Abstract
Drug-induced liver injury (DILI) is common and nearly all classes of medications can cause liver disease. Most cases of DILI are benign, and improve after drug withdrawal. It is important to recognize and remove the offending agent as quickly as possible to prevent the progression to chronic liver disease and/or acute liver failure. There are no definite risk factors for DILI, but pre-existing liver disease and genetic susceptibility may predispose certain individuals. Although most patients have clinical symptoms that are identical to other liver diseases, some patients may present with symptoms of systemic hypersensitivity. Treatment of drug and herbal-induced liver injury consists of rapid drug discontinuation and supportive care targeted to alleviate unwanted symptoms.
Keywords: Drug-induced liver injury (DILI), drug-induced hepatitis, drug-induced cholestasis, acetaminophen, vanishing bile duct syndrome, herbal toxicity
Adverse drug reactions are an important cause of liver injury that may require discontinuation of the offending agent, hospitalization, or even liver transplantation.1 Indeed, drug-induced hepatotoxicity is the most frequent cause of acute liver failure in US.2 Because the liver is responsible for concentrating and metabolizing a majority of medications, it is a prime target for medication-induced damage. Among hepatotoxic drugs, acetaminophen (paracetamol) is the most often studied. However, a broad range of different pharmacological agents can induce liver damage, including anesthetics, anticancer drugs, antibiotics, antituberculosis agents, antiretrovirals, and cardiac medications. In addition, a plethora of traditional medical therapies and herbal remedies may also be hepatotoxic.
Depending on the duration of injury and the histological location of damage, drug-induced liver injury (DILI) is categorized as acute or chronic, and either as hepatitis, cholestatic, or a mixed pattern of injury. The hepatitis pattern is characterized by hepatocyte necrosis and is associated with a poor prognosis. There are three types of acute cholestatic drug-induced injury: bland cholestasis is the result of abnormal biliary secretion, and is not accompanied by significant hepatocellular damage; cholestatic hepatitis (mixed type) refers to cholestasis with concomitant hepatic parenchymal damage; and the third form of acute cholestasis is defined by the presence of bile duct injury or cholangiolitis. Medications may cause chronic cholestasis through two additional mechanisms: through the obliteration of bile ducts, also known as the vanishing bile duct syndrome, or by extrahepatic biliary obstruction, known as secondary sclerosing cholangitis.3–6
Mechanisms of Drug-induced Liver Injury
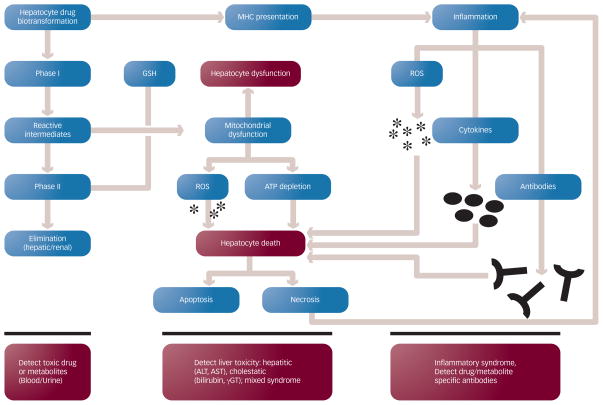
DILI may be the result of direct toxicity from the administered drug or their metabolites, or injury may result from immune-mediated mechanisms (see Figure 1). Although these mechanisms are distinct, they may also be interconnected; for example, initial hepatocyte destruction due to direct drug toxicity may be further enhanced by the subsequent inflammatory reaction. It is also important to recognize that oral medications with significant hepatic metabolism are more likely to result in DILI.7
Figure 1. Mechanisms of Drug-induced Liver Injury.
Drugs are metabolized by the liver p450 system in a series of phase I and phase II reactions (left column). Toxic intermediates can illicit hepatocyte damage and death by inducing apoptosis or necrosis (center column). Drugs that bind to cellular membranes can elicit an immunologic reaction upon presentation to major histocompatibility complex (MHC) particles, resulting in inflammation (right column).
The vast majority of drugs are liposoluble and metabolized in the liver and excreted in bile or urine. The first step of drug metabolism is known as a phase I reaction and is mediated by enzymes of the hepatic cytochrome p450 system.8 Intermediate bioactive products generated in this step may interact with various cellular organelles (e.g. mitochondria) leading to hepatocyte dysfunction and cellular demise.9 These potentially toxic intermediate products are then inactivated through glucurono-, glutathione- or sulfa-conjugation in subsequent phase II reactions. In order to limit hepatotoxicity, the generation rate for phase I products should not exceed the liver’s capacity to inactivate them. Depletion or deficiency of the compounds responsible for the phase II conjugation reactions may result in accumulation of toxic metabolites. Such is the case in patients who abuse alcohol and ingest acetaminophen.10 In this example, even low-dose acetaminophen can result in severe liver damage.11
One of the earliest events in DILI is the inhibition of the mitochondrial respiratory chain, resulting in increased reactive oxygen species (ROS) and depletion of adenosine triphosphate (ATP).12 There are several mechanisms contributing to mitochondrial dysfunction: the mitochondrial respiratory chain may be inhibited, diminishing ATP production and resulting in increased ROS levels.12 Furthermore, certain drugs, such as amiodarone, may inhibit-oxidation of fatty acids, resulting in steatosis or steatohepatitis.13 Dideoxynucleotide analogs, often used in the treatment of HIV, may impair mitochondrial DNA replication.13,14 Drug toxicity may also result from the opening of the mitochondrial permeability transition pore (MPTP), which is strongly associated with cell death.15
ROS generation, ATP depletion, and the aforementioned mitochondrial insults may combine to induce intracellular damage. Ultimately, hepatocytes commit to apoptosis, but this process requires energy (ATP), which may not be available due to mitochondrial dysfunction and depleted ATP stores. In this instance, hepatocyte death occurs through the necrotic pathway, which may enhance hepatic inflammation.16
Immune-mediated injury is also an important mechanism of DILI and may be characterized by a prolonged interval between administration of the drug and recognized liver toxicity. The liver contains components of both the innate and adaptive immune system. Bioactive drug metabolites bind to cellular proteins and are exposed to major histocompatibility complex (MHC) molecules on antigen presenting cells.17 This interaction triggers an immune response directed against the hepatocyte. Halothane, for example, triggers the generation of antibodies directed against cytochrome p450 CYP2E1. Thus, identifying drug-induced antibodies in patients’ blood may help in the diagnosis. Apart from antibody-mediated cell death, locally released cytokines and ROS also enhance hepatic injury.18 As classically described with halogenated anesthetics, immune-mediated DILI may be much more pronounced and severe after repeated exposures to the medication.19 Thus, a careful medication history may reveal important information regarding reactions that appeared after previous administration of the respective drug.
Risk Factors for Drug-induced Liver Injury
In men, advanced age is correlated with cholestatic forms of DILI. Women are more prone to developing hepatitis, and are more likely to progress to acute liver failure.20,21
Pre-existing liver pathology predisposes one to higher toxicity from drugs that are metabolized by the liver. For example, hepatitis B or C may augment the severity of inflammatory reactions to antituberculosis medication.22 Chronic alcohol consumption is also known to exacerbate drug toxicity.23 Furthermore, acetaminophen is particularly toxic in heavy alcohol drinkers due to increased activation of the cytochrome p450 system, which leads to generation of the toxic metabolite acetaldehyde.24 It is also recognized that non-alcoholic fatty liver disease (NAFLD) can also increase susceptibility for DILI.25 Thus, particular attention is needed when medicating patients with liver disease (reviewed by Gupta and Lewis).26 However, the presence of pre-existing liver disease does not mean that potentially hepatotoxic medications cannot be used. For example, statins are commonly used in patients with NAFLD. Genetic factors predisposing patients to DILI have been attributed to polymorphisms of the cytochrome p450 enzymes that either slow the metabolism of toxic drugs or accelerate the generation of bioreactive drug metabolites.27–29 Human leukocyte antigen (HLA) phenotype also plays a role in idiosyncratic, immune-mediated reactions to drugs.30–32
Clinical, Laboratory and Histopathological Features of Drug-induced Liver Injury
The majority of cases of DILI are acute illnesses that resolve quickly after the offending medication is stopped. The clinical symptoms are similar to other forms of hepatitis or cholestasis where fatigue, nausea, malaise, pruritus, and jaundice predominate. In some circumstances, abdominal pain that is indistinguishable from acute cholecystitis may be present.33 Concurrent with the theory that immunologic mechanisms are responsible for certain forms of DILI, symptoms of systemic hypersensitivity may be occasionally seen (e.g. fever, rash and eosinophilia).
