SUMMARY
Angiogenin is a stress-activated ribonuclease that cleaves tRNA within anticodon loops to produce tRNA-derived stress-induced fragments (tiRNAs). Transfection of natural or synthetic tiRNAs inhibits protein synthesis and triggers the phospho-eIF2α independent assembly of stress granules (SGs), essential components of the stress response program. We show that selected tiRNAs inhibit protein synthesis by displacing eIF4G/eIF4A from uncapped>capped RNAs. tiRNAs also displace eIF4F, but not eIF4E:4EBP1, from isolated m7G cap. We identify a terminal oligoguanine motif that is required to displace the eIF4F complex, inhibit translation, and induce SG assembly. We show that the tiRNA-associated translational silencer YB-1 contributes to angiogenin-, tiRNA-, and oxidative stress-induced translational repression. Our data reveal some of the mechanisms by which stress-induced tRNA cleavage inhibits protein synthesis and activates a cytoprotective stress response program.
INTRODUCTION
Small non-protein-coding RNAs (ncRNAs; e.g., miRNA, piRNA) play important roles in regulating gene expression. A subset of these regulatory RNAs is derived from constitutive or stress-induced ribonucleolytic processing of mature tRNAs. Constitutive tRNA-derived 19–22 nucleotide ncRNAs are products of DICER (Cole et al., 2009) and the tRNA 3′-end processing enzyme RNase Z (Haussecker et al., 2010; Lee et al., 2009). Stress-induced tRNA-derived ~30 and ~40 nucleotide ncRNAs (designated 5′- and 3′-tiRNAs, respectively) are products of secreted ribonucleases (e.g., RNY1 in yeast (Thompson and Parker, 2009b) and angiogenin in human (Fu et al., 2009; Yamasaki et al., 2009)) that cleave within the anticodon loops of mature tRNAs (Thompson and Parker, 2009a). The phenomenon of stress-induced tRNA cleavage has been described in nutrient-deprived Tetrahymena thermophila (Lee and Collins, 2005), Streptomyces coelicolor (Haiser et al., 2008), and Trypanosoma cruzi (Garcia-Silva et al.), as well as in serum-deprived Giardia lamblia (Li et al., 2008), spore-forming Aspergillus fumigatus (Jochl et al., 2008), phosphate-depleted Arabidopsis thaliana (Hsieh et al., 2010), and oxidatively-stressed Saccharomyces cerevisiae (Thompson et al., 2008), and Homo sapiens (Thompson et al., 2008; Yamasaki et al., 2009).
Several observations suggest that stress-induced tRNA fragments can directly inhibit protein synthesis: 1) tRNA fragments found in the phloem sap of pumpkin plants inhibit in vitro translation in wheat germ extracts (Zhang et al., 2009), 2) transfection of 5′-, but not 3′-, tRNA fragments inhibit global translation in human U2OS cells (Yamasaki et al., 2009), and 3) transfection of 5′-, but not 3′-, tRNA fragments trigger the assembly of stress granules, cytoplasmic foci that are induced by inhibitors of translation initiation (Emara et al., 2010). The finding that angiogenin contributes to stress-induced translational repression (Yamasaki et al., 2009) suggests that tiRNAs help to reprogram protein translation during stress. Although the mechanism by which tiRNAs inhibit protein synthesis is not known, their ability to induce the assembly of stress granules (Emara et al., 2010) suggests that they target the translation initiation machinery.
Stress-induced translational repression is achieved by inhibiting the assembly of distinct pre-initiation complexes that combine to form the 48S initiation complex (Yamasaki and Anderson, 2008). Assembly of the 43S pre-initiation complex is inhibited by stress-activated kinases that phosphorylate eIF2α, a component of the eIF2:GTP:tRNAMet ternary complex. Assembly of the eIF4F pre-initiation complex is inhibited by stress-induced inactivation of the PI3K-mTOR pathway that phosphorylates 4E-BP1 to liberate the cap-binding protein eIF4E (Sonenberg and Hinnebusch, 2009). We have found that tiRNAs contribute to the displacement of eIF4G/A from capped and uncapped mRNA and eIF4E/G/A (eIF4F) from the m7G cap. Moreover, we have implicated YB-1, a translational repressor known to displace eIF4G from RNA and eIF4E/G/A from the m7G cap (Evdokimova et al., 2001; Nekrasov et al., 2003), in this process. We propose that tiRNAs cooperate with YB-1 to re-program translation in stressed cells.
RESULTS
Endogenous 5′-, but not 3′-, tiRNAs inhibit translation
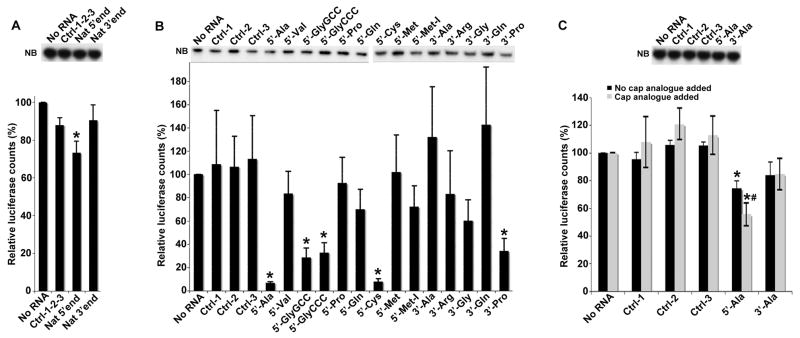
We previously showed that transfection of natural 5′-, but not 3′-, tiRNAs inhibits global translation in U2OS cells (Yamasaki et al., 2009). Similarly, stress-induced tRNA fragments found in the phloem sap of pumpkin plants (Cucurbita maxima) inhibit translation in vitro in wheat germ extracts (Zhang et al., 2009). We found that natural 5′-, but not 3′-, tiRNAs gel purified from angiogenin-treated U2OS cells significantly inhibit translation of uncapped luciferase transcripts in rabbit reticulocyte lysates (RRL) (Figure 1A). Northern blotting analysis confirmed that luciferase transcripts are not degraded under these conditions (Figure 1A, NB).
Figure 1.
5′-tiRNAs inhibit translation of mRNA reporters in vitro.
A. Natural 5′-tiRNAs. Uncapped Firefly luciferase mRNA (Promega) was translated in RRL in the presence of control RNA mix (Ctrl-1-2-3; derived from piRNAs or random sequences), natural 5′-tiRNAs (Nat 5′end) or natural 3′-tiRNAs (Nat 3′end). Luciferase expression is relative to that in the absence of RNA (No RNA =100%). Means and standard deviations are from three independent experiments (*-p = 0.01–0.02 (Table S1), Student’s t-test, n=3). NB: Northern Blotting for the luciferase mRNA reporter. For P-values, see Table S1.
B. Synthetic tiRNAs. Uncapped Firefly luciferase expression in the presence of synthetic 5′- and 3′-tiRNAs (100 picomoles/10 μl) as described in Fig. 1A. Means and standard deviations are from four independent experiments (*- p < 0.05 (Table SI), Student’s t-test, n=4). These reactions contain 50 ng/10 μl of uncapped Firefly RNA. NB: Northern Blotting for the luciferase mRNA reporter. See also Figure S1.
C. Synthetic 5′-tiRNAAla inhibits translation of capped Firefly luciferase mRNA. Capped Firefly luciferase RNA (10 ng/10 μl) was translated in RRL in the presence of indicated RNAs (100 picomoles/10 μl) in the absence (dark bars) or presence (light bars) of cap analogue (m7GpppG, 0.1mM). Means and standard deviations are from three independent experiments (*-p < 0.05 (Table SI); # p=0.04, comparing 5′-tiRNAAla in the absence or presence of the cap analogue, Student’s t-test, n=3). Cap analogue reduces the basal level of capped mRNA translation by ~2.5 fold. NB: Northern Blotting for the capped luciferase mRNA reporter.
