Abstract
The spindle assembly checkpoint (SAC) restrains anaphase until all chromosomes become bi-oriented on the mitotic spindle. The SAC protein Mad2 can fold into two distinct conformers, open (O) and closed (C), and can asymmetrically dimerize. Here, we describe a monoclonal antibody that specifically recognizes the dimerization interface of C-Mad2. This antibody revealed several conformation-specific features of Mad2 in human cells. Notably, we show that Mad2 requires association with Mad1 to adopt the closed conformation and that the activity of the Mad1:C-Mad2 complex undergoes regulation by p31comet-dependent ‘capping’. Furthermore, C-Mad2 antibody microinjection caused an abrupt termination of the SAC and accelerated mitotic progression. Remarkably, microinjection of a Mad1-neutralizing antibody triggered a comparable mitotic acceleration. Our study provides direct in vivo evidence for the model that a kinetochore complex of Mad1:C-Mad2 acts as a template to sustain the SAC and it challenges the distinction between SAC and mitotic timer.
Keywords: closed-Mad2, Mad2 template model, mitotic timing, p31comet, spindle assembly checkpoint
Introduction
Eukaryotic cells have evolved a surveillance mechanism, called spindle assembly checkpoint (SAC), that ensures faithful segregation of the replicated genome during mitosis. This pathway is devoted to restraining anaphase onset until chromosome bi-orientation has occurred. The molecular components of the SAC are conserved among eukaryotes and include Mad1, Mad2, Bub1, Bub3, BubR1/Mad3 and Mps1 (see Musacchio and Salmon, 2007 for review). The target of the SAC is Cdc20, a cofactor of the anaphase promoting complex/cyclosome (APC/C), that functions as a ubiquitin ligase for degradation of Cyclin B1 and Securin (Peters, 2006). During mitosis, both checkpoint proteins and Cdc20 associate with kinetochores (KTs), where Mad2, BubR1 and Bub3 display high turnover (Howell et al, 2004). The same checkpoint proteins can also be found to physically associate with Cdc20 in the cytosol (Sudakin et al, 2001). This and other evidence (Rieder et al, 1995) led to the model that unattached KTs act as catalytic platforms to generate the SAC signal (Musacchio and Salmon, 2007).
The mechanistic aspects of the KT-dependent catalytic generation of an anaphase inhibitor remain to be understood. Biochemical approaches indicate that both Mad2 and BubR1 directly interact with Cdc20 and can inhibit APC/CCdc20 activity in vitro (Fang et al, 1998; Tang et al, 2001; Fang, 2002). Furthermore, Mad2 and BubR1 (together with Bub3) can coexist in the same complex, called the mitotic checkpoint complex (MCC) (Sudakin et al, 2001), but distinct Cdc20 complexes comprising either Mad2 or BubR1:Bub3 have also been described (Tang et al, 2001; Fang, 2002). Recent evidence suggests that, during checkpoint action, KT-activated Mad2 entraps Cdc20 first, thereby priming it for subsequent binding of BubR1:Bub3. The latter proteins would then sequester Cdc20 through a complex in which Mad2 is substoichiometric (Nilsson et al, 2008; Kulukian et al, 2009).
While the exact nature of the anaphase inhibitor remains a matter of debate, our understanding of the role of Mad2 in SAC signalling has benefited greatly from structural analysis (Luo et al, 2002, 2004; Sironi et al, 2002; Mapelli et al, 2007). The Mad2 protein can in fact adopt two distinct natively folded states: ‘open’ (or ‘N1’) and ‘closed’ (or ‘N2’) (O- and C-Mad2, respectively). The two conformers differ in the orientation of a C-terminal β-sheet that in the closed conformation surrounds the polypeptide chain of a Mad2 interaction partner (or ‘ligand’) in a structure reminiscent of a safety belt (Sironi et al, 2002). Best-known ligands of C-Mad2 are Mad1 and Cdc20. Central to a prevailing model of SAC signalling, the so-called ‘template model’, is the ability of Mad2 to dimerize asymmetrically, that is O-Mad2 can bind C-Mad2 (De Antoni et al, 2005; Mapelli et al, 2006, 2007). Moreover, reconstitution experiments have shown that a complex of Mad1:C-Mad2 can, through Mad2 asymmetric dimerization, enhance the ability of O-Mad2 to bind Cdc20, thereby generating a structurally equivalent C-Mad2:Cdc20 complex (De Antoni et al, 2005; Nasmyth, 2005; Vink et al, 2006; Kulukian et al, 2009; Lad et al, 2009). Some aspects of this template model find experimental support also in the cellular environment: Mad1 clearly is the KT receptor of Mad2 (Chen et al, 1998; Luo et al, 2002; Martin-Lluesma et al, 2002) and interfering with Mad1 affects the ability of Mad2 to bind Cdc20 (Hwang et al, 1998; Hardwick et al, 2000; Fraschini et al, 2001). Furthermore, FRAP experiments performed in mammalian cells revealed a biphasic recovery after photobleaching of KT-associated Mad2, indicating the existence of two distinct Mad2 populations (Shah et al, 2004). While one population showed a slow turnover reminiscent of the recovery kinetics of Mad1 (Shah et al, 2004), the other Mad2 population turned over much faster, similar to the behaviour of Cdc20 at KTs (Howell et al, 2004).
Chromosome bi-orientation leads to SAC silencing and this in turn allows anaphase onset. While several pathways involved in SAC silencing have been described, two are thought to be crucial in mammalian cells. The first is based on the dynein-dependent ‘stripping’ from KTs of SAC components and other proteins, notably Spindly, upon microtubule (MT) attachment (Howell et al, 2001; Gassmann et al, 2010). The second is based on a binding partner of Mad2, known as p31comet, which associates selectively with the dimerization interface of C-Mad2 in vitro (Xia et al, 2004; Mapelli et al, 2007; Yang et al, 2007). Depletion of p31comet from cells interferes with efficient recovery from a SAC-dependent arrest, whereas overexpression of p31comet causes a SAC override (Habu et al, 2002; Xia et al, 2004; Yang et al, 2007). Although these data strongly implicate p31comet in SAC silencing, a mechanistic understanding of its action remains elusive. In particular, it is unknown how the p31comet:Mad2 interaction is regulated in time and space in vivo.
At present, the template model is supported by several lines of evidence, but direct experimental proof for its operation within cells is lacking. Furthermore, some observations, particularly the purported Mad1-independent function of Mad2 in regulating mitotic timing (Meraldi et al, 2004), do not readily find a mechanistic explanation in the template model. Here, we characterize a monoclonal antibody (mAb) raised against human Mad2 and demonstrate that it specifically recognizes the dimerization interface of the closed conformer of Mad2. By using this mAb we show, first, that Mad2 requires the association with Mad1 to adopt the closed conformation and, second, that the Mad1:C-Mad2 complex undergoes spatio-temporal regulation by p31comet-dependent ‘capping’ of C-Mad2's dimerization interface. Finally, we demonstrate that neutralization of the Mad1:C-Mad2 complex by microinjection of antibodies directed against either C-Mad2 or Mad1 abolishes the SAC and causes mitotic acceleration, thus challenging the notion that cytoplasmic Mad2 controls mitotic timing independently of Mad1. Collectively, our study provides direct in vivo evidence to support the model that the Mad1:C-Mad2 complex acts as a template at KTs to trigger SAC signalling in human cells.