Certain medications may also cause chronic cholestasis, with clinical features remarkably similar to primary biliary cirrhosis (PBC). Prolonged jaundice, xanthomas, and pruritus have been described in patients taking a variety of different medications. Features that help to distinguish between PBC and drug-induced cholestasis are the lack of circulating anti-mitochondrial antibodies in the latter.34 While PBC may result in end-stage liver disease (ESLD) and death, chronic cholestasis caused by medications is usually reversible and considered benign. Some forms of chronic medication-induced cholestasis are associated with destruction of the intra-hepatic bile ducts. Although the clinical features of this vanishing bile duct syndrome are similar to other forms of chronic cholestasis, the ductopenia is often irreversible and may lead to cirrhosis.
The diagnosis of DILI is typically made by establishing a temporal relationship between drug exposure and development of signs and symptoms of liver disease. Exclusion of infectious, autoimmune or other forms of liver disease is essential. A thorough medical history and a high clinical suspicion is the basis for a correct diagnosis. The astute clinician should actively investigate the chronological relationship between drug administration and onset of pathology. Drugs that have a dose-dependent toxicity usually elicit clinical features within hours to days, while immune-mediated reactions may manifest weeks after administering the drug. Another important feature that helps to confirm DILI is improvement after drug withdrawal. A rechallenge test may be confirmatory; however, re-administration of the drug is not practical in the clinical setting due to safety concerns, and is not recommended. The diagnosis of DILI is associated with increased levels of hepatic enzymes and bilirubin. The pattern of these abnormalities may be hepatocellular, cholestatic, or mixed (see Table 1). The hepatocellular pattern is characterized by increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which reflects hepatocyte destruction and is potentially associated with a worse prognosis. Toxic levels of acetaminophen can elevate liver enzymes above 20,000IU/L. Alkaline phosphatase elevation is the predominant laboratory feature of cholestatic DILI. Histopathological findings of DILI are not specific. The extent of hepatocyte necrosis may portend a worse outcome, while eosinophilia is potentially a marker of better prognosis.35
Table 1.
Types of Drug-induced Liver Injury
| Type | Enzymatic profile | Prognosis |
|---|---|---|
| Hepatocellular | ALT > 2ULN Serum ALT/Serum Alk. Phos ≥ 5* |
More severe prognosis |
| Cholestatic | Alk Phos ≥ 2ULN Serum ALT/Serum Alk Phos ≤ 2* |
More prone to chronic disease |
| Mixed | ALT > 2 ULN Serum ALT/Serum Alk Phos between 2 and 5* |
More prone to chronic disease |
The values in the ratios are expressed as ULN multiples.
ALT = alanine aminotransferase; ULN = upper limit of normal; Alk Phos = alkaline phosphatase.
As the main challenge is to establish a causal relationship between a certain medication and liver injury, several clinical scales have been developed. The scoring criteria are based on the chronological relationship between drug intake/drug withdrawal and clinical effect, clinical course of reaction, exclusion of other potential causes, and rechallenge.
The Rousse Uclaf Causality Assessment Method of the Council of International Organization of Medical Science (RUCAM/CIOMS) is the most frequently used criteria set for the diagnosis of DILI.36 In addition to the aforementioned criteria, the RUCAM/CIOMS scale scores several risk factors (age, alcohol consumption, and pregnancy) and separates DILI into the three patterns described above: hepatocellular, cholestatic, and mixed.37,38
The Maria and Victorino (M&V) Scoring system simplifies the approach by using only five of the seven criteria of the RUCAM/CIOMS scale, but also considers the presence of extra-hepatic manifestations such as fever, rash, arthralgia, eosinophilia, or cytopenia.39 A major critique of the M&V scale is the omission of the liver injury pattern. In addition, the M&V scale is not sensitive to diagnosing chronic forms of DILI and fulminant drug-induced hepatitis.40,41
In clinical practice these scales are not consistently used for the diagnosis of DILI. In order to obtain better data concerning drug hepatotoxicity and to provide access to a case registry, the National Institutes of Health (US) has sponsored an on-going research consortium titled the Drug-Induced Liver Injury Network (DILIN).42–44
Management of DILI
The management of DILI is based upon proper diagnosis, recognition of the offending agent, and its withdrawal. The decision to discontinue the medication is based on the values of liver enzymes. Drug administration should be stopped whenever ALT > 8 x upper limit of normal (ULN), ALT > 5 x ULN for three weeks, ALT > 3 x ULN + bilirubin > 2 x ULN, prothrombin time/international normalized ratio (PT-INR) > 1.5 x ULN or in the presence of symptoms suggesting liver injury.36 Even after stopping the drug, the outcome may vary from complete resolution to acute liver failure and death. With the exception of N-acetylcysteine employed in acetaminophen intoxication, no other specific antidotes are currently employed.
Severe cases that progress to acute liver failure may require liver transplantation. Several different scoring systems have been proposed to determine candidates for this procedure. The King’s College Criteria for acute liver failure divides patients in two classes, depending on the etiology (see Table 2), and is useful to predict which patients will survive and which patients will require liver transplantation.45
Table 2.
King’s College Criteria for Liver Transplantation
| Acetaminophen | Non-acetaminophen |
|---|---|
| pH<7.3 | INR>6.5/PT>100 seconds or any three of the following: |
| Lactic acid>3.5mM at 8 hours | • INR>3.4/PT >50 seconds |
| Lactic acid>3.0mM at 12 hours | • Bilirubin >17.5mg/dl |
| Creatinine>3.4mg/dl | • Jaundice for >7 days |
| INR>6.5/PT>100sec | • Age between 10 and 40 |
| Encephalopathy grade 3 or 4 | • Etiology: drug reaction or unknown |
The model for end-stage liver disease (MELD) criteria, which uses bilirubin, creatinine, and INR can also be used to assess the risk of developing fulminant hepatic failure following acetaminophen intoxication.46 More recently, computed tomography (CT)-obtained hepatic volumetric analysis has been suggested as a novel parameter in predicting prognosis in DILI patients.47 Additional factors associated with a poor prognosis are concurrent hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV infection.
Specific Examples of DILI
Nearly every class of medication can illicit liver injury; listed below are some examples, but the list is not meant to be conclusive. Consultation with a pharmaceutical reference such as Micromedix is recommended if DILI is suspected.
