Abstract
Genomic instability due to telomere dysfunction and defective repair of DNA double-strand breaks (DSBs) is an underlying cause of ageing-related diseases. 53BP1 is a key factor in DNA DSBs repair and its deficiency is associated with genomic instability and cancer progression. Here, we uncover a novel pathway regulating the stability of 53BP1. We demonstrate an unprecedented role for the cysteine protease Cathepsin L (CTSL) in the degradation of 53BP1. Overexpression of CTSL in wild-type fibroblasts leads to decreased 53BP1 protein levels and changes in its cellular distribution, resulting in defective repair of DNA DSBs. Importantly, we show that the defects in DNA repair associated with 53BP1 deficiency upon loss of A-type lamins are due to upregulation of CTSL. Furthermore, we demonstrate that treatment with vitamin D stabilizes 53BP1 and promotes DNA DSBs repair via inhibition of CTSL, providing an as yet unsuspected link between vitamin D action and DNA repair. Given that CTSL upregulation is a hallmark of cancer and progeria, regulation of this pathway could be of great therapeutic significance for these diseases.
Keywords: DNA repair, genomic instability, protein degradation, vitamin D
Introduction
Alterations in telomere biology and defects in DNA double-strand breaks (DSBs) repair are among the leading causes of genomic instability, and are clear contributors to ageing and cancer phenotypes. DNA DSBs are dangerous lesions, since their inefficient repair can result in genetic translocations, deletions, and chromosome fusions or loss. Cells respond to DSBs by activating the DNA damage response (DDR), which blocks cell-cycle progression until the damage is repaired or initiates apoptosis/senescence programmes if the damage is beyond repair (Zhou and Elledge, 2000; Khanna and Jackson, 2001; Pierce et al, 2001). There are two main pathways for repair of DNA DSBs: non-homologous end-joining (NHEJ), an error-prone repair pathway predominant during G1 and S phases of the cell cycle, and homologous recombination (HR), error-free repair pathway active primarily in late S and G2 phases of the cycle (Hartlerode and Scully, 2009). The less characterized alternative NHEJ pathway provides a backup repair mechanism in cells defective in classical NHEJ.
Signalling through the DDR pathway involves a plethora of sensor, mediator, transducer, and effector proteins (Kastan and Bartek, 2004; d’Adda di Fagagna, 2008; Jackson and Bartek, 2009). Recruitment of the mediator/adaptor protein 53BP1 to DNA DSBs is a key event in the DDR pathway (Schultz et al, 2000; Wang et al, 2002; FitzGerald et al, 2009), which is mediated by interaction with MDC1 (Stewart et al, 2003a) and dimethylated lysine 20 of histone 4 (Botuyan et al, 2006), and promoted by sumoylating (Galanty et al, 2009) and ubiquitylating enzymes (Kolas et al, 2007; Mailand et al, 2007). Defective recruitment of 53BP1 to DNA DSBs is associated with defects in the DDR pathway. Accordingly, 53BP1-deficient cells exhibit increased genomic instability and radiosensitivity and 53BP1-null mice are tumour prone (Schultz et al, 2000; DiTullio et al, 2002; Ward et al, 2003). A number of studies have provided evidence for a role of 53BP1 in DNA repair by NHEJ. In particular, loss of 53BP1 hinders the processing of dysfunctional telomeres induced by expression of a dominant-negative form of TRF2 (Dimitrova et al, 2008) as well as the rate of class switch recombination and long-range V(D)J recombination (Manis et al, 2004; Ward et al, 2004; Difilippantonio et al, 2008). Importantly, recent studies indicated that regulation of 53BP1 levels might impact tumour progression. In particular, downregulation of 53BP1 in triple-negative and BRCA-mutant breast tumours was suggested to promote the viability of these cancer cells (Bouwman et al, 2010; Bunting et al, 2010). To date, very limited information is available about how the levels of 53BP1 are regulated in normal or cancer cells.
In a previous study we showed that A-type lamins participate in the stabilization of 53BP1 in part by preventing its degradation, although the molecular mechanisms remain unknown. In eukaryotic cells, the two main protein degradation pathways are the ubiquitin–proteasome system and the endosomal/lysosomal pathway. Cathepsin L (CTSL), one of the most abundant proteases in the endosomal/lysosomal compartment, has recently been identified in the nucleus, where it processes in a regulated manner the N-terminal tail of histone H3 and the transcription factor CDP/Cux (Goulet et al, 2004; Duncan et al, 2008). Intriguingly, a marked upregulation of CTSL mRNA levels was observed in a mouse model of progeria, characterized by defective processing of lamin A (Varela et al, 2005). Upregulation of CTSL is also a hallmark of metastatic cancers (Jedeszko and Sloane, 2004; Gocheva and Joyce, 2007). We previously showed that 53BP1 is degraded by the proteasome in wild-type (WT) and A-type lamins-deficient (Lmna−/−) mouse embryonic fibroblasts (MEFs). However, inhibition of the proteasome in Lmna−/− MEFs did not restore the levels of 53BP1 to that of WT cells, suggesting that additional mechanisms are responsible for the destabilization of 53BP1 upon loss of A-type lamins (Gonzalez-Suarez et al, 2009).
Here, we addressed the molecular mechanisms by which A-type lamins stabilize 53BP1 protein levels, promoting NHEJ repair and preserving the integrity of the genome. Our studies unravel a novel pathway regulating the levels of 53BP1 and the repair of DNA DSBs by NHEJ that has as a central player the cysteine protease CTSL, which is impacted upon by the loss of A-type lamins and can be regulated by treatment with vitamin D.
Results
A-type lamins stabilize 53BP1 via regulation of CTSL expression
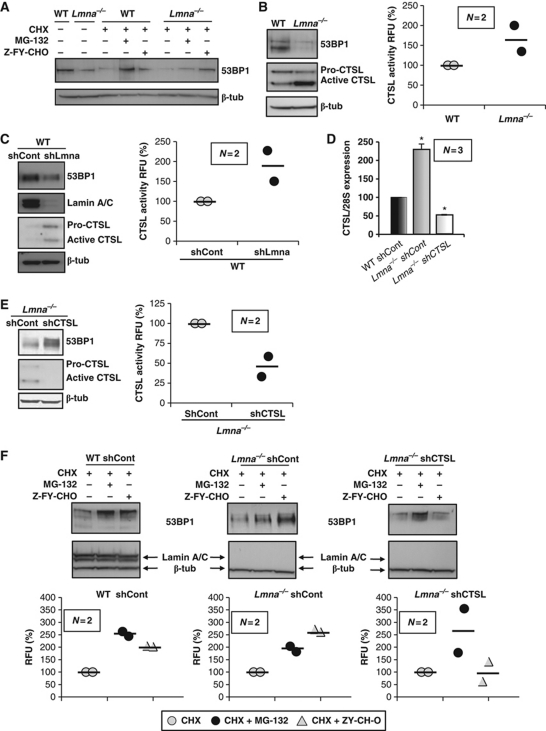
We determined the extent to which the endosomal/lysosomal degradation pathway, and specifically CTSL, is involved in the regulation of 53BP1 levels. As shown in Figure 1A, incubation of WT and Lmna−/− MEFs with either a proteasome inhibitor (MG-132) or a specific CTSL inhibitor (Z-FY-CHO) stabilizes 53BP1 protein, indicating that both pathways are involved in the degradation of the protein. Interestingly, while MG-132 treatment blocked 53BP1 degradation to a larger extent in WT MEFs, the CTSL inhibitor had a more profound effect in Lmna−/− MEFs, restoring 53BP1 protein to near normal levels. These results suggest that loss of A-type lamins preferentially promotes CTSL-mediated degradation.
Figure 1.
