Abstract
Rationale
Pharmacological manipulations of the type 1 cannabinoid receptor (CB1) suggest a role for CB1 in morphine-induced antinociception, but studies utilizing CB1 knockout (KO) mice do not support this conclusion. Since studies using CB1 KO mice to study morphine’s antinociceptive effects have only examined thermal nociception, this study examines these interactions in models that employ a chemical stimulus.
Objectives
To determine whether the findings obtained with thermal pain models extend to other models, the effects of morphine on acetic acid-induced writhing were examined in CB1 KO and wildtype (WT) mice. Behaviors that decrease in response to acid injection, feeding and wheel-running, were also examined, and investigations were carried out in the thermal hotplate assay. The CB1 antagonist SR141716A was also examined in these assays.
Results
Morphine completely blocked acid-induced writhing (1.0 – 10.0 mg/kg) and increased response latencies in the hotplate (10.0 - 32.0 mg/kg) in both genotypes. Morphine (3.2 mg/kg) significantly attenuated the suppression of wheel-running but did not completely prevent this effect in either genotype. Morphine did not alter pain-suppressed feeding. In each of these assays, morphine’s effects were not altered in CB1 KO mice compared to WT mice, however, SR141716A attenuated morphine’s effects in C57BL/6 mice.
Conclusions
The effects of morphine do not differ in CB1 KO and WT mice in preclinical pain models using thermal and chemical stimuli. Since SR141716A did attenuate the effects of morphine, it is possible that CB1 KO mice undergo developmental changes that mask the role of CB1 receptors in morphine’s antinociceptive effects.
Keywords: CB1, cannabinoid, knockout, morphine, opioid, pain, antagonist, antinociception
INTRODUCTION
The cannabinoid and opioid systems include G-protein-coupled receptors, type 1 cannabinoid (CB1) and μ-opioid receptors, respectively, that have parallel distribution throughout the CNS. The activation of these receptors produce similar effects such as decreased cyclic AMP and inhibition of synaptic activity (Pertwee 2006; Waldhoer et al. 2004). Further, agonists of CB1 receptors and μ-opioid receptors produce similar behavioral effects that include antinociception (Cox and Welch 2004), alterations of locomotor activity (Pascual et al. 2005; Smith et al. 2009), food consumption (Jarbe and DiPatrizio 2005; Li et al. 2006), and thermoregulation (Diaz et al. 2009; Wang et al. 2009). Moreover, agonists at these receptors serve as reinforcers in self-administration paradigms (Justinova et al. 2008; Negus and Rice 2009).
Numerous studies have examined the interactions of these systems, and antinociception is one area in which interactions have been consistently reported. The cannabinoid agonist delta-9-tetrahydrocannabinol (Δ9-THC) potentiates the effects of morphine in the mouse tail-flick test (Cichewicz and McCarthy 2003) and the rat paw pressure test (Cox et al. 2007). There is also evidence that endogenous cannabinoids modulate the effects of opioids. The CB1 antagonist AM251 blocks morphine-induced antinociception as measured by an inflammatory pain model (Pacheco et al. 2008) and the tail-flick test in mice (Pacheco et al. 2009). Methylarachidonoylflurophosphate (MAFP), which inhibits the degradation of the endogenous cannabinoids anandamide (AEA), and 2-arachidonoylethanolamine (2-AG), enhances the effects of morphine (Pacheco et al. 2009), as does administration of AEA in combination with the fatty acid amide hydrolase (FAAH) inhibitor URB597 (Haller et al. 2008).
Although studies utilizing pharmacological manipulations of cannabinoid signaling suggest a role for endogenous cannabinoids in the antinociceptive effects of opioids, the results of research with genetic models do not. Specifically, morphine is equally effective in CB1 knockout (KO) and wildtype (WT) mice in the hotplate and tail immersion assays (Ledent et al. 1999; Valverde et al. 2000). It is noteworthy that a variety of pain models have been used in studies utilizing pharmacological manipulations to examine cannabinoid-opioid interactions (Cox et al. 2007; Pacheco et al. 2008, 2009) and in studies phenotyping CB1 KO mice (Ledent et al. 1999; Zimmer et al. 1999). In contrast, studies assessing the antinociceptive effects of morphine in CB1 knockout mice have only examined behaviors that occur in response to acute presentation of thermal noxious stimuli (Ledent et al. 1999; Valverde et al. 2000).
The mechanisms underlying nociception and antinociception depend on factors such as the modality and duration of the noxious stimuli (Mogil 2009) as well as the behavioral endpoints that are used to measure the pain response (Le Bars et al 2001). This being the case, the current studies extend the existing research on interactions between the cannabinoid and opioid systems by examining CB1 KO and WT mice in preclinical tonic pain assays that employ chemical noxious stimuli and measure behavioral responses that either decreased or increased in response to exposure to noxious stimuli. Specifically, we examined the effects of morphine on the behavioral consequences of intraperitoneal injection of acetic acid in CB1 KO and WT mice. We sought to determine the role of the CB1 receptor in the effects of morphine on the writhing response to acetic acid injection, and on behaviors that decrease in response to acetic acid injection, namely feeding and wheel-running (pain-suppressed behaviors). CB1 KO and WT were also used to determine the effects of morphine in a thermal (hotplate) assay for comparison.
In addition to the noxious stimulus modality and duration, another variable that might account for the dissimilar results obtained from studies using CB1 KO mice and antagonists is that constitutive inactivity of CB1 in the KO animals might produce changes that mask the role of this receptor in the effects of morphine. Accordingly, SR141716A, a CB1 antagonist, was used to determine the effects of acute disruption of CB1 activity on morphine antinociception.