Acetaminophen is the classic example of acute, dose-related DILI, and is responsible for the largest number of cases. Acetaminophen is either glucuronylated or sulfa-conjugated to compounds that are excreted in urine. A fraction of the drug is metabolized by CYP2E1, CYP1A2 and CYP3A4 to a toxic intermediate metabolite (N-acetyl-p-benzo-quinone imine, NAPQI) that can interact with intracellular proteins and induce hepatocyte death.9 Generated NAPQI is rapidly bound by glutathione (GST), which prevents the toxic effects. Hepatotoxicity occurs when GST is depleted or when NAPQI generation exceeds GST binding capacity. It is important to recognize that both GST depletion and increased generation of NAPQI occur in alcoholics, and these patients can develop severe liver injury even with low (2–4g/day) doses of acetaminophen.48 Symptoms in the first 24 hours post ingestion typically consist of nausea, vomiting and malaise (phase 1). These symptoms usually abate for 24 hours (phase 2). Then, hepatocellular destruction occurs between 72 and 96 hours post ingestion and is associated with abdominal pain and jaundice, accompanied by nausea and vomiting. Coagulopathy, hepatic encephalopathy and renal failure characterize severe cases, potentially resulting in death. Rising serum levels of AST and ALT reflect hepatocyte destruction. Centrilobular necrosis in zone 3 is classically observed on liver biopsy. Clinical suspicion and a good history provide the diagnosis, which is confirmed by serum acetaminophen levels. The acetaminophen concentration (plotted on the Rumack–Matthew nomogram) and the King’s College Criteria are used to predict prognosis.49 Initial therapeutic measures include gastric emptying by lavage or ipecac syrup and activated charcoal administration within four hours of ingestion. N-acetyl-cysteine is the specific antidote and can be administered orally or intravenously. Patients that recover from acetaminophen toxicity have no long-term hepatic sequelae. Severe acetaminophen intoxication cases may progress to acute liver failure, and need for liver transplantation is predicted by the King’s College Criteria.
Anesthetics
Halothane-induced DILI usually occurs after multiple exposures and is thought to be driven by immunologic mechanisms.50,51 The conversion of halothane to trifluoroacetylchloride by the cytochrome p450 (especially by CYP2E1) results in the formation of trifluoroacetylated proteins that serve as neoantigens and drive the production of autoantibodies (anti-CYP2E1) that mediate hepatic destruction.52–54 Clinical history may reveal fever and jaundice after previous administration(s). Hepatocyte destruction is reflected by elevated serum transaminases, while eosinophilia suggests the immune reaction. Biopsy findings may range from leukocyte infiltration to massive hepatic necrosis. Most of the cases are mild, but acute liver failure may occur, potentially requiring liver transplant.51 Although case reports exist, liver toxicity from newer-generation halogenated anesthetics, such as isofluorane or sevofluorane, is uncommon.
Non-steroidal Anti-inflammatory Drugs
Due to their extensive use, non-steroidal anti-inflammatory drugs (NSAIDs) are also an important cause of hepatotoxicity.55,56 Diclofenac, the most studied in this class, is glucuronylated and also subjected to cytochrome p450-mediated reactions that result in bioactive products.57,58 Both reactive metabolites and immune mechanisms mediate toxicity. Decreased prostaglandin synthesis due to cyclooxygenase (COX) inhibition may also enhance injury. Chronic diclofenac administration may result in elevated ALT levels in the first four–six months of therapy, but severe toxicity has also been reported.59 Besides diclofenac, bromfenac, nimesulide and sulindac are the NSAIDs most frequently associated with hepatotoxicity.56,60 Nimesulide administration has been reported to illicit severe toxicity resulting in acute liver failure.61 Sulindac and ibuprofen are associated with cholestatic DILI that is reversible after drug withdrawal, although fatal cases have also been reported.62,63
Antimicrobial Medications
Antibiotic-induced hepatotoxicity is responsible for 25–45% of DILI cases.43,64,65 Antituberculosis drugs are reported to be hepatotoxic in up to 35% of patients receiving these medications.66–69 The American Thoracic Society has published formal guidelines on how to monitor these patients for DILI.70 Isoniazid (INH) is metabolized in the liver mainly to mono- and diacetylhydrazine and several other compounds. Genetic variations in rates of INH metabolism exist; slow metabolizers are likely to develop high transaminase levels in response to INH administration. Co-administration of drugs that increase cytochrome p450 activity has an additional effect: rifampin, for example, enhances the toxicity of INH.71,72 Most patients recover in several weeks after discontinuing the drug, while continuing the medication may result in severe hepatotoxicity (potentially leading to acute liver failure).21,70,73 Rifampin alone seldom induces DILI (in up to 2.7% of patients); however, it may occur in patients with pre-existing liver disease.72,74–76 Mechanistically, rifampin competes with bilirubin for the bile salt export pump, and results in hyperbilirubinemia and cholestatic liver disease.77 In addition, drug-induced hypersensitivity may also be responsible for a minority of cases.
Pirazinamide is generally not toxic per se, but when administered in combination with other drugs (INH, rifampin, ethambutol or quinolones) the risk of hepatic adverse reaction is significantly increased.78–82 Therefore, rifampin is no longer combined with pirazinamide for treating latent tuberculosis infections.70
Other Antibiotics
Beta-lactams, such as penicillins and cephalosporins, are commonly associated with DILI. The presence of beta-lactamase inhibitors (clavulanic acid) significantly increases the frequency of adverse reactions leading to cholestasis or a mixed pattern of liver injury. DILI induced by clavulanic acid compounds typically is manifested by reversible jaundice, but severe cases requiring liver transplant or resulting in fatal outcomes have been reported.30,83–85 Penicillinase-resistant penicillins are also associated with cholestatic forms of DILI.86–89 Macrolides are generally associated with reversible cholestatic liver injury. The risk of erythromycin inducing DILI is estimated at 3.6/100,000 cases.90 Patients present with abdominal pain, anorexia, nausea, and vomiting two–four weeks following the initial administration or after two–three days if re-challenged, suggesting a hypersensitivity mechanism. Liver abnormalities generally subside within two–five weeks after stopping the drug.91 In rare cases, cholestasis persists for up to six months.
Sulfonamide-induced liver injury occurs within the first month of administering the medication. Most forms of liver injury are cholestatic, but inflammation and necrosis may also occur. The patients usually recover within several weeks after stopping the antibiotic, although chronic cholestasis or enhanced severity has been reported.92–95 Macrodantin is well recognized to induce both acute and chronic liver disease, and may be indistinguishable from autoimmune hepatitis.96,97
Antifungals
Ketoconazole and other azoles are associated with an increased risk of hepatotoxicity. Liver injury generally presents as increased transaminase levels that are usually reversible.98 Although the hepatitis pattern is the most common, cholestatic and mixed forms have been observed.99,100 Patients on antifungal therapy require careful monitoring, and administration should be abruptly stopped if the liver enzymes become elevated. Failure to do so can result in severe liver damage, and death.100,101 Oral terbinafine rarely induces DILI (1/45,000 to 1/54,000); however, severe cases have been reported, thus monitoring liver enzymes at baseline and after four–six weeks of treatment may assess for the potential hepatotoxicity of the drug.102,103
HIV Antiretroviral Therapy
Up to 18% of patients treated with highly active antiretroviral therapy (HAART) develop DILI. The risk is increased by alcohol consumption, older age, and female gender. In addition, HBV and HCV co-infection enhances both the frequency and the severity of liver injury.104–109 Successful treatment of the HCV infection results in reduced hepatic toxicity of antiretroviral drugs.110,111 Drug combinations employed in HAART complicate the attempts to clearly identify the hepatotoxic potential of each individual medication. Clinical manifestations range from asymptomatic patients to acute liver failure and death. Although all antiretrovirals may induce hepatototoxicity, non-nucleoside analog reverse transcriptase inhibitors are the most likely culprits. Hypersensitivity and idiosyncratic mechanisms are implicated in the liver toxicity caused by these agents. Nevirapine is associated with a high incidence of liver toxicity,112 and the risk is associated with HLA–DRB*0101 and low body mass index (BMI).113–119 Clinically, hepatotoxicity due to nevirapine occurs either early or after several months of therapy and it has a mixed pattern of liver injury.120 A risk factor for abacavir-induced DILI is HLA-B*5701 positivity, thus patients should be screened for this phenotype prior to abacavir treatment.121,122 Liver toxicity occurs through mitochondrial damage resulting in lactic acidosis and hepatic steatosis.123–125 Protease inhibitors induce DILI in 6–11% of patients, but the incidence is significantly increased in HBV or HCV co-infections and alcohol consumption.126,127 Among this class of drugs, ritonavir is the most frequently associated with hepatotoxicity. Interestingly, simultaneously employing two protease inhibitors does not augment liver toxicity.128,129 Although many protease inhibitors increase unconjugated bilirubin levels, liver injury is not reported with this abnormality.130
Oral Hypoglycemics
The first drug of the thiazolidinedione class, troglitazone, was withdrawn due to its potential to cause severe hepatotoxicity.131,132 Rarely, rosiglitazone and pioglitazone have been reported to cause hepatotoxicity, including cases of hepatic failure.133–137 Among sulfonylureas, glimepiride is associated with cholestatic DILI.138–140
Lipid-lowering Agents
Statins induce a reversible, dose-dependent rise in aminotransferase levels and very rarely result in liver failure.2,141,142 Monitoring the liver tests at the beginning and during statin therapy is under debate.143,144 Autoimmune-like hepatitis has been reported in several cases and may be correlated with HLA-DR3, DR4, or DR5.145–148 Interestingly, HCV infection did not significantly increase aminotransferase levels during statin treatment.149,150
Ezetimibe (which inhibits the intestinal absorbtion of cholesterol) was initially reported to be safe.151–153 However, ezetimibe was recently shown to induce cholestatic DILI or an autoimmune-like hepatitis when employed alone or in combination with a simvastatin.154 Despite these associations, statins can often be safely used in patients with chronic liver disease.