Regulation of 53BP1 protein levels by CTSL. (A) WT and Lmna−/− MEFs were treated with cycloheximide (CHX) to inhibit protein synthesis and with either a proteasome inhibitor (MG-132) or a CTSL inhibitor (Z-FY-CHO). Global levels of 53BP1 were monitored by western blot. β-Tubulin was used as loading control. Note the stabilization of 53BP1 by both treatments. (B) Western blots showing higher levels of active CTSL and lower levels of 53BP1 in Lmna−/− MEFs with respect to WT. Graph showing CTSL enzymatic activity in WT and Lmna−/− MEFs. (C) Acute depletion of A-type lamins by lentiviral transduction with an shRNA followed by western blots to monitor the levels of A-type lamins, 53BP1, and CTSL. Graph showing CTSL enzymatic activity upon depletion of A-type lamins. Note how acute depletion of A-type lamins leads to increased levels of CTSL activity and decreased levels of 53BP1. (D) Levels of CTSL mRNA detected by northern blot. Average of three independent experiments is shown. (E) Western blots showing that acute depletion of CTSL by lentiviral transduction with an shRNA leads to stabilization of 53BP1 protein in Lmna−/− MEFs. Graph shows the decrease in CTSL enzymatic activity. (F) WT and Lmna−/− MEFs transduced with an shRNA control and Lmna−/− MEFs depleted of CTSL were incubated with cycloheximide and either proteasome or CSTL inhibitors. The levels of 53BP1 were monitored by western blots. Graphs show the quantitation of two independent experiments. Note how depletion of CTSL prevents stabilization of 53BP1 by the CTSL inhibitor, but not by the proteasome inhibitor. Values in bar graphs are expressed as mean±s.e.m. In ‘bee swarm’ plots, horizontal bar indicates the average value. N represents the number of independent experiments. *P-value of statistical significance (P⩽0.05). RFU stands for relative fluorescence units and RU stands for relative units (normalization to control).
Next, we assessed whether loss of A-type lamins specifically impacts CTSL activity. CTSL is synthesized as an inactive propeptide that undergoes autoproteolytic processing to release active enzyme (Katunuma, 1989). These two different forms of CTSL can be distinguished by western blot. As shown in Figure 1B, the levels of active CTSL are higher in Lmna−/− MEFs than in WT counterparts. This CTSL upregulation was accompanied by an increase in CTSL activity (Figure 1B) without changes in the overall cysteine protease activity of the cell (Supplementary Figure S1). Our findings indicate that the effect of loss of A-type lamins on CTSL is specific and not the result of a global deregulation of protease activity. Furthermore, acute depletion of A-type lamins by means of a specific shRNA (Gonzalez-Suarez et al, 2009) resulted in increased CTSL levels and activity and destabilization of 53BP1 (Figure 1C), without affecting total protease activity (Supplementary Figure S1). To elucidate the mechanism behind the increase in CTSL levels, we analysed CTSL gene expression by northern blot. As shown in Figure 1D and Supplementary Figure S2, CTSL mRNA levels are significantly higher in Lmna−/− fibroblasts when compared with WT, indicating that A-type lamins regulate the steady-state levels of CTSL mRNA.
To ascertain whether CTSL upregulation was specifically responsible for the decrease in 53BP1 levels in A-type lamins-deficient cells, we monitored the levels of 53BP1 in Lmna−/− fibroblasts upon acute depletion of CTSL with a specific shRNA. Strikingly, depletion of CTSL in Lmna−/− fibroblasts was sufficient to restore the levels of 53BP1 to those of a WT cell (Figure 1E), indicating that A-type lamins destabilize 53BP1 primarily via upregulation of CTSL activity. Acute depletion of CTSL inhibited CTSL activity (Figure 1E), without changes in total protease activity (Supplementary Figure S1).
Taken together, the data indicate that both the proteasome and CTSL can induce the degradation of 53BP1. Although they could represent two independent degradation pathways, it is also possible that there is some coordination between them. Upon loss of A-type lamins, CTSL could process nuclear 53BP1 to be degraded by the proteasome. In this scenario, depletion of CTSL would prevent degradation of 53BP1 by the proteasome, and thus hinder the ability of MG-132 to restore 53BP1 protein levels. To test whether proteasome and CTSL degradation are two independent processes, we treated WT, Lmna−/− fibroblasts and CTSL-depleted Lmna−/− fibroblasts with either MG-132 or Z-FY-CHO in the presence of cycloheximide to block protein synthesis. As expected, in WT and Lmna−/− cells, both the proteasome and the CTSL inhibitors were able to increase 53BP1 levels. Upon depletion of CTSL, Z-FY-CHO was no longer able to restore 53BP1 levels, demonstrating the specificity of Z-FY-CHO towards CTSL. Interestingly, MG-132 restored 53BP1 levels in Lmna−/− cells depleted of CTSL (Figure 1F), indicating that, at least partially, the proteasome component of 53BP1 degradation is independent of CTSL.
A-type lamins regulate NHEJ by using CTSL as an effector
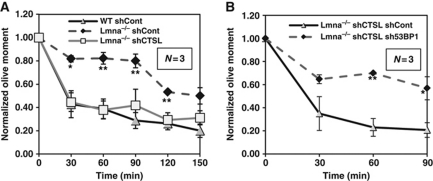
Loss of A-type lamins impairs NHEJ and thus the ability of cells to process dysfunctional telomeres (Gonzalez-Suarez et al, 2009) and ionizing-radiation (IR)-induced DNA DSBs. Moreover, such defects are specifically due to the decrease in 53BP1 levels (Redwood et al, 2011). To determine if the recovery of 53BP1 levels observed in CTSL-depleted Lmna−/− fibroblasts was able to restore DNA repair by NHEJ, we performed neutral comet assays in IR-treated cells (Olive et al, 1990). As shown in Figure 2A and Supplementary Figure S3A, whereas Lmna−/− fibroblasts presented defects in the fast phase of repair corresponding to NHEJ, depletion of CTSL was sufficient to restore the repair of IR-induced DSBs to a degree that was indistinguishable from that of WT cells. Most importantly, the rescue of defective NHEJ by depletion of CTSL was specifically mediated by 53BP1. As shown in Figure 2B and Supplementary Figure S3B and C, depletion of 53BP1 with a specific shRNA prevents the rescue of NHEJ induced by the loss of CTSL. These results demonstrate an unprecedented role for CTSL in the regulation of 53BP1 levels and thus the ability of cells to repair DNA DSBs by NHEJ. A-type lamins regulate the levels of 53BP1 and NHEJ by using CTSL as an effector.
Figure 2.
Effect of CTSL on the repair of IR-induced DNA DSBs. (A) Neutral comet assays after irradiation with 8 Gy shows defects in the fast phase of repair of DNA DSBs in Lmna−/− MEFs with respect to WT, both transduced with an shRNA control. Defects in DNA repair are rescued by acute depletion of CTSL with a specific shRNA. (B) Neutral comet assays in Lmna−/− MEFs depleted of CTSL and transduced with either an shRNA specific for depletion of 53BP1 or an shRNA control. Note how depletion of 53BP1 prevents the rescue of defects in DNA repair induced by depletion of CTSL. Values are expressed as mean±s.e.m. N represents the number of independent experiments; *, **P-value of statistical significance (*P⩽0.05 and **P⩽0.001).
Upregulation of CTSL upon loss of A-type lamins alters the cellular distribution of 53BP1
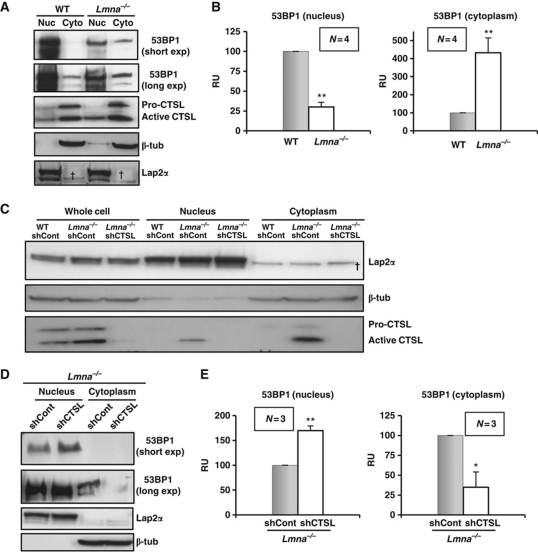
Seeking a deeper understanding of the mechanism for 53BP1 degradation, we performed subcellular fractionation of WT and Lmna−/− MEFs. As expected, in both cell types, 53BP1 protein was found primarily in the nuclear fraction (Figure 3A and B). However, a marked increase in cytoplasmic 53BP1 was observed in Lmna−/− cells when compared with WT cells. Similarly, acute depletion of A-type lamins with a specific shRNA also resulted in decreased levels of 53BP1 in the nuclear fraction, and increased protein in the cytoplasm (Supplementary Figure S4). These results indicate that A-type lamins regulate the cellular distribution of 53BP1.