METHODS
Subjects
Male and female CB1 KO mice and age-matched WT littermates were used for these experiments (total animal numbers: female KO = 49, male KO = 52, female WT = 51, and male WT = 51). The distribution of males and females was balanced across groups except in instances where there were an odd number of mice, in which case males outnumbered females by 1. Mice were generated on a full C57BL/6 background (Zimmer et al. 1999) and heterozygous breeding pairs were obtained from Virginia Commonwealth University. Due to limited access to CB1 KO and WT mice, male C57BL/6 mice were used in studies examining morphine in combination with SR141716A. All mice were bred and housed in the animal facilities of the Department of Psychology at the University of North Carolina at Chapel Hill. Mice were group housed after weaning and were subsequently individually housed where specified by the experimental protocols. Mean (SEM) mouse weights at the time of testing were as follows: female KO = 19.89 (0.69) g, male KO = 24.09 (0.57) g, female WT = 21.42 (0.30) g and male WT = 26.73 (0.57) g. Mean (SEM) weight for C57BL/6 mice was 27.83 (0.43) g.
Mice had free access to food and water except where specified by the experimental protocols below. Lights were programmed on a 12 hour light/dark cycle with lights off at 7:00am. All experiments took place during the dark cycle. Animal protocols were approved by the institutional animal care and use committee, and the methods were in accord with the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Research, Division on Earth and Life Studies, National Research Council, 2010)
Experimental Procedures
Assays of pain-elicited behavior
Hotplate
Mice were group housed and had free access to food and water for these experiments. Prior to testing mice were habituated to the testing room and handling for two days. On the test day antinociception was assessed using a hotplate analgesia meter (25.3 X 25.3 cm; Columbus Instruments, Columbus, OH). During the hotplate assay the mouse was placed onto the surface of the apparatus and the latency to lick or flutter the hind paws, or to jump from the hotplate surface was recorded. Responses were measured to the nearest 0.1 s and predetermined cutoff time of 20 s was defined as the maximum trial duration in order to prevent tissue damage. Immediately following termination of a trial, whether due to an animal’s response or the fact that the cutoff time elapsed, mice were removed from the apparatus and returned to the home cage.
Baseline latencies were assessed in CB1 KO and WT mice (n = 12 of each genotype) across a range of hotplate temperatures (44–56±0.1°C). Temperatures were tested in ascending order and 15 min elapsed between trials. One week later the same mice were used to assess the effects of morphine using a hotplate temperature of 56±0.1°C. Responses were measured 30 and 15 min prior to drug administration and the latencies from these trials were averaged to yield one baseline value. During dose-effect determination, cumulative doses of morphine were administered 30 min apart in half log increments. Temperatures and doses were examined in a repeated measures fashion. The effect of each dose of morphine is expressed as the percentage of the maximum possible effect (%MPE): [postdrug latency (s) – baseline latency (s)]/[20 – baseline latency (s)].
To determine the consequences of pretreatment with the CB1 antagonist SR141716A on morphine antinociception, C57BL/6 mice were tested at 56º C as described above. Morphine (32.0 mg/kg) and SR141716A (3.0 mg/kg) were administered alone and in combination 30 and 60 min prior to testing, respectively. These doses were selected based on prior work in our laboratory with these compounds in this mouse strain.
Acetic acid-induced writhing
Mice were group housed and had free access to food and water until 12 and 5 h prior to testing, respectively. Mice were habituated to the testing room and handling for two days prior to testing. In addition, mice were given a 30 min session with free access to 32% liquid nutrition (Vanilla flavor, CVS brand) on the day prior to testing. Food and water deprivation and access to liquid nutrition occurred due to the fact that data for acetic acid-suppressed feeding were collected in these animals during the same session (see below).
On the test day, separate groups of mice were injected with saline or morphine (0.1 – 10.0 mg/kg) 45 min prior to the start of the test session (n = 5–6 of each genotype per group). Immediately prior to the test session, mice were injected with saline or 0.56% acetic acid. Following the second injection mice were immediately placed into clean polycarbonate mouse cages (29.91 X 19.05 X 12.7 cm; Allentown Inc, Allentown, NJ) containing dishes of 32% liquid nutrition, and their behavior was videotaped for 30 min. Following the session, the tapes were viewed and the number of writhes was recorded. Writhes were operationally defined as an elongation of the body with simultaneous extension of the hind limbs. The effect of each dose of morphine is expressed as percent inhibition of writhing (% Inhibition): [((writhes in saline treated mice/writhes in morphine treated mice)/writhes in saline treated mice) X 100].
To determine the consequences of pretreatment with the CB1 antagonist SR141716A on morphine antinociception in the writhing assay, C57BL/6 mice were tested as described above. Morphine (3.2 mg/kg) and SR141716A (3.0 mg/kg) were administered alone and in combination 45 and 60 min prior to testing, respectively. These doses were selected based on prior work in our laboratory with these compounds in this mouse strain.
Assays of pain-suppressed behavior
Acetic acid-suppressed feeding
Mice were group housed and had free access to food and water until 12 and 5 h prior to testing, respectively. Mice were habituated to the testing room and handling for two days prior to testing. In addition, 24 h prior to testing, mice were given a 30 min session of free access to approximately 14 ml of 32% liquid nutrition (Vanilla flavor, CVS brand; composition as used: protein = 0.36 g/oz, carbohydrate = 1.6 g/oz, and fat = 0.24 g/oz) in order to reduce the novelty of the substance. During this session, individual mice were placed into bedding-free polycarbonate mouse cages (29.91 X 19.05 X 12.7 cm; Allentown Inc, Allentown, NJ) that contained a glass dish containing liquid nutrition and were allowed to explore the testing environment and consume the liquid nutrition.
On the test day, separate groups of mice were injected with saline or morphine (0.1 – 10.0 mg/kg) 45 min prior to the start of the test session (n = 5–6 of each genotype per group). Immediately prior to the test session, mice were injected with 0.56% acetic acid or saline. Following the second injection mice were immediately placed into clean polycarbonate mouse cages (29.91 X 19.05 X 12.7 cm; Allentown Inc, Allentown, NJ) containing liquid nutrition and the amount of liquid consumed was measured for 30 min. Consumption was quantified by subtracting the weight of the liquid-containing dishes after the session from the weight obtained prior to the session. Because body size might influence consumption independent of other variables, this value was divided by the animals’ weight (grams of liquid consumed per gram of body weight). In the event of spillage, the data were discarded. The effect of each dose of morphine on pain-suppressed consumption is expressed as percent of non-suppressed consumption (% Control): [(acetic acid-suppressed consumption (g/g)/non-suppressed consumption (g/g)) X 100].
