 5As counseling strategy). 14,15 After receiving brief advice from the case managers, participants were randomized.
Maintenance Counseling Group (MC)
Following randomization, the Intervention Coordinator reviewed the patient’s exercise prescription received at CR discharge. The participant was given home logs to monitor exercise participation and a pedometer (Digiwalker, Yamax Corporation, Tokyo, Japan) to wear during exercise activities that involved walking. Each participant received calls over 6 months (weekly over the first 2 months, bi-weekly for the next 2 months, and monthly for the last 2 months, a total of 14 calls) from the Intervention Coordinator to promote adherence to prescribed aerobic exercise. Activity counseling was based on the Transtheoretical Model 16 and Social Cognitive Theory 17 and tailored to each participant’s motivational readiness. 18 Action stage was defined as exercising at levels consistent with the exercise prescription provided at CR discharge. Specific components from motivational interviewing 19 were also included in the calls.
Participants reported on the exercise recorded on home logs and received feedback. After the 6-month intervention, bi-monthly phone calls were provided to prompt and reinforce regular physical activity. Participants were mailed an informational tip-sheet on exercise and one on cardiovascular health for each call during the 6-month program. Finally, a feedback letter summarizing the participants’ exercise progress and supporting motivation was sent to them at Weeks 4, 8, 12, 16, and 20. See Appendix A for details (available online at www.ajpmonlin.org).
Contact Control Group (CC)
To control for frequency of contact with the two groups, these participants also received calls from the Intervention Coordinators at the same intervals as MC participants over the entire study period. During these calls, the Symptom Questionnaire 20 was administered to monitor general health problems. The group also received tip-sheets on cardiovascular health (the same as those provided to MC participants). After completing the 12-month assessment, participants received the exercise tip-sheets. See Appendix A (available online at www.ajpmonline.org) for details.
Intervention delivery
All telephone calls to participants were audio-taped and 25% of these tapes were reviewed to ensure fidelity to intervention content and process.
Measures
At baseline, demographic information was obtained and disease and treatment variables were extracted from medical records. Participants’ body weight and height were measured on a calibrated scale to determine BMI. Participants received $20 for completing the assessments at each time-point. At baseline and follow-ups, they completed the following measures:
-
1a
Seven-Day Physical Activity Recall (7-Day PAR). 21 This valid interviewer-administered measure was developed for the Stanford Five City Project 22 and was administered by a RA blinded to participants’ group assignment. Weekly caloric expenditures are estimated based on the METs of tasks for the different activity classes. The primary outcome was the weekly minutes of at least moderate-intensity exercise which was analyzed as a continuous outcome and as a dichotomous indicator of whether study participants were able to meet physical activity guidelines (150 minutes/week). 12, 13
-
1b
Accelerometer Data: The IM Systems-3 dimensional accelerometer, the “Biotrainer-Pro” (Individual Monitoring Systems, Baltimore, MD) was used as an objective measure of exercise to validate the 7-Day PAR. 23 At baseline, 6 months and 12 months, participants were instructed to wear the monitor at the waist over the right hip, on 3 consecutive days (including at least 1 weekend day) during all waking hours, except when bathing or swimming. Exercise counts were converted to caloric expenditure after adjusting for participants’ weight.
-
2
Maximal Exercise Stress Test. All participants were asked to complete a graded maximal exercise stress test at study entry and at 6 months. These tests were conducted by staff blind” to the participants’ group assignment. The tests were administered on a treadmill using a Bruce protocol 24 with a Quinton exercise electrographic recorder for continuous ECG monitoring. 25, 26 Estimated peak oxygen uptake (peak VO2) was used to assess changes in fitness from study entry to 6 months.
-
3
Stage of Motivational Readiness for Exercise. This measure assesses an individual’s motivational readiness for exercise, is reliable and has concurrent validity with the 7-Day PAR. 27 It allows individuals to be classified into one of five stages: precontemplation, contemplation, preparation, action, and maintenance. Because participants were provided individualized exercise prescriptions that varied from physical activity guidelines for U.S. adults, 12, 13 regular activity was defined individually per the exercise prescription provided to the participant at CR discharge.