Selected synthetic 5′-tiRNAs are potent inhibitors of translation
Since natural tiRNA preparations are contaminated with ribosomal and mRNA fragments (Thompson et al., 2008; Thompson and Parker, 2009b), we compared the activity of synthetic 5′-end phosphorylated 5′-tiRNAs (5′-tiRNAs) and unphosphorylated 3′-tiRNAs (3′-tiRNAs) (Emara et al., 2010) in this in vitro translation assay (Figure 1B). Although several tiRNAs significantly inhibit translation, 5′-tiRNAAla and 5′-tiRNACys are particularly potent translational repressors (Figure 1B). Northern blotting analysis confirmed that luciferase transcripts are not degraded under these conditions (Figure 1B, NB). A dose response analysis comparing 5′- and 3′-tiRNAAla reveals that 5′-tiRNAAla represses translation at low micromolar concentrations (Figure S1). Since the abundance of tiRNAs in angiogenin- or arsenite-treated cells is ~10 fold less than that of tRNA (intracellular tRNA concentration is estimated to be ~10–200 μM depending on cell type (Dr. Tao Pan, personal communication)), similar inhibitory concentrations of tiRNAs are likely to be found in stressed cells. Our calculations predict that the ratio of tiRNAs to mRNAs in stressed cells is ~8, consistent with their ability to repress translation in vivo (see legend to Figure S1). However, actual ratios of tiRNA, mRNA and translation initiation factors found in stressed cells is not known. 5′-tiRNAAla (but not 3′-tiRNAAla) also significantly inhibits the translation of capped luciferase transcripts (Figure 1C), and addition of cap analogue, which inhibits cap-dependent translation, significantly increases the relative translational repression. Northern blotting analysis confirmed that luciferase transcripts are not degraded under these conditions (Figure 1C, NB). Since translation of uncapped mRNAs, or capped mRNAs in the presence of cap analogue, depends on eIF4G binding to the 5′ terminus of mRNA (De Gregorio et al., 1998), these results suggest that 5′-tiRNAAla interferes with some functions of eIF4G (e.g., binding to mRNA or/and eIF4E).
EMCV-IRES mediated translation
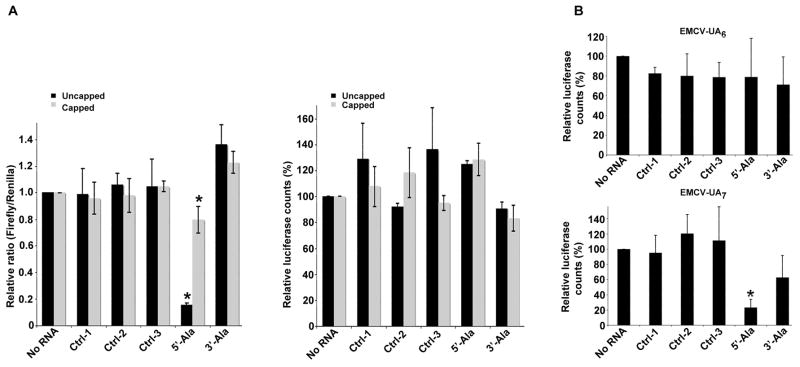
The EMCV-IRES initiates cap-independent translation by recruiting eIF4G and eIF4A to a site adjacent to the AUG initiation codon (Kolupaeva et al., 2003; Lomakin et al., 2000). To clarify the role of eIF4G in tiRNA-mediated translational repression, we quantified translation of capped or uncapped bicistronic reporter transcripts encoding an upstream Firefly luciferase and downstream EMCV IRES-driven Renilla luciferase in the absence or presence of tiRNAs (Figure 2A and Supplementary Table IB). 5′-, but not 3′-, tiRNAAla significantly reduces the Firefly/Renilla ratio (Figure 2A, left panel) without affecting the translation of Renilla luciferase (Figure 2A, right panel). This result indicates that 5′-tiRNAAla does not prevent the recruitment of eIF4G to this EMCV IRES. Because this result was not consistent with the ability of tiRNAs to preferentially inhibit the eIF4G-dependent translation of uncapped RNA, we extended this analysis to include an EMCV IRES variant that has reduced affinity for eIF4G and less efficient translation initiation (Bochkov and Palmenberg, 2006; Kaminski and Jackson, 1998). The recruitment of eIF4G to the EMCV IRES is critically dependent upon binding to the J-K domain which includes a UA6 bifurcation loop located upstream from the AUG start site (Hoffman and Palmenberg, 1996; Kaminski et al., 1994; Pestova et al., 1996). A variant bifurcation loop (UA7) reduces the binding of eIF4G to reduce translation efficiency and infectivity of encephalomyocarditisvirus (Hoffman and Palmenberg, 1996; Kaminski et al., 1994; Pestova et al., 1996). We compared the ability of control RNAs and tiRNAs to inhibit the translation of luciferase from monocistronic constructs expressing the wild type (UA6) or mutant (UA7) EMCV IRES (Figure 2B). Whereas 5′-tiRNAAla fails to inhibit translation from the wild type (UA6) IRES (Figure 2B, upper panel), it potently inhibits translation from the mutant (UA7) IRES (Figure 2B, lower panel). These results suggest that 5′-tiRNAAla-mediated translational repression is a function of the strength of eIF4G:RNA interactions.
Figure 2.
Effect of tiRNAs on EMCV IRES-mediated translation initiaton.
A. Synthetic 5′-tiRNAAla does not inhibit EMCV IRES-driven translation. Uncapped (dark bars) or capped (light bars) pF/R bicistronic transcripts were translated in RRL. Left panel: The relative ratio of Firefly to Renilla counts. Means and standard deviations are from 3–4 independent experiments (*-p <0.05, compared to no RNA and three control RNAs (ctrl-1,-2,-3) (Tables S1 and S2, Student’s t-test, n=3–4). Right panel: counts derived from EMCV IRES-driven translation of Renilla ORF relative to the no RNA control (100%). For actual luciferase counts, see Table S2.
B. Synthetic 5′-tiRNAAla inhibits translation of EMCV IRES UA7 variant. Uncapped monocistronic transcripts encoding Renilla luciferase under control of different EMCV IRES variants (WT EMCV-UA6 (upper panel) and EMCV-UA7 (lower panel)) were translated in RRL in the presence of control RNAs (ctrl-1,-2,-3), 5′-tiRNAAla or 3′-tiRNAAla. Means and standard deviations are from three independent experiments *-p <0.05, compared to no RNA and three control RNAs (ctrl-1,-2,-3) (Table SI, Student’s t-test, n=3). See also Figure S2. For actual luciferase counts, see Table S2.
The ability of tiRNAs to inhibit EMCV-IRES (UA7) translation makes it unlikely that eIF4E is a primary target for translational repression. To further support this conclusion, we determined whether recombinant eIF4E competitively inhibits tiRNAAla-induced translational repression in RRL (Figure S2). As previously reported (Tarun and Sachs, 1997), recombinant eIF4E inhibits the translation of uncapped mRNA by ~50%. This is presumably due to the fact that eIF4E:eIF4G complexes do not efficiently bind to the 5′ end of uncapped RNA (Tarun and Sachs, 1997). Importantly, recombinant eIF4E does not competitively inhibit tiRNAAla-induced translational repression (Figure S2). In fact, the combination of tiRNAAla and recombinant eIF4E results in 2.5-fold more inhibition than tiRNAAla alone, suggesting that these two factors act independently to inhibit translation.