Herbal or Traditional Remedies
Herbal remedies are widely used for a multitude of purposes and evidence about their hepatotoxicity is accumulating.155 Although the use of herbal products has been consistently rising, no regulatory guidelines or standards are issued for their composition.156,157 These factors make it difficult to clearly establish their hepatic toxicities. A notable case of herbal toxicity is reported for Herbalife® products. Interestingly, Herbalife ingestion has resulted in different patterns of liver injury including one case of fulminant hepatic failure.158 Further research revealed that contamination with Bacillus subtilis was thought to be responsible for the liver toxicity of Herbalife.159 Surprisingly, Spirulina, taken for its broad range of protective effects, was also recorded as the culprit for one case of DILI.160
Several other more common examples of liver injury due to herbal compounds are provided in Table 3. Further complicating the identification of individual toxic components, alternative medicine frequently employs mixtures of several components. For example, acute hepatitis and liver failure were reported for LipoKinetix, a weight loss product containing norephedrine, caffeine, yohimbine, diiodothyronine and sodium usniate, leading to its withdrawal from the US market in 2001.161,162 Chinese herbal medicines comprised of multiple compounds have been shown to result in liver injury; for example, Dai-Saiko-To and Sho-Saiko-To can induce acute hepatitis.163–165
Table 3.
Hepatotoxicity Associated with Herbal Medications
| Herbal supplement | Use | Type of Liver Injury | Refs. |
|---|---|---|---|
| Chaparral-Larrea Tridentata | Multiple | Cholestasis, zone 3 necrosis, chronic hepatitis | 166, 167 |
| Comfrey–Symphytum Officinale | Anti-inflammatory | Veno-occlusive disease, cholestasis in some cases | 168 |
| Greater Celandine - Chelidonium Majus | Irritable bowel syndrome Biliary diskinesia |
Cholestasis, autoimmune reaction | 169, 170 |
| Kava root | Anxiety, depression, sleeping aid | Necrosis, cholestasis, fulminant hepatic failure | 171 |
| Cascara Sagrada-Rhamnus Prusiana | Laxative | Cholestatic hepatitis | 172 |
| Germander - Teucrium Chamaedrys | Multiple | Cholestatic hepatitis, chronic hepatitis, cirrhosis | 173–176 |
| Jin Bu Huan Lycopodium Serratum | Sedative, analgesic | Acute and chronic hepatitis | 177–179 |
| Ma Huang - Ephedra Sinica | Weight reduction | Acute hepatitis | 180–182 |
Conclusions
Hepatotoxicity is a potential complication of nearly all classes of medication. Most cases of DILI are benign, and improve after drug withdrawal. It is important to recognize and remove the offending agent as quickly as possible to prevent the progression to chronic liver disease and/or fulminant hepatic failure. There are no definite risk factors for DILI, but pre-existing liver disease and genetic susceptibility may predispose certain individuals. Although most patients have clinical symptoms that are identical to other liver diseases, some patients may present with symptoms of systemic hypersensitivity. Treatment of drug-and herbal-induced liver injury consists of rapid drug discontinuation and supportive care targeted to alleviate unwanted symptoms.
Acknowledgments
James P Hamilton, MD, is supported by a Clinician Scientist Award funded by the Johns Hopkins University School of Medicine.
Biographies
 James P Hamilton, MD, is an Assistant Professor of medicine in the Division of Gastroenterology and Hepatology at Johns Hopkins University School of Medicine. His clinical specialties include liver transplantation, hepatocellular carcinoma, and acute and chronic viral, alcoholic, non-alcoholic, autoimmune, and cholestatic liver disease. His research interests are the molecular genetics of liver fibrosis and hepatocellular carcinoma and early detection biomarkers for these diseases. Dr. Hamilton attended medical school at the University of Maryland in Baltimore, MD. He completed a residency in internal medicine at Northwestern University in Chicago, IL, and returned to the University of Maryland for a Fellowship in gastroenterology and hepatology. He then completed a Fellowship in advanced hepatology at The Johns Hopkins Hospital in Baltimore.
James P Hamilton, MD, is an Assistant Professor of medicine in the Division of Gastroenterology and Hepatology at Johns Hopkins University School of Medicine. His clinical specialties include liver transplantation, hepatocellular carcinoma, and acute and chronic viral, alcoholic, non-alcoholic, autoimmune, and cholestatic liver disease. His research interests are the molecular genetics of liver fibrosis and hepatocellular carcinoma and early detection biomarkers for these diseases. Dr. Hamilton attended medical school at the University of Maryland in Baltimore, MD. He completed a residency in internal medicine at Northwestern University in Chicago, IL, and returned to the University of Maryland for a Fellowship in gastroenterology and hepatology. He then completed a Fellowship in advanced hepatology at The Johns Hopkins Hospital in Baltimore.
Stefan David, MD, is Research Fellow at Johns Hopkins School of Medicine, Department of Gastroenterology. He received his MD from the Carol Davila University in Romania. His current research focuses on identifying perturbed mechanisms controlling cell proliferation, differentiation migration and death that can be used as biomarkers for predicting gastrointestinal neoplastic disease. Dr David presented his results at international scientific meetings (FASEB, AACR, DDW) and published in the area of neurodegeneration and GI tract carcinogenesis.