-
4
Lipids and inflammatory markers. Blood draws were conducted at baseline, 6 months and 12 months to obtain plasma lipid concentrations (low-density lipoprotein, high-density lipoprotein and total cholesterol) and inflammatory markers (c-reactive protein) to assess the effects of sustained exercise on these cardiac risk factors.
-
5
MOS 36-Item Short Form Health Survey (SF-36) 28, 29 assesses eight health concepts (e.g., physical functioning, bodily pain). Of these, the Physical Functioning subscale (PF) was analyzed as improvements on this subscale have been associated with exercise. 30
Analyses
Significance of the findings was consistently evaluated using 2-tailed tests conducted at the 5% significance level, with no adjustment for multiplicity. T-tests for continuous variables and χ2 tests for categoric variables were conducted to determine the success of the randomization procedure in balancing participant characteristics and baseline values of outcomes across groups (see Table 1). Similar analyses were used to compare retained participants versus dropouts. To monitor the intervention dose that was delivered, Wilcoxon rank-sum tests and t-tests were conducted to determine if there were significant group differences in the frequency and duration of telephone contact with research staff.
Table 1.
Sample characteristics at baseline (N=130)
| Characteristic | Category | Group
|
p-val | |||
|---|---|---|---|---|---|---|
| Maintenance Counseling | Control | |||||
| n | % | n | % | |||
| Gender | Male | 50 | 78.1 | 53 | 80.3 | 0.93 |
| Female | 14 | 21.9 | 13 | 19.7 | ||
| Race/Ethnicity | Non-Hispanic white | 61 | 95.3 | 61 | 92.4 | 0.86 |
| Non-Hispanic black | 2 | 3.1 | 2 | 3.0 | ||
| Other | 1 | 1.6 | 3 | 4.6 | ||
| Marital Status | Single | 3 | 4.7 | 1 | 1.5 | 0.65 |
| Married/Living with Partner | 47 | 73.4 | 51 | 77.3 | ||
| Divorced/Separated | 8 | 12.5 | 10 | 15.2 | ||
| Widowed | 6 | 9.4 | 4 | 6.0 | ||
| Educational Level | High School Diploma or Less | 11 | 17.2 | 16 | 24.2 | 0.67 |
| Vocational/Trade School | 3 | 4.7 | 6 | 9.1 | ||
| Some College | 19 | 29.7 | 16 | 24.2 | ||
| Bachelor Degree | 14 | 21.9 | 14 | 21.2 | ||
| Graduate School | 17 | 26.5 | 14 | 21.2 | ||
| Employment Status | Employed Full-Time | 28 | 43.8 | 25 | 37.9 | 0.56 |
| Employed Part-Time | 5 | 7.8 | 7 | 10.6 | ||
| Unemployed | 2 | 3.1 | 0 | 0.0 | ||
| Homemaker/Medical Leave | 5 | 7.8 | 7 | 10.6 | ||
| Retired | 24 | 37.5 | 27 | 40.9 | ||
| Household Income ($) | <39,999 | 18 | 31.0 | 18 | 29.5 | 0.24 |
| 40,000–79,999 | 14 | 24.2 | 23 | 37.7 | ||
| >80,000 | 26 | 44.8 | 20 | 32.8 | ||
| Age (years) | M (SD) | 62.9 | (9.3) | 64.3 | (10.0) | 0.42 |
| Motivational Readiness | Contemplation/Preparation | 12 | 18.8 | 10 | 15.5 | 0.75 |
| Action/Maintenance | 52 | 81.2 | 56 | 84.5 | ||
| physical activity guidelines | <150 PAR minutes/week | 20 | 31.3 | 27 | 40.9 | 0.34 |
| ≥ 150 PAR minutes/week | 44 | 68.7 | 39 | 59.1 | ||
| 7-Day PAR Exercise (minutes/week) | M (SD) | 233 | (199) | 199 | (138) | 0.25 |
| 7-Day PAR METs (kcals/week) | M (SD) | 234 | (13) | 233 | (11) | 0.42 |
| SF-36 PF | M (SD) | 80.1 | (19.2) | 77.6 | (18.0) | 0.45 |
| Stress Test VO2 Peak (ml/kg/min) | M (SD) | 30.6 | (6.4) | 30.1 | (5.3) | 0.65 |
| BMI | M (SD) | 29.0 | 5.3 | 29.2 | 5.1 | 0.81 |
| HDL | M (SD) | 38.3 | (9.3) | 40.9 | (10.3) | 0.14 |