Displacement of eIF4G
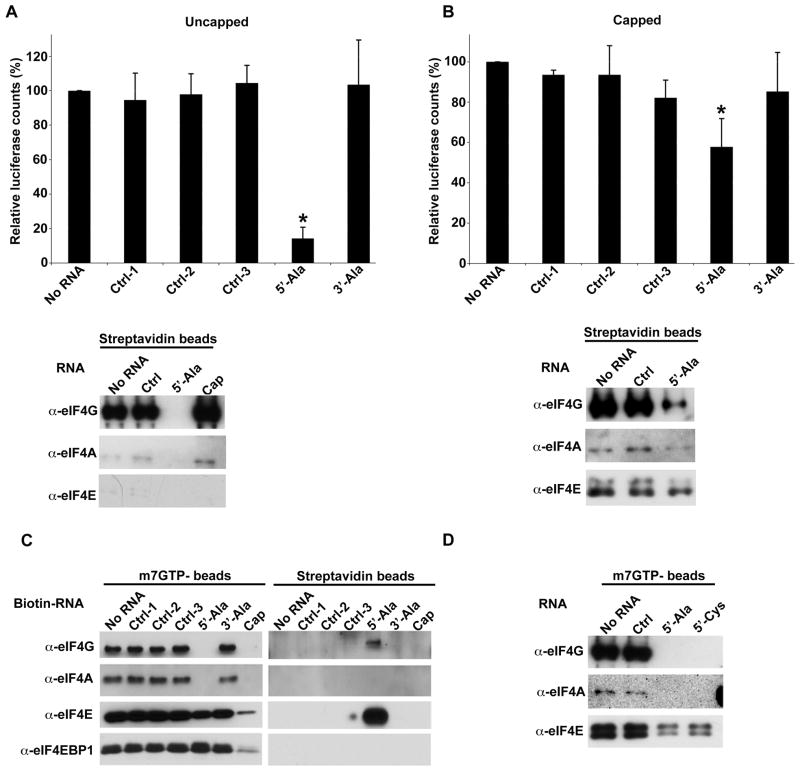
To test the hypothesis that 5′-tiRNAs can displace eIF4G from RNA, we added biotin-tagged capped or uncapped luciferase transcripts to heterologous (80% RRL + 20% U2OS) lysates containing control RNA or tiRNAs (Figure 3A–B). After streptavidin pull down, reporter RNA-bound proteins were quantified by immunoblotting. Uncapped RNA is bound to eIF4G, but not eIF4E (Figure 3A, lower panel), consistent with the ability of eIF4G to bind to the 5′ end of uncapped transcripts (De Gregorio et al., 1998). 5′-tiRNAAla but not control RNAs or cap analogue, quantitatively displaces eIF4G from these uncapped transcripts (Figure 3A, lower panel). In contrast, biotin-tagged capped RNA is bound to eIF4G and eIF4E (Figure 3B, lower panel). 5′-tiRNAAla only partially displaces eIF4G (~75% displacement) suggesting that stabilization of eIF4G conferred by cap-bound eIF4E may inhibit tiRNA-induced displacement (Figure 3B). 5′-tiRNAAla also modestly displaces eIF4E suggesting that its effect on eIF4G may reduce the affinity of eIF4E:cap interactions.
Figure 3.
5′-tiRNAs target eIF4F complex.
A. Synthetic 5′-tiRNAAla displaces eIF4G from uncapped mRNA. Upper panel: Uncapped polyA-biotinylated transcript encoding Renilla luciferase was translated in RRL supplemented with U2OS extract in the presence of the indicated control RNAs or tiRNAs. Luciferase expression is relative to that in the absence of RNA (No RNA =100%). Means and standard deviations are from three independent experiments. *-p <0.05, compared to no RNA and three control RNAs (ctrl-1,-2,-3) (Table S1, Student’s t-test, n=3). Lower panel: Streptavidin pull-down of uncapped polyA-biotinylated transcript. In vitro translation was performed as indicated in the upper panel in the presence of control RNA (Ctrl), 5′-tiRNAAla or cap analogue (Cap, m7GpppG, 0.1mM). Streptavidin beads were used to pull down reporter RNA-protein complexes. Bound proteins were identified by western blotting using indicated antibodies. For p values, see Table S1.
B. Synthetic 5′-tiRNAAla displaces eIF4G from capped mRNA. Upper panel: Capped polyA-biotinylated transcript encoding Renilla luciferase was translated in RRL supplemented with U2OS extract in the presence of the indicated control RNAs or tiRNAs. Luciferase expression is relative to that in the absence of RNA (No RNA =100%). Means and standard deviations are from three independent experiments. *-p <0.05, compared to no RNA and three control RNAs (ctrl-1,-2,-3) (Table S1, Student’s t-test, n=3). Lower panel: Streptavidin pull-down of capped polyA-biotinylated transcript as in Fig. 3A.
C. 5′-tiRNAAla displaces the eIF4F complex from m7GTP-Sepharose. The indicated 3′-end biotinylated RNAs or cap analogue (Cap, m7GpppG, 0.1mM) were added to m7GTP-sepharose complexes and displaced components were quantified by western blot (left panel). Streptavidin beads were used to pull down displaced RNA-bound proteins and were identified by western blotting (right panel).
D. eIF4F complex was assembled on m7GTP-Sepharose from RRL supplemented with U2OS extract. No RNA, control RNA (ctrl), 5′-tiRNAAla or 5′-tiRNACys were added to m7GTP-sepharose complexes. Integrity of eIF4F complex was monitored by western blotting using anti-eIF4E, -eIF4G and –eIF4A antibodies.
Displacement of eIF4F from m7G cap
To quantify the effect of 5′-tiRNAs on eIF4F:cap interactions, we assembled eIF4E-containing complexes (e.g., eIF4F (eIF4E:eIF4A:eIF4G) and eIF4E:4E-BP1) on m7GTP-Sepharose from U2OS cell lysates. Sepharose-bound complexes were incubated with 3′-end biotinylated control or tiRNAs before analyzing retained components of the eIF4E-containing complexes using Western blotting (Figure 3C). Although control RNAs do not displace initiation factors from m7GTP-Sepharose, 5′-, but not 3′-, tiRNAAla completely displaces eIF4G and eIF4A and partially displaces (~50%) eIF4E from the beads (Figure 3C, left panel). In contrast, 4E-BP1 is not displaced from the beads, suggesting that the retained eIF4E is complexed with 4E-BP1. As expected, cap analogue displaces both eIF4F and eIF4E:4E-BP1 from m7GTP-sepharose (Figure 3C, left panel: cap). The supernatants containing displaced initiation factors were collected and streptavidin beads were used to capture biotin-RNA oligos and their associated proteins. Western blot analysis reveals that biotin-5′-tiRNAAla is in a stable complex with eIF4G and eIF4E (Figure 3C, right panel). This result suggests that 5′-tiRNAAla binds, directly or indirectly, to an eIF4E:eIF4G complex and causes its dissociation from m7G cap. It is notable that 5′-tiRNAAla displaces eIF4G from m7GTP-Sepharose in U2OS lysate much more efficiently than it displaces eIF4G from capped mRNA in RRL (Figure 3B). Similar results were obtained using heterologous lysate (80% RRL + 20% U2OS extract, Figure 3D) suggesting that this difference is not conferred by the nature of the lysate.