Results
A mAb specific for C-Mad2
The mAbs represent powerful tools to visualize and characterize physiologically and/or pathophysiologically relevant conformers of particular proteins (see for example, Gannon et al, 1990; Korth et al, 1997). The mAb 107–276 readily recognizes human Mad2 by immunoprecipitation (IP) and immunofluorescence, but it does not detect the denatured protein in western blots (WBs) (Chan et al, 2009). In addition, an attempt to identify the epitope of this mAb using a 12-mer peptide array encompassing the entire Mad2 sequence failed to reveal reactivity, suggesting that this mAb recognizes a conformational rather than a linear epitope (Supplementary Figure S1A–C). To determine whether the mAb 107–276 displays a preference for a specific Mad2 conformer, we purified His-tagged Mad2 from Escherichia coli in either its wild-type form (Mad2WT) or as mutants (Mad2V193N and Mad2L13Q, respectively) that are known to mimic the two distinct conformers, O-Mad2 and C-Mad2 (Mapelli et al, 2007; see also Supplementary Figure S1G and H for a summary of the mutants’ characteristics). In IP, the mAb displayed increased reactivity towards C-Mad2 when compared with Mad2WT or O-Mad2 (Figure 1A). These results demonstrate that the mAb 107–276 is specific for the closed conformer of Mad2; therefore, it will hereafter be referred to as ‘CM2276’ (C-Mad2, clone 276). Note that a Mad2 polyclonal antibody that did not display preference for any Mad2 conformer in denaturing conditions (Figure 1A, lower panel) was used for all WB experiments throughout this work.
Figure 1.
Biochemical characterization of the C-Mad2 mAb (CM2276). (A) Purified Mad2WT (WT), Mad2L13Q (closed) and O-Mad2V193N (open) were immunoprecipitated (IP) with CM2276. Inputs and IPs were analysed by Coomassie Brilliant Blue staining (CBB) and WB using a rabbit polyclonal Mad2 antibody. IgG HC and LC indicate heavy and light IgG chains, respectively. (B) IPs were performed with unspecific IgGs or CM2276 from HeLaS3 lysate obtained from cells arrested with nocodazole. (C) IPs were performed from HeLaS3 cells released from nocodazole for 2.5 h in the presence of MG132 using rabbit unspecific IgGs and a rabbit Mad2 antibody (IP (rabbit)) or with mouse unspecific IgGs and CM2276 (IP (mouse)). Input and IPs in (B, C) were analysed by WB using the indicated antibodies. (D) Purified CM2276, p31comet or a mixture of constant amount of Mad2 mAb with increasing concentrations of p31comet were incubated with Mad2:GST–Cdc20111−138 complex preadsorbed on GSH beads. (E) Purified CM2276, Mad2ΔC or a mixture of Mad2ΔC with increasing concentrations of CM2276 were treated as described in (D). Asterisks in (D, E) indicate a contaminant of the GST–Cdc20111−138 preparation with electrophoretic mobility similar to the IgG light chain. Inputs and GST pull downs in (D, E) were analysed by Coomassie Brilliant Blue staining. The arrow and the circle in (D, E) mark the species used at constant or increasing concentrations, respectively. (F) Purified Mad2ΔC was added to Mad2:GST–Cdc20111−138 complex adsorbed on GSH beads, following preincubation with buffer (−) or CM2276 (+). GST pull downs were analysed by Coomassie Brilliant Blue staining. The arrow marks Mad2ΔC. (G) Purified Mad2WT (WT) and Mad2RQ (R133E–Q134A) were immunoprecipitated with CM2276. Inputs and IPs were analysed by Coomassie Brilliant Blue staining. (H) Schematic representation of the CM2276 epitope.
CM2276 recognizes C-Mad2 on its dimerization interface
A prediction from the above results is that CM2276 should be able to coprecipitate the known C-Mad2 interaction partners. Therefore, we asked whether the CM2276 can coprecipitate Mad1, Cdc20 and/or p31comet from a human cell lysate. Both Mad1 and Cdc20 were enriched in the immune complexes obtained after immunoprecipitating C-Mad2 from SAC-arrested HeLaS3 cells, whereas p31comet was not (Figure 1B). We also used CM2276 to immunoprecipitate C-Mad2 from a lysate of cells synchronized in metaphase by the proteasome inhibitor MG132. Again, p31comet was not detected in CM2276 immune complexes, although it was present in immunoprecipitates that were prepared using a polyclonal Mad2 antibody (Figure 1C; see also Supplementary Figure S1D for a more extensive panel of WBs). These results demonstrate that CM2276 is unable to coimmunoprecipitate p31comet with Mad2, even though a Mad2:p31comet complex is present in metaphase-arrested cells.
p31comet is known to interact with C-Mad2 through direct contact with the dimerization interface of Mad2 (Mapelli et al, 2006; Yang et al, 2007). Thus, we asked whether CM2276 competes with p31comet for C-Mad2 binding. Specifically, we performed GST pull-down assays, using GST–Cdc20111−138 that had been preadsorbed on glutathione-sepharose beads (GSH) and bound to His-tagged WT-Mad2 (Mad2WT), thereby generating a C-Mad2:GST–Cdc20111−138 complex (Mapelli et al, 2006). As expected, this complex readily pulled down both CM2276 and His-tagged p31comet, provided that the two proteins were added separately (Figure 1D, lanes 1 and 2). In striking contrast, coincubation of CM2276 with increasing concentrations of p31comet decreased the amount of CM2276 that was bound by C-Mad2 (Figure 1D, lanes 3–5), clearly demonstrating competition between p31comet and CM2276 for binding to C-Mad2. Attesting to the specificity of this competition, increasing concentrations of the unrelated protein His–hSpindly1−444 (Chan et al, 2009) did not affect the association of CM2276 to C-Mad2 in this assay (Supplementary Figure S1E). One prediction of the observed competition between p31comet and CM2276 is that the antibody should interfere with Mad2 conformational dimerization (Mapelli et al, 2006, 2007; Yang et al, 2007). Indeed, coincubation of CM2276 with Mad2ΔC, known to adopt the open conformation (Supplementary Figure S1G), also resulted in a competitive behaviour in that CM2276 abolished the ability of Mad2ΔC to bind to C-Mad2:GST–Cdc20111−138 (Figure 1E, lanes 3–5). In contrast, equal concentrations of a Mad1 mAb did not interfere with the binding between Mad2ΔC and C-Mad2, demonstrating specificity of this competition (Supplementary Figure S1F). Binding of Mad2ΔC was also prevented upon prior adsorption of CM2276 to C-Mad2:GST–Cdc20111−138 beads (Figure 1F). To further demonstrate that CM2276 binds to the C-Mad2 dimerization interface, we also tested its ability to recognize a Mad2 protein that carries the double mutation R133E–Q134A (Mad2RQ), which is known to affect residues crucial for Mad2 dimerization and p31comet binding (De Antoni et al, 2005; Yang et al, 2007) (Supplementary Figure S1G). As shown in Figure 1G, Mad2RQ was barely able to bind to CM2276 when compared with Mad2WT. The most likely interpretation of these data is that CM2276 recognizes a conformational epitope on the dimerization interface of C-Mad2 and that this epitope is critical for both conformational dimerization and p31comet binding (Figure 1H).