| LDL | M (SD) | 74.05 | (22.6) | 82.3 | (29.7) | 0.09 |
| Total Cholesterol | M (SD) | 134.8 | (31.0) | 146.4 | (34.1) | 0.05 |
| Triglycerides | M (SD) | 111.8 | (60.4) | 113.1 | (54.9) | 0.91 |
| C-Reactive Protein | M (SD) | 3.1 | (5.9) | 3.9 | (10.0) | 0.61 |
| Anti-Lipid Medication | Prescribed | 54 | 84.4 | 56 | 84.8 | 0.87 |
| Not Prescribed | 10 | 5.6 | 10 | 5.2 | ||
| Anti-Hypertensive Medication | Prescribed | 55 | 85.9 | 56 | 84.8 | 0.94 |
| Not Prescribed | 9 | 14.1 | 10 | 15.2 | ||
| Type 2 Diabetes | Yes | 15 | 23.4 | 18 | 27.3 | 0.76 |
| No | 49 | 76.6 | 48 | 72.7 | ||
Note: Percentages have been calculated on study participants with available data.
Longitudinal trajectory modeling of continuous outcomes was conducted using Linear Mixed Effects (LME) models (Splus 8.2, Insightful Corporation, 2007). Continuous variables were standardized by subtracting their baseline mean and dividing by their baseline SD, so that regression coefficients would have an effect size interpretation, 31 allowing them to be compared across time points and outcomes. In these analyses, study group was coded as a binary indicator (CC=0, MC=1), while time was coded as a 2-level factor (6 months, 12 months). Time-specific effects of baseline values of the outcome and of study group were calculated at each follow-up. Subject-specific intercepts were used to accommodate within-subject correlation across time (see Table 2). Effect sizes were converted to the original measurement scale in Appendix B (available online at www.ajpmonline.org), which shows predicted longitudinal trajectories by study arm of “typical” participants assumed to have started with baseline values on the 7-Day PAR equal to the overall sample mean.
Table 2.
Longitudinal linear regression models predicting exercise, physical functioning, and fitness outcomes at follow-up
| Outcome
|
Regression Coefficient
|
Follow-up
|
|||||
|---|---|---|---|---|---|---|---|
| 6 Months
|
12 Months
|
||||||
| Value | SE | p-val | Value | SE | p-val | ||
| PAR Exercise | Control | −0.22 | 0.11 | 0.046 | −0.33 | 0.11 | 0.006 |
| Counseling vs Control | 0.19 | 0.16 | 0.26 | 0.47 | 0.17 | 0.008 | |
| Baseline Exercise | −0.48 | 0.08 | <0.001 | −0.49 | 0.08 | <0.001 | |
| PAR METs | Control | −0.16 | 0.09 | 0.06 | −0.29 | 0.09 | 0.003 |
| Counseling vs Control | 0.23 | 0.13 | 0.08 | 0.43 | 0.14 | 0.003 | |
| Baseline MET | −0.40 | 0.06 | <0.001 | −0.40 | 0.06 | <0.001 | |
| SF-36 PF | Control | 0.05 | 0.12 | 0.67 | −0.26 | 0.12 | 0.03 |
| Counseling vs Control | 0.06 | 0.18 | 0.71 | 0.42 | 0.18 | 0.02 | |
| Baseline SF-36 PF | −0.23 | 0.09 | 0.02 | −0.32 | 0.10 | 0.001 | |
| Stress Test VO2 Peak | Control | −0.02 | 0.06 | 0.74 | |||
| Counseling vs Control | 0.04 | 0.09 | 0.65 | ||||
| Baseline VO2 Peak | −0.06 | 0.05 | 0.17 | ||||
Note: Both baseline and follow-up values of all continuous have been standardized by subtracting off the baseline mean and dividing by the baseline SD of the entire sample. Standardized regression coefficients can be interpreted as effect sizes, and are comparable across outcomes and time-points. Control group estimates correspond to standardized within-group changes over time for “typical” participants reporting 7-Day PAR values at baseline equal to the overall sample mean (216 minutes).