Structure:function analysis
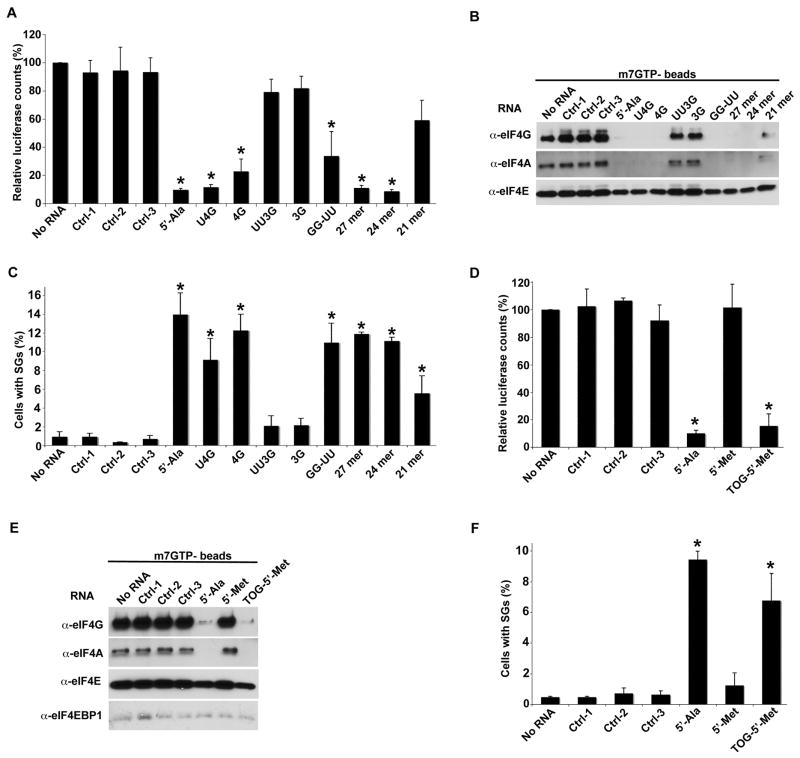
tRNAAla and tRNACys are the only human tRNAs with terminal oligo-guanine (TOG) motifs (4–5 guanine residues) at their 5′ ends (up-to-date alignments for H.sapiens tRNA can be found at http://lowelab.ucsc.edu/GtRNAdb/Hsapi19/Hsapi19-align.html). This restriction is extremely well conserved among all vertebrates, insects and worms (>95% of all tRNAAla and tRNACys genes), well conserved in plants (>95% of all tRNAAla genes), and less conserved in fungi and protozoa (<50% of all tRNAAla genes). In contrast, 5′-TOG motifs are extremely rare in all other tRNAs. To determine whether this structural feature is required for translational repression (Figure S3A–B), we compared the ability of truncation and substitution mutants (Figure S3C) to inhibit the translation of reporter mRNA in RRL (Figure 4A). Whereas 5′-tiRNAAla mutants with a singly truncated (4G) or G→U substituted (U4G) guanine residue retain their activity, mutants with doubly truncated (3G) or GG→UU substituted (UU3G) guanine residues are completely inactive. Thus, at least four guanines at or near to the 5′-end of tiRNAs are absolutely required for translational repression. In contrast, substitution of two invariant guanine residues within the D-loop region (GG→UU) does not prevent translational repression. RNAfold predicts that 5′-tiRNAs are composed of a single stranded 5′-TOG motif followed by a stem-loop structure resembling the D-loop of tRNA, followed by a single stranded 3′ region (Figure S3A–B). Truncations from the 3′ end of 5′-tiRNAAla retain their activity until the region of the tRNA D-loop is encroached upon (i.e., 21-mer (Figure 4A; structures shown in Figure S3C)) suggesting that the structure formed by the D-loop region may contribute to translational repression.
Figure 4.
Structure/function analysis of tiRNAs.
A. In vitro translation in RRL. Uncapped Firefly luciferase mRNA was translated in RRL in the presence of synthetic control RNAs (ctrl-1,-2,-3), 5′-tiRNAAla, or the indicated mutants. Luciferase expression is relative to that produced in the absence of any RNA (No RNA = 100%). Means and standard deviations are from four independent experiments (*- p < 0.05 (Table SI), Student’s t-test, n=4). See also Figures S3 and S4. For p values, see Table S1.
B. Displacement of eIF4F complexes from m7GTP-sepharose. eIF4F complex was assembled on m7GTP-sepharose in U2OS lysates. The indicated synthetic RNAs were added to beads and analyzed as described in Figure 3C.
C. SG assembly. U2OS cells were transfected with indicated RNAs. The percentage of U2OS cells with SGs was quantified by counting 200 cells/experiment. Error bars reflect standard deviations of the mean (*-p-value < 0.05 (Table S1), relative to control without any RNA, Student’s t-test, n=3).
D. Addition of 5′-TOG motif “activates” 5′-tiRNAMet to inhibit translation in RRL as in Figure 3C. Error bars reflect standard deviations of the mean (*-p-value < 0.05 (Table S1), relative to control without any RNA, Student’s t-test, n=3).
E. 5′-TOG-containing tiRNAs displace eIF4F. Western blot analysis was used to quantify the binding of eIF4F complexes and eIF4E-BP1 as in Figure 4B.
F. Addition of 5′-TOG “activates” 5′-tiRNAMet to induce the assembly of SGs as in Figure 3C. Error bars reflect standard deviations of the mean (*-p-value < 0.05 (Table S1), relative to control without any RNA, Student’s t-test, n=3).
Importantly, the ability of 5′-tiRNAAla and its mutants to inhibit mRNA translation closely correlates with their ability to displace eIF4F from m7GTP-Sepharose (Figure 4B) and to induce the assembly of SGs when transfected into U2OS cells (Figure 4C). We also confirmed that transfection of wild type or mutant 5′-tiRNAAla does not induce the phosphorylation of eIF2α, a classical trigger of SG assembly (Figure S4). Since SG assembly is triggered by impaired translation initiation, this reveals that 5′-tiRNAAla inhibits translational initiation in live cells in which most of the target transcripts are presumably capped. The close correlation between translational repression in RRL, displacement of eIF4F from m7GTP-Sepharose and SG assembly suggests that eIF4F is a target for tiRNA-induced translational repression.
Addition of 5′-TOGs “activates” 5′-tiRNAMet
To confirm the importance of the 5′-TOG motif for tiRNA activity, we substituted the first 5 nucleotides of non-TOG-containing 5′-tiRNAMet with 5 guanine residues (Figure S3C). Although 5′-tiRNAMet does not inhibit translation in RRL (Figure 4D), displace eIF4F from m7G-Sepharose (Figure 4E) or induce the assembly of SGs (Figure 4F), addition of a 5′-TOG motif is sufficient to confer these activities on this tRNA fragment (Figure 4D–F).
Identification of tiRNA-interacting proteins
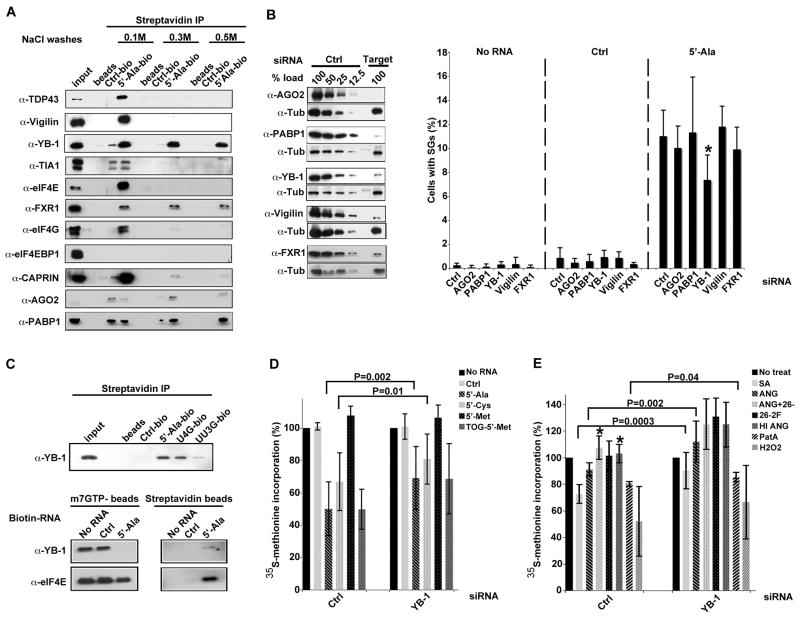
Pull-down of RNA-protein complexes using biotinylated control RNA or 5′-tiRNAAla immobilized on streptavidin beads was used to purify proteins that interact with these RNAs. RNA-bound proteins were identified by mass spectrometry (Supplemental Table II). Independent pull-down experiments confirmed that several proteins involved in the regulation of RNA metabolism, including TDP-43 (TARDBP), Vigilin (HDLBP), YB-1 (YBX1), eIF4E, FXR1, eIF4G, CAPRIN1 (CAPRIN), Argonaute-2 (EIF2C2), and PABP1 (PABPC1) bind to 5′-tiRNAAla more strongly than control RNA (Figure 5A). Some of these interactions (e.g., YB-1, FXR-1, PABP1) are maintained under high salt wash conditions.