p31comet masks the presence of C-Mad2 at nuclear pore complexes and spindle poles
Mad1 is known to form a constitutive protein complex with Mad2 throughout the cell cycle (Chen et al, 1998; Campbell et al, 2001; De Antoni et al, 2005). The two proteins also display identical localizations. They decorate the nucleoplasmic side of the nuclear pore complex (NPC) during interphase (Campbell et al, 2001) and then relocalize to KTs upon mitotic entry. Upon KT–MT attachment, they are ‘stripped’ from KTs in a dynein and MT-dependent manner and transiently accumulate at spindle poles (Li and Benezra, 1996; Gorbsky et al, 1998; Howell et al, 2001). These exact localization patterns were seen when endogenous Mad1 was stained with a mAb in HeLaS3 cells (Figure 2A; see Supplementary Figure S2 for characterization of Mad1 mAbs). However, contrary to expectation, costaining of the same cells with CM2276 revealed only partial colocalization with Mad1. Although CM2276 staining revealed clear KT localization from prophase to prometaphase, C-Mad2 could barely be detected by this antibody at NPCs and spindle poles (Figure 2A; see Supplementary Figure S3 for results using methanol as alternative cell fixation method). One possible explanation for this result is that Mad2 adopts the open conformation at NPCs and spindle poles. Alternatively, one or more proteins might mask the dimerization interface of C-Mad2 at NPCs and spindle poles. As p31comet localizes to both NPCs (Tighe et al, 2008; A Musacchio, personal communication) and the mitotic spindle (Habu et al, 2002), and we have previously demonstrated that p31comet can mask the CM2276 epitope (Figure 1D), we asked whether depletion of p31comet could restore the ability of CM2276 to detect Mad2 at NPCs and spindle poles. Indeed, depletion of p31comet by two independent siRNA oligonucleotides readily allowed the detection of C-Mad2 by CM2276 at both NPCs and spindle poles (Figure 2C and D, respectively; for efficiency of p31comet depletion see Figure 2B). To substantiate this conclusion, we also used a HeLa cell line expressing LAPGFP-mouse-Mad2 (Poser et al, 2008; Hubner et al, 2010), which allowed us to study the localization of Mad2 through the monitoring of GFP fluorescence. As predicted, GFP fluorescence at the nuclear envelope and at spindle pole staining was indistinguishable, regardless of the presence or absence of p31comet (Supplementary Figure S4A–C).
Figure 2.
Subcellular localization of C-Mad2. (A) HeLaS3 cells arrested with thymidine for 24 h were released for 10 h and fixed with PTEMF. After fixation, C-Mad2 was stained with CM2276, followed by secondary antibody incubation (red). After extensive washing, Mad1 was costained with a Mad1 mAb directly coupled to Alexa Fluor 488 (green), DNA was visualized with DAPI (blue). White arrows indicate spindle pole staining. (B) Cells were transfected with the indicated siRNA oligonucleotides for a total of 48 h, and synchronized as described in (A). Lysates were obtained and analysed by WB with the indicated antibodies. (C, D) Cells transfected with the indicated siRNA oligonucleotides as in (B) were treated as described in (A). Scale bars=10 μm. (E–G) Box-and-whisker plots showing the average pixel intensities at single KTs expressed as the Mad1/CREST fluorescence ratio in (E), the CM2276/Mad1 ratio in (F) and the LAPGFP-mouse-Mad2 (LAP-mMad2)/Mad1 ratio in (G) either in Gl2 or in p31comet-1 siRNA-treated cells. a.u.=arbitrary units, n⩾80 KTs from four independent cells for (E, F) and n⩾180 KTs from eight independent cells for (G). Lower and upper whiskers represent 10th and 90th percentiles, respectively.
Most interestingly, we also observed that p31comet depletion caused a detectable increase in CM2276 signal at KTs, without affecting the level of resident Mad1:C-Mad2 complex (Figure 2D and E). Quantification (Figure 2F) indicates that p31comet masks ∼50% of C-Mad2 at KTs. Furthermore, a significant increase in GFP signal at KTs could be observed in the cell line expressing LAPGFP-mouse-Mad2 (Figure 2G), suggesting that additional Mad2, almost certainly in the open conformation, had been recruited upon depletion of p31comet. Taken together, these data indicate that Mad2 can adopt the closed conformation at multiple subcellular sites, but that the dimerization interface of Mad2 undergoes masking by p31comet at NPCs, spindle poles and, partially, also at KTs. The data also indicate that unmasking of C-Mad2 leads to recruitment of O-Mad2 selectively at KTs, implying that asymmetric dimer formation is differentially regulated at KTs, NPCs and spindle poles.
p31comet localizes to KTs in a C-Mad2-dependent manner
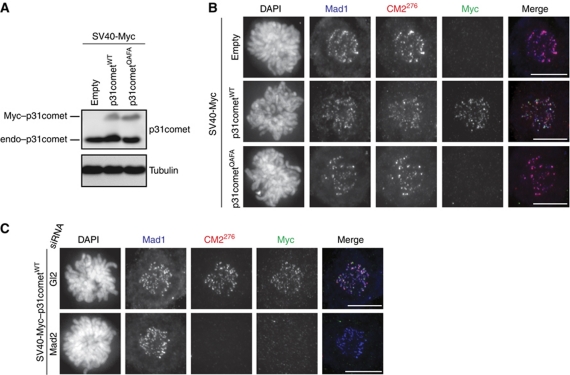
The observation that a sizeable population of KT-associated C-Mad2 could be unmasked by depletion of p31comet predicts that p31comet associates with KTs already during prometaphase, when the SAC is active, and that this KT association should depend on C-Mad2. To explore this point, we expressed Myc–p31comet in its wild-type form or as a version carrying two point mutations (Q83A–F191A, abbreviated as p31cometQAFA) that are known to abolish binding to C-Mad2 (Yang et al, 2007). Importantly, the use of a SV40 promoter allowed us to express p31comet at subendogenous levels (Figure 3A). Following cytosolic preextraction, Myc–p31cometWT, but not Myc–p31cometQAFA, was readily visualized at C-Mad2-positive KTs (Figure 3B). In addition, depletion of endogenous Mad2 by siRNA completely prevented Myc–p31cometWT to localize to KTs (Figure 3C). Taken together, these data show that p31comet can localize at KTs during an active SAC and that the Mad1:C-Mad2 complex acts as its main KT receptor.
Figure 3.
p31comet localizes at KTs in a Mad2-dependent manner. (A) HeLaS3 cells were transfected with the indicated SV40-Myc expression vectors for a total of 36 h. At 12 h after transfection, cells were arrested with thymidine for 14 h and subsequently released for 10 h. Lysates were obtained and analysed by WB with the indicated antibodies. Bands corresponding to endogenous and ectopically expressed p31comet are indicated (‘endo–p31comet’ and ‘Myc–p31comet’, respectively). (B) Cells transfected with the indicated expression vectors were treated as described in (A). CM2276 and rabbit-Myc antibodies were detected by secondary antibody staining (red and green, respectively), whereas the Mad1 mAb was directly coupled to Alexa Fluor 647 (displayed in blue). (C) Cells transfected with either Gl2 or Mad2 siRNA for a total of 48 h were subsequently transfected with the SV40-Myc–p31cometWT construct, synchronized and treated as described in (B). Scale bars=10 μm.
Only liganded Mad2 adopts the closed conformation throughout the cell cycle
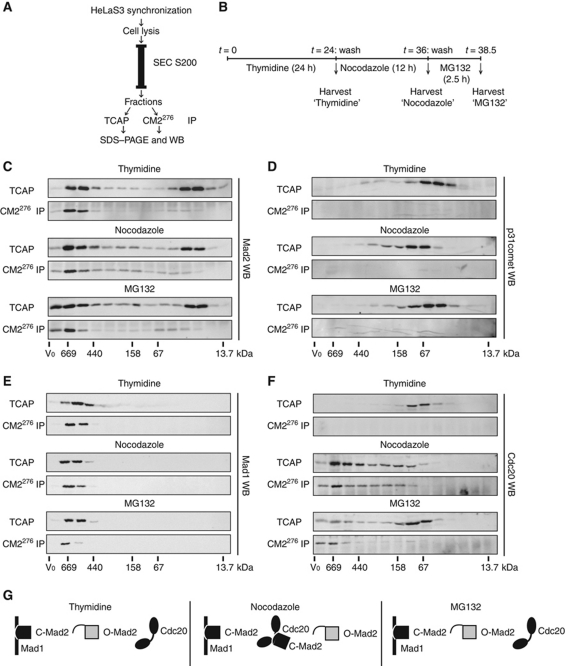
The template model predicts not only that Mad2 adopts two distinct conformations in the cell, open and closed, but also that O-Mad2 should freely diffuse in the cytoplasm and, by definition, be devoid of ligand, whereas C-Mad2 should tightly bind Mad1 and therefore be a resident component of unattached KTs. Furthermore, during SAC signalling, the Mad1:C-Mad2 complex is predicted to exert a catalytic function by triggering the conversion (through asymmetric dimerization) of O-Mad2 to C-Mad2 (with Cdc20 as ligand). Although this model is appealing and strongly supported by structural data (Mapelli and Musacchio, 2007; Luo and Yu, 2008), the lack of tools to discriminate between O-Mad2 and C-Mad2 has so far precluded the analysis of Mad2 conformation in a cellular context. To monitor the two-state behaviour of Mad2 in cells, we fractionated cell lysates by size exclusion chromatography (SEC) and analysed each fraction for the presence of C-Mad2, using CM2276 for IP (Figure 4A). In order to analyse transitions between interphase and mitosis as well as cells with both an active and an inactive SAC, we synchronized HeLaS3 cells in G1/S phase, prometaphase and metaphase (by addition of thymidine, nocodazole and MG132, respectively; Figure 4B). The use of a Superdex 200 column resulted in a Mad2 elution profile that showed two peaks of comparable intensity; these reflect Mad2 associated with its interaction partners (>440 kDa) and monomeric Mad2 (ca. 25 kDa), respectively (Figure 4C, ‘TCAP’ panels; see also Materials and methods). CM2276 IP on all fractions invariably revealed the ability of this mAb to recognize Mad2 bound to its interaction partners, but not free Mad2 (Figure 4C, ‘CM2276 IP’ panels). These data confirm that Mad2 adopts the closed conformation when in complex with other proteins. Moreover, they indicate that free Mad2 adopts the open conformation at all cell cycle stages examined.