Analyses of longitudinal binary outcomes were based on the Generalized Estimating Equation (GEE) capabilities available in the Correlated Data library (Splus 8.2, Insightful Corporation, 2007). Logistic regression models with a working independence correlation matrix were used to estimate time-specific effects of baseline exercise levels and of study group on the odds of meeting or exceeding physical activity guidelines at follow-ups (Table 3). These odds were then converted to the probability scale for participants who either met or failed to meet physical activity guidelines at baseline (Appendix C, available online at www.ajpm-online.org) allowing intervention effects to be assessed in both relative and absolute terms. Analyses of movement into Action/Maintenance were based on the same GEE procedure but were stratified on baseline stage of change (Contemplation/Preparation vs Action/Maintenance).
Table 3.
Longitudinal logistic regression models predicting odds of meeting physical activity guidelines and achieving Action/Maintenance at follow-up
| Outcome
|
Regression Coefficient
|
Follow-up
|
|||
|---|---|---|---|---|---|
| 6 Months
|
12 Months
|
||||
| AOR | 95% CI | AOR | 95% CI | ||
| physical activity guidelines | Control | 0.35 | (0.17, 0.73) | 0.40 | (0.18, 0.92) |
| Counseling vs Control | 1.50 | (0.69, 3.26) | 2.23 | (0.89, 5.60) | |
| Baseline PAR ≥150 | 5.30 | (2.33, 12.06) | 4.97 | (1.95, 12.68) | |
| Stage | Control: Men | 0.46 | (0.17, 1.26) | 0.22 | (0.07, 0.72) |
| Control: Women | 0.15 | (0.04, 0.58) | 0.24 | (0.06, 0.88) | |
| Counseling vs Control | 1.92 | (0.89, 4.16) | 2.57 | (1.12, 5.89) | |
| Baseline Stage = A/M vs Con/Prep | 2.35 | (0.87, 6.40) | 2.63 | (0.81, 8.53) | |
Note: A/M, Action/Maintenance; Con/Prep, Contemplation/Preparation physical activity guidelines analyses use baseline PAR <150 minutes/week as the reference stratum.
Stage of motivational readiness for exercise analyses use Contemplation/Preparation as the reference stratum.
Control group estimates correspond to odds of a positive outcome within the corresponding reference stratum.
For missing data, the LME models used likelihood-based estimation methods that use all available data to produce consistent estimates of the regression coefficients. 32, 33 Although these approaches are sensitive to drop out patterns that depend on the missing outcome itself, they are superior to completers-only analyses or ITT approaches that assign a pre-specified score to the missing data.
Results
Sample Characteristics
One hundred and thirty participants (mean age =63.6 years, 20.8% female, 75.4% married/partnered, 93.8% non-Hispanic white) were randomized to either MC (n=64) or CC (n=66). At baseline, there were no significant between-group differences on demographic variables, and primary and secondary outcomes (see Table 1).
There were no instances of adverse effects related to study participation in either group. Thirty-four participants withdrew or were dropped from the trial (n=20 in MC, n=14 in CC). Attrition in the CC group was quite low across follow-ups (n=8 at 6 months, n=6 at 12 months). In contrast, the MC group experienced larger dropout at 6 months (n=15) with fewer losses thereafter (n=5). At 6 months, within-group comparisons of MC dropouts with retained participant showed that gender was the only variable approaching significance, with attrition among women more than double that of men (42.9% vs 18.0%, p=0.06). Hence, gender and its interactions with follow-ups were included as additional covariates in the regression models of all outcomes. However, these effects did not attain significance for any outcome except stage of motivational readiness (all other p’s >0.10) and were omitted from the presentation of results.
Validation of Self-reported physical activity
7-Day PAR weekly exercise of at least moderate intensity were compared to the corresponding measures collected via the Biotrainer. Significant Spearman rank correlations were obtained at all three assessments, ranging from 0.50 at baseline to 0.22 and 0.32 respectively at 6 months and 12 months (all p’s <0.03).