To determine the consequences of pretreatment with the CB1 antagonist SR141716A on morphine antinociception in the pain-suppressed feeding assay, C57BL/6 mice were tested as described above. Morphine (3.2 mg/kg) and SR141716A (3.0 mg/kg) were administered alone and in combination 45 and 60 min prior to testing, respectively. The effects of 3.0 mg/kg SR141716A on non-suppressed feeding were also determined. These doses were selected based on prior work in our laboratory with these compounds in this mouse strain.
Acetic acid-suppressed wheel-running
Mice were grouped housed in standard polycarbonate mouse cages (29.91 X 19.05 X 12.7 cm; Allentown Inc, Allentown, NJ) until the start of these experiments. At this time mice were individually housed in polycarbonate cages (35.56 X 26.67 X 13.97 cm; Tecniplast USA Inc., Exton, PA) containing running wheels (ENV-044, Med Associates, St. Albans, VT). Testing occurred after three weeks of habituation to handling and acquisition of wheel-running behavior. On the day prior to testing (control session) mice were injected with saline followed 45 min later by a second injection of saline. The next day (test session), 24 h after the control session, separate groups of mice were injected with morphine (0.32 – 3.2 mg/kg) or saline and 45 min later were injected with 0.56% acetic acid or saline (n = 6–7 of each genotype per group). Wheel-running was recorded for 30 min following the second injection (acetic acid or saline) during the control and test sessions. The effect of each dose of morphine on pain-suppressed wheel-running is expressed as percent of non-suppressed wheel-running (% Control): [(acetic acid-suppressed running/non-suppressed running) X 100].
To determine the consequences of pretreatment with the CB1 antagonist SR141716A on morphine antinociception in the pain-suppressed wheel-running assay, C57BL/6 mice were tested as described above. Morphine (1.0 mg/kg) and SR141716A (3.0 mg/kg) were administered alone and in combination 45 and 60 min prior to testing, respectively. The effects of morphine and SR141716A on non-suppressed running were also determined. These doses were selected based on prior work in our laboratory with these compounds in this mouse strain.
Drugs
Morphine sulphate and SR141716A were provided by the National Institute on Drug Abuse (Bethesda, MD, USA). Morphine was dissolved in 0.9% saline and SR141716A was dissolved in a vehicle of 100% ethanol, Alkamuls EL-620 (Rhodia, Cranbury, New Jersey) and saline in a ratio of 1:1:18. Acetic acid was purchased from Fischer Scientific and diluted in 0.9% saline. Morphine and SR141716A were injected subcutaneously and acetic acid was injected intraperitoneally at a volume of 0.1 ml/10 g.
Data Analysis
No sex differences were detected in these studies and all data analyses that follow were conducted with groups collapsed across this variable. Data are presented as raw values (±SEM) or expressed as mean (±SEM) % MPE, % Inhibition, or % Control depending on the assay (see above). The morphine dose required to produce a 50% maximal effect (ED50) was derived using log-linear interpolation when possible, and differences in morphine potency between genotypes were determined and expressed as a potency ratio with 95% confidence limits. Two-factor analyses of variance (ANOVA) were performed to determine the effects of genotype and dose. Student’s t-tests were used when appropriate to determine genotypic differences and the effects of SR141716A under control conditions. In studies examining the effects of pretreatment with SR141716A on morphine antinociception, one-way ANOVA with Bonferoni comparisons were used to determine treatment effects. All statistical analyses were conducted with an alpha level of significance set at p < 0.05.
RESULTS
Fig. 1 (top) shows the response latencies for CB1 KO and WT mice across a range of temperatures (44–56±0.1°C) on the hotplate. Two-factor ANOVA revealed temperature dependent decreases in response latencies [F(4,110) = 296.66, p < 0.05]. However, there were no genotypic differences in response latencies and there was no interaction between genotype and temperature. Baseline responses at 56 º did not differ between assessments during the temperature effect curve or morphine dose-effect curve. Morphine (Fig. 1 bottom) produced dose-dependent antinociception on the 56º C hotplate [F(3,66) = 174.18, p < 0.05] in CB1 KO [ED50 (95% CL) = 7.35 (5.99–9.01)] and WT mice [ED50 (95% CL) = 8.37 (6.97–10.05)] but there were no differences in the effects of morphine between genotypes [potency ratio = 1.11 (0.85–1.45)], and no significant interaction of morphine and genotype.
Fig. 1.
Hotplate assay for CB1 KO and WT mice. Top, temperature-effect curves (44–56 ºC) in the absence of morphine. Abscissa: hotplate temperature (ºC). Ordinate: mean response latency in seconds. Bottom, morphine dose-effect curves (1.0 – 32.0 mg/kg) on 56º C hotplate. Abscissa: dose of morphine in milligrams per kilogram. Ordinate: percent maximum possible effect
Fig. 2 shows the effects of morphine (32.0 mg/kg) and SR141716A (3.0 mg/kg) alone and in combination in the hotplate assay at 56º C in C57BL/6 mice. One-way ANOVA revealed a significant effect of treatment [F(3,32) = 111.02, p < 0.05] and bonferoni comparisons show that SR141716A had no effect on hotplate latency. Morphine produced a 100% maximum possible effect in all animals tested (p < .05 vs saline) whereas pretreatment with SR141716A significantly attenuated the effects of morphine (p < .05 vs morphine alone), though there was still a significant antinociceptive effect (p < .05 vs saline).
Fig. 2.