Figure 4.
Distribution of C-Mad2 among ‘free’ and ‘bound’ pools of the protein. (A) Schematic representation of the approach followed to study the distribution of C-Mad2. Synchronized HeLaS3 cells were lysed and fractionated by SEC. SEC was performed with a Superdex 200 16/60 column, followed by collection of 4 ml fractions through the 120-ml of elution volume. Fourteen fractions covering size ranges from the upper limit of the column to 13.7 kDa were further processed in parallel by TCA precipitation (TCAP) and CM2276 IP. Resulting samples were analysed by WB. (B) Timeline of the synchronization used for experiments shown in (C–E); ‘MG132’ cells were first arrested in thymidine for 24 h and were incubated in the presence of nocodazole for 12 h after release, collected by mitotic shake off and released for 2.5 h in the presence of MG132. ‘thymidine’ and ‘nocodazole’ cells were harvested following thymidine and nocodazole block, respectively. (C–F) WBs on samples obtained from TCA precipitations (TCAP) and CM2276 IP on fractions obtained as described in (A), following synchronization as described in (B). WBs were performed with rabbit Mad2 polyclonal antibody (C), p31comet antibody (D), Mad1 mAb (E) and Cdc20 antibody (F). (G) Schematic summary of the results displayed in (C–F).
Theoretically, one could argue that the free Mad2 could actually be in the closed conformation but not be recognized by CM2276, due to epitope masking by p31comet. However, this is clearly not the case as p31comet did not extensively comigrate with free Mad2 (Figure 4D). We also note that the elution profile of p31comet did not show major changes during the different cell cycle stages analysed (Figure 4D). At first glance, this may appear surprising in view of our cell biological data (shown above), which indicate that p31comet undergoes cell-cycle-regulated interactions with Mad1:C-Mad2 complexes. Although the absence of p31comet in the CM2276 IPs is readily explained by antibody competition for the p31comet-binding site (see Figure 1D and H), it is more difficult to explain the absence of p31comet in the high molecular weight fractions of total cell lysates. We interpret this to indicate that only a very minor fraction of the total cellular p31comet interacts with Mad1:C-Mad2 complexes at any given stage. In support of this conclusion, semi-quantitation of cellular protein levels indicates that p31comet is approximately twice as abundant as total Mad2 (Supplementary Figure S5B).
WBs for the other known Mad2 ligands revealed that Mad1 comigrated with C-Mad2 and could be coimmunoprecipitated by CM2276 throughout the cell cycle (Figure 4E). In stark contrast, Cdc20 migrated as a monomeric species in interphase and did not display any interaction with C-Mad2 (Figure 4F, upper panels), arguing against the existence of a sizeable pool of MCC during interphase. During SAC activation, Cdc20 shifted to the higher molecular weight fractions, where it clearly interacted with C-Mad2 (Figure 4F, middle panels). Upon SAC silencing, however, the bulk of Cdc20 was again released as a monomeric entity and only a minor population, still engaged in protein complexes, could be coimmunoprecipitated by CM2276 (Figure 4F, lower panels). Interestingly, the association of C-Mad2 with Cdc20 during prometaphase was not reflected in a major change in the overall elution profile of total Mad2 (Figure 4C), consistent with the emerging notion that the inhibitory action of Mad2 on Cdc20 is likely to be catalytic rather than stoichiometric (Nilsson et al, 2008; Kulukian et al, 2009). A similar experiment performed with a Superose 6 column also revealed the presence of C-Mad2 in both the MCC and the APC/C+MCC complexes (Supplementary Figure S5C–E). Collectively, our results show that liganded Mad2 adopts the closed conformation in human cells, whereas free Mad2 remains in the open conformation during the cell cycle (Figure 4G; see also Luo et al, 2004).
Mad1 is required for the formation of C-Mad2 in interphase
Our data show that, within cells, Mad1 is a constitutive ligand of C-Mad2, whereas Cdc20 is entrapped into Mad2's safety belt only during checkpoint activation (Figure 4). As C-Mad2 is the energetically favoured conformer in vitro (Luo et al, 2004), we asked whether Mad2 can possibly adopt the closed conformation in vivo, in the absence of any known ligand. To this end, we used siRNA to deplete Mad1 from interphase-arrested cells, in which Mad1 is the only known ligand of C-Mad2, before samples were analysed using the assay described above. When compared with the Gl2 control, Mad1 siRNA caused a significant (albeit not complete) reduction in Mad1 protein levels (Figure 5A, B and E). Under these conditions, a marked shift of Mad2 from the liganded population to the free population was observed (Figure 5A, B and E), confirming that Mad1 is the major binding partner of Mad2 in interphase cells. Importantly, while CM2276 was able to immunoprecipitate the Mad1:C-Mad2: complex, but not free Mad2, from Gl2 control cells (Figure 5C–E), the amount of C-Mad2 that could be precipitated by CM2276 from Mad1-depleted cells was decreased in proportion to the reduction in Mad1 levels, without a corresponding increase of free Mad2 in the CM2276 IP (Figure 5D and E). These data clearly demonstrate that the association with Mad1 is required for interphase Mad2 to adopt the closed conformation; furthermore, they suggest that, in vivo, C-Mad2 does not accumulate to significant levels in the absence of ligand (Figure 5F and G).
Figure 5.
Requirement of Mad1 for C-Mad2 formation in interphase. (A) SEC analysis of a lysate obtained from HeLaS3 cells treated with Gl2 siRNA for 60 h and arrested with thymidine for 24 h. Samples were concentrated by TCA precipitation (TCAP). WBs were performed with the indicated antibodies. (B) Samples obtained as in (A) but following Mad1 siRNA-mediated depletion. (C, D) Samples obtained by CM2276 IP against the same fractions used in (A, B), respectively. WBs were performed with indicated antibodies. (E) Fractions highlighted as large and small in (A–D) were pooled and WBs were performed with the indicated antibodies. (F) Schematic representation of the Mad2 subcomplexes found in interphase: Mad2 is either bound to Mad1, adopting the closed conformation or free, adopting the open conformation. (G) In the absence of Mad1, Mad2 cannot adopt the closed conformation during interphase, and the bulk is thereby released as open free Mad2.