Longitudinal Regression Models
At 6 months, group differences in weekly minutes of moderate-intensity exercise (7-Day PAR) were nonsignificant (MC exceeded CC by δ=0.19 standard units, p=0.26) (Table 2). This initially weak intervention effect was strengthened to moderate levels at 12 months (δ=0.47, p=0.008). The CC group showed significant decreases in physical activity from baseline to 6 months (δ= −0.22, p=0.046) that accelerated at 12 months (δ= −0.33, p=0.006) for study participants reporting average exercise levels at baseline (M=216, SD=171). Combining the estimates of CC changes from baseline and MC versus CC differences at each follow-up to obtain MC changes from baseline (not shown in Table 2) indicates that exercise levels of typical MC participants remained stable at 6 months (δ= −0.04, p=0.77) and showed a small increase at 12 months (δ=0.14, p=0.26).
These effect sizes translate into between-group differences of 32 minutes at 6 months (95% CI= −23, 86) that increased to 80 minutes at 12 months (95% CI= 22, 137) (Appendix B, available online at www.ajpmonline.org). Additionally, participants with low baseline levels of exercise benefitted disproportionally from study participation, with minutes of exercise at follow-up increasing by δ=0.48 standard units at 6 months for every standard unit by which a participant’s baseline exercise fell below the overall sample mean (p<0.001). These baseline physical activity main effects remained unchanged at 12 months (δ= −0.49, p<0.001). The related PAR energy expenditure measure (METs) also showed weak intervention effects at 6 months (δ=0.23, p=0.08) that reached moderate levels at 12 months (δ=0.43, p=0.003).
Given that analyses based on mean exercise levels are sensitive to the presence of outliers, a longitudinal logistic regression model was used, in which the binary response was an indicator of whether a study participant was able to meet or exceed physical activity guidelines (150 minutes/week) (Table 3). Although dichotomization of a continuous outcome reduced power and led to rather wide CIs, these analyses provide suggestive evidence that MC participants increased the odds of meeting physical activity guidelines at 6 months (AOR=1.50, 95% CI=0.69, 3.26; p=0.31) and 12 months (AOR=2.23, 95% CI=0.89, 5.60; p=0.09), after adjustment for differences in baseline physical activity.
In contrast to the 7-Day PAR results, participants meeting physical activity guidelines at baseline were five times more likely to do so at follow-up (AOR=5.30–4.97 by time-point). Although participants with initially high exercise levels regressed toward the baseline mean of 216 minutes/week at follow-up, they were still very likely to remain above 150 minutes/week. Logistic regression results were converted to the probability scale (Appendix C, available online at www.ajpmonline.org) to reveal intervention effects of 8%–9% at 6 months that increased to 15%–18% at 12 months, depending on initial exercise levels. Among participants who did not meet physical activity guidelines at baseline, 35% could be expected to achieve guidelines at 6 months if randomized to MC versus 26% if randomized to CC; at 12 months the corresponding proportions were 47% versus 29%.
Our final exercise outcome was stage of motivational readiness. Since a majority of participants entered the study in Action/Maintenance, the focus of MC was to prevent stage regression. Analyses were conducted after stratifying by baseline stage (Contemplation/Preparation versus Action/Maintenance) and controlling for gender effects across time: the latter was found to be significant at 6 months (p=0.037) but not at 12 months, (p=0.857). Table 3 reveals that the intervention improved the odds of a participant being in Action/Maintenance at 6 months (AOR=1.92, 95% CI=0.89, 4.16; p=0.099) after adjustment for baseline stage and gender, and that these beneficial intervention effects strengthened further at 12 months (AOR=2.57, 95% CI=1.12, 5.89; p=0.025).
Additionally, participants in Action/Maintenance at study entry were more than twice as likely to be in that stage at follow-up, compared to those in Contemplation/Preparation at study entry (AOR=2.35–2.65 by time-point). Translating these findings into a probability scale revealed a between-group difference in 12-month success rates of 18% (MC=36% vs CC=18%) for those in Contemplation/Preparation at baseline vs 23% (MC=59% vs CC=36%) for those in Action/Maintenance at baseline. Success rates among women were lower than among men at 6 months across groups but became statistically indistinguishable at 12 months.