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
References
- 1.Smith DA, et al. Drug withdrawals and the lessons within. Curr Opin Drug Discov Devel. 2006;9:38–46. [PubMed] [Google Scholar]
- 2.Ostapowicz G, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Erlinger S. Drug-induced cholestasis. J Hepatol. 1997;26(Suppl 1):1–4. doi: 10.1016/s0168-8278(97)82326-4. [DOI] [PubMed] [Google Scholar]
- 4.Alazmi WM, et al. Chemotherapy-induced sclerosing cholangitis: long-term response to endoscopic therapy. J Clin Gastroenterol. 2006;40:353–7. doi: 10.1097/01.mcg.0000210098.28876.66. [DOI] [PubMed] [Google Scholar]
- 5.Sandrasegaran K, et al. Chemotherapy-induced sclerosing cholangitis. Clin Radiol. 2006;61:670–8. doi: 10.1016/j.crad.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Chitturi S, et al. Drug-induced cholestasis. Semin Gastrointest Dis. 2001;12:113–24. [PubMed] [Google Scholar]
- 7.Lammert C, et al. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology. 2010;51:615–20. doi: 10.1002/hep.23317. [DOI] [PubMed] [Google Scholar]
- 8.Antoine DJ, et al. Understanding the role of reactive metabolites in drug-induced hepatotoxicity: state of the science. Expert Opin Drug Metab Toxicol. 2008;4:1415–27. doi: 10.1517/17425255.4.11.1415. [DOI] [PubMed] [Google Scholar]
- 9.Park BK, et al. The role of metabolic activation in drug-induced hepatotoxicity. Annu Rev Pharmacol Toxicol. 2005;45:177–202. doi: 10.1146/annurev.pharmtox.45.120403.100058. [DOI] [PubMed] [Google Scholar]
- 10.Wu D, et al. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–54. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman HJ, et al. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22:767–73. [PubMed] [Google Scholar]
- 12.Berson A, et al. Uncoupling of rat and human mitochondria: a possible explanation for tacrine-induced liver dysfunction. Gastroenterology. 1996;110:1878–90. doi: 10.1053/gast.1996.v110.pm8964414. [DOI] [PubMed] [Google Scholar]
- 13.Fromenty B, et al. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther. 1995;67:101–54. doi: 10.1016/0163-7258(95)00012-6. [DOI] [PubMed] [Google Scholar]
- 14.Setzer B, et al. Pyrimidine nucleoside depletion sensitizes to the mitochondrial hepatotoxicity of the reverse transcriptase inhibitor stavudine. Am J Pathol. 2008;172:681–90. doi: 10.2353/ajpath.2008.070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AU, et al. Mechanism of azathioprine-induced injury to hepatocytes: roles of glutathione depletion and mitochondrial injury. J Hepatol. 2001;35:756–64. doi: 10.1016/s0168-8278(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 16.Leist M, et al. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481–6. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Njoku DB, et al. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth Analg. 2002;94:243–9. doi: 10.1097/00000539-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Holt MP, et al. Mechanisms of drug-induced liver injury. Aaps J. 2006;8:E48–54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZX, et al. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–74. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 20.Lucena MI, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49:2001–9. doi: 10.1002/hep.22895. [DOI] [PubMed] [Google Scholar]
- 21.Russo MW, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 22.Lee BH, et al. Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy. Chest. 2005;127:1304–11. doi: 10.1378/chest.127.4.1304. [DOI] [PubMed] [Google Scholar]
- 23.Seeff LB, et al. Acetaminophen hepatotoxicity in alcoholics. A therapeutic misadventure. Ann Intern Med. 1986;104:399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt LE, et al. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity. Hepatology. 2002;35:876–82. doi: 10.1053/jhep.2002.32148. [DOI] [PubMed] [Google Scholar]
- 25.Tarantino G, et al. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410–5. doi: 10.1111/j.1872-034X.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta NK, et al. The use of potentially hepatotoxic drugs in patients with liver disease. Aliment Pharmacol Ther. 2008;28:1021–41. doi: 10.1111/j.1365-2036.2008.03822.x. [DOI] [PubMed] [Google Scholar]
- 27.Tarantino G, et al. Drug-induced liver injury: is it somehow foreseeable? World J Gastroenterol. 2009;15:2817–33. doi: 10.3748/wjg.15.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang YS. Genetic polymorphisms of drug-metabolizing enzymes and the susceptibility to antituberculosis drug-induced liver injury. Expert Opin Drug Metab Toxicol. 2007;3:1–8. doi: 10.1517/17425255.3.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Daly AK, et al. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology. 2007;132:272–81. doi: 10.1053/j.gastro.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 30.O’Donohue J, et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–20. doi: 10.1136/gut.47.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrade RJ, et al. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology. 2004;39:1603–12. doi: 10.1002/hep.20215. [DOI] [PubMed] [Google Scholar]
- 32.Daly AK, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–9. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- 33.Zafrani ES, et al. Cholestatic and hepatocellular injury associated with erythromycin esters: report of nine cases. Dig Dis Sci. 1979;24:385–96. doi: 10.1007/BF01297126. [DOI] [PubMed] [Google Scholar]
- 34.Mohi-ud-din R, et al. Drug- and chemical-induced cholestasis. Clin Liver Dis. 2004;8:95–132. vii. doi: 10.1016/S1089-3261(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 35.Bjornsson E, et al. The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2007;25:1411–21. doi: 10.1111/j.1365-2036.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- 36.Tajiri K, et al. Practical guidelines for diagnosis and early management of drug-induced liver injury. World J Gastroenterol. 2008;14:6774–85. doi: 10.3748/wjg.14.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–6. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 38.Danan G, et al. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 39.Maria VA, et al. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664–9. doi: 10.1002/hep.510260319. [DOI] [PubMed] [Google Scholar]
- 40.Aithal GP, et al. Clinical diagnostic scale: a useful tool in the evaluation of suspected hepatotoxic adverse drug reactions. J Hepatol. 2000;33:949–52. doi: 10.1016/s0168-8278(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 41.Lucena MI, et al. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33:123–30. doi: 10.1053/jhep.2001.20645. [DOI] [PubMed] [Google Scholar]
- 42.Hoofnagle JH. Drug-induced liver injury network (DILIN) Hepatology. 2004;40:773. doi: 10.1002/hep.20445. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani N, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. 1934, e1–4. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Grady JG, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–45. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt LE, et al. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789–96. doi: 10.1002/hep.21503. [DOI] [PubMed] [Google Scholar]
- 47.Yamagishi Y, et al. A new prognostic formula for adult acute liver failure using computer tomography-derived hepatic volumetric analysis. J Gastroenterol. 2009;44:615–23. doi: 10.1007/s00535-009-0045-7. [DOI] [PubMed] [Google Scholar]
- 48.Slattery JT, et al. The complex interaction between ethanol and acetaminophen. Clin Pharmacol Ther. 1996;60:241–6. doi: 10.1016/S0009-9236(96)90050-8. [DOI] [PubMed] [Google Scholar]
- 49.Rumack BH, et al. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55:871–6. [PubMed] [Google Scholar]
- 50.Kenna JG. Immunoallergic drug-induced hepatitis: lessons from halothane. J Hepatol. 1997;26(Suppl 1):5–12. doi: 10.1016/s0168-8278(97)82327-6. [DOI] [PubMed] [Google Scholar]