Figure 5.
Identification of tiRNA-interacting proteins.
A. Validation of selected candidate 5′-tiRNAAla-binding proteins. Proteins bound to biotinylated control RNA and 5′-tiRNAAla were purified using streptavidine beads, subjected to the indicated salt washes (0.1M, 0.3M and 0.5M), eluted, then analyzed by Western blotting using corresponding antibodies. For list of interacting proteins, see Table S3.
B. tiRNA-binding proteins required for 5′-tiRNAAla-induced SG assembly. U2OS cells treated with indicated siRNAs, were mock transfected (No RNA) or transfected with control RNA (Ctrl) or 5′-tiRNAAla (5′-Ala) before processing for immunofluorescence microscopy to quantify SGs. Left panel: Efficiency of knock down was assessed by comparing the expression of each protein from siRNA control transfections (Ctrl, serial dilutions) to targeted siRNA transfections (Target). Tubulin was used as a specificity and loading control. Right panel: Percentage of cells with SGs was quantified by counting 200 cells/experiment. Results show the means and standard deviations from the indicated number of experiments (AGO2 siRNA, n=7; PABP1 siRNA, n=3; YB-1 siRNA, n=9; Vigilin siRNA, n=3; FXR1 siRNA, n=3). *-p-value = 0.0001 when comparing the percentage of cells with SGs in control and YB-1 siRNA treated cells; no other treatment reached statistical significance.
C. YB-1 is a part of complex displaced by 5′-tiRNAAla from m7GTP-sepharose. Upper panel: Biotinylated control RNA (Ctrl-bio), 5′-tiRNAAla (5′-Ala-bio) or its mutants (U4G-bio and UU3G-bio) were immobilized on streptavidin beads and used to pull down protein complexes as described in Fig. 4A. Bound complexes were analyzed by Western blotting using anti-YB-1 antibodies. Lower panel: eIF4E-containing complexes were assembled on m7GTP-Sepharose and incubated with no RNA, biotinylated control RNA (Ctrl) or biotinylated 5′-tiRNAAla (5′Ala). Beads were washed, bound proteins were eluted and analyzed by Western blot using antibodies against eIF4E and YB-1 (left panel). Proteins associated with biotin-RNAs were pulled down using streptavidin beads, eIF4E and YB-1 were detected by western blotting (right panel).
D. YB-1 is required for 5′-tiRNA-induced translational repression. U2OS cells treated with control or YB-1 siRNAs were transfected with Ctrl RNA, 5′-tiRNAAla, 5′-tiRNACys, 5′-tiRNAMet, and TOG-5′-Met and then labeled with (35S)-methionine. (35S)-methionine incorporation in cells transfected with no RNA is reported as 100%. Error bars show means and standard deviations (5′-tiRNAAla, p=0.002, n=10; 5′-tiRNACys, p=0.01, n=4; when comparing inhibition of global protein synthesis by tiRNAs in control and YB-1 siRNA treated cells; no other treatments reached statistical significance).
E. YB-1 is required for stress- and angiogenin-induced translational repression. U2OS treated with control or YB-1 siRNAs were labeled with (35S)-methionine in the presence of sodium arsenite (SA), Pateamine A (PatA), hydrogen peroxide (H2O2), angiogenin (ANG), heat inactivated ANG (HI ANG), angiogenin-neutralizing antibody (26-2F) alone or in combination with ANG (ANG+26-2F). (35S)-methionine incorporation in untreated cells (No treat) is reported as 100%. Error bars show means and standard deviations (SA, p=0.0003, n=8; ANG, p=0.002, n=8; and PatA, p=0.04, n=3 when comparing inhibition of global protein synthesis by those treatments in control and YB-1 siRNA treated cells; *-p-value = 0.003; n=4 and 0.01; n=3 when comparing inhibition of global protein synthesis by ANG+26-2F and HI ANG to angiogenin-treated cells, respectively).
YB-1 promotes tiRNA- and stress-induced translational repression
To determine whether these 5′-tiRNAAla-binding proteins are required for translational repression, we knocked down AGO2, PABP1, YB-1, Vigilin, and FXR1 (Figure 5B, left panel) prior to quantifying tiRNA-induced SG assembly in U2OS cells (Figure 5B, right panel). YB-1, a protein that inhibits translation of both uncapped and capped mRNAs by displacing eIF4G from the eIF4F complex (Nekrasov et al., 2003) and displacing eIF4E from the m7G cap (Evdokimova et al., 2001), was the only 5′-tiRNAAla-binding protein required for the assembly of SGs (Figure 5B). The effect of YB-1 knockdown was only partial, however, suggesting that tiRNAs may have YB-1 independent functions as well. Moreover, binding of YB-1 to 5′-tiRNAAla and its mutants (Figure 5C, upper panel) directly correlates with the ability of these RNAs to inhibit translation, displace eIF4F complex from cap and trigger SG formation (Figure 4). YB-1 is also part of the 5′-tiRNAAla complex that is displaced from m7GTP-Sepharose by 5′-tiRNAAla (Figure 5C, lower panel). These results suggest that tiRNAs and YB-1 are components of a complex that inhibits protein translation.
Consistent with the role of YB-1 in tiRNA-mediated translational repression, YB-1 knockdown significantly reverses 5′-tiRNAAla- and 5′-tiRNACys-induced translational repression (Figure 5D). It also partially reverses the translational repression conferred by 5′-TOG-tiRNAMet (but not WT 5′-tiRNAMet) although this did not reach statistical significance (Figure 5D). Moreover, translational repression induced by sodium arsenite (SA), angiogenin (ANG), and pateamine A (PatA) was significantly reversed by YB-1 knockdown (Figure 5E), supporting a common role for tiRNAs and YB-1 in these processes. In contrast, the translational repression induced by H2O2 is not significantly reversed by YB-1 knockdown (Figure 5E). The specificity of the angiogenin effect was confirmed by the addition of a neutralizing antibody (26-2F) and by heat inactivation (HI), both of which reversed the inhibition of translation (Figure 5E). In these cases, YB-1 knockdown further enhanced protein translation, but this was not statistically significant. Nevertheless, it is possible that YB-1 constitutively dampens protein translation. Whether this is dependent upon endogenous tiRNAs remains to be determined.
DISCUSSION
In stressed cells, tRNA cleavage results in the production of 5′ and 3′ tRNA fragments in a variety of organisms (Thompson and Parker, 2009a). We have previously reported that transfection of 5′-, but not 3′-, tiRNAs inhibits protein synthesis and promotes the assembly of stress granules (Emara et al., 2010; Yamasaki et al., 2009). These effects are relatively modest (~20% inhibition) suggesting that a subset of tiRNAs maybe responsible for translational repression and/or a subset of mRNAs are susceptible to translational repression. Both of these conjectures have been confirmed by comparing the activity of individual synthetic tiRNAs in RRL. We find that synthetic 5′-tiRNAAla and 5′-tiRNACys are significantly more potent than other 5′-tiRNAs. Structure:function analysis reveals that this is due to presence of 5′-TOG motifs that are uniquely found in these tRNAs. Evolutionary conservation of this motif in tRNAAla and tRNACys implies a conserved biological function for these sequences. In RRL, 5′-tiRNAAla/Cys preferentially inhibit the translation of uncapped>EMCV-IRES (UA7)>capped>EMCV-IRES (UA6) RNAs identifying preferred substrates for translational repression. These results suggest that tiRNAs may play a role in reprogramming protein translation in stressed cells.