Results of fitness tests conducted at 6 months showed no group differences in peak VO2 (Tables 2 and Appendix B). There were no significant group differences in lipids (HDL, LDL, total) and c-reactive protein at 6 months and 12 months (data not shown).
Finally, similar to the 7-Day PAR results, intervention effects on physical functioning (SF-36) were weak at 6 months (δ=0.06, p=0.71) but strengthened considerably at 12 months (δ=0.42, p=0.02). Table 2 reveals that between-group differences were due primarily to a significant deterioration in the CC group (δ= −0.26, p=0.03), compared with small improvement in the MC group (δ=0.16, p=0.23).
Intervention Delivery
There were no significant group differences in frequency of calls (p=0.149). As expected, the average call duration for the MC group was 15.8 minutes (SD=5.8), whereas calls to the CC group averaged 7.9 minutes (SD=2.5) (p<0.001).
Discussion
The goals of this study were to test the efficacy of a telephone-based exercise counseling program that targeted maintenance of exercise participation among patients who had completed CR. There was partial support for the current hypothesis that the intervention would promote increased maintenance, as mean group differences in exercise participation were nonsignificant at 6 months (32 minutes.), but became stronger and significant at 12 months (mean group differences=80 minutes.). The intervention was associated with a stronger likelihood of achieving physical activity guidelines and protection against regression in motivational readiness at 6 months and 12 months. Results did not support significant group differences in fitness at 6 months and in lipid measures and inflammatory markers at 6 months and 12 months.
At baseline, both groups reported exercise participation exceeding physical activity guidelines of 150 minutes/week. 12, 13 At 6 months and 12 months, the MC group reported a mean of 210 minutes and 240 minutes (regression adjusted values) versus 178 minutes and 160 minutes in the CC group. These mean values exceed physical activity guidelines but the differences became significant at 12 months indicating that as time from CR discharge increased, the CC group’s exercise decreased, as has been found in previous studies. 5,6,7 The correlations between accelerometer and 7- Day PAR interview data validated the self-reported exercise.
In comparing the current results with prior studies, baseline exercise among current participants was lower than in another trial where patients reported 275–300 minutes/week at baseline. 9 In that trial, a significant mean group difference of 130 minutes/week favoring the intervention group (which received two exercise consultations and two phone calls) was found at 12 months. In another trial testing the effects of a pedometer-based intervention (mean baseline exercise 324.2–366.8 minutes/week), an effect size of 0.43 for exercise participation was obtained at 6 months. 10 In the current study, the effect size at 6 months was 0.19 (nonsignificant), but did increase to 0.47 at 12 months. Thus, a more intensive intervention in the current study produced smaller-sized effects at follow-ups.
When considering physical activity guidelines (150 minutes/week), it appears that the individualized phone counseling did help patients achieve these recommendations, particularly among those who did not meet the guidelines at study entry. The latter subgroup was 35% more likely to exercise per guidelines at 6 months if assigned to MC (vs 26% if assigned to CC). These strong results contrast with another trial, where only 29% of intervention participants versus 27% of usual care group met physical activity guidelines at 12 months (baseline percentages of 44% and 34% respectively). 8
The current study targeted patients who had already achieved Action/Maintenance as a result of the exercise training they received in CR. Hence, the goal was to prevent regression. The intervention which was based on the Transtheoretical Model of behavior change and Social Cognitive Theory was clearly effective in preventing such regression. If assigned to MC, the participants’ odds for being in Action/Maintenance at 6 months were twice as high as those in CC. The effects were further strengthened in favor of MC at 12 months. CC participants regressed in their motivational readiness despite receiving an equivalent number of calls from the research staff.
Consistent with prior studies that also failed to detect significant group differences in fitness at 6 months 10 and at 12 months, 9 group differences in fitness at 6 months were not found. One might speculate that such differences might have been detected had participants repeated fitness tests at 12 months, the time-point at which group differences in exercise participation, meeting physical activity recommendations and motivational readiness were stronger.