Effect of the CB1 antagonist SR141716A on morphine antinociception in the hotplate assay in C57BL/6 mice. Abscissa: treatment. Ordinate: mean response latency in seconds. Asterisk denotes statistical significance compared with saline. Double asterisk denotes statistical significance compared with morphine alone
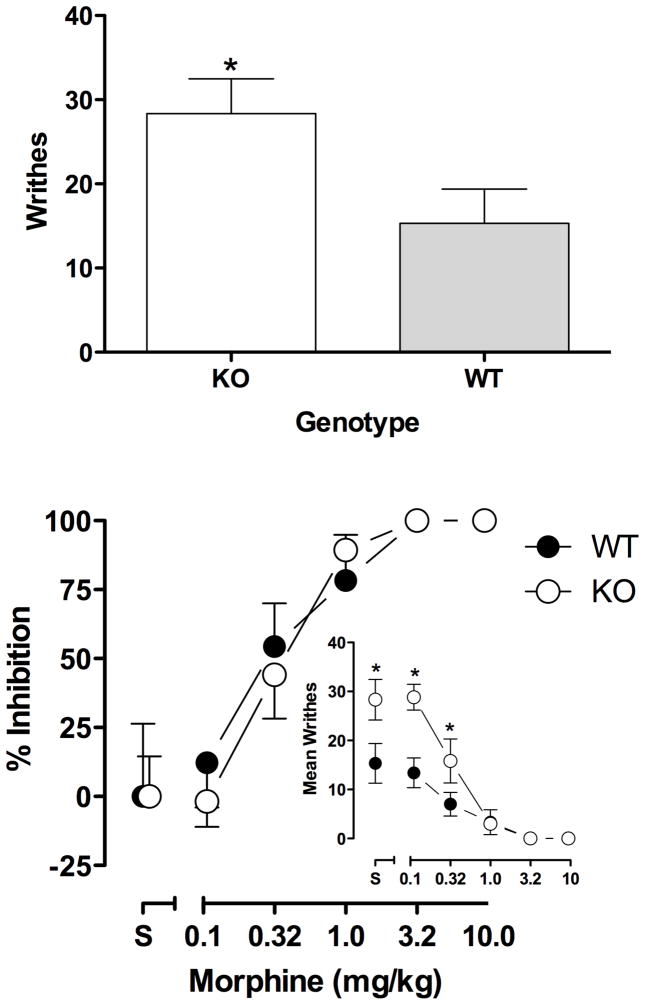
Fig. 3 (top) shows the number of writhes produced by acetic acid in CB1 KO and WT mice in the absence of morphine. KO mice writhed significantly more [28±4.1] than WT mice [15±4.1] in response to acetic acid [t(10) = 2.2, p < 0.05]. The raw data (number of writhes; Fig. 2 bottom inset) indicate that these genotypic differences persisted when morphine was administered prior to acetic acid [F(1,50) = 16.47, p < 0.05]. However, when basal genotypic differences were taken into account by expressing the data as percent inhibition of writhing (Fig. 3 bottom), morphine dose-dependently inhibited writhing [F(4,50) = 28.99, p < 0.05] in CB1 KO [ED50 (95% CL) = 0.37 (0.27–0.51)] and WT mice [ED50 (95% CL) = 0.34 (0.17–0.66)] and there were no differences in the effects of morphine between genotypes [potency ratio = 1.13 (0.61–2.13)]; there was no significant interaction of genotype and dose.
Fig. 3.
Acetic acid-induced writhing in CB1 KO and WT mice. Top, writhing in response to intraperitoneal injection of 0.56% acetic acid. Ordinate: mean number of writhes. Asterisk denotes statistical significance compared with WT (p<0.05). Bottom, effects of morphine on writhing expressed as percent inhibition of writhing. Abscissa: dose of morphine in milligrams per kilogram. Ordinate: percent inhibition of writhing. Bottom inset, effects of morphine on writhing (raw data)
Fig. 4 shows the effects of 3.2 mg/kg morphine and 3.0 mg/kg SR141716A, alone and in combination on acetic acid induced writhing in C57BL/6 mice. One-way ANOVA revealed a significant effect of treatment [F(3,20) = 23.90, p < 0.05] such that SR141716 produced a slight but non-significant increase in writhes. Morphine significantly inhibited writhing (p < .05 vs saline) and this effect was attenuated by pretreatment with SR141716A (p < 0.05 vs saline).
Fig. 4.
Effect of the CB1 antagonist SR141716A on morphine antinociception in the writhing assay in C57BL/6 mice. Abscissa: treatment. Ordinate: mean number of writhes. Asterisk denotes statistical significance compared with saline. Double asterisk denotes statistical significance compared with morphine alone
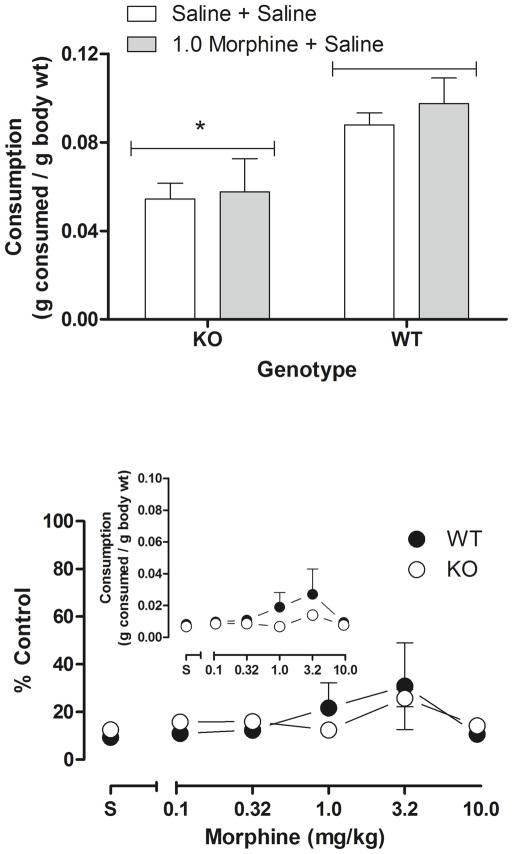
Fig. 5 (top) shows the effects of saline and 1.0 mg/kg morphine on liquid nutrition consumption in CB1 KO and WT in the absence of acetic acid. There was a main effect of genotype [F(1,21) = 9.16, p < 0.05] where WT mice consumed more liquid nutrition than KO mice, but 1.0 mg/kg morphine had no effect on non-suppressed feeding and there was not a significant interaction between genotype and treatment. Intraperitoneal injection of acetic acid almost completely suppressed consumption in both genotypes [WT: 0.008±0.002 g/g; KO: 0.007±0.001 g/g]. Morphine (Fig. 5 bottom) was not effective at attenuating acetic acid-induced suppression of consumption. Table 1 shows raw consumption (ml) for each genotype.