- 51.Lo SK, et al. Halothane-induced acute liver failure: continuing occurrence and use of liver transplantation. Eur J Gastroenterol Hepatol. 1998;10:635–9. [PubMed] [Google Scholar]
- 52.Njoku D, et al. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth Analg. 1997;84:173–8. doi: 10.1097/00000539-199701000-00031. [DOI] [PubMed] [Google Scholar]
- 53.Eliasson E, et al. Cytochrome P450 2E1 is a cell surface autoantigen in halothane hepatitis. Mol Pharmacol. 1996;50:573–82. [PubMed] [Google Scholar]
- 54.Bourdi M, et al. Human cytochrome P450 2E1 is a major autoantigen associated with halothane hepatitis. Chem Res Toxicol. 1996;9:1159–66. doi: 10.1021/tx960083q. [DOI] [PubMed] [Google Scholar]
- 55.Manov I, et al. Hepatotoxicity of anti-inflammatory and analgesic drugs: ultrastructural aspects. Acta Pharmacol Sin. 2006;27:259–72. doi: 10.1111/j.1745-7254.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 56.Aithal GP, et al. Nonsteroidal anti-inflammatory drug-induced hepatotoxicity. Clin Liver Dis. 2007;11:563–75. vi–vii. doi: 10.1016/j.cld.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Shen S, et al. Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac. Chem Res Toxicol. 1999;12:214–22. doi: 10.1021/tx9802365. [DOI] [PubMed] [Google Scholar]
- 58.Kenny JR, et al. Syntheses and characterization of the acyl glucuronide and hydroxy metabolites of diclofenac. J Med Chem. 2004;47:2816–25. doi: 10.1021/jm030891w. [DOI] [PubMed] [Google Scholar]
- 59.Laine L, et al. How common is diclofenac-associated liver injury? Analysis of 17,289 arthritis patients in a long-term prospective clinical trial. Am J Gastroenterol. 2009;104:356–62. doi: 10.1038/ajg.2008.149. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez-Matienzo D, et al. Hepatic disorders in patients treated with COX-2 selective inhibitors or nonselective NSAIDs: a case/noncase analysis of spontaneous reports. Clin Ther. 2006;28:1123–32. doi: 10.1016/j.clinthera.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Walker SL, et al. Nimesulide associated fulminant hepatic failure. Pharmacoepidemiol Drug Saf. 2008;17:1108–12. doi: 10.1002/pds.1665. [DOI] [PubMed] [Google Scholar]
- 62.Wood LJ, et al. Sulindac hepatotoxicity: effects of acute and chronic exposure. Aust N Z J Med. 1985;15:397–401. doi: 10.1111/j.1445-5994.1985.tb02758.x. [DOI] [PubMed] [Google Scholar]
- 63.Tarazi EM, et al. Sulindac-associated hepatic injury: analysis of 91 cases reported to the Food and Drug Administration. Gastroenterology. 1993;104:569–74. doi: 10.1016/0016-5085(93)90428-f. [DOI] [PubMed] [Google Scholar]
- 64.Sgro C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 65.Andrade RJ, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Ohkawa K, et al. Risk factors for antituberculous chemotherapy-induced hepatotoxicity in Japanese pediatric patients. Clin Pharmacol Ther. 2002;72:220–6. doi: 10.1067/mcp.2002.126175. [DOI] [PubMed] [Google Scholar]
- 67.Sharifzadeh M, et al. Evaluation of patient-related factors associated with causality, preventability, predictability and severity of hepatotoxicity during antituberculosis [correction of antituberclosis] treatment. Pharmacol Res. 2005;51:353–8. doi: 10.1016/j.phrs.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 68.Fernandez-Villar A, et al. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8:1499–505. [PubMed] [Google Scholar]
- 69.Tostmann A, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23:192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 70.Saukkonen JJ, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 71.Sarma GR, Venkatesan P, et al. Rifampin-induced release of hydrazine from isoniazid. A possible cause of hepatitis during treatment of tuberculosis with regimens containing isoniazid and rifampin. Am Rev Respir Dis. 1986;133:1072–5. doi: 10.1164/arrd.1986.133.6.1072. [DOI] [PubMed] [Google Scholar]
- 72.Steele MA, et al. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–71. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 73.Idilman R, et al. Antituberculous therapy-induced fulminant hepatic failure: successful treatment with liver transplantation and nonstandard antituberculous therapy. Liver Transpl. 2006;12:1427–30. doi: 10.1002/lt.20839. [DOI] [PubMed] [Google Scholar]
- 74.Polesky A, et al. Rifampin preventive therapy for tuberculosis in Boston’s homeless. Am J Respir Crit Care Med. 1996;154:1473–7. doi: 10.1164/ajrccm.154.5.8912767. [DOI] [PubMed] [Google Scholar]
- 75.Villarino ME, et al. Rifampin preventive therapy for tuberculosis infection: experience with 157 adolescents. Am J Respir Crit Care Med. 1997;155:1735–8. doi: 10.1164/ajrccm.155.5.9154885. [DOI] [PubMed] [Google Scholar]
- 76.Fountain FF, et al. Rifampin hepatotoxicity associated with treatment of latent tuberculosis infection. Am J Med Sci. 2009;337:317–20. doi: 10.1097/MAJ.0b013e31818c0134. [DOI] [PubMed] [Google Scholar]
- 77.Byrne JA, et al. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649–58. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- 78.Chang KC, et al. Hepatotoxicity of pyrazinamide: cohort and case-control analyses. Am J Respir Crit Care Med. 2008;177:1391–6. doi: 10.1164/rccm.200802-355OC. [DOI] [PubMed] [Google Scholar]
- 79.Jasmer RM, et al. Short-course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Ann Intern Med. 2002;137:640–7. doi: 10.7326/0003-4819-137-8-200210150-00007. [DOI] [PubMed] [Google Scholar]
- 80.Younossian AB, et al. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–4. doi: 10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 81.Papastavros T, et al. Adverse events associated with pyrazinamide and levofloxacin in the treatment of latent multidrug-resistant tuberculosis. CMAJ. 2002;167:131–6. [PMC free article] [PubMed] [Google Scholar]
- 82.Ridzon R, et al. Asymptomatic hepatitis in persons who received alternative preventive therapy with pyrazinamide and ofloxacin. Clin Infect Dis. 1997;24:1264–5. doi: 10.1093/clinids/24.6.1264. [DOI] [PubMed] [Google Scholar]
- 83.Garcia Rodriguez LA, et al. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327–32. doi: 10.1001/archinte.1996.00440110099013. [DOI] [PubMed] [Google Scholar]
- 84.Lucena MI, et al. Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–6. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 85.Fontana RJ, et al. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785–90. doi: 10.1007/s10620-005-2938-5. [DOI] [PubMed] [Google Scholar]
- 86.Hautekeete ML. Hepatotoxicity of antibiotics. Acta Gastroenterol Belg. 1995;58:290–6. [PubMed] [Google Scholar]
- 87.Barrio J, et al. Hepatotoxicity caused by cloxacillin. Rev Esp Enferm Dig. 1997;89:559–60. [PubMed] [Google Scholar]
- 88.Presti ME, et al. Nafcillin-associated hepatotoxicity. Report of a case and review of the literature. Dig Dis Sci. 1996;41:180–4. doi: 10.1007/BF02208602. [DOI] [PubMed] [Google Scholar]
- 89.Andrews E, et al. Flucloxacillin-induced liver injury. Toxicology. 2008;254:158–63. doi: 10.1016/j.tox.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Derby LE, et al. Erythromycin-associated cholestatic hepatitis. Med J Aust. 1993;158:600–2. doi: 10.5694/j.1326-5377.1993.tb137625.x. [DOI] [PubMed] [Google Scholar]
- 91.Braun P. Hepatotoxicity of erythromycin. J Infect Dis. 1969;119:300–6. doi: 10.1093/infdis/119.3.300. [DOI] [PubMed] [Google Scholar]
- 92.Tonder M, et al. Sulfonamide-induced chronic liver disease. Scand J Gastroenterol. 1974;9:93–6. [PubMed] [Google Scholar]
- 93.Kowdley KV, et al. Prolonged cholestasis due to trimethoprim sulfamethoxazole. Gastroenterology. 1992;102:2148–50. doi: 10.1016/0016-5085(92)90346-z. [DOI] [PubMed] [Google Scholar]
- 94.Kouklakis G, et al. Cholestatic hepatitis with severe systemic reactions induced by trimethoprim-sulfamethoxazole. Ann Hepatol. 2007;6:63–5. [PubMed] [Google Scholar]