Conformational dimerization of Mad2 is essential to initiate and maintain a SAC-dependent arrest
The key prediction made by the template model is that C-Mad2 is required for the catalytic activation of a cytosolic pool of O-Mad2 and that this conversion is in turn indispensable for SAC signalling. The ability of CM2276 to interfere with Mad2 conformational dimerization afforded a unique opportunity to test this model within the cellular context. In a series of experiments, mAbs were microinjected into HeLaS3 stably expressing histone H2B-GFP and progression through mitosis was monitored by time-lapse video microscopy. We first asked whether C-Mad2 is required to initiate SAC signalling. To this end, cells were injected in interphase and treated with nocodazole before progress through mitosis was monitored (Figure 6A). As expected, the microinjection of a Myc mAb, used as negative control, did not interfere with the ability of HeLaS3 cells to respond to nocodazole, as all injected cells underwent mitotic arrest for at least 6 h without signs of apoptosis (Figure 6B and C; Supplementary Movie S1). In stark contrast, the majority of the CM2276-injected cells escaped the nocodazole block in <1 h, resulting in cells with decondensed chromatin and aberrantly shaped nuclei, a phenotype typical of SAC override (Figure 6B and C; Supplementary Movie S2). Importantly, the microinjection phenotype caused by CM2276 injection depended exclusively on C-Mad2 recognition, as coinjection of the antibody with Mad2L13Q but not Mad2V193N (mimicking C- and O-Mad2, respectively), abolished the observed phenotype (Supplementary Figure S6). Microinjection of a Mad1 mAb (117–468; Supplementary Figure S2), performed as a positive control, resulted in a nearly identical phenotype (Figure 6B and C; Supplementary Movie S3). These results strongly indicate that the conformational dimerization of Mad2 is required for SAC signalling, as predicted by the template model and supported by experiments conducted in budding yeast (Nezi et al, 2006).
Figure 6.
C-Mad2 is required for initiating a SAC-dependent arrest. (A) Schematic representation of the microinjection protocol followed to assess the ability of cells to respond to nocodazole upon CM2276 injection. Interphase HeLaS3 stably expressing histone H2B-GFP cells were subjected to microinjection 7 h after thymidine release and analysed by time-lapse video microscopy in the presence of nocodazole. (B) Movie stills from representative cells injected with the indicated mAbs. Time in h:min is indicated. T=0 was defined as the time point at which NEBD became evident. Scale bars=10 μm. (C) Histogram showing cells microinjected with the indicated mAbs, subdivided into the displayed categories according to the time elapsed between NEBD and nocodazole override (SAC override). Percentage of cells is shown (n=total number of cells counted).
Next, we asked whether Mad2 conformational dimerization is also required to maintain an already established SAC arrest. This question was of particular interest, as several lines of recent evidence indicate that Mad2 acts epistatically upstream of BubR1/Mad3 (Hardwick et al, 2000; Burton and Solomon, 2007; King et al, 2007; Nilsson et al, 2008; Kulukian et al, 2009). To examine this issue, we injected CM2276 into mitotic HeLaS3 cells in which the SAC had been activated by treatment with nocodazole and analysed the injected cells 20, 40 and 60 min later by fluorescence microscopy (Figure 7A). Already 20 min after CM2276 injection, levels of Cyclin B1 were decreased to background levels in approximately half of the cells, and by 60 min all injected cells had overcome the nocodazole-induced arrest (Figure 7B and C), while injection of Myc mAb for control did not trigger a SAC override, as expected (Figure 7B and C). We have also analysed the kinetics of SAC override in response to microinjection of the anti-Mad1 mAb. This antibody triggered an even faster SAC override than CM2276 (Figure 7B and C). These results demonstrate, first, that C-Mad2 (and hence conformational dimerization) is required to maintain an already established SAC-dependent arrest, supporting and extending previous data obtained with a polyclonal Mad2 antibody (Gorbsky et al, 1998). They do not contradict an important role for BubR1 in APC/CCdc20 inhibition, but demonstrate a continuous requirement for C-Mad2 for sustaining this inhibition. Second, they also bear on a model of SAC signalling that invokes a contribution of an autocatalytic loop based on cytosolic C-Mad2 (De Antoni et al, 2005). Specifically, our side-by-side comparison of the consequences of anti-Mad1 mAb and CM2276 injection argues that cytosolic C-Mad2 alone cannot provide sufficient C-Mad2:Cdc20 to maintain a SAC arrest.
Figure 7.
The Mad1:C-Mad2 complex is required for maintaining a SAC-dependent arrest. (A) Schematic representation of the protocol followed to assess the capability of cells to maintain an already established SAC-dependent arrest upon microinjection of CM2276 and Mad1 mAb. Asynchronous HeLaS3 cells were treated with nocodazole for 5 h and mitotic cells were selectively microinjected with the mAbs. After injection, cells were incubated for 20, 40 or 60 min in the presence of nocodazole and fixed with PTEMF. Cyclin B1 was stained with a mAb directly coupled to Alexa Fluor 488. (B) Representative cells treated as described in (A). Cyclin B1 (green), DNA was visualized with DAPI (blue) and the injection marker Texas Red dextran (red) are displayed. Scale bar=10 μm. (C) Histogram showing cells microinjected with the indicated mAbs, subdivided into the displayed categories according to the absence (−) or presence (+) of Cyclin B1 staining. Percentage of cells is indicated (n=total number of cells counted).
The Mad1:C-Mad2 complex is required to regulate proper mitotic timing
In addition to being an essential component of the SAC, Mad2 has been proposed to constitute a key component of a mitotic timer operating in the cytosol (Meraldi et al, 2004). In support of this notion, the siRNA-mediated knockdown of Mad2 and BubR1 caused not only SAC silencing but also a striking acceleration of mitotic progression, whereas the knockdown of other Mad or Bub components was reported to abolish the SAC without accelerating the traverse of mitosis (Meraldi et al, 2004).
A mechanistic understanding of the proposed KT-independent mitotic timer is presently elusive, but the fact that Mad1 was reportedly dispensable (Meraldi et al, 2004) suggested the non-catalytic generation of a C-Mad2:Cdc20 complex. To clarify the relationship between the proposed Mad2-dependent mitotic timer and the SAC in human cells, we took advantage of CM2276 and asked whether interfering with conformational dimerization (and therefore C-Mad2 catalysis) would cause a similar mitotic acceleration, as the generalized Mad2 knockdown. We injected interphase HeLaS3 stably expressing histone H2B-GFP with either CM2276 or Myc mAb, as control, and analysed their progression through an unperturbed mitosis by time-lapse video microscopy (Figure 8A). Injection of Myc mAb (or buffer alone) caused a noticeable delay in the elapsed time between nuclear envelope breakdown (NEBD) and anaphase onset (54±8.1 and 69±6.1 min in average±s.d., respectively), when compared with control non-injected cells (41.7±3.7 min) (Figure 8B and C; Supplementary Movies S4–S6), most likely reflecting the cellular stress caused by the microinjection procedure. More importantly, injection of CM2276 led to a drastic mitotic acceleration (22.4±1.5 min) (Figure 8B and C; Supplementary Movie S7); in addition, CM2276-injected cells invariably showed signs of chromosome missegregation indicative of SAC abrogation (i.e. chromosome bridges and/or lagging chromosomes) (Figure 8B). These results clearly show that conformational dimerization of Mad2 is also involved in regulating mitotic timing.
Figure 8.
The Mad1:C-Mad2 complex is required for regulating mitotic timing. (A) Schematic representation of the microinjection protocol followed to analyse the progression through an unperturbed mitosis after mAb microinjection. Interphase HelaS3 stably expressing histone H2B-GFP cells were subjected to microinjection 7 h after thymidine release and analysed by time-lapse video microscopy. (B) Movie stills from representative cells injected with the indicated mAbs as described in (A). Control cells correspond to non-injected cells. Time in h:min is indicated. T=0 was defined as the time point at which NEBD became evident. Scale bar=10 μm. (C) Box-and-whisker plot showing the elapsed time (min) between NEBD and anaphase onset for individual cells microinjected as described in (A). Analysis performed on >40 cells per condition, from three independent experiments. (D) Box-and-whisker plot showing the elapsed time (min) between NEBD and anaphase onset for individual cells microinjected as described in Supplementary Figure S7. Analysis performed on ⩾10 cells. Lower and upper whiskers in (C, D) represent 10th and 90th percentiles, respectively.