Fig. 5.
Acetic acid-suppressed feeding in CB1 KO and WT mice. Top, non-suppressed feeding after saline and 1.0 mg/kg morphine. Ordinate: mean grams consumed per gram of body weight. Asterisk denotes statistical significance compared with WT (p<0.05). Bottom, effects of morphine on acetic acid-suppressed feeding expressed as percent control. Abscissa: dose of morphine in milligrams per kilogram. Ordinate: percent control consumption. Bottom inset, effects of morphine on acetic acid-suppressed feeding (raw data)
Table 1.
Mean (SEM) liquid nutrition consumption (ml), unadjusted for weight, by CB1 KO and WT mice.
| Treatment | KO | WT |
|---|---|---|
| Control | 1.26 (0.18) | 2.05 (0.01) |
| Saline | 0.15 (0.02) | 0.22 (0.05) |
| 0.1 mg/kg | 0.25 (0.03) | 0.22 (0.05) |
| 0.32 mg/kg | 0.18 (0.02) | 0.25 (0.03) |
| 1.0 mg/kg | 0.15 (0.02) | 0.47 (0.23) |
| 3.2 mg/kg | 0.26 (0.03) | 0.58 (0.32) |
| 10.0 mg/kg | 0.17 (0.02) | 0.23 (0.03) |
Pretreatment with 3.0 mg/kg SR141716A (Fig. 6 (top)) decreased consumption of ensure by C57BL/6 mice in the absence of acetic acid (i.e. consumption not suppressed by pain; t (5) = 2.38, p < .05). Figure 6 (bottom) shows that neither 3.2 mg/kg morphine nor 3.0 mg/kg SR141716A, alone or in combination, altered consumption that was suppressed by acetic acid injection.
Fig. 6.
Effect of the CB1 antagonist SR141716A on morphine antinociception in the feeding assay in C57BL/6 mice. Top, non-suppressed feeding after saline and 3.0 mg/kg SR141716A. Abscissa: treatment. Ordinate: mean grams consumed per gram of body weight. Asterisk denotes statistical significance compared with saline. Bottom, morphine alone and in combination with SR141716A. Abscissa: treatment. Ordinate: mean grams consumed per gram of body weight
Fig. 7 (top) shows the effects of saline and 3.2 mg/kg morphine on wheel-running behavior in CB1 KO and WT mice in the absence of acetic acid. There was a main effect of genotype [F(1,20) = 9.36, p < 0.05] where WT mice displayed higher levels of wheel-running than KO mice, however 3.2 mg/kg morphine had no effect on non-suppressed wheel-running and there was no significant interaction between genotype and treatment. Fig. 7 (bottom inset) shows that intraperitoneal injection of acetic acid completely suppressed wheel-running in both genotypes. Morphine significantly attenuated the suppression of wheel-running by injection of acetic acid [F(4,53) = 6.03, p < 0.05] and the genotypic differences seen with non-suppressed wheel-running persisted with WT mice displaying higher levels of wheel-running than KO mice [F(1,53) = 5.10, p < 0.05]; there was no significant interaction of morphine and genotype. When basal genotypic differences in wheel-running were taken into account by expressing wheel-running as percentage of control running, the effects of morphine on acetic acid-suppressed wheel running did not differ between genotypes. Morphine ED50 (95% CL) = 1.72 (0.77–3.79) for KO mice and 1.38 (0.84–2.25) for WT mice [potency ratio = 1.18 (0.51–2.94)].
Fig. 7.
Acetic acid-suppressed wheel-running in CB1 KO and WT mice. Top, non-suppressed wheel-running after saline and 3.2 mg/kg morphine. Ordinate: mean wheel revolutions. Asterisk denotes statistical significance compared with WT (p<0.05). Bottom, effects of morphine on acetic acid-suppressed wheel-running expressed as percent control. Abscissa: dose of morphine in milligrams per kilogram. Ordinate: percent control running. Bottom inset, effects of morphine on acetic acid-suppressed wheel-running (raw data)
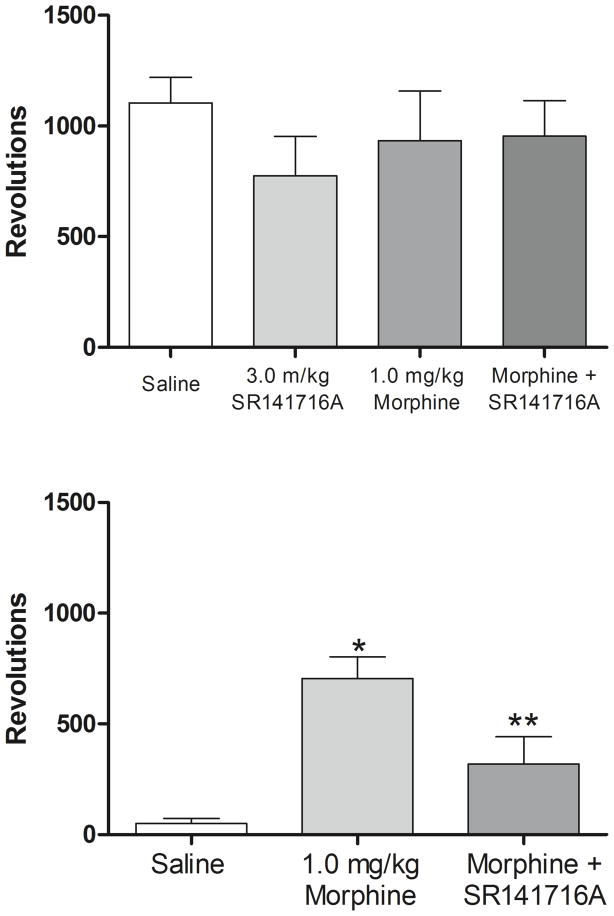
Fig. 8 (top) shows that when administered in the absence of acetic acid, morphine and SR141716A, alone and in combination, do not alter wheel running behavior in C57BL/6 mice. The bottom portion of Fig.8 shows the effects of morphine, alone and in combination with SR141716A, on pain-suppressed wheel running. One-way ANOVA revealed a significant effect of treatment [F(2,19) = 10.44, p < 0.05], such that mice treated with morphine produced significantly more wheel revolutions than mice treated with saline (p < .05 vs saline). Pretreatment with SR141716A blocked this effect (p < 0.5 vs morphine alone).