- 95.Zaman F, et al. Successful orthotopic liver transplantation after trimethoprim-sulfamethoxazole associated fulminant liver failure. Clin Transplant. 2003;17:461–4. doi: 10.1034/j.1399-0012.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 96.Iwarson S, et al. Nitrofurantoin-induced chronic liver disease. Clinical course and outcome of five cases. Scand J Gastroenterol. 1979;14:497–502. [PubMed] [Google Scholar]
- 97.Stricker BH, et al. Hepatic injury associated with the use of nitrofurans: a clinicopathological study of 52 reported cases. Hepatology. 1988;8:599–606. doi: 10.1002/hep.1840080327. [DOI] [PubMed] [Google Scholar]
- 98.Garcia Rodriguez LA, et al. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol. 1999;48:847–52. doi: 10.1046/j.1365-2125.1999.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stricker BH, et al. Ketoconazole-associated hepatic injury. A clinicopathological study of 55 cases. J Hepatol. 1986;3:399–406. doi: 10.1016/s0168-8278(86)80495-0. [DOI] [PubMed] [Google Scholar]
- 100.Chien RN, et al. Hepatic injury during ketoconazole therapy in patients with onychomycosis: a controlled cohort study. Hepatology. 1997;25:103–7. doi: 10.1002/hep.510250119. [DOI] [PubMed] [Google Scholar]
- 101.Findor JA, et al. Ketoconazole-induced liver damage. Medicina (B Aires) 1998;58:277–81. [PubMed] [Google Scholar]
- 102.Perveze Z, et al. et al. Terbinafine-induced hepatic failure requiring liver transplantation. Liver Transpl. 2007;13:162–4. doi: 10.1002/lt.21034. [DOI] [PubMed] [Google Scholar]
- 103.Gupta AK, et al. Shear NH, Hepatitis associated with terbinafine therapy: three case reports and a review of the literature. Clin Exp Dermatol. 1998;23:64–7. doi: 10.1046/j.1365-2230.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 104.Bonnet F, et al. A cohort study of nevirapine tolerance in clinical practice: French Aquitaine Cohort, 1997–1999. Clin Infect Dis. 2002;35:1231–7. doi: 10.1086/343046. [DOI] [PubMed] [Google Scholar]
- 105.Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44:S132–9. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 106.Servin-Abad L, et al. Liver enzymes elevation after HAART in HIV-HCV co-infection. J Viral Hepat. 2005;12:429–34. doi: 10.1111/j.1365-2893.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 107.Lai AR, et al. Antiretroviral medication considerations for individuals coinfected with HIV and hepatitis C virus. AIDS Patient Care STDS. 2006;20:678–92. doi: 10.1089/apc.2006.20.678. [DOI] [PubMed] [Google Scholar]
- 108.den Brinker M, et al. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- 109.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 110.Labarga P, et al. Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J Infect Dis. 2007;196:670–6. doi: 10.1086/520092. [DOI] [PubMed] [Google Scholar]
- 111.Shah T, et al. Resolution of hepatitis C virus-induced steatosis improves tolerability of antiretroviral drugs associated with hepatotoxicity in an HIV-infected individual. J Infect Dis. 2008;197:932–3. doi: 10.1086/528800. [DOI] [PubMed] [Google Scholar]
- 112.Martinez E, et al. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15:1261–8. doi: 10.1097/00002030-200107060-00007. [DOI] [PubMed] [Google Scholar]
- 113.Martin AM, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97–9. doi: 10.1097/00002030-200501030-00014. [DOI] [PubMed] [Google Scholar]
- 114.Dieterich DT, et al. Drug-induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clin Infect Dis. 2004;38(Suppl 2):S80–9. doi: 10.1086/381450. [DOI] [PubMed] [Google Scholar]
- 115.Sanne I, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–9. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 116.Torti C, et al. Analysis of severe hepatic events associated with nevirapine-containing regimens: CD4+ T-cell count and gender in hepatitis C seropositive and seronegative patients. Drug Saf. 2007;30:1161–9. doi: 10.2165/00002018-200730120-00008. [DOI] [PubMed] [Google Scholar]
- 117.De Lazzari E, et al. Hepatotoxicity of nevirapine in virologically suppressed patients according to gender and CD4 cell counts. HIV Med. 2008;9:221–6. doi: 10.1111/j.1468-1293.2008.00552.x. [DOI] [PubMed] [Google Scholar]
- 118.Knobel H, et al. Risk of side effects associated with the use of nevirapine in treatment-naive patients, with respect to gender and CD4 cell count. HIV Med. 2008;9:14–8. doi: 10.1111/j.1468-1293.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- 119.Medrano J, et al. Risk for immune-mediated liver reactions by nevirapine revisited. AIDS Rev. 2008;10:110–5. [PubMed] [Google Scholar]
- 120.de Maat MM, et al. Case series of acute hepatitis in a non-selected group of HIV-infected patients on nevirapine-containing antiretroviral treatment. AIDS. 2003;17:2209–14. doi: 10.1097/00002030-200310170-00009. [DOI] [PubMed] [Google Scholar]
- 121.Mallal S, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–32. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 122.Phillips EJ, et al. HLA and drug-induced toxicity. Curr Opin Mol Ther. 2009;11:231–42. [PubMed] [Google Scholar]
- 123.Johnson AA, et al. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J Biol Chem. 2001;276:40847–57. doi: 10.1074/jbc.M106743200. [DOI] [PubMed] [Google Scholar]
- 124.Sulkowski MS, et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–92. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 125.Miller KD, et al. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann Intern Med. 2000;133:192–6. doi: 10.7326/0003-4819-133-3-200008010-00010. [DOI] [PubMed] [Google Scholar]
- 126.Sulkowski MS, et al. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 127.Aceti A, et al. Hepatotoxicity development during antiretroviral therapy containing protease inhibitors in patients with HIV: the role of hepatitis B and C virus infection. J Acquir Immune Defic Syndr. 2002;29:41–8. doi: 10.1097/00042560-200201010-00005. [DOI] [PubMed] [Google Scholar]
- 128.Sulkowski MS, et al. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. AIDS. 2004;18:2277–84. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 129.Cooper CL, et al. Hepatotoxicity associated with antiretroviral therapy containing dual versus single protease inhibitors in individuals coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2002;34:1259–63. doi: 10.1086/339867. [DOI] [PubMed] [Google Scholar]
- 130.Zucker SD, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci U S A. 2001;98:12671–6. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Graham DJ, Green L, Senior JR, Nourjah P. Troglitazone-induced liver failure: a case study. Am J Med. 2003;114:299–306. doi: 10.1016/s0002-9343(02)01529-2. [DOI] [PubMed] [Google Scholar]
- 132.Chan KA, et al. A cohort study of the incidence of serious acute liver injury in diabetic patients treated with hypoglycemic agents. Arch Intern Med. 2003;163:728–34. doi: 10.1001/archinte.163.6.728. [DOI] [PubMed] [Google Scholar]
- 133.Marcy TR, et al. Second-generation thiazolidinediones and hepatotoxicity. Ann Pharmacother. 2004;38:1419–23. doi: 10.1345/aph.1E072. [DOI] [PubMed] [Google Scholar]
- 134.Al-Salman J, et al. Hepatocellular injury in a patient receiving rosiglitazone. A case report. Ann Intern Med. 2000;132:121–4. doi: 10.7326/0003-4819-132-2-200001180-00006. [DOI] [PubMed] [Google Scholar]
- 135.Forman LM, et al. Hepatic failure in a patient taking rosiglitazone. Ann Intern Med. 2000;132:118–21. doi: 10.7326/0003-4819-132-2-200001180-00005. [DOI] [PubMed] [Google Scholar]
- 136.Farley-Hills E, et al. Fatal liver failure associated with pioglitazone. BMJ. 2004;329:429. doi: 10.1136/bmj.329.7463.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chase MP, et al. Pioglitazone-associated fulminant hepatic failure. Am J Gastroenterol. 2002;97:502–3. doi: 10.1111/j.1572-0241.2002.05516.x. [DOI] [PubMed] [Google Scholar]