From the perspective of the proposed timer model, allegedly dependent on Mad2 but not Mad1, the above results create a conundrum. It implies that either C-Mad2 can be generated in a Mad1-independent manner or, alternatively, Mad1 is also part of the mitotic timer mechanism. To distinguish between these possibilities, we used the Mad1 mAb, which was able to neutralize the SAC (Figure 6; Supplementary Movie S3), to carry out microinjection experiments. Remarkably, microinjection of this anti-Mad1 mAb led to a striking acceleration of unperturbed mitosis (19.1±1.2 min), causing also a strong chromosome missegregation phenotype (Figure 8B and C; Supplementary Movie S8), similar to the results obtained when injecting CM2276. This indicates that Mad1 also contributes to mitotic timing regulation.
In order to address to which extent the regulation of mitotic timing relies on the formation of an APC/C inhibitor during interphase, we undertook two different but complementary approaches. First, antibodies were microinjected into cells that had been arrested at the G2/M boundary with the Cdk1 inhibitor RO-3306 (Vassilev et al, 2006), and mitotic progression was then monitored following inhibitor wash out (Supplementary Figure S7A). The injection of either CM2276 or the anti-Mad1 mAb caused a striking mitotic acceleration (NEBD-to-anaphase onset; 24.4 and 22.3 min on average, respectively) when compared with non-injected cells (68.5 min on average) (Figure 8D; Supplementary Figure S7B). Second, we took advantage of the fact that the SAC kinase Mps1, amenable to chemical inhibition and required for Mad1:C-Mad2 loading to KTs, has also been recently implicated in the regulation of mitotic timing (Hewitt et al, 2010; Maciejowski et al, 2010; Santaguida et al, 2010; Sliedrecht et al, 2010). Specifically, we monitored the timing of mitotic progression in cells that experienced an Mps1 inhibition already during S, G2 and M phase or only during M phase (Supplementary Figure S8A). Remarkably, Mps1 inhibition led to an indistinguishable mitotic acceleration, regardless of the timing of inhibitor addition (Supplementary Figure S8B and C). These results lend no support to the idea that Mps1 affects the duration of mitosis independently of the SAC.
Taken together, the above data identify the KT Mad1:C-Mad2 complex as a prominent regulator of mitotic timing in human cells and they call for a reconsideration of the distinction between SAC and mitotic timer in human cells.
Discussion
In this study, we have used a conformation-specific mAb (CM2276) to study mechanistic aspects of SAC signalling in living human cells. We demonstrate that CM2276 selectively recognizes one of the two Mad2 conformers, namely C-Mad2. This powerful tool provided a unique opportunity to probe Mad2 conformation and directly validate several aspects of the template model for SAC signalling in a cellular context. In particular, the CM2276 antibody allowed us to reveal a role for p31comet in capping C-Mad2 at particular subcellular locations, and to demonstrate that Mad1 is required for the generation of C-Mad2 in vivo, thus providing strong support for the template model. Finally, we demonstrate that mitotic timing also depends on the Mad1:C-Mad2 template. This latter observation suggests that, from a mechanistic perspective, the SAC and the proposed mitotic timer are closely related if not identical.
The two-state behaviour of Mad2 in the cell
The template model of SAC signalling hinges crucially on the existence and interconversion of two conformers of Mad2 (Mapelli and Musacchio, 2007; Luo and Yu, 2008). Using CM2276, we have been able to directly visualize the proposed two-state behaviour of Mad2 within living cells. The fact that the C-Mad2 mAb could only immunoprecipitate Mad2 when bound to interaction partners strongly supports the concept that liganded but not free Mad2 adopts the closed conformation, and our data indicate that this conclusion holds true throughout the cell cycle. As we show here, most of Mad2 was released as a free moiety when Mad1 was depleted, and this free Mad2 existed in the open conformation (as it was not recognized by CM2276). Our data demonstrate that Mad1 is strictly required for Mad2 to adopt the closed conformation in vivo. Previous work had already suggested that free Mad2 adopts the open conformation in cells (Luo et al, 2004), and this important notion had been incorporated in the template model (De Antoni et al, 2005). However, a recent in vitro reconstitution study has also suggested that Mad1:C-Mad2 is able to generate unliganded C-Mad2, which then bound Cdc20 with higher efficiency than O-Mad2 (Yang et al, 2008). In our study, we did not detect significant levels of unliganded C-Mad2, suggesting that, in vivo, the lifetime of this species must be short, if it exists at all. As C-Mad2 is the energetically favoured conformer in vitro (Luo et al, 2004), this raises the possibility that in the cytoplasm of living cells an as yet unknown mechanism actively promotes the open conformation of Mad2.
Regulation of the Mad1:C-Mad2 complex by p31comet
Considering the central role of the Mad1:C-Mad2 complex in SAC signalling, it is clearly important to understand its regulation in time and space. On the one hand, KTs provide an essential microenvironment for the activity of the Mad1:C-Mad2 complex, as indicated by the observation that the complex is removed from KTs in a dynein-dependent manner upon SAC silencing (Howell et al, 2001) and interference with this removal generates a SAC-dependent arrest (Griffis et al, 2007; Gassmann et al, 2010). On the other hand, SAC signalling is known to be inhibited by p31comet (Habu et al, 2002; Xia et al, 2004). Our present study provides further insight into the role of this protein. Immunostaining with CM2276 revealed that the accessibility of C-Mad2 is controlled by p31comet at multiple locations. In particular, our siRNA experiments show that the dimerization interface of C-Mad2 is masked by p31comet not only at NPCs and spindle poles, but also, in part, at KTs. In support of this conclusion, p31comet could be detected at KTs (Habu et al, 2002; this study). This raises the question of why a protein implicated in silencing of the SAC associates with the catalytic source of the inhibitory SAC signal already during propmetaphase. One possible explanation is that p31comet begins to cap and functionally inactivate individual C-Mad2 proteins as soon as a threshold of bipolar MT attachment to KTs has been reached, perhaps in preparation for subsequent stripping of the Mad1:C-Mad2:p31comet complex from KTs. Alternatively, it would be premature to exclude that p31comet may have not only an inhibitory but also an activating role in SAC signalling, similar to the dual role of securin in the regulation of separase (Hornig et al, 2002). Future studies will have to explore these possibilities. In addition, it will be important to better understand the role of posttranslational modifications in the formation and activity of KT-associated complexes (Burke and Stukenberg, 2008; Hewitt et al, 2010). Finally, it is noteworthy that the regulation of the p31comet interaction with the Mad1:C-Mad2 complex could not be visualized by analysing bulk populations through biochemical approaches (Supplementary Figures S1D and S5A), suggesting that this regulation concerns only a minor subpopulation of the two proteins (notably the fraction of the complex that localizes at KTs). This further emphasizes the importance of tools such as CM2276 for uncovering local regulation.