Figure 3.
Effect of A-type lamins and CTSL in the cellular distribution of 53BP1. (A) Subcellular fractionation of WT and Lmna−/− MEFs followed by western blots to monitor the levels of 53BP1 and CTSL in the nucleus and the cytoplasm. β-Tubulin was used as marker of cytoplasmic fraction and LAP2α as marker of nuclear fraction. (B) Graphs show the quantitation of four independent experiments. Note how loss of A-type lamins leads to an increase of 53BP1 in the cytoplasm. (C) Western blots showing the distribution of CTSL in WT and Lmna−/− MEFs transduced with an shRNA control and in Lmna−/− MEFs depleted of CTSL. Note the presence of active CTSL in the nuclear fraction in A-type lamins-deficient cells. (D) Subcellular fractionation of Lmna−/− fibroblasts lentivirally transduced with an shRNA for depletion of CTSL or an shRNA control. Note how depletion of CTSL restores the normal distribution of 53BP1 protein. (E) Graphs show the quantitation of three independent experiments. In all cases, 100 μg of total cell lysate, 60 μg of nuclear extract, and 120 μg of cytoplasmic extract were loaded per well. N represents the number of independent experiments; *, **P-value of statistical significance (*P⩽0.05 and **P⩽0.001). Values are expressed as mean±s.e.m. †Non-specific band detected with LAP2α antibody. RU stands for relative units (normalization to control).
The accumulation of 53BP1 in the cytoplasm could result from impaired import of the protein into the nucleus or from leakage of 53BP1 out of the nucleus as a consequence of the loss of A-type lamins. As cellular scaffolds, lamins are known to interact with multiple protein complexes (Zastrow et al, 2004; Dechat et al, 2008). Thus, A-type lamins could promote 53BP1 retention within the nucleus. On the other hand, two groups have independently identified CTSL in the nucleus of eukaryotic cells (Goulet et al, 2004; Duncan et al, 2008). We also detected the active form of CTSL in the nucleus especially in Lmna−/− MEFs (Figure 3C). Thus, it is possible that the accumulation of nuclear CTSL participates in the targeting of 53BP1 to be exported out of the nucleus. To distinguish between these possibilities, we performed subcellular fractionation in Lmna−/− fibroblasts proficient or deficient in CTSL. Interestingly, whereas in Lmna−/− cells, 53BP1 accumulated in the cytoplasm, depletion of CTSL in this context was sufficient to restore the normal 53BP1 distribution within the cell (Figure 3D and E), with undetectable 53BP1 levels in the cytoplasm. These results demonstrate that it is the increase in CTSL and not the loss of A-type lamins that is responsible for the increase in cytoplasmic 53BP1. Thus, CTSL has an active role in the degradation of 53BP1 as well as in the entry or retention of the protein inside the nucleus.
Effect of upregulation of CTSL in WT cells
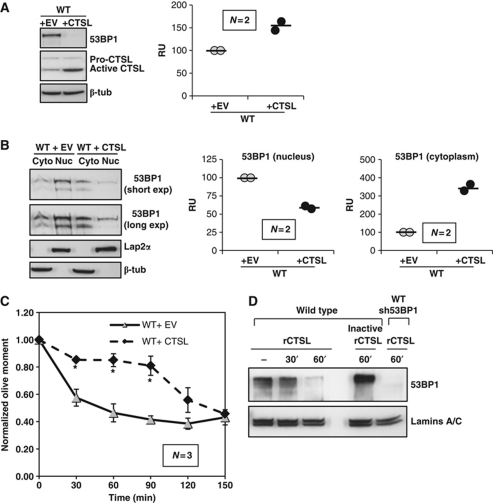
Altogether, our data demonstrate that the upregulation of CTSL activity upon loss of A-type lamins leads to degradation of 53BP1 and defective repair of DNA DSBs by NHEJ. Next, we determined if CTSL-mediated regulation of 53BP1 protein levels is a general mechanism, taking place also in WT cells. We retrovirally transduced WT fibroblasts with a CTSL-expressing construct or an empty vector control. As shown in Figure 4A, CTSL overexpression led to increased levels of the active form of the enzyme and increased CTSL activity, as well as a decrease in 53BP1 levels. CTSL overexpression did not affect total cysteine protease activity (Supplementary Figure S1). Furthermore, overexpression of CTSL in WT fibroblasts results in a cellular distribution of 53BP1 that resembles that of A-type lamins-deficient cells—decreased levels of 53BP1 in the nucleus and increased levels of the protein in the cytoplasm (Figure 4B). To ascertain whether overexpression of CTSL in WT cells impacts on the repair of IR-induced DNA DSBs by NHEJ, we performed neutral comet assays. As shown in Figure 4C and Supplementary Figure S3D, CTSL overexpression led to defects in DNA repair by NHEJ in WT fibroblasts. Our results demonstrate for the first time that upregulation of CTSL in WT cells leads to degradation of 53BP1 and defective repair of DNA DSBs repair, which in turn could contribute to the genomic instability that drives malignancy, especially in CTSL-overexpressing tumours.
Figure 4.
Effect of overexpression of CTSL in WT cells. (A) Overexpression of CTSL in WT MEFs by retroviral transduction leads to degradation of 53BP1 (western blots) and increased CTSL enzymatic activity (graph). (B) Subcellular fractionation of WT MEFs retrovirally transduced with a CTSL-expressing vector or an empty vector control, followed by western blots to monitor the levels of 53BP1 in the nucleus and the cytoplasm. β-Tubulin was used as marker of cytoplasmic fraction and LAP2α as marker of nuclear fraction. Graphs show the quantitation of two independent experiments. Note how overexpression of CTSL leads to an increase of 53BP1 in the cytoplasm. (C) Neutral comet assays showing that overexpression of CTSL in WT MEFs leads to defects in the fast phase of repair of DNA DSBs induced by IR. (D) In vitro degradation of 53BP1 obtained from WT MEFs nuclear extracts. Incubation with recombinant CTSL leads to a time-dependent degradation of nuclear 53BP1. Heat inactivation of CTSL prevents 53BP1 degradation. 53BP1-depleted nuclear extracts were used as control for antibody specificity. Lamins A/C were used as loading control. Values in bar graphs are expressed as mean±s.e.m. In ‘bee swarm’ plots, horizontal bar indicates the average value. N represents the number of independent experiments. *P-value of statistical significance (P⩽0.05). RFU stands for relative fluorescence units and RU stands for relative units (normalization to controls).
Next, we determined whether 53BP1 is a direct target of CTSL by performing an in vitro degradation assay. Nuclei from WT fibroblasts were isolated and subjected to mild solubilization to extract soluble nucleoplasmic proteins. Nuclei were then incubated in the presence of recombinant CTSL for increasing periods of time, and the levels of 53BP1 monitored by western blot. As shown in Figure 4D, incubation of nuclei with recombinant CTSL leads to degradation of 53BP1 in a time-dependent manner. Importantly, heat inactivation of recombinant CTSL prevented the degradation of 53BP1. As control, the levels of A-type lamins were not affected by CTSL. We conclude that CTSL can degrade 53BP1 when the proteins are in contact such is the case of Lmna-deficient cells.