Fig. 8.
Effect of the CB1 antagonist SR141716A on morphine antinociception in the wheel-running assay in C57BL/6 mice. Top, non-suppressed running after saline, 1.0 mg/kg morphine and 3.0 mg/kg SR141716A. Abscissa: treatment. Ordinate: mean wheel revolutions. Bottom, morphine alone and in combination with SR141716A. Abscissa: treatment. Ordinate: mean wheel revolutons. Asterisk denotes statistical significance compared with saline. Double asterisk denotes statistical significance compared with morphine alone
DISCUSSION
The primary goal of these experiments was to investigate the role of the CB1 receptor in the antinociceptive effects of morphine in CB1 KO mice. Previous studies have shown that CB1 KO and WT mice respond similarly in models of acute thermal pain. In the present study, we extended these findings by determining the effects of morphine in CB1 KO and WT mice in models examining pain resulting from i.p. injection of acetic acid. These models allow for the assessment of both pain-elicited behaviors (writhing) and pain-suppressed behaviors (food consumption and wheel-running). In addition, we compared the results seen in CB1 KO and WT mice with results obtained with the CB1 antagonist, SR141716A in C57BL/6 mice.
The two genotypes did not differ in their responses on the hotplate across temperatures that ranged from innocuous to noxious (44–56±0.1°C). This suggests that CB1 receptors are not integral to this behavioral response to thermal noxious stimuli. Previous research has revealed mixed results with regard to nociceptive responding in the hotplate assay between CB1 KO and WT mice. In a study utilizing mice on the same background as used in the present study (C57BL/6), CB1 KO mice were found to have increased latencies (interpreted as hypoalgesia) relative to WT mice (Zimmer et al. 1999). Consistent with the data obtained in the present study, studies utilizing mice on a CD1 background showed no differences between genotypes (Ledent et al. 1999; Valverde et al. 2000).
Previous studies have obtained mixed results when examining the effects of CB1 antagonists on responses to acute thermal pain with some studies revealing no effect (Cravatt et al. 2001) and others suggesting a hyperalgesic effect (Richardson et al. 1998). The present findings are consistent with the former in that, at the dose tested, SR141716A did not alter hotplate latencies.
That there were no differences between CB1 KO and WT mice in response to morphine in the hotplate assay suggests that the absence of functioning CB1 receptors does not alter the effects of morphine in this context. Pretreatment with SR141716A, however, did produce a modest but significant attenuation of the antinociceptive effects of morphine in the hotplate assay. This finding is consistent with other data that suggest that CB1 receptors do modulate the effects of morphine (Pacheco et al. 2008, 2009). Taken together, these findings suggest that CB1 KO and WT mice respond similarly to morphine because of differences in the consequences of constitutive inactivity of CB1 and the acute effects of a CB1 receptor antagonist. It might be the case that the knockout mice undergo unidentified developmental changes that mask the role of CB1 receptors in the effects of morphine.
In order to determine if the findings of studies using acute thermal pain extend to other stimulus modalities and durations, experiments were conducted with acetic acid serving as a tonic, chemical noxious stimulus. Acetic acid (0.56%) produced more writhes in CB1 KO mice than in CB1 WT mice, suggesting a role for CB1 receptors in the writhing response to acetic acid. Other findings also suggest a role for CB1 receptors in responses to chemical noxious stimuli. For instance, disruption of FAAH activity by pharmacological or genetic means inhibits acetic acid-induced writhing (Naidu et al. 2009), and inhibition of CB1 signaling results in hyperalgesia in the formalin test (Calignano et al. 1998). On the other hand, studies utilizing CB1 KO and WT mice on a CD1 background (Ledent et al. 1999; Valverde et al. 2000) found no differences between genotypes in the writhing test. At the dose tested in the present experiments, the CB1 antagonist SR141716A did not alter the number of writhes elicited by acetic acid which is consistent with other findings (Booker et al. 2009).
Morphine dose-dependently decreased the number of writhes that resulted from intraperitoneal injection of acetic acid, and there were no genotypic differences in the effects of morphine in this assay. This suggests that results obtained in other studies utilizing acute thermal pain to study the antinociceptive effects of morphine in CB1 knockout mice extend to this commonly used model of tonic pain. On the other hand, pretreatment with SR141716A attenuated the ability of morphine to inhibit the writhing response, again suggesting that morphine’s effects are modulated by CB1, but this role is masked in CB1 knockout mice.