- 138.Sitruk V, et al. Acute cholestatic hepatitis induced by glimepiride. Gastroenterol Clin Biol. 2000;24:1233–4. [PubMed] [Google Scholar]
- 139.Heurgue A, et al. Glimepiride-induced cute cholestatic hepatitis. Ann Endocrinol (Paris) 2004;65:174–5. doi: 10.1016/s0003-4266(04)95666-1. [DOI] [PubMed] [Google Scholar]
- 140.Chounta A, et al. Cholestatic liver injury after glimepiride therapy. J Hepatol. 2005;42:944–6. doi: 10.1016/j.jhep.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 141.Tolman KG. The liver and lovastatin. Am J Cardiol. 2002;89:1374–80. doi: 10.1016/s0002-9149(02)02355-x. [DOI] [PubMed] [Google Scholar]
- 142.Law M, et al. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 143.Talbert RL. Safety issues with statin therapy. J Am Pharm Assoc. 2006;46:479–88. doi: 10.1331/154434506778073637. quiz 488–90. [DOI] [PubMed] [Google Scholar]
- 144.Cohen DE, et al. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 145.Alla V, et al. Autoimmune hepatitis triggered by statins. J Clin Gastroenterol. 2006;40:757–61. doi: 10.1097/00004836-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 146.Pelli N, et al. Autoimmune hepatitis revealed by atorvastatin. Eur J Gastroenterol Hepatol. 2003;15:921–4. doi: 10.1097/00042737-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 147.Wolters LM, et al. Rosuvastatin-associated hepatitis with autoimmune features. Eur J Gastroenterol Hepatol. 2005;17:589–90. doi: 10.1097/00042737-200505000-00019. [DOI] [PubMed] [Google Scholar]
- 148.Graziadei IW, et al. Drug-induced lupus-like syndrome associated with severe autoimmune hepatitis. Lupus. 2003;12:409–12. doi: 10.1191/0961203303lu313cr. [DOI] [PubMed] [Google Scholar]
- 149.Khorashadi S, et al. Incidence of statin hepatotoxicity in patients with hepatitis C. Clin Gastroenterol Hepatol. 2006;4:902–7. doi: 10.1016/j.cgh.2006.03.014. quiz 806. [DOI] [PubMed] [Google Scholar]
- 150.Segarra-Newnham M, et al. Effectiveness and hepatotoxicity of statins in men seropositive for hepatitis C virus. Pharmacotherapy. 2007;27:845–51. doi: 10.1592/phco.27.6.845. [DOI] [PubMed] [Google Scholar]
- 151.Dujovne CA, Ettinger MP, McNeer JF, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1092–7. doi: 10.1016/s0002-9149(02)02798-4. [DOI] [PubMed] [Google Scholar]
- 152.Gagne C, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002;90:1084–91. doi: 10.1016/s0002-9149(02)02774-1. [DOI] [PubMed] [Google Scholar]
- 153.Pandor A, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Intern Med. 2009;265:568–80. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]
- 154.Stolk MF, et al. Severe hepatic side effects of ezetimibe. Clin Gastroenterol Hepatol. 2006;4:908–11. doi: 10.1016/j.cgh.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 155.Seeff LB. Herbal hepatotoxicity. Clin Liver Dis. 2007;11:577–96. vii. doi: 10.1016/j.cld.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 156.Kessler RC, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 157.Stickel F, et al. Herbal hepatotoxicity. J Hepatol. 2005;43:901–10. doi: 10.1016/j.jhep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 158.Schoepfer AM, et al. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J Hepatol. 2007;47:521–6. doi: 10.1016/j.jhep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 159.Stickel F, et al. Severe hepatotoxicity following ingestion of Herbalife nutritional supplements contaminated with Bacillus subtilis. J Hepatol. 2009;50:111–7. doi: 10.1016/j.jhep.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 160.Iwasa M, et al. pirulina-associated hepatotoxicity. Am J Gastroenterol. 2002;97:3212–3. doi: 10.1111/j.1572-0241.2002.07145.x. [DOI] [PubMed] [Google Scholar]
- 161.Favreau JT, et al. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann Intern Med. 2002;136:590–5. doi: 10.7326/0003-4819-136-8-200204160-00008. [DOI] [PubMed] [Google Scholar]
- 162.Estes JD, et al. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch Surg. 2003;138:852–8. doi: 10.1001/archsurg.138.8.852. [DOI] [PubMed] [Google Scholar]
- 163.Itoh S, et al. Liver injuries induced by herbal medicine, syo-saiko-to (xiao-chai-hu-tang) Dig Dis Sci. 1995;40:1845–8. doi: 10.1007/BF02212712. [DOI] [PubMed] [Google Scholar]
- 164.Hsu LM, et al. Acute hepatitis induced by Chinese hepatoprotective herb, xiao-chai-hu-tang. J Chin Med Assoc. 2006;69:86–8. doi: 10.1016/S1726-4901(09)70119-4. [DOI] [PubMed] [Google Scholar]
- 165.Kamiyama T, et al. Autoimmune hepatitis triggered by administration of an herbal medicine. Am J Gastroenterol. 1997;92:703–4. [PubMed] [Google Scholar]
- 166.Gordon DW, et al. Chaparral ingestion. The broadening spectrum of liver injury caused by herbal medications. JAMA. 1995;273:489–90. doi: 10.1001/jama.273.6.489. [DOI] [PubMed] [Google Scholar]
- 167.Levy C, et al. Drug-induced cholestasis. Clin Liver Dis. 2003;7:311–30. doi: 10.1016/s1089-3261(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 168.Stickel F, et al. The efficacy and safety of comfrey. Public Health Nutr. 2000;3:501–8. doi: 10.1017/s1368980000000586. [DOI] [PubMed] [Google Scholar]
- 169.Benninger J, et al. Acute hepatitis induced by greater celandine (Chelidonium majus) Gastroenterology. 1999;117:1234–7. doi: 10.1016/s0016-5085(99)70410-5. [DOI] [PubMed] [Google Scholar]
- 170.Stickel F, et al. Acute hepatitis induced by Greater Celandine (Chelidonium majus) Scand J Gastroenterol. 2003;38:565–8. doi: 10.1080/00365520310000942. [DOI] [PubMed] [Google Scholar]
- 171.Stickel F, et al. Hepatitis induced by Kava (Piper methysticum rhizoma) J Hepatol. 2003;39:62–7. doi: 10.1016/s0168-8278(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 172.Nadir A, et al. Cascara sagrada-induced intrahepatic cholestasis causing portal hypertension: case report and review of herbal hepatotoxicity. Am J Gastroenterol. 2000;95:3634–7. doi: 10.1111/j.1572-0241.2000.03386.x. [DOI] [PubMed] [Google Scholar]
- 173.Castot A, et al. Hepatitis observed during a treatment with a drug or tea containing Wild Germander. Evaluation of 26 cases reported to the Regional Centers of Pharmacovigilance. Gastroenterol Clin Biol. 1992;16:916–22. [PubMed] [Google Scholar]
- 174.Larrey D, et al. Hepatitis after germander (Teucrium chamaedrys) administration: another instance of herbal medicine hepatotoxicity. Ann Intern Med. 1992;117:129–32. doi: 10.7326/0003-4819-117-2-129. [DOI] [PubMed] [Google Scholar]
- 175.Pauwels A, Thierman-Duffaud D, Azanowsky JM, et al. Acute hepatitis caused by wild germander. Hepatotoxicity of herbal remedies. Two cases. Gastroenterol Clin Biol. 1992;16:92–5. [PubMed] [Google Scholar]
- 176.Sundaresan PR, et al. Isolation and characterisation of selected germander diterpenoids from authenticated Teucrium chamaedrys and T. canadense by HPLC, HPLC-mS and NMR. Phytochem Anal. 2006;17:243–50. doi: 10.1002/pca.912. [DOI] [PubMed] [Google Scholar]
- 177.Woolf GM, et al. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121:729–35. doi: 10.7326/0003-4819-121-10-199411150-00001. [DOI] [PubMed] [Google Scholar]
- 178.Horowitz RS, et al. The clinical spectrum of Jin Bu Huan toxicity. Arch Intern Med. 1996;156:899–903. [PubMed] [Google Scholar]
- 179.Picciotto A, et al. Chronic hepatitis induced by Jin Bu Huan. J Hepatol. 1998;28:165–7. doi: 10.1016/s0168-8278(98)80217-1. [DOI] [PubMed] [Google Scholar]
- 180.Nadir A, et al. Acute hepatitis associated with the use of a Chinese herbal product, ma-huang. Am J Gastroenterol. 1996;91:1436–8. [PubMed] [Google Scholar]
- 181.Borum ML. Fulminant exacerbation of autoimmune hepatitis after the use of ma huang. Am J Gastroenterol. 2001;96:1654–5. doi: 10.1111/j.1572-0241.2001.03827.x. [DOI] [PubMed] [Google Scholar]
- 182.Neff GW, et al. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol. 2004;41:1062–4. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]