Vitamin D regulates 53BP1 levels through inhibition of CTSL activity
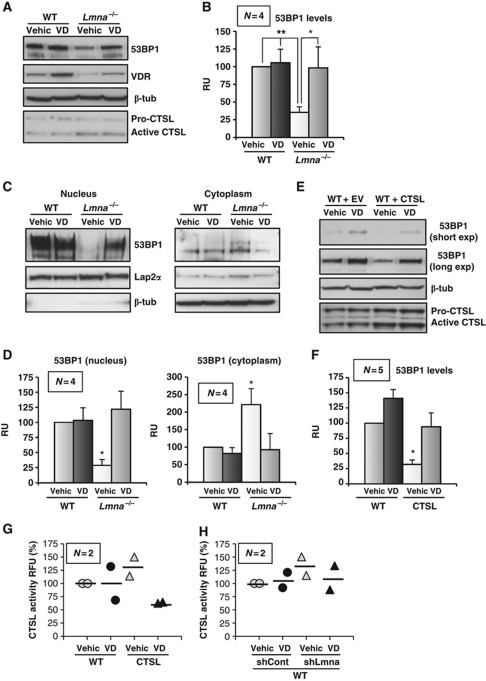
Deregulation of 53BP1 function contributes to genomic instability and disrupts cell homeostasis. The ability to exogenously manipulate this pathway and restore the cellular ability to repair DNA damage could be of potential critical relevance in the context of both cancer and laminopathies. In a recent report, vitamin D treatment was shown to induce the expression of Cystatin D, an endogenous inhibitor of CTSL, in human colon cancer cells (Alvarez-Diaz et al, 2009). We hypothesized that vitamin D could impact on 53BP1 stability, by blocking the activity of CTSL. To test this hypothesis, we incubated WT and Lmna−/− fibroblasts with 1,25(OH)2 vitamin D or calcitriol, the active metabolite of vitamin D. As previously reported, vitamin D treatment resulted in an increase in the levels of vitamin D receptor (VDR) (Dusso et al, 2005) (Figure 5A). Remarkably, vitamin D was able to restore 53BP1 levels in Lmna−/− MEFs to that of WT cells, without altering CTSL protein levels, suggesting a Cystatin-mediated effect (Figure 5A and B). These results were confirmed by immunofluorescence studies. As shown in Supplementary Figure S5, acute depletion of A-type lamins leads to a marked decrease in the nuclear levels of 53BP1 and vitamin D treatment restores the intensity of labelling of nuclear 53BP1. Moreover, subcellular fractionation experiments in Lmna−/− MEFs showed that vitamin D treatment restores the nuclear/cytoplasmic distribution of 53BP1 to that of normal cells, similarly to CTSL depletion and without any effect on WT cells (Figure 5C and D). Furthermore, overexpression of CTSL in WT cells led to degradation of 53BP1, and treatment with vitamin D prevented the CTSL-mediated degradation of the protein (Figure 5E and F). Vitamin D treatment also inhibited the activity of exogenously expressed CTSL (Figure 5G), as well as the increased CTSL activity due to depletion of A-type lamins (Figure 5H). Overall, our results indicate that vitamin D promotes the stability of 53BP1 by inhibiting CTSL enzymatic activity.
Figure 5.
Effect of vitamin D in the CSTL-mediated degradation of 53BP1. (A) WT and Lmna−/− MEFs were treated with calcitriol (1,25 dihydroxy vitamin D) or vehicle for 24 h and the levels of 53BP1, VDR, and CTSL are monitored by western blot. β-Tubulin was used as loading control. VDR levels monitored to ascertain efficacy of vitamin D treatment. (B) Graph shows the quantitation of four independent experiments. Note how vitamin D treatment stabilizes 53BP1 in Lmna−/− MEFs. (C) Subcellular fractionation of WT and Lmna−/− MEFs upon treatment with vitamin D or vehicle, followed by western blots to monitor localization of 53BP1. (D) Graphs show the quantitation of four independent experiments. Note how Lmna−/− MEFs treated with vitamin D revert to a normal cellular distribution of 53BP1. (E) Western blots to monitor levels of 53BP1 upon overexpression of CTL in WT MEFs, followed by treatment with vitamin D or vehicle. (F) Graph shows the quantitation of five independent experiments. Note that vitamin D treatment restores 53BP1 levels in CTSL-overexpressing cells. (G) Graph showing inhibition of exogenously expressed CTSL enzymatic activity by treatment with vitamin D. (H) Graph showing partial inhibition of CTSL enzymatic activity by vitamin D in lamins-deficient cells. Values in bar graphs are expressed as mean±s.e.m. In ‘bee swarm’ plots, horizontal bar indicates the average value. N represents the number of independent experiments; *, **P-value of statistical significance (*P⩽0.05 and **P⩽0.001).
Vitamin D rescues some of the phenotypes associated with loss of A-type lamins
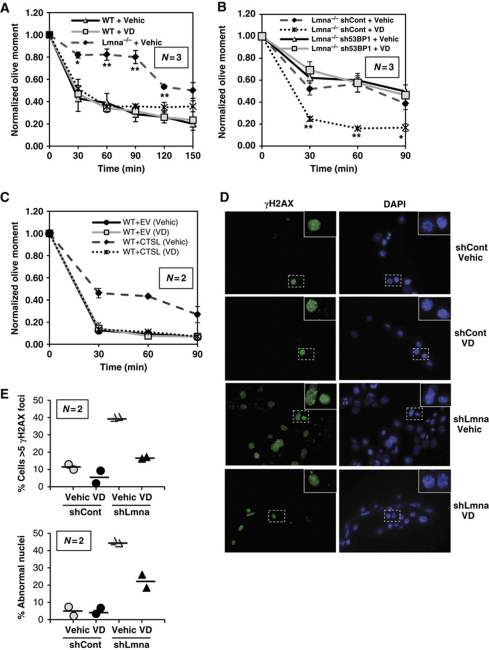
Given the ability of vitamin D to stabilize 53BP1 protein levels, we evaluated whether vitamin D treatment was able to improve the defects in repair of IR-induced DNA DSBs by NHEJ in Lmna−/− fibroblasts. As shown in Figure 6A and Supplementary Figure S6A, vitamin D treatment allows Lmna−/− MEFs to efficiently repair IR-induced DSBs by NHEJ. Furthermore, the vitamin D-induced rescue of NHEJ defects is due specifically to its ability to inhibit CTSL and stabilize 53BP1 protein. As shown in Figure 6B and Supplementary Figure S6B, depletion of 53BP1 inhibits the ability of vitamin D to rescue NHEJ in Lmna−/− MEFs. Moreover, the defects in NHEJ due to overexpression of CTSL in WT cells are rescued by vitamin D treatment (Figure 6C; Supplementary Figure S6C). Thus, CTSL and 53BP1 are mediators of the effect of vitamin D on DNA repair.
Figure 6.
Effect of vitamin D on the phenotype of lamins-deficient cells. (A) Neutral comet assays in WT and Lmna−/− MEFs after treatment with vitamin D or vehicle. Note how the defects in the fast phase of DNA repair in Lmna−/− MEFs are rescued by vitamin D. (B) Neutral comet assays in Lmna−/− MEFs lentivirally transduced with an shRNA specific for depletion of 53BP1 or an shRNA control, followed by treatment with vitamin D or vehicle. Note how depletion of 53BP1 in Lmna−/− MEFs prevents the vitamin D-induced rescue of defects in DNA repair. (C) Neutral comet assays in WT MEFs overexpressing CTSL and treated with vitamin D or vehicle. Note how overexpression of CTSL leads to defects in the fast phase of repair that are rescued by vitamin D treatment. (D) Immunofluorescence with γH2AX antibody in WT and lamins-deficient cells treated with either vehicle of vitamin D for 72 h. DAPI was used as counterstaining. (E) Graphs showing quantification of γH2AX-positive cells (⩾5 foci) and nuclear abnormalities in WT and lamins-deficient cells treated with vitamin D or vehicle control. Note how VD treatment decreases the number of γH2AX-positive cells and nuclear abnormalities in lamins-deficient cells. A minimum of 800 cells were analysed in each case. Values in graphs are expressed as mean±s.e.m. In ‘bee swarm’ plots, horizontal bar indicates the average value. N represents the number of independent experiments; *, ** P-value of statistical significance (*P⩽0.05 and **P⩽0.001). Bars, standard error.