Nociception elicits pain-related behaviors and suppresses other behaviors. Research has demonstrated the utility of preclinical pain assays that measure pain-suppressed behavior in addition to traditional assays that measure pain-elicited behavior (Martin et al. 2005; Matson et al. 2007; Negus et al. 2010; Pereira Do Carmo et al. 2009; Stevenson et al. 2006, 2009). Consequently, the present studies also used CB1 KO and WT mice to determine the role of the endogenous cannabinoid system in morphine’s effects on behaviors that are suppressed by exposure to noxious stimuli.
Consumption of palatable food, a behavior that occurs at a high rate under control conditions, is decreased upon exposure to noxious stimuli (Stevenson et al. 2006). In the present studies, control consumption was decreased in mice lacking CB1. This finding, along with the finding that SR141716A decreased non-suppressed food consumption is consistent with research indicating that the cannabinoid system is an important mediator of feeding behaviors. Other studies assessing feeding behavior in CB1 KO mice have revealed results similar to those found here (Cota et al. 2003). Moreover, it has been shown that CB1 agonists and antagonists produce hyperphagia (Miller et al. 2004) and hypophagia (Ward et al. 2009), respectively.
In contrast to findings obtained from studies utilizing C57BL/6J mice (Stevenson et al. 2006), in the current study morphine was not effective at attenuating the suppression of feeding by i.p. injections of acetic acid. That morphine had no effect on non-suppressed consumption suggests that it is unlikely that this result is due to suppression of consumption by morphine. The reasons for the discrepancies between the present studies and the Stevenson et al. (2006) study are unclear, but there were numerous differences in experimental parameters between the two studies (e.g. amount of exposure to acetic acid and food) that might contribute to the dissimilar findings.
Wheel-running also occurs at a high rate in rodents, and is susceptible to suppression by noxious stimuli. Under baseline conditions, CB1 KO mice exhibited less wheel-running behavior than WT mice which is consistent with other findings (Dubreucq et al. 2010). The CB1 antagonist CB1 antagonist, SR141716A, did not reduce wheel-running when administered to C57BL/6 mice though it has been shown to do so in rats (Keeney et al. 2008). The findings in CB1 KO mice might suggest a role of the endocannabinoid system in the reinforcing effects of wheel-running, but it might also be the case that the differences seen in the present studies are related to other effects of CB1 knockout. For instance, the decreased caloric intake by CB1 KO mice, demonstrated by others (Cota et al. 2003) and suggested by the present feeding experiments, might lead to decreased wheel-running behavior.
Unlike the results observed in the pain-suppressed feeding assay, morphine dose-dependently attenuated the suppression of wheel-running behavior after i.p. injection of acetic acid. These findings are consistent with previous reports showing that opioids are effective at blocking decreases in a variety of behaviors that result from exposure to noxious stimuli (Martin et al. 2005; Matson et al. 2007; Negus et al. 2010; Pereira Do Carmo et al. 2009; Stevenson et al. 2006, 2009). This suggests that under these conditions, morphine reduces nociception in a manner that not only decreases reflexive responses to i.p. injection of acetic acid (i.e. writhing), but restores rates of pain-suppressed spontaneous behavior. In addition, 3.2 mg/kg morphine, which produced the peak effect on pain-suppressed wheel-running in CB1 KO and WT mice, had no effect on non-suppressed wheel-running. This suggests that the present results are not due to non-specific increases in wheel-running as a result of morphine administration. As stated above, there were no genotypic differences in the effects of morphine on suppressed or non-suppressed wheel-running. On the other hand, as was the case in the other models used here, SR141716A significantly attenuated the ability of morphine to block the suppression of wheel-running by acetic acid injection, again suggesting a role for CB1 receptors in the antinociceptive effects of morphine in this assay.
The findings of the present studies are consistent with research demonstrating that CB1 KO and WT mice do not respond differently to morphine on endpoints related to antinociception (Ledent et al. 1999; Valverde et al. 2000). Moreover, the findings with SR141716A are consistent with other studies that demonstrate a reduction of the antinociceptive effects of morphine upon administration of CB1 antagonists (Pacheco et al. 2008, 2009). Combined administration of anandamide and a selective inhibitor of its degradation enhanced the effects of morphine (Haller et al. 2008), and Pacheco et al. (2009) also showed that MAFP, which non-selectively inhibits the catabolism of AEA and 2-AG, enhanced the effects of morphine. These authors also demonstrated that a CB2 antagonist did not alter the effects of morphine, and it has been demonstrated that the effects of MAFP are mediated via CB1 (Ates et al. 2003).
In the context of the literature, the present results demonstrating attenuation of morphine’s effects by the CB1 antagonist, SR141716A, suggest that endogenous cannabinoids are involved in the antinociceptive effects of morphine in models of acute thermal pain and chemical pain. The mechanisms of these interactions are not clear. Researchers have hypothesized that one factor in cannabinoid/opioid interactions is that exposure to cannabinoids and opioids results in reciprocal changes in receptor expression and/or function, and there is evidence that these systems do interact in this manner (e.g. Vigano et al. 2005). Another possible mechanism behind opioid/cannabinoid interactions is that activity at opioid and cannabinoid receptors may elevate levels of endogenous cannabinoids and opioids, respectively. For instance, there is evidence that cannabinoid agonists elevate dynorphin levels (Mason et al. 1999) and that morphine elevates anandamide levels (Vigano et al 2004), but more data are needed to determine the significance of these effects. The finding that morphine-induced antinociception does not differ in CB1 KO and WT mice indicates that the constitutive absence of CB1 signaling may result in compensatory changes that mask this receptor’s role in the antinociceptive effects of morphine.
Acknowledgments
Supported by NIH grants R01-DA002749, T32-DA00724, and F31-DA025446.
The authors thank Dr. Steve Negus for his important comments and advice prior to the initiation of these studies.