The absence of group differences in lipids was not surprising because the baseline lipid values were quite low reflecting floor effects that may be associated with lipid-lowering medications. Consistent with exercise participation, self-reported physical functioning did not differ significantly between groups at 6 months, but the MC group reported significantly improved physical functioning at 12 months versus the CC group. Given the mean age of CR patients and associated comorbidities, it is noteworthy that physical functioning was enhanced by sustained levels of exercise.
Overall study attrition was 26.1% (n=34) with medical issues (e.g., repeat surgeries, re-hospitalizations) accounting for 41% of the attrition. Attrition in MC was higher in the first 6 months (n=15 vs n=8 in CC) and this may reflect the demands of the intervention. Other trials with less intensive intervention had lower attrition at 12 months (6%–19%). 8, 9, 11
The strengths of the study include a theory-based intervention delivered at home that was directed toward exercise maintenance, a conservative comparison group that controlled for frequency of contact, and the use of well-established measures of physical activity, fitness and motivational readiness. The current sample was relatively homogenous (race/ethnicity and education) thereby limiting the generalizability of results. As is typical of CR populations, female representation in the sample was low (20%) and more women than men dropped out in MC over the first 6 months. Although a telephone-delivered intervention places fewer burdens on participants, 54.5% of those who received a study invitation did not respond or were not interested in study participation. This suggests a limited reach of the intervention.
In sum, given the accelerating decline in exercise among patients after completing CR, there is a need to offer interventions for exercise maintenance to help offset the risk of future cardiac events in this population. The current home-based intervention that was delivered as designed helped to maintain exercise among MC participants, prevented regression in motivational readiness and improved their physical functioning at 12 months. Sustained exercise after CR will require interventions that have both efficacy and maximize participant retention in this patient population.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of the research staff (Lisa Breault, B.A., Kelly Greenwood, B.A., Cary Garcia, MBA, Jennifer Correia, B.A., and Carl Robitaille, BS. Loren Stabile, M.S. and the case managers at the Cardiac Fitness Center at the Miriam Hospital (RI), and Dr. Mark Gabry and the case managers at the Cardiac Fitness Center, St. Anne’s Hospital (MA). The study was approved by the IRB at the Miriam Hospital in 2003 and at St. Anne’s Hospital in 2007.
The study was funded by the National Heart, Lung and Blood Institute (HL 76734 to Dr. Pinto). The study is registered in Clinicaltrials.gov (NCT 00230724).
Footnotes
No financial disclosures were reported by the authors of this paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, DeSimone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics 2009 Update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;27;119(3):480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Wenger NK, Forelicher RN, Smith LK, Ades PA, Berra K, Blumenthal JA, et al. Cardiac rehabilitation as secondary prevention (AHCPR Publication No. 96–0673) Rockville, MD: DHHS; 1995. Clinical practice guidelines for clinicians: No. 17. [PubMed] [Google Scholar]
- 3.Balady GJ, Ades PA, Comoss P, Pina IL, Southard D, Williams MA, Bazzarre T. Core components of cardiac rehabilitation/secondary prevention programs: a statement for healthcare professionals from the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation Writing Group. Circulation. 2000;102(9):1069–73. doi: 10.1161/01.cir.102.9.1069. [DOI] [PubMed] [Google Scholar]
- 4.Thompson PD, Buchner D, Pina I, Balady GJ, Williams MA, Blair SN, Marcus BH, et al. Exercise and physical activity in cardiovascular disease: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, subcommittee on Exercise, Rehabilitation and Prevention and the Council on Nutrition, Physical Activity and Metabolism, subcommittee on Physical Activity. Circulation. 2003;107(24):e9053–4. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 5.Ades PA, Savage PD, Tischler MD, Poehlman ET, Dee J, Niggel J. Determinants of disability in older coronary patients. American Heart J. 2002;143 (1):151–6. doi: 10.1067/mhj.2002.119379. [DOI] [PubMed] [Google Scholar]