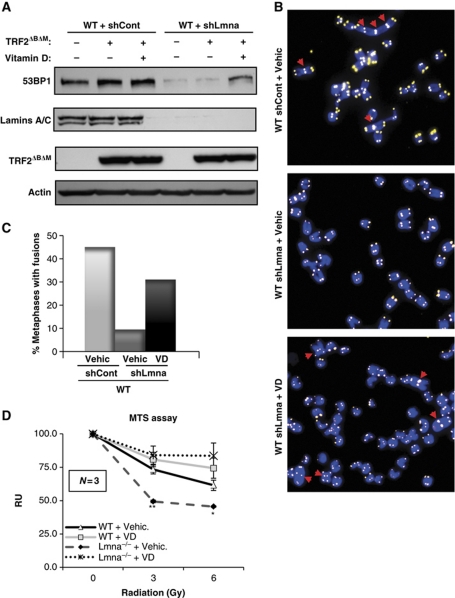
Next, we tested whether vitamin D can rescue other phenotypes associated with the loss of A-type lamins, such as nuclear morphological abnormalities, degree of unrepaired DNA damage, and processing of dysfunctional telomeres by NHEJ. As shown in Figure 6D and E, acute depletion of A-type lamins leads to a marked increase in the percentage of cells presenting γH2AX foci, indicative of DNA damage, and nuclear morphological abnormalities. Importantly, treatment with vitamin D for 3 days reduced significantly the percentage of cells both with γH2AX foci and with nuclear abnormalities. To test the effect of vitamin D in NHEJ of dysfunctional telomeres, lamins-proficient and -deficient cells were retrovirally transduced with a dominant-negative form of the telomere protein TRF2 (TRF2ΔBΔM) (Figure 7A). Expression of this mutant protein induces massive chromosome end-to-end fusions via 53BP1-dependent classical NHEJ (Rai et al, 2010). As previously reported (Gonzalez-Suarez et al, 2009; Redwood et al, 2011), loss of A-type lamins leads to decreased levels of 53BP1 (Figure 7A), and defective processing of dysfunctional telomeres by NHEJ (Figure 7B and C). Interestingly, treatment with vitamin D rescues the levels of 53BP1 (Figure 7A) and restores the ability of lamins-deficient cells to process dysfunctional telomeres by NHEJ (Figure 7B and C). Altogether our results show that vitamin D rescues at least partially some of the phenotypes associated with the loss of A-type lamins, which are expected to contribute to the maintenance of genome integrity.
Figure 7.
Effect of vitamin D in NHEJ of dysfunctional telomeres and radiation sensitivity. (A) Western blots showing the levels of 53BP1 and lamins upon ectopic expression of TRF2ΔBΔM in cells proficient or deficient in A-type lamins (lentivirally transduced with shControl or shLmna, respectively). The two cell lines were treated with vitamin D or vehicle control for 24 h. Actin was used as a loading control. (B) Representative images of metaphase spreads from the different samples analysed. Telomeres are labelled with a specific PNA probe (yellow). Chromosomes are stained with DAPI (blue). Red arrows indicate fusions. (C) Quantification of the percentage of metaphases presenting chromosome end-to-end fusions in cells treated with either vitamin D or vehicle control after retroviral transduction with TRF2ΔBΔM. Note how VD treatment restores 53BP1 levels and normal processing of dysfunctional telomeres in lamins-deficient cells, thus leading to increased number of fusions. WT MEFs retrovirally transduced with TRF2ΔBΔM were used as a positive control. A minimum of 20 metaphases were analysed per sample. (D) MTS assays to monitor radiation sensitivity of WT and Lmna−/− MEFs treated with vitamin D or vehicle. Note the decreased viability of Lmna−/− MEFs, which is rescued by vitamin D treatment. N represents the number of independent experiments; *, **P-value of statistical significance (*P⩽0.05 and **P⩽0.001). Bars, standard error.
To evaluate the functional consequences of vitamin D treatment, we monitored radiation sensitivity in Lmna−/− fibroblasts treated with vehicle control or with vitamin D. As previously observed, Lmna−/− fibroblasts exhibit increased radiosensitivity (Redwood et al, 2011) (Figure 7D). Treatment with vitamin D induces radioprotection in Lmna−/− cells, putatively via stabilization of 53BP1 protein. Our data reveal an unprecedented role for vitamin D in the maintenance of mechanisms of DNA repair.
Discussion
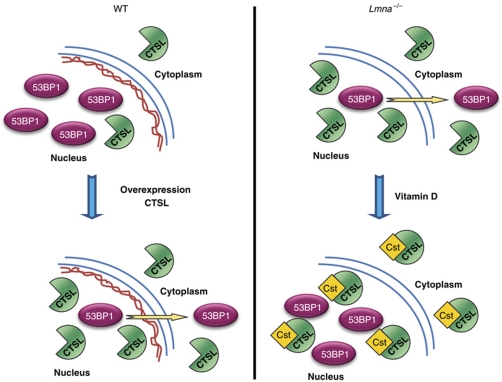
Our study demonstrates that 53BP1 stability and cellular distribution are regulated by CTSL, a cysteine protease that has a key role in the endosomal/lysosomal degradation pathway, and that has also been shown to function in the nucleus (Goulet et al, 2004; Duncan et al, 2008; Lankelma et al, 2010). Overexpression of CTSL in WT fibroblasts leads to degradation of 53BP1, and as a consequence, to defects in the fast phase of repair of DNA DSBs corresponding to NHEJ. In addition, we show that the 53BP1 deficiency associated with the loss of A-type lamins is due to the upregulation of the steady-state levels of CTSL mRNA and accumulation of the active form in the nucleus. Moreover, we find that vitamin D treatment of cells with upregulated CTSL activity, either Lmna−/− or CTSL-overexpressing fibroblasts, stabilizes 53BP1 and rescues DNA DSBs repair via inhibition of CTSL activity (Figure 8). Interestingly, vitamin D treatment also rescues to certain extent the basal levels of DNA damage, the nuclear abnormalities, and the defects in NHEJ of dysfunctional telomeres caused by loss of A-type lamins. We speculate that the nuclear form of CTSL, previously shown to participate in the cleavage of Histone H3 tail (Duncan et al, 2008) and the transcription factor CDP/Cux (Goulet et al, 2004) is likely to be responsible for the degradation of 53BP1, a protein found almost exclusively in the nucleus of WT cells. This notion is supported by the data showing that CTSL accumulates in the nucleus primarily in A-type lamins-deficient cells and that CTSL is actively involved in the accumulation of 53BP1 in the cytoplasm. In this context, vitamin D inhibits CTSL activity, ameliorating some of the phenotypes of lamins-deficient cells.
Figure 8.
Model of novel pathway regulating 53BP1 stability. (Left) A-type lamins stabilize 53BP1 levels by preventing its degradation by the proteasome and by downregulating the steady-state levels of CTSL mRNA. Most of the 53BP1 protein is localized in the nucleus. Maintenance of 53BP1 levels ensures proper repair of DNA DSBs induced by IR, thus preserving genomic stability. Overexpression of CTSL promotes the transport of 53BP1 out of the nucleus and its degradation. Cells with upregulated CTSL have defects in DNA DSBs repair. (Right) Loss of A-type lamins leads to upregulation of CTSL and accumulation of the active enzyme in the nucleus, which in turn induces the degradation of 53BP1 and its accumulation out of the nucleus, without any detectable changes in mRNA levels. 53BP1 deficiency results in defects in the fast phase of repair of DNA DSBs corresponding to NHEJ, and thus genomic instability. Treatment of A-type lamins-deficient cells with vitamin D inhibits CTSL activity, putatively via upregulation of Cystatins, and rescues defects in DNA repair.