References
- Ates M, Hamza M, Seidel K, Kotalla CE, Ledent C, Guhring H. Intrathecally applied flurbiprofren produces an endocannabinoid-dependent antinociception in the rat formalin test. Eur J Neurosci. 2003;17:597–604. doi: 10.1046/j.1460-9568.2003.02470.x. [DOI] [PubMed] [Google Scholar]
- Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH. Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug and Alcohol Dependence. 2009;105:42–47. doi: 10.1016/j.drugalcdep.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parson LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsican G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipgenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP. Synergy between Δ9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacology. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Cox ML, Welch SP. The antinociceptive effect of Delta-9-tetrahydrocannabinol in the arthritic rat. Eur J Pharmacology. 2004;493:65–84. doi: 10.1016/j.ejphar.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. PNAS. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Mariscano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Haller VL, Stevens DL, Welch SP. Modulation of opioids via protection of anandamide degradation by fatty acid amide hydrolase. Eur J Pharmacol. 2008;600:50–58. doi: 10.1016/j.ejphar.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research, Division on Earth and Life Studies, National Research Council. Guide for the care and use of laboratory animals. Washington DC: National Academy Press; 2010. [Google Scholar]
- Jarbe TU, DiPatrizio NV. Delta9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist delta9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol. 2005;16:373–380. doi: 10.1097/00008877-200509000-00009. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Munzar P, Panlilio LV, Yasar S, Redhi GH, Tanda G, Goldberg SR. Blockade of THC-seeking behavior and relapse in monkeys by the cannabinoid CB(1)-receptor antagonist rimonabant. Neuropsychopharmacol. 2008;33:2870–2877. doi: 10.1038/npp.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney BK, Raichlen DA, Meek TH, Wijeratne RS, Middleton KM, Gerdeman GL, Garland TJ. Differential responses to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behavior. Behav Pharmacol. 2008;19:812–820. doi: 10.1097/FBP.0b013e32831c3b6b. [DOI] [PubMed] [Google Scholar]
- Li D, Olszewski PK, Shi MK, Billington CJ, Kotz CM, Levine AS. Effect of opioid receptor ligands injected into the rostral lateral hypothalamus on c-fos and feeding behavior. Brain Res. 2006;1096:120–124. doi: 10.1016/j.brainres.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:310–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–692. [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petetet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Zhang Y, Buechler N, Conklin DR, Eisenach JC. Intrathecal morphine and ketorlac analgesia after surgery: Comparison of spontaneous and elicited responses in rats. Pain. 2005;113:376–385. doi: 10.1016/j.pain.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Lowe J, Welch SP. A diminution of delta9-tetrahydrocannabinol modulation of dynorphin A-(1–17) in conjunction with tolerance development. Eur J Pharmacol. 1999a;381:105–111. doi: 10.1016/s0014-2999(99)00542-7. [DOI] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: A novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards FG. Cannabinoid agonst, CP 55940, facilitates intake of palatable foods when injected into the hindbrain. Physiology and Behavior. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: Progress and challenges. Nature Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Booker L, Cravatt BF, Lichtman AH. Synergy between enzyme inhibitors of fatty acid amide hydrolase and cyclooxygenase in visceral nociception. J Pharmacol Exp Ther. 2009;329:48–56. doi: 10.1124/jpet.108.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology. 2010 doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacological modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacol. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco DF, Klein A, Perez AC, Pacheco CMF, Francischi JN, Duarte ID. The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Brit J Pharmacol. 2008;154:1143–1149. doi: 10.1038/bjp.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco DF, Klein A, Perez AC, Pacheco CMF, Francischi JN, Reis GML, Duarte IDG. Central antinociception induced by μ-opioid receptor agonist morphine, but not δ- or κ- is mediated by cannabinoid CB1 receptor. Brit J Pharmacol. 2008;158:225–231. doi: 10.1111/j.1476-5381.2009.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Suardiaz M, Martin MI. A cannabinoid agonist, WIN 55,212–2, reduces neuropathic nociception induced by paclitaxel in rats. Pain. 2005;118:23–34. doi: 10.1016/j.pain.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obesity. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Smith MA, Greene-Naples JL, Lyle MA, Iordanou JC, Felder JN. The effects of repeated opioid administration on locomotor activity: I. Opposing actions of μ and κ receptors. J Pharmacol Exp Ther. 2009;330:468–475. doi: 10.1124/jpet.108.150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Mathews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacol. 2005;30:2046–57. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57Bl/6J mice. J Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Ledent C, Beslot F, Parmentier M, Roques BP. Reduction of stress-induced analgesia but not of exogenous opioid effects in mice lacking CB1 receptors. Eur J Neurosci. 2000;12:533–539. doi: 10.1046/j.1460-9568.2000.00929.x. [DOI] [PubMed] [Google Scholar]
- Vigano D, Grazia Cascio M, Rubino T, Fezza F, Vaccani A, Di Marzo V, Parolaro D. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacol. 2003;28:1160–1167. doi: 10.1038/sj.npp.1300117. [DOI] [PubMed] [Google Scholar]
- Vigano D, Valenti M, Cascio MG, Di Marzo V, Parolaro D, Rubino T. Changes in endocannabinoid levels in a rat model of behavioral sensitization. Eur J Neurosci. 2004;20:1849– 1857. doi: 10.1111/j.1460-9568.2004.03645.x. [DOI] [PubMed] [Google Scholar]
- Viganò D, Rubino T, Vaccani A, Bianchessi S, Marmorato P, Castiglioni C, Parolaro D. Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology (Berl) 2005;182:527–536. doi: 10.1007/s00213-005-0114-4. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo J, Wang S, Fang Q, He F, Wang R. Neuropeptide FF receptors antagonist, RF9, attenuates opioid-evoked hypothermia in mice. Peptides. 2009;29:1183–1190. doi: 10.1016/j.peptides.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Rawls SM, Whiteside GT, Walker EA. Age-dependent effects of the cannabinoid CB1 antagonist SR141716A on food intake, body weight change, and pruritus in rats. Psychopharmacology. 2009;206:155–165. doi: 10.1007/s00213-009-1592-6. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]