The Mad1:C-Mad2 complex is required to both initiate and maintain the SAC
Several predictions of the template model find strong support in the microinjection experiments carried out in our study. The injection of CM2276 abolished SAC activation, thus confirming the template model's prediction that pinpoints the dimerization interface as the active site of C-Mad2 catalysis (De Antoni et al, 2005; Kulukian et al, 2009; Lad et al, 2009; Simonetta et al, 2009). One interesting emerging view is that the C-Mad2:Cdc20 complex generated at KTs is not the final inhibitory component of the SAC, but that C-Mad2 acts catalytically—rather than stoichiometrically—to promote Cdc20 binding to BubR1 (Nilsson et al, 2008; Kulukian et al, 2009). From this perspective, it is interesting that interference with the Mad1:C-Mad2 KT template by injection of CM2276 into cells in which the BubR1:Cdc20 complex was already functional, also caused an abrupt extinction of the SAC. This indicates that the continuous production of new C-Mad2:Cdc20 is required for maintaining a SAC-dependent arrest, even after BubR1 had been loaded onto Cdc20. This in turn suggests that Mad2 is required for SAC maintenance by exerting a BubR1-independent inhibitory action on Cdc20 and/or by continuously replenishing an intrinsically unstable BubR1:Cdc20 complex (Burton and Solomon, 2007; King et al, 2007; Nilsson et al, 2008).
One interesting hypothesis in the SAC field is that cytoplasmic C-Mad2:Cdc20 can promote its own formation through an autocatalytic loop (De Antoni et al, 2005). This idea finds experimental support through in vitro experiments (Lad et al, 2009; Simonetta et al, 2009), but the in vivo contribution of a cytosolic branch of Mad2 template activity remains to be tested. From this perspective, it is interesting that microinjection (into nocodazole-arrested cells) of an anti-Mad1 mAb (targeting exclusively the Mad1:C-Mad2 complex) was at least as efficient in causing a SAC override as injection of CM2276 (targeting both KT-associated Mad1:C-Mad2 and cytosolic C-Mad2:Cdc20 complexes). Although these quantitative differences may well reflect a difference in the function-neutralizing potency of the injected antibodies, our observation clearly argues that a C-Mad2:Cdc20 autocatalytic loop is not in itself sufficient to maintain a SAC arrest.
KT-dependent control of mitotic timing
Careful live-cell studies in siRNA-treated human cells have led to the proposal that anaphase onset depends on a mitotic timer that involves Mad2 and BubR1, but not Mad1 (Meraldi et al, 2004). Genetic analyses in Drosophila are consistent with such a timer model, although the contributions of Mad2 and BubR1 to mitotic timing in flies appear to be minor and, remarkably, the SAC is not strictly required for undisturbed mitoses in this organism (Buffin et al, 2007; Rahmani et al, 2009; Emre et al, 2011). In the present study, injection of cells with antibodies directed at either C-Mad2 or Mad1 triggered a striking mitotic acceleration. These results cannot readily be reconciled with the proposal that Mad2 (together with BubR1) is part of a ‘cytoplasmic mitotic timer’, which works independently of the SAC (Meraldi et al, 2004). Although we cannot formally exclude that functional neutralization by mAb microinjection triggers a different (and even stronger) phenotype than the reduction of the corresponding proteins by siRNA-mediated depletion, we suspect that the reported differences in the phenotypes observed after siRNA-mediated depletion of Mad1 or Mad2 (Meraldi et al, 2004; our unpublished data) reflect differences in the functionality of residual protein levels. In particular, Mad2 depletion simultaneously affects both the KT-associated Mad1-bound Mad2 population (C-Mad2) and the cytoplasmic pool of Mad2 that awaits catalytic conversion (O-Mad2). Hence, even partial reduction of Mad2 readily causes acceleration of traverse through mitosis (Hubner et al, 2010). Conversely, Mad1 depletion affects exclusively the catalyst (the Mad1:C-Mad2 complex) and, therefore, minor amounts of residual Mad1 might be sufficient to partly support the checkpoint, thus preventing mitotic acceleration. A recent study has also implicated the SAC kinase Mps1 in the regulation of mitotic timing, suggesting that Mps1 is also required during interphase to form an anaphase inhibitor in a KT-independent manner (Maciejowski et al, 2010). However, as our present data demonstrate that functional abrogation of Mad1:C-Mad2 also accelerates mitotic progression, we would predict that complete neutralization of any SAC component, including Mps1, will translate into faster progression through mitosis. Most importantly, the consequences of Mps1 inhibition were indistinguishable, regardless of whether the kinase was inhibited already during interphase or only during mitosis. Thus, interphase activity of Mps1 does not significantly contribute to the timing of mitotic progression. Collectively, our results on the role of Mad1:C-Mad2 and Mps1 should prompt a reconsideration of the distinction between SAC and mitotic timer.
Materials and methods
Generation of CM2276
CM2276 (clone 107–276) has been described previously (Chan et al, 2009). Briefly, CM2276 (IgG1k) was generated against GST-tagged full-length human Mad2 by repeated subcutaneous injection of 100 μg of antigen into Balb/c mice using aluminium hydroxide as an adjuvant. Spleen cells were fused with PAIB3Ag81 mouse myeloma cells. Supernatant screening was performed by ELISA.
Competition assays
For GST pull-down experiments in Figure 1D–F and Supplementary Figure S1E and F, a complex between GST–Cdc20111−138 and His-tagged Mad2WT was generated on glutathione-sepharose 4B beads (GE Healthcare) as described before (Mapelli et al, 2007). Competition in binding to C-Mad2 was assessed by adding CM2276 to the beads at 0.2 μM together with p31comet at 0.6, 3.6 or 9 μM (Figure 1D). The corresponding negative control (Supplementary Figure S1E) was performed by replacing p31comet with equal concentrations of hSpindly1−444. In Figure 1E, Mad2ΔC was added to the beads at a concentration of 0.35 μM together with CM2276 at concentrations of 0.15, 0.45 or 1.35 μM. The corresponding negative control (Supplementary Figure S1F) was performed by replacing CM2276 with equal concentrations of Mad1 mAb (117–468). For the experiment in Figure 1F, Mad2ΔC and CM2276 were used at concentrations of 0.35 and 0.9 μM, respectively.
SEC followed by IP
Synchronized HeLaS3 cells were lysed as described in the Supplementary data. A measure of 1 ml of clarified lysate containing 10 mg of total protein extract was loaded on a Superdex 200 16/60 column (GE) using an Äkta Explorer FPLC system (Amersham). An isocratic elution was performed at 4°C applying a constant flow of 1.1 ml/min of 20 mM Tris–HCl, pH 7.4, 150 mM NaCl. Fractions were collected every 4 ml; protease and phosphatase inhibitors were added to each fraction as to the lysis buffer. Each fraction was subdivided in a 1.3-ml part and a 2.7-ml part, destined to trichloroacetic acid (TCA) precipitation and IP, respectively. IP was performed for 2 h at 4°C using 20 μl of solid protein A beads (Bio-Rad), previously coupled and cross-linked to the CM2276 at 1 μg/μl. Beads were washed three times with 0.5 ml of elution buffer and resuspended in 30 μl of Laemmli buffer. TCA-precipitated protein pellets were resuspended in 80 μl of Laemmli buffer. A total of 10 μl was further analysed by SDS–PAGE and WB.
Antibody microinjection
Antibody injection was performed using Femtotips II capillaries operated on a FemtoJet microinjector (Eppendorf). Antibodies were injected at a needle concentration of 1–2 mg/ml in PBS in the presence of 1.66 mg/ml Texas Red dextran or Alexa Fluor 488 dextran (both 10.000 MW; Invitrogen). Each injection was performed for 0.2–0.3 s with injection and compensation pressures of 15 hPa. To assess the SAC functionality and effects on mitotic timing upon mAb microinjection, HeLaS3 H2B-GFP (Sillje et al, 2006) cells or HeLa Kyoto H2B-mCherry (Neumann et al, 2010) were seeded on 35 mm μ-Dishes or coverslips and synchronized as described in the corresponding Figure legends 6–8. Different mAbs were injected at different grid coordinates within the same plate or on different coverslips and phenotypes were analysed by live-cell imaging or immunofluorescence microscopy.