Regulation of 53BP1 protein levels and disease
In recent years, emphasis has been placed on understanding the molecular mechanisms modulating the recruitment of 53BP1 protein to DNA repair foci, as well as its function during repair. Recruitment and maintenance of 53BP1 at these foci involves interactions and post-translational modifications of a variety of proteins (Stewart et al, 2003a; Botuyan et al, 2006; Kolas et al, 2007; Mailand et al, 2007; Galanty et al, 2009). 53BP1 recruitment to DNA damaged sites has been shown to promote NHEJ by inhibiting the end-resection of DSBs, which is required for HR (Bunting et al, 2010). Thus, 53BP1 is considered to have a critical role in the choice of mechanism used to repair DNA DSBs: NHEJ or HR. Our previous studies showed that loss of A-type lamins does not affect the recruitment of 53BP1 to DNA repair foci. However, the decreased global levels of the protein resulted in lower accumulation at these foci, which was sufficient to hinder repair of IR-induced DNA DSBs by NHEJ (Redwood et al, 2011). A conclusion that emanates from these findings is that cells are very sensitive to the levels of 53BP1, with only limited changes in protein levels rendering a significant NHEJ deficiency. Interestingly, recent studies in breast cancer cells showed that triple-negative and BRCA1-deficient tumours are characterized by a reduction in 53BP1 levels. This reduction in 53BP1 prevents the profound genomic instability induced by the loss of BRCA1 function, and it is thought to promote viability of the cancer cells allowing tumour progression (Bouwman et al, 2010; Bunting et al, 2010; Kass et al, 2010). However, how the levels of 53BP1 are regulated in normal or cancer cells remains largely unexplored. It is possible that CTSL upregulation is one of the mechanisms participating in the downregulation of 53BP1 in cancer, especially in the context of triple-negative and BRCA1-deficient breast tumour cells. Upregulation of CTSL mRNA levels and defective recruitment of 53BP1 to DNA repair foci have also been shown in a mouse model of progeria (Zmpste24 knockout mice), which exhibits genomic instability (Liu et al, 2005; Varela et al, 2005). However, the stability of 53BP1 was not monitored in this model. Future studies will need to determine if CTSL upregulation contributes to the defects in DNA repair and the genomic instability characteristic of some laminopathies.
Novel role of CTSL in the regulation of DNA repair mechanisms
Upregulation of CTSL is a hallmark of a variety of cancers and has been correlated with increased invasiveness, metastasis, and overall degree of malignancy (Jedeszko and Sloane, 2004; Skrzydlewska et al, 2005; Gocheva and Joyce, 2007). Thus, inhibition of CTSL activity, which contrary to other Cathepsins is exclusively elevated in malignant cells, is considered a promising strategy for cancer treatment. However, the results of in vitro and in vivo studies using CTSL inhibition as monotherapy or in combination with conventional chemotherapy remain inconclusive (Lankelma et al, 2010). In addition to the previously reported effects of CTSL upregulation on the degradation of extracellular matrix components and cell-adhesion molecules, our study suggests that CTSL upregulation in cancer could inhibit mechanisms of DNA repair. Thus, it is tempting to speculate that upregulation of CTSL either by loss of A-type lamins or by other means would cause genomic instability, which in turn could contribute to the development of ageing-related pathologies, especially cancer. On the other hand, CTSL-overexpressing tumours might exhibit increased sensitivity to treatment with radiation and chemotherapeutic agents. In the light of the unsuspected role for CTSL in the maintenance of 53BP1 protein levels and mechanisms of DNA repair, the use of CTSL inhibitors in cancer therapy needs to be revisited. The most benefit in cancer treatment could be achieved by inhibiting specifically the CTSL secreted form without affecting the nuclear form. This strategy could potentially ameliorate the metastatic potential of the cancer cells, while maintaining their sensitivity to DNA damaging therapeutic compounds. Further understanding of the role of nuclear and secreted forms of CTSL in cancer cells will be fundamental for the design of the best anti-CTSL therapeutic strategy.
Vitamin D link with DNA repair
Our results indicate that vitamin D treatment can counteract the destabilization of 53BP1 upon upregulation of CTSL activity, rescuing the associated defects in NHEJ. In addition, we show that vitamin D can reduce the degree of unrepaired DNA damage (γH2AX foci) as well as the extent of nuclear abnormalities characteristic of lamins-deficient cells. Previous studies in MDA-MB-231 breast cancer cells showed that the CTSL gene is a target of the active metabolite of vitamin D, 1,25(OH)2D3, which causes a decrease in CTSL expression (Swami et al, 2003). Similarly, vitamin D treatment of colon cancer cells activates the expression of Cystatins, endogenous inhibitors of cysteine proteases from the Cathepsin family. In particular, treatment with vitamin D activated the expression of Cystatin D, inhibiting the activity of CTSL in these tumour cells (Alvarez-Diaz et al, 2009). We found that the protective effect of vitamin D in fibroblasts with upregulated CTSL does not involve changes in the levels of CTSL, but rather an inhibition of CTSL activity, suggesting a Cystatin-mediated effect. The mouse genome encodes 13 different Cystatins, with no orthologue of Cystatin D. Interestingly, we found that a number of Cystatin genes have vitamin D-responsive elements (data not shown), and thus it is possible that some members of the Cystatin family are mediating the effect of vitamin D on the stability of 53BP1.
The unprecedented action of vitamin D on the maintenance of mechanisms of DNA repair and genomic stability is of critical importance for understanding ageing-related pathologies. Vitamin D deficiency is prevalent among older adults and is associated with risk for cardiovascular disease, cancer, autoimmune diseases, infectious diseases, and even symptoms of depression and cognitive deficits (Dusso et al, 2005; Holick, 2007). Our findings suggest that in the context of vitamin D deficiency, cells could be hindered in their ability to deal with endogenous sources of DNA damage, which can lead over time to genetic alterations promoting tumourigenesis and other ageing-related diseases. Thus, supplementation with vitamin D in older adults might prevent upregulation of CTSL and 53BP1 deficiency, promoting the maintenance of mechanisms of DNA repair and the stability of the genome. Interestingly, our results indicate that vitamin D is particularly effective in rescuing the phenotypes of cells with aberrant upregulation of CTSL activity, either A-type lamins-deficient cells or cells exogenously expressing CTSL. In contrast, no profound effects on CTSL activity or 53BP1 levels were observed in WT cells. Vitamin D could represent a safe and efficient strategy to ameliorate the disease phenotypes specifically of CSTL-overexpressing cells. Future studies need to determine the extent to which treatment with vitamin D affects DNA repair in transformed cells. These types of studies will be fundamental for investigating the use of vitamin D for the treatment of specific types of cancer.
Summary
Overall, our studies provide novel links between expression of A-type lamins, transcriptional regulation of CTSL, stabilization of 53BP1, and maintenance of mechanisms of DNA repair. A number of studies have shown correlations between either silencing of A-type lamins, overexpression of CTSL, or downregulation of 53BP1 with poor prognosis in different types of tumours (Broers et al, 1993; Agrelo et al, 2005; Prokocimer et al, 2006, 2009; Bouwman et al, 2010; Bunting et al, 2010; Kass et al, 2010; Lankelma et al, 2010). We provide evidence of a functional interplay between these different cancer phenotypes. Our findings could be relevant for the design of therapeutic strategies to ameliorate the genomic instability characteristic of ageing and cancer.
Materials and methods
MEF culture
WT and Lmna−/− MEFs were generated in the laboratory of Colin L Stewart as described (Sullivan et al, 1999) and allowed to spontaneously immortalize in culture. All lines were maintained in DMEM-Glutamax (Invitrogen) supplemented with 10% bovine growth serum (BGS; Hyclone), antibiotics, and antimycotics.
Immunoblotting
Cells were lysed in RIPA buffer (50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.2% SDS) supplemented with PMSF and protease and phosphatase inhibitors (Sigma). Protein detection was carried out with Lamin A/C (SC-6215, Santa Cruz), VDR (SC-1008, Santa Cruz), CTSL (SC-6498, Santa Cruz), 53BP1 (NB-100-304, Novus Biologicals), Lap2α (ab 5112, Abcam), and β-tubulin (T0198, Sigma).