Our results shed light on the mechanism by which tiRNAs inhibit protein translation. The ability of tiRNAs to inhibit translation in RRL correlates with their ability to displace eIF4G/A from mRNA. In pull-down experiments using biotin-RNA, tiRNAs quantitatively displace eIF4G/A from uncapped mRNA, but only partially displace eIF4G/A from capped mRNA (Figure 3). Displacement of eIF4G/A from capped mRNA results in a minor displacement of eIF4E, suggesting that a conformational change in eIF4G may reduce the affinity of eIF4E for m7G cap (Haghighat and Sonenberg, 1997). This may be the mechanism by which tiRNAs inhibit translation of capped mRNA in RRL and in eukaryotic cells. The correlation between translational repression and eIF4G/A displacement is supported by studies of EMCV-IRES-mediated translation in which tiRNAAla inhibits the translation of the UA7-IRES (low affinity eIF4G/A binding), but not the UA6-IRES (high affinity eIF4G/A binding). Taken together, these results suggest that tiRNAs bind, directly or indirectly, to eIF4G, eIF4A, or the eIF4G/A complex to inhibit translation.
We also find that 5′-tiRNAs can displace eIF4F, but not eIF4E/4E-BP-1, from m7GTP-Sepharose. Under conditions of nutrient stress, dephosphorylated 4E-BP displaces eIF4G from eIF4E to inhibit protein synthesis (Sonenberg and Hinnebusch, 2009). Binding of 4E-BP alters the conformation of eIF4E, increasing its affinity for the m7G cap (Ptushkina et al., 1999). Thus, stress-induced 5′-tiRNAs may promote translational repression by favoring eIF4E:4E-BP complexes over eIF4F complexes. This may be the mechanism by which tiRNAs promote phospho-eIF2α independent translational repression in stressed cells (Yamasaki et al., 2009). The ability of 5′-tiRNAs to quantitatively displace eIF4F from m7GTP-Sepharose is surprising since they only partially displace eIF4G and very modestly displace eIF4E from capped mRNA. This difference may be due to more stable interactions between these initiation factors and the 5′ end of the capped RNA. This probably explains why tiRNAs only modestly inhibit the translation of capped mRNA in RRL and in eukaryotic cells. In stressed cells, a modest reprogramming of protein synthesis may play an important role in the cellular stress response program. Moreover, tiRNAs may preferentially modulate the translation of mRNAs initiated by IRES elements, some of which play important roles in the stress response program (King et al., 2011; Komar and Hatzoglou, 2011).
Pull-down analysis reveals that tiRNAs directly or indirectly interact with proteins involved in several aspects of mRNA metabolism. In addition to known components of the initiation complex (e.g., eIF4E, eIF4G, PABP1), tiRNAs bind to a complex containing AGO2, a key component of the RNA-induced silencing complex. This raises the possibility that stress-induced tiRNA production may interfere with miRNA-mediated regulation of gene expression. As AGO2 is not required for tiRNA-induced stress granule assembly (Fig. 5B), it is unlikely that tiRNA:argonaute complexes directly repress protein synthesis.
YB-1 is the only tiRNA-interacting protein that is required for tiRNA-induced stress granule assembly (Fig. 5B). Since stress granule assembly is a consequence of impaired translation initiation, this result suggests that YB-1 cooperates with tiRNAs to inhibit translation initiation in cells. YB-1 is also the protein that binds most strongly to tiRNAs in the presence of 0.5M NaCl (Fig. 5A). YB-1 is a cold shock domain-containing protein that regulates both transcription and translation (Evdokimova et al., 2006). In reticulocyte lysates, YB-1 at low concentrations promotes translation and at high concentrations inhibits translation (Nekrasov et al., 2003). Translational repression is mediated by two distinct domains: the cold shock domain displaces eIF4E from the m7G cap, whereas the carboxyl-terminus interferes with eIF4G binding (Nekrasov et al., 2003). These domain-specific activities allow YB-1 to potently inhibit the translation of uncapped transcripts (eIF4G-dependent initiation) and less efficiently inhibit the translation of capped transcripts (eIF4E/eIF4G-dependent initiation) (Evdokimova et al., 2001; Nekrasov et al., 2003). The finding that tiRNAs inhibit the translation of uncapped mRNA more efficiently than capped mRNA (Fig. 1) is consistent with a role for YB-1 in tiRNA-induced translational repression. The finding that both tiRNAs and YB-1 displace eIF4F complexes from the m7G cap is also consistent with a role for YB-1 in tiRNA-induced translational repression. Our results suggest that tiRNAs and YB-1 cooperate to prevent eIF4G/A from initiating translation.
The ability of YB-1 knockdown to significantly reverse the inhibitory effects of angiogenin, sodium arsenite, and pateamine A is consistent with a role for tiRNAs and YB-1 in the process of stress-induced translational repression. These effects are relatively modest, however, suggesting that additional inhibitory mechanisms are likely to contribute to this process. Nevertheless, our results implicate these tRNA-derived small non-coding RNAs in the stress-response program in mammalian cells.
EXPERIMENTAL PROCEDURES
Tissue culture, cell treatments and metabolic labeling of cells
U2OS cells were maintained at 37°C in a CO2 incubator in Minimal Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Sigma) and 1% of penicillin/streptomycin (Sigma). Cell were treated with the indicated doses of sodium arsenite (Sigma, 70 μM), Pateamine A (15 nM) and angiogenin (0.5 μg/ml) as described in (Kedersha et al., 2008), and with hydrogen peroxide (Sigma, 15 μM, 1 hour). To prepare heat-inactivated angiogenin, recombinant angiogenin (0.5 μg/ml) was boiled for 5 minutes. For neutralization of angiogenin by the angiogenin-specific 26-2F antibody, angiogenin and the 26-2F antibodies were mixed (ratio 1:5) for 30 minutes prior treatment. (35S)-methionine protein labeling was done as described in (Emara et al., 2010).
Cell transfections
Cells were transfected with RNA oligos using Lipofectamine 2000 (Invitrogen). Before transfection, RNA-complexes were pre-incubated in serum free medium (Opti-MEM medium, Invitrogen) for 20 minutes at room temperature. U2OS cells (1 × 105/well) were plated in 24 well plates for 24 hr and then transfected with 750 nM synthetic tiRNAs using 2.5 μl lipofectamine.
Isolation of tiRNAs
Extraction of tiRNAs from ANG treated cells was done as previously described (Yamasaki et al., 2009).
Antibodies
The following antibodies were used in this study: Goat polyclonal anti-eIF3b, goat polyclonal anti-eIF4A, goat polyclonal anti-FXR1, goat polyclonal anti-TIA1, rabbit polyclonal anti-eIF4G, mouse monoclonal anti-YB-1, mouse monoclonal anti-eIF4E and mouse monoclonal anti-Vigilin antibodies were purchased from Santa Cruz Biotechnology. Rabbit polyclonal anti-eIF4EBP1 and rabbit polyclonal anti-TDP43 antibodies were from Cell Signaling. Mouse monoclonal antibody against Ago2 was from Wako BioProducts. Mouse polyclonal anti-G3BP was from Biomedical Biosciences. Rabbit polyclonal anti-CAPRIN1 antibody was from Proteintech. Rabbit polyclonal phospho specific anti-eIF2α (serine 52) was from Assay Designs. The mouse monoclonal antibody 26-2F against human angiogenin was a gift from Dr. Guo-fu Hu (Tufts University Medical Center). Anti-mouse, anti-goat and anti-rabbit secondary antibodies conjugated with horseradish peroxidase (HRP) were from GE Healthcare. Cy2-, Cy3-, and Cy5-HRP-conjugated secondary antibodies were purchased from Jackson Immunoresearch Labs.