- 6.Bock BC, Carmona-Barros RE, Esler JL, Tilkemeier PL. Program participation and physical activity maintenance after cardiac rehabilitation. Behav Modif. 2003;27 (1):37–53. doi: 10.1177/0145445502238692. [DOI] [PubMed] [Google Scholar]
- 7.Hellman EA. Use of the stages of change in exercise adherence model among older adults with a cardiac diagnosis. J Cardiopulm Rehabil. 1997;17(3):145–55. doi: 10.1097/00008483-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Moore SM, Charvat JM, Gordon NH, Pashkow F, Ribisl P, Roberts BL, Rocco M. Effects of a CHANGE intervention to increase exercise maintenance following cardiac events. Ann Behav Med. 2006;31(1):53–62. doi: 10.1207/s15324796abm3101_9. [DOI] [PubMed] [Google Scholar]
- 9.Hughes AR, Mutrie N, MacIntyre PD. Effect of an exercise consultation on maintenance of physical activity after completion of phase III exercise-based cardiac rehabilitation. European J Cardiovascular Prevention Rehabil. 2007;14:114–121. doi: 10.1097/HJR.0b013e3280116485. [DOI] [PubMed] [Google Scholar]
- 10.Butler L, Furber S, Phongsavan P, Mark A, Bauman A. Effects of a pedometer-based intervention on physical activity levels after cardiac rehabilitation: A randomized controlled trial. J Cardiopulm Rehabil Prev. 2009;29(2):105–14. doi: 10.1097/HCR.0b013e31819a01ff. [DOI] [PubMed] [Google Scholar]
- 11.Arrigo I, Brunner-LaRocca H, Lefkovits M, Pfisterer M, Hoffmann A. Comparative outcome 1 year after formal cardiac rehabilitation: the effects of a randomized intervention to improve exercise adherence. European J Cardiovascular Prev Rehab. 2008;15:306–311. doi: 10.1097/HJR.0b013e3282f40e01. [DOI] [PubMed] [Google Scholar]
- 12.DHHS. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: CDC, National Center for Chronic Disease Prevention and Health Promotion. U.S. Government Printing Office; 1996. [Google Scholar]
- 13.DHHS. 2008 Physical Activity Guidelines for Americans. 2008 Retrieved August 25, 2009, from www.health.gov/paguidelines.
- 14.Goldstein MG, Pinto BM, Marcus BH, Jette AM, Rakowski W, McDermott S, et al. Physician-based activity counseling for middle-aged and older adults: A randomized trial. Ann Behav Med. 1999;21 (1):40–7. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 15.Pinto BM, Goldstein MG, Ashba J, Jette A, Sciamanna C. Physical activity counseling for older primary care patients. Am J Prev Med. 2005;29 (4):247–255. doi: 10.1016/j.amepre.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: Toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 18.Marcus BH, Simkin L. The stages of exercise behavior. J Sports Med. 1993;33:83–88. [PubMed] [Google Scholar]
- 19.Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 2000. [Google Scholar]
- 20.Winningham M. Developing the Symptom Activity 27: An instrument to evaluate perception of symptom effects on activity. Oncol Nurs Forum. 1993;20:330. [Google Scholar]
- 21.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a 7-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122 (5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 22.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS., Jr Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 23.Moser CD, Andersen RE, Verde TJ, et al. The use of a three dimensional (Bio-trainer) accelerometer to measure graded walking in obese women. Med Sci Sports Exerc. 1996;29:S44. [Google Scholar]
- 24.Bruce RA, McDonough JR. Stress testing in screening for cardiovascular disease. Bull N Y Acad Med. 1969;45 (12):1288–1305. [PMC free article] [PubMed] [Google Scholar]
- 25.American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Program: American Association of Cardiovascular and Pulmonary Rehabilitation. 3. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- 26.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 8. Baltimore: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- 27.Marcus BH, Rossi JS, Selby VC, Niaura RS, Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychol. 1992;11 (6):386–395. doi: 10.1037//0278-6133.11.6.386. [DOI] [PubMed] [Google Scholar]
- 28.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 30.Pinto BM, Trunzo JJ, Reiss P, Shiu S. Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psycho-Oncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: L. Erlbaum; 1988. [Google Scholar]
- 32.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York, NY: J. Wiley; 2002. [Google Scholar]
- 33.Daniels MJ, Hogan JW. Missing data in longitudinal studies. Boca Raton: FL: Chapman & Hall/CRC; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.