Northern blot
Total RNA was isolated using the TRI Reagent™ (Sigma) and following the manufacturer's instructions. A total of 5 μg per well were separated in 1.2% agarose-formaldehyde gels, blotted onto a Hybond N+ membrane and hybridized with CTSL or 28S rRNA probes. Quantification of the signal was performed with the ImageQuant software (Molecular Dynamics).
Treatments with proteasome and CTSL inhibitors and with vitamin D
Proteasome and CTSL inhibitors. Cells were incubated for 6 h in the presence of cycloheximide (10 μg/ml) to inhibit protein synthesis, and in the presence of the proteasome inhibitor MG-132 (30 μM, 474790, EMD), the CTSL-specific inhibitor Z-FY-CHO (50 nM, 219426, EMD) or vehicle (ethanol). Cells were collected after the treatments and processed for immunoblotting.
Vitamin D. When indicated, cells were treated with 10−7 M 1α,25-dihydroxyvitamin D3 (Calcitriol, D1530, Sigma). Briefly, aliquots of 1 nmol of calcitriol were resuspended in 1 ml of BGS and diluted in DMEM-Glutamax with antibiotics and antimycotics. Working media contained 10% BGS and 10−7 M calcitriol. In all, 10% BGS in DMEM was used as control media. Treatment with vitamin D was carried out for 24 h unless indicated otherwise.
Constructs and viral transduction
Retroviral and lentiviral transductions were performed as previously described (Gonzalez-Suarez et al, 2009). Briefly, 293T cells were transfected with viral packaging (pUMVC3 or pHR′8.2ΔR) and envelope plasmids (pCMV-VSV-G) (Stewart et al, 2003b) along with the appropriate vector containing the cDNA or shRNA of interest. After 48 h, virus-containing media was harvested and used to infect target cells. Retroviral transductions were carried out as two 4–6 h infections on sequential days and lentiviral as one 4 h infection. Cells were allowed to recover for 48 h, and treated with the appropriate selective drugs. Viral envelope and packaging plasmids were gifts from Sheila Stewart (Washington University, St Louis, MO). The shRNA targeting mouse lamin A/C was a gift from Didier Hodzic (Washington University). The shRNA targeting 53BP1 was a gift from Barry Sleckman (Washington University). The CTSL overexpression plasmid was generated by PCR using as template a pMSCV-CTSL-GFP construct generated by Emily Cheng. Briefly, primers were designed to introduce a stop codon and a XhoI endonuclease restriction site at the end of the codifying sequence of the CTSL cDNA. The PCR fragment was purified, digested with BglII and XhoI restriction endonucleases and subcloned into a pMSCV-puro vector (primers: Fw:CCCTTGAACCTCCTCGTT; Rv:ATATATATACTGGAGTCAATCAATCAATTCACGACAGGATAGCT).
Subcellular fractionation
Nuclear and cytoplasmic extracts were prepared by using the Nuclear Extract Kit (Active Motif # 40010) following the manufacturer's instructions. Briefly, 5 × 106 cells per sample were trypsinized and washed 1 × in PBS and 1 × in PBS with phosphatase inhibitors. The cytoplasmic fraction was collected by centrifugation at 14 000 r.p.m. for 10 min after treating the cells with 1% NP-40 in Hypotonic Buffer supplemented with PMSF and protease inhibitors. Nuclear stability was determined at the microscope by Trypan blue staining. Pellet (nuclear extract) was washed 3 × in PBS containing 0.05% NP-40. Nuclear proteins were extracted in Complete Lysis Buffer supplemented with 1 mM DTT, PMSF, and protease inhibitors. Samples were incubated in buffer for 20 min, sonicated for 5 min, and centrifuged at 14 000 r.p.m. for 10 min. After protein quantification, 80–200 μg of protein were loaded per well.
Comet assays
To assess DNA DSBs repair, neutral comet assays were performed using CometSlide assay kits (Trevigen). WT and Lmna−/− MEFs were irradiated with 8 Gy, and cells were incubated at 37°C for different periods of time (0, 30, 60, 90, 120, and 150 min) to allow for DNA damage repair. Cells were embedded in agarose, lysed, and subjected to neutral electrophoresis. Immediately before image analysis, cells were stained with ethidium bromide and visualized under a fluorescence microscope. Olive comet moment was calculated by multiplying the percentage of DNA in the tail by the displacement between the means of the head and tail distributions, as described (Olive et al, 1990). We used the program CometScore™ Version 1.5 (TriTek) to calculate Olive Comet Moment. A total of 25–30 comets were analysed per sample in each experiment
CTSL activity
CTSL and total Cathepsin activity was measured by using the fluorogenic substrate Z-Phe-Arg-AMC from the InnoZyme™ Cathepsin L Activity Kit (EMD Chemicals) following the manufacturer's instructions. Briefly, 106 cells were lysed in Lysis Buffer (400 mM Na phosphate buffer pH 6, 75 mM NaCl, 4 mM EDTA, 0.25% Triton X-100). A total of 20–80 μg of protein extract was used per sample. Samples were measured in duplicate. Cleaved Z-Phe-Arg-AMC substrate was detected by fluorescence emission (Exc. max: 360 nm; Emi. max: 460 nm). CTSL activity was determined in the presence of CA 074, a specific Cathepsin B inhibitor. As a negative control, the CTSL inhibitor Z-FY(t-but)-DMK was used. Total cysteine activity was determined as the cleavage of the substrate in the absence of inhibitors.
Immunofluorescence
Cells growing in coverslips were fixed for 10 min in 3.7% formaldehyde/0.2% Tx-100/PBS at RT, followed by three washes in PBS. After blocking in 10% BGS/PBS for 1 h, cells were incubated with γH2AX (07-164, Upstate) or 53BP1 (NB-100-304, Novus Biologicals) antibodies for 1 h at 37°C, washed three times and incubated 1 h at 37°C with secondary antibodies. After washing three times in PBS, cells were counterstained with DAPI and coverslips mounted on slides. Cells were considered positive for γH2AX if contained more than five nuclear foci. In each case, at least 800 cells were accounted per condition.
53BP1 degradation assay
Nuclear protein extracts were obtained from WT MEFs by using the Nuclear Extract Kit (Active Motif) and following the manufacturer's instructions. A total of 20 × 106 cells were used per experiment. Aliquots containing 60 μg of protein were incubated with 60 ng of human recombinant CTSL (EMD Biosciencies) for 0, 30, or 60 min in CTSL Buffer (400 mM sodium phosphate buffer pH 6, 75 mM NaCl, 4 mM EDTA, 0.25% Triton X-100) at 37°C. As a negative control for protein degradation, recombinant CTSL was heat inactivated 15 min at 95°C and then incubated with the nuclear protein extract for 60 min at 37°C. A nuclear extract from 53BP1-depleted cells was used as a negative control. Samples were loaded onto SDS–PAGE gels and blotted for 53BP1 and lamins (loading control).
Processing of dysfunctional telomeres by NHEJ
A dominant-negative TRF2 mutant (TRF2ΔBΔM) along with packaging and envelope plasmids pUMVC and pCMV-VSV-G were transfected in 293T packaging cells using Fugene. At 48 h post-transfection, virus-containing media was used to infect WT MEFs containing either shLmna or shControl. A second round of infection was repeated 24 h later. At 48 h after the second infection, cells were treated with 10−7 M vitamin D or vehicle for 24 h. We prepared metaphase stage chromosomal spreads for FISH and hybridized them with a telomeric probe (Gonzalez-Suarez et al, 2009). Fluorescent images were taken using a Nikon 90i upright microscope. At least 20 metaphases were analysed in all cases.
Statistical analysis
A ‘two-tailed’ Student's t-test was used to calculate the statistical significance of the observed differences. Microsoft Excel v.2007 was used for the calculations. In all cases, differences were considered statistically significant when P<0.05. Unless otherwise indicated, values represent mean±s.e.m.