Preparation of RNA transcripts for in vitro translation
Plasmid pF/R (Bochkov and Palmenberg, 2006) was linearized by digesting with HpaI (New England Biolabs); plasmids pCDNA3-EMCV-R-luc (EMCV-UA6), pRL-5boxB and pEMCV-RL-5boxB (EMCV-UA7; Kiriakidou et al, 2007) were linearized by XbaI (New England Biolabs). Cut plasmids were separated on a 1% agarose gel and then purified from the gel with QIAquick Gel Extraction Kit (Qiagen). Riboprobe T7 in vitro transcription System (Promega) was used to synthesize pF/R RNA using T7 RNA Polymerase according to the manufacture’s recommendations. Subsequently, in vitro transcribed RNA was purified using Trizol (Invitrogen) extraction followed by isopropanol precipitation. Purified RNA was analyzed for purity on a formaldehyde gel and quantified by spectrophotometry (Beckman DU 640).
To prepare polyA-biotinylated mRNAs used for streptavidin pull-down assays, capped or uncapped pRL-5boxB RNAs were polyadenylated using Poly (A) Polymerase Tailing Kit (EPICENTRE Biotechnologies) according to the manufacture’s recommendation but in the presence of 10 nM biotin-ATP (PerkinElmer).
To prepare capped Firefly luciferase mRNA, 2 μg of commercial uncapped Firefly luciferase mRNA (Promega) was capped by Vaccinia Virus Capping enzyme using ScriptCap m7G Capping System (EPICENTRE Biotechnologies) according to the manufacture’s recommendations. Capped mRNA was purified by standard ethanol precipitation, and quantified by spectrophotometry (Beckman DU 640 instrument).
In vitro translation of mRNA reporters in Rabbit Reticulocyte Lysates (RRLs)
Flexi Rabbit Reticulocyte Lysate System (Promega) was used for the in vitro analysis of mRNA translation according to the manufacture’s recommendations with some modifications. In all cases, translation reactions (10 μl final volume) contained 70% of reticulocyte lysates or mixtures of RRL supplemented with 20% of microccocal nuclease-treated U2OS extract (RRL+20% U2OS lysate) and 8 units of RNasin Ribonuclease Inhibitor (Promega). Typically, 10 ng of capped Firefly mRNA, 50 ng of uncapped Firefly RNA (Promega) or 100 ng of pF/R bicistronic mRNA were used per translation reaction. 100 picomoles of control RNAs or tiRNAs were added to translation reactions, mixed and incubated for 30 minutes at 30°C. Reactions were stopped at 4°C, and activities of Firefly and Renilla luciferase were measured using 1/5 of translation reactions with Dual-Luciferase Reporter Assay System (for pF/R transcript), or with Luciferase Assay System (Promega) (for Firefly luciferase) according to the manufacture’s recommendations (2 second measurement delay followed by 10 second measurement read).
7-Methyl GTP Sepharose Chromatography
7-Methyl-GTP-Sepharose 4B (m7GTP-Sepharose, GE Healthcare) suspension was washed twice with ice-cold RNAse-free Buffer A (15mM Tris HCl, pH 7.0, 100mM NaCl, 1 mM EDTA) to remove sodium azide. U2OS cells were grown until 70–80% confluence in 15cm dishes under standard conditions, and then collected by scraping with Lysis Buffer (50mM Tris-HCl, pH 7.2, 100mM NaCl, 1mM EDTA, 0.5% NP-40) supplemented with protease inhibitors (Protease Inhibitor Cocktail “Complete”, Roche) into Eppendorf tubes followed by tumbling at 4°C for 15 minutes. Cell debris and nuclei were removed by centrifugation (20 minutes, 13200 rpm, 4°C), and the cytoplasmic fraction (supernatant) was applied to pre-washed m7GTP-Sepharose, and incubated for 1 hour at 4°C. Typically, we use 10–20 μl of m7GTP-Sepharose suspension per one sample of synthetic RNA (40nM final concentration). After incubation, m7GTP-Sepharose was washed three times with Lysis Buffer and m7GTP-bound protein complexes were divided into equal parts. Synthetic RNAs (50 or 100 pmoles, final concentrations 20–40 nM) were added to the complexes and incubated for 1 hour at 4°C. Unbound proteins were removed by washing once with Lysis Buffer, and proteins bound to m7GTP-Sepharose were eluted with 60 μl of 1×SDS PAGE Loading Buffer. Eluted proteins were analyzed by Western Blotting using protein-specific antibodies.
Protein precipitation using biotinylated RNAs
3′-end-biotinylated RNAs (control RNAs or tiRNAs) were synthesized by and purchased from Integrated DNA Technology. Streptavidin agarose beads (Invitrogen) (40μL per sample) were washed twice with RNAse-free Biotin Binding Buffer (10mM Tris HCl, pH 7.2, 100mM NaCl, 1mM EDTA, 0.1% NP-40). 500 pmoles of biotinylated RNAs were added to streptavidin beads and incubated for 1 hour at room temperature with rotation in 0.75 ml of Biotin Binding Buffer. After incubation, immobilized biotinylated RNA-streptavidin complexes were washed twice with RNAse-free Wash Buffer II (15mM Tris HCl. pH7.2, 750 mM NaCl, 1mM EDTA, 0.1% NP-40) and once with ice-cold RNAse-free Wash Buffer I (15mM Tris HCl. pH7.2, 150 mM NaCl, 1mM EDTA, 0.1% NP-40%) at room temperature to remove unbound RNA. Pre-cleared U2OS lysates were added to the biotinylated RNA-streptavidin bead complexes, incubated for 2 hours at 4°C with rotation and washed 3 times with Wash Buffers (15mM Tris HCl. pH7.2, 1mM EDTA, 0.1% NP-40) containing different NaCL concentrations (0.1M, 0.3M or 0.5M). Proteins were eluted using 60 μl of 1×SDS PAGE Loading Buffer.
For pull-down of biotinylated polyA mRNAs, 200 ng of capped or uncapped pRL-5boxB mRNAs were used for in vitro translation in rabbit reticulocyte lysate supplemented with 20% U2OS extract under conditions described above. This heterologous in vitro translation system (RRL with U2OS cell extract) is capable to translate mRNAs with similar efficiency as Flexi Rabbit Reticulocyte Lysate System and allows to detect eIF4G (human) by Western Blotting (our antibodies do not detect eIF4G of rabbit origin). 100 picomoles of control RNAs or tiRNAs were added to translation reactions. After completion of translation, streptavidin agarose beads (Invitrogen) (40 μL per sample) were added to reactions and incubated at 4°C for 30 minutes. Streptavidin beads were precipitated by centrifugation (1000 rpm, 5 min), supernatants were removed and beads were washed once with ice-cold Wash buffer (15mM Tris HCl. pH7.2, 150 mM NaCl, 1mM EDTA, 0.1% NP-40%). Resulting mRNA-protein complexes were eluted from the beads using 60 μl of 1×SDS PAGE Loading Buffer.
Mass-spectrometry identification of tiRNA-binding proteins
For mass spectrometry, protein solutions from affinity purification using 3′-end biotinylated RNAs were precipitated with TCA (Trichloroacetic acid). Briefly, eluted protein samples were adjusted to 20% final volume of TCA using 100% TCA (in water) solution. The resulting mixture was placed on ice for 20 minutes followed by centrifugation (20 minutes, 13200 rpm, 4°C). Protein pellet was washed once with 1 ml of cold (−20°C) acetone (HPLC grade, Sigma) followed by centrifugation (20 minutes, 13200 rpm, 4°C). Supernatant was carefully removed, and pellet was air-dried for 10 minutes at room temperature. Identification of tiRNA-binding proteins has been done by Taplin Mass Spectrometry Facility according to standard protocols (Harvard Medical School).
Supplementary Material
HIGHLIGHTS.
Selected 5′-tiRNAs target eIF4G/A to inhibit translation in reticulocyte lysates
A terminal oligoguanine motif is required for tiRNA-induced translational repression
5′-tiRNAs cooperate with YB-1 to promote stress granule assembly
YB-1 is required for optimal 5′-tiRNA- and arsenite-induced translational repression
Acknowledgments
We thank N. Kedersha, S. Yamasaki, N. Dawra, D. Sciaranghella and V. Ivanova for technical support and helpful comments, and Prof. Tao Pan (University of Chicago) for help in estimation of intracellular tRNA levels. We thank Prof. Ann Palmenberg (University of Wisconsin-Madison) for kind gift of pF/R plasmid and Prof. Zissimos Mourelatos (University of Pennsylvania School of Medicine) for pEMCV-RL-5boxB and pRL-5boxB plasmids. We thank Prof. Guo-fu Hu and Dr. Wenhao Yu (Tufts University School of Medicine) for providing recombinant angiogenin and 26-2F antibodies; Prof. Gerhard Wagner, Dr. Ricard Rodriguez and Dr. Melissa Leger (Harvard Medical School) for providing recombinant eIF4E protein; Prof. Jun Liu (John Hopkins School of Medicine). This work was supported by NIH grants AI033600 and AI065858 (P.A.) and a Research Development Grant from the Muscular Dystrophy Association (ID158521, P.I.).
Footnotes
Additional experimental procedures are found in the supplemental information.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bochkov YA, Palmenberg AC. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. BioTechniques. 2006;41:283–284. 286, 288. doi: 10.2144/000112243. passim. [DOI] [PubMed] [Google Scholar]
- Cole C, Sobala A, Lu C, Thatcher SR, Bowman A, Brown JW, Green PJ, Barton GJ, Hutvagner G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA (New York, NY. 2009;15:2147–2160. doi: 10.1261/rna.1738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Preiss T, Hentze MW. Translational activation of uncapped mRNAs by the central part of human eIF4G is 5′ end-dependent. RNA (New York, NY. 1998;4:828–836. doi: 10.1017/s1355838298980372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara M, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu G, Anderson P. Angiogenin-induced tiRNAs promote stress-induced stress granule assembly. The Journal of biological chemistry. 2010;285:10959–10968. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evdokimova V, Ovchinnikov LP, Sorensen PH. Y-box binding protein 1: providing a new angle on translational regulation. Cell cycle (Georgetown, Tex. 2006;5:1143–1147. doi: 10.4161/cc.5.11.2784. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Ruzanov P, Imataka H, Raught B, Svitkin Y, Ovchinnikov LP, Sonenberg N. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. The EMBO journal. 2001;20:5491–5502. doi: 10.1093/emboj/20.19.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Garcia-Silva MR, Frugier M, Tosar JP, Correa-Dominguez A, Ronalte-Alves L, Parodi-Talice A, Rovira C, Robello C, Goldenberg S, Cayota A. A population of tRNA-derived small RNAs is actively produced in Trypanosoma cruzi and recruited to specific cytoplasmic granules. Mol Biochem Parasitol. 171:64–73. doi: 10.1016/j.molbiopara.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Haghighat A, Sonenberg N. eIF4G dramatically enhances the binding of eIF4E to the mRNA 5′-cap structure. The Journal of biological chemistry. 1997;272:21677–21680. doi: 10.1074/jbc.272.35.21677. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Karginov FV, Hannon GJ, Elliot MA. Developmentally regulated cleavage of tRNAs in the bacterium Streptomyces coelicolor. Nucleic acids research. 2008;36:732–741. doi: 10.1093/nar/gkm1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussecker D, Huang Y, Lau A, Parameswaran P, Fire AZ, Kay MA. Human tRNA-derived small RNAs in the global regulation of RNA silencing. RNA (New York, NY. 2010;16:673–695. doi: 10.1261/rna.2000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MA, Palmenberg AC. Revertant analysis of J-K mutations in the encephalomyocarditis virus internal ribosomal entry site detects an altered leader protein. Journal of virology. 1996;70:6425–6430. doi: 10.1128/jvi.70.9.6425-6430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LC, Lin SI, Kuo HF, Chiou TJ. Abundance of tRNA-derived small RNAs in phosphate-starved Arabidopsis roots. Plant Signal Behav. 2010;5 doi: 10.4161/psb.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochl C, Rederstorff M, Hertel J, Stadler PF, Hofacker IL, Schrettl M, Haas H, Huttenhofer A. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic acids research. 2008;36:2677–2689. doi: 10.1093/nar/gkn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Belsham GJ, Jackson RJ. Translation of encephalomyocarditis virus RNA: parameters influencing the selection of the internal initiation site. The EMBO journal. 1994;13:1673–1681. doi: 10.1002/j.1460-2075.1994.tb06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Jackson RJ. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA (New York, NY. 1998;4:626–638. doi: 10.1017/s1355838298971898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Tisdale S, Hickman T, Anderson P. Real-time and quantitative imaging of mammalian stress granules and processing bodies. Methods Enzymol. 2008;448:521–552. doi: 10.1016/S0076-6879(08)02626-8. [DOI] [PubMed] [Google Scholar]
- King HA, Cobbold LC, Willis AE. The role of IRES trans-acting factors in regulating translation initiation. Biochemical Society transactions. 2011;38:1581–1586. doi: 10.1042/BST0381581. [DOI] [PubMed] [Google Scholar]
- Kolupaeva VG, Lomakin IB, Pestova TV, Hellen CU. Eukaryotic initiation factors 4G and 4A mediate conformational changes downstream of the initiation codon of the encephalomyocarditis virus internal ribosomal entry site. Molecular and cellular biology. 2003;23:687–698. doi: 10.1128/MCB.23.2.687-698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell cycle (Georgetown, Tex. 2011;10:229–240. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Collins K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. The Journal of biological chemistry. 2005;280:42744–42749. doi: 10.1074/jbc.M510356200. [DOI] [PubMed] [Google Scholar]
- Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luo J, Zhou H, Liao JY, Ma LM, Chen YQ, Qu LH. Stress-induced tRNA-derived RNAs: a novel class of small RNAs in the primitive eukaryote Giardia lamblia. Nucleic acids research. 2008;36:6048–6055. doi: 10.1093/nar/gkn596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin IB, Hellen CU, Pestova TV. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Molecular and cellular biology. 2000;20:6019–6029. doi: 10.1128/mcb.20.16.6019-6029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov MP, Ivshina MP, Chernov KG, Kovrigina EA, Evdokimova VM, Thomas AA, Hershey JW, Ovchinnikov LP. The mRNA-binding protein YB-1 (p50) prevents association of the eukaryotic initiation factor eIF4G with mRNA and inhibits protein synthesis at the initiation stage. The Journal of biological chemistry. 2003;278:13936–13943. doi: 10.1074/jbc.M209145200. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU, Shatsky IN. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Molecular and cellular biology. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptushkina M, von der Haar T, Karim MM, Hughes JM, McCarthy JE. Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state. The EMBO journal. 1999;18:4068–4075. doi: 10.1093/emboj/18.14.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun SZ, Jr, Sachs AB. Binding of eukaryotic translation initiation factor 4E (eIF4E) to eIF4G represses translation of uncapped mRNA. Molecular and cellular biology. 1997;17:6876–6886. doi: 10.1128/mcb.17.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA (New York, NY. 2008 doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Parker R. Stressing out over tRNA cleavage. Cell. 2009a;138:215–219. doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. The Journal of cell biology. 2009b;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Anderson P. Reprogramming mRNA translation during stress. Current opinion in cell biology. 2008;20:222–226. doi: 10.1016/j.ceb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Ivanov P, Hu GF, Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. The Journal of cell biology. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Sun L, Kragler F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 2009;150:378–387. doi: 10.1104/pp.108.134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.