Supplementary Material
Acknowledgments
We thank B Sleckman and D Hodzic at Washington University for the gifts of sh53BP1 and shLmna, respectively. We acknowledge Rachel Waller for her assistance in this project. Work performed in the laboratory of SG was supported by NIH Grant RO1 GM094513-01 and Research Development Award from Siteman Cancer Center, St Louis, MO. IGS is a recipient of a postdoctoral fellowship from the American Heart Association.
Author contributions: IGS performed most of the experiments and contributed to the writing of the manuscript. ABR performed the experiments on NHEJ of dysfunctional telomeres. DEG performed experiments not included in the manuscript. MN provided technical assistance. EC provided essential reagents such as CTSL inhibitor, and lentiviral and retroviral vectors for overexpression and depletion of CTSL. CLS generated the Lmna−/− mouse model and provided the MEFs used in this study. AD provided reagents and seminal advice and helpful discussions during the progression of the project, especially related to vitamin D treatment and actions. SG supervised the research and the preparation of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agrelo R, Setien F, Espada J, Artiga MJ, Rodriguez M, Perez-Rosado A, Sanchez-Aguilera A, Fraga MF, Piris MA, Esteller M (2005) Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J Clin Oncol 23: 3940–3947 [DOI] [PubMed] [Google Scholar]
- Alvarez-Diaz S, Valle N, Garcia JM, Pena C, Freije JM, Quesada V, Astudillo A, Bonilla F, Lopez-Otin C, Munoz A (2009) Cystatin D is a candidate tumor suppressor gene induced by vitamin D in human colon cancer cells. J Clin Invest 119: 2343–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G (2006) Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman P, Aly A, Escandell JM, Pieterse M, Bartkova J, van der Gulden H, Hiddingh S, Thanasoula M, Kulkarni A, Yang Q, Haffty BG, Tommiska J, Blomqvist C, Drapkin R, Adams DJ, Nevanlinna H, Bartek J, Tarsounas M, Ganesan S, Jonkers J (2010) 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat Struct Mol Biol 17: 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broers JL, Raymond Y, Rot MK, Kuijpers H, Wagenaar SS, Ramaekers FC (1993) Nuclear A-type lamins are differentially expressed in human lung cancer subtypes. Am J Pathol 143: 211–220 [PMC free article] [PubMed] [Google Scholar]
- Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A, Bothmer A, Feldhahn N, Fernandez-Capetillo O, Cao L, Xu X, Deng CX, Finkel T, Nussenzweig M, Stark JM, Nussenzweig A (2010) 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna F (2008) Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8: 512–522 [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22: 832–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difilippantonio S, Gapud E, Wong N, Huang CY, Mahowald G, Chen HT, Kruhlak MJ, Callen E, Livak F, Nussenzweig MC, Sleckman BP, Nussenzweig A (2008) 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature 456: 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, Chen YC, Spector DL, de Lange T (2008) 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature 456: 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTullio RA Jr, Mochan TA, Venere M, Bartkova J, Sehested M, Bartek J, Halazonetis TD (2002) 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4: 998–1002 [DOI] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD (2008) Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell 135: 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289: F8–F28 [DOI] [PubMed] [Google Scholar]
- FitzGerald JE, Grenon M, Lowndes NF (2009) 53BP1: function and mechanisms of focal recruitment. Biochem Soc Trans 37: 897–904 [DOI] [PubMed] [Google Scholar]
- Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP (2009) Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462: 935–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocheva V, Joyce JA (2007) Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle 6: 60–64 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, Morgado-Palacin L, Gapud EJ, Sleckman BP, Sullivan T, Sage J, Stewart CL, Mai S, Gonzalo S (2009) Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J 28: 2414–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, Bogyo M, Nepveu A (2004) A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell 14: 207–219 [DOI] [PubMed] [Google Scholar]
- Hartlerode AJ, Scully R (2009) Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J 423: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266–281 [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461: 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedeszko C, Sloane BF (2004) Cysteine cathepsins in human cancer. Biol Chem 385: 1017–1027 [DOI] [PubMed] [Google Scholar]
- Kass EM, Moynahan ME, Jasin M (2010) Loss of 53BP1 is a gain for BRCA1 mutant cells. Cancer Cell 17: 423–425 [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432: 316–323 [DOI] [PubMed] [Google Scholar]
- Katunuma N (1989) Mechanisms and regulation of lysosomal proteolysis. Revis Biol Cell 20: 35–61 [PubMed] [Google Scholar]
- Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27: 247–254 [DOI] [PubMed] [Google Scholar]
- Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D (2007) Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318: 1637–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma JM, Voorend DM, Barwari T, Koetsveld J, Van der Spek AH, De Porto AP, Van Rooijen G, Van Noorden CJ (2010) Cathepsin L, target in cancer treatment? Life Sci 86: 225–233 [DOI] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, Pei D, Pendas AM, Cadinanos J, Lopez-Otin C, Tse HF, Hutchison C, Chen J, Cao Y, Cheah KS, Tryggvason K et al. (2005) Genomic instability in laminopathy-based premature aging. Nat Med 11: 780–785 [DOI] [PubMed] [Google Scholar]
- Mailand N, Bekker-Jensen S, Faustrup H, Melander F, Bartek J, Lukas C, Lukas J (2007) RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131: 887–900 [DOI] [PubMed] [Google Scholar]
- Manis JP, Morales JC, Xia Z, Kutok JL, Alt FW, Carpenter PB (2004) 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat Immunol 5: 481–487 [DOI] [PubMed] [Google Scholar]
- Olive PL, Banath JP, Durand RE (1990) Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the ‘comet’ assay. Radiat Res 122: 86–94 [PubMed] [Google Scholar]
- Pierce AJ, Stark JM, Araujo FD, Moynahan ME, Berwick M, Jasin M (2001) Double-strand breaks and tumorigenesis. Trends Cell Biol 11: S52–S59 [DOI] [PubMed] [Google Scholar]
- Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar D, Barkan R, Meshorer E, Gruenbaum Y (2009) Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med 13: 1059–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M, Margalit A, Gruenbaum Y (2006) The nuclear lamina and its proposed roles in tumorigenesis: projection on the hematologic malignancies and future targeted therapy. J Struct Biol 155: 351–360 [DOI] [PubMed] [Google Scholar]
- Rai R, Zheng H, He H, Luo Y, Multani A, Carpenter PB, Chang S (2010) The function of classical and alternative non-homologous end-joining pathways in the fusion of dysfunctional telomeres. EMBO J 29: 2598–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwood AB, Perkins SM, Vanderwaal RP, Feng Z, Biehl KJ, Gonzalez-Suarez I, Morgado-Palacin L, Shi W, Sage J, Roti-Roti JL, Stewart CL, Zhang J, Gonzalo S (2011) A dual role for A-type lamins in DNA double-strand breaks repair. Cell Cycle 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz LB, Chehab NH, Malikzay A, Halazonetis TD (2000) p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol 151: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S (2005) Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol 11: 1251–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ (2003a) MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature 421: 961–966 [DOI] [PubMed] [Google Scholar]
- Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD (2003b) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9: 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B (1999) Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147: 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D (2003) Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat 80: 49–62 [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, Lopez-Otin C (2005) Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 437: 564–568 [DOI] [PubMed] [Google Scholar]
- Wang B, Matsuoka S, Carpenter PB, Elledge SJ (2002) 53BP1, a mediator of the DNA damage checkpoint. Science 298: 1435–1438 [DOI] [PubMed] [Google Scholar]
- Ward IM, Minn K, van Deursen J, Chen J (2003) p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol 23: 2556–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IM, Reina-San-Martin B, Olaru A, Minn K, Tamada K, Lau JS, Cascalho M, Chen L, Nussenzweig A, Livak F, Nussenzweig MC, Chen J (2004) 53BP1 is required for class switch recombination. J Cell Biol 165: 459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastrow MS, Vlcek S, Wilson KL (2004) Proteins that bind A-type lamins: integrating isolated clues. J Cell Sci 117: 979–987 [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.