Supplementary Material
Acknowledgments
We are especially grateful to A Musacchio for reagents, insight on the project and comments on the manuscript. We also thank J Ellenberg, AA Hyman, I Poser, NS Gray, A Uldschmid and YW Chan for reagents and A Wehner and E Nigg for technical help. We thank all past and present members of our laboratory for insightful discussion. This work was supported by the Max Planck Society, the University of Basel and the Experimental Network for Functional Integration (ENFIN) Contract LSHG-CT-2005-518254 funded by the European Commission within its FP6 Programme. LLF was partly supported by the International Max Planck Research School for Molecular and Cellular Life Sciences.
Author contributions: LLF, EAN and AS conceived and designed the experiments. LLF and MK carried out the experiments. LLF and AS analysed the data. LLF, EAN and AS wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Buffin E, Emre D, Karess RE (2007) Flies without a spindle checkpoint. Nat Cell Biol 9: 565–572 [DOI] [PubMed] [Google Scholar]
- Burke DJ, Stukenberg PT (2008) Linking kinetochore-microtubule binding to the spindle checkpoint. Dev Cell 14: 474–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton JL, Solomon MJ (2007) Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev 21: 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MS, Chan GK, Yen TJ (2001) Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J Cell Sci 114(Pt 5): 953–963 [DOI] [PubMed] [Google Scholar]
- Chan YW, Fava LL, Uldschmid A, Schmitz MH, Gerlich DW, Nigg EA, Santamaria A (2009) Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J Cell Biol 185: 859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Shevchenko A, Mann M, Murray AW (1998) Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J Cell Biol 143: 283–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Antoni A, Pearson CG, Cimini D, Canman JC, Sala V, Nezi L, Mapelli M, Sironi L, Faretta M, Salmon ED, Musacchio A (2005) The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 15: 214–225 [DOI] [PubMed] [Google Scholar]
- Emre D, Terracol R, Poncet A, Rahmani Z, Karess RE (2011) A mitotic role for Mad1 beyond the spindle checkpoint. J Cell Sci 124(Pt 10): 1664–1671 [DOI] [PubMed] [Google Scholar]
- Fang G (2002) Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol Biol Cell 13: 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW (1998) The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev 12: 1871–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, Beretta A, Sironi L, Musacchio A, Lucchini G, Piatti S (2001) Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J 20: 6648–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon JV, Greaves R, Iggo R, Lane DP (1990) Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J 9: 1595–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Holland AJ, Varma D, Wan X, Civril F, Cleveland DW, Oegema K, Salmon ED, Desai A (2010) Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev 24: 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky GJ, Chen RH, Murray AW (1998) Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J Cell Biol 141: 1193–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis ER, Stuurman N, Vale RD (2007) Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol 177: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu T, Kim SH, Weinstein J, Matsumoto T (2002) Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J 21: 6419–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Johnston RC, Smith DL, Murray AW (2000) MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J Cell Biol 148: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS (2010) Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J Cell Biol 190: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig NC, Knowles PP, McDonald NQ, Uhlmann F (2002) The dual mechanism of separase regulation by securin. Curr Biol 12: 973–982 [DOI] [PubMed] [Google Scholar]
- Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED (2001) Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol 155: 1159–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED (2004) Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol 14: 953–964 [DOI] [PubMed] [Google Scholar]
- Hubner NC, Wang LH, Kaulich M, Descombes P, Poser I, Nigg EA (2010) Re-examination of siRNA specificity questions role of PICH and Tao1 in the spindle checkpoint and identifies Mad2 as a sensitive target for small RNAs. Chromosoma 119: 149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW (1998) Budding yeast Cdc20: a target of the spindle checkpoint. Science 279: 1041–1044 [DOI] [PubMed] [Google Scholar]
- King EM, van der Sar SJ, Hardwick KG (2007) Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS One 2: e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B (1997) Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature 390: 74–77 [DOI] [PubMed] [Google Scholar]
- Kulukian A, Han JS, Cleveland DW (2009) Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell 16: 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad L, Lichtsteiner S, Hartman JJ, Wood KW, Sakowicz R (2009) Kinetic analysis of Mad2-Cdc20 formation: conformational changes in Mad2 are catalyzed by a C-Mad2-ligand complex. Biochemistry 48: 9503–9515 [DOI] [PubMed] [Google Scholar]
- Li Y, Benezra R (1996) Identification of a human mitotic checkpoint gene: hsMAD2. Science 274: 246–248 [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Rizo J, Yu H (2002) The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol Cell 9: 59–71 [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Xia G, Wassmann K, Matsumoto T, Rizo J, Yu H (2004) The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat Struct Mol Biol 11: 338–345 [DOI] [PubMed] [Google Scholar]
- Luo X, Yu H (2008) Protein metamorphosis: the two-state behavior of Mad2. Structure 16: 1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J, George KA, Terret ME, Zhang C, Shokat KM, Jallepalli PV (2010) Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J Cell Biol 190: 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli M, Filipp FV, Rancati G, Massimiliano L, Nezi L, Stier G, Hagan RS, Confalonieri S, Piatti S, Sattler M, Musacchio A (2006) Determinants of conformational dimerization of Mad2 and its inhibition by p31comet. EMBO J 25: 1273–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli M, Massimiliano L, Santaguida S, Musacchio A (2007) The Mad2 conformational dimer: structure and implications for the spindle assembly checkpoint. Cell 131: 730–743 [DOI] [PubMed] [Google Scholar]
- Mapelli M, Musacchio A (2007) MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr Opin Struct Biol 17: 716–725 [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma S, Stucke VM, Nigg EA (2002) Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297: 2267–2270 [DOI] [PubMed] [Google Scholar]
- Meraldi P, Draviam VM, Sorger PK (2004) Timing and checkpoints in the regulation of mitotic progression. Dev Cell 7: 45–60 [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Nasmyth K (2005) How do so few control so many? Cell 120: 739–746 [DOI] [PubMed] [Google Scholar]
- Neumann B, Walter T, Heriche JK, Bulkescher J, Erfle H, Conrad C, Rogers P, Poser I, Held M, Liebel U, Cetin C, Sieckmann F, Pau G, Kabbe R, Wunsche A, Satagopam V, Schmitz MH, Chapuis C, Gerlich DW, Schneider R et al. (2010) Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464: 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezi L, Rancati G, De Antoni A, Pasqualato S, Piatti S, Musacchio A (2006) Accumulation of Mad2-Cdc20 complex during spindle checkpoint activation requires binding of open and closed conformers of Mad2 in Saccharomyces cerevisiae. J Cell Biol 174: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Yekezare M, Minshull J, Pines J (2008) The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol 10: 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7: 644–656 [DOI] [PubMed] [Google Scholar]
- Poser I, Sarov M, Hutchins JR, Heriche JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, Pelletier L, Kittler R, Hua S, Naumann R, Augsburg M, Sykora MM, Hofemeister H, Zhang Y, Nasmyth K, White KP et al. (2008) BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods 5: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Z, Gagou ME, Lefebvre C, Emre D, Karess RE (2009) Separating the spindle, checkpoint, and timer functions of BubR1. J Cell Biol 187: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G (1995) The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol 130: 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Tighe A, D’Alise AM, Taylor SS, Musacchio A (2010) Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol 190: 73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JV, Botvinick E, Bonday Z, Furnari F, Berns M, Cleveland DW (2004) Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr Biol 14: 942–952 [DOI] [PubMed] [Google Scholar]
- Sillje HH, Nagel S, Korner R, Nigg EA (2006) HURP is a Ran-importin beta-regulated protein that stabilizes kinetochore microtubules in the vicinity of chromosomes. Curr Biol 16: 731–742 [DOI] [PubMed] [Google Scholar]
- Simonetta M, Manzoni R, Mosca R, Mapelli M, Massimiliano L, Vink M, Novak B, Musacchio A, Ciliberto A (2009) The influence of catalysis on mad2 activation dynamics. PLoS Biol 7: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Mapelli M, Knapp S, De Antoni A, Jeang KT, Musacchio A (2002) Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J 21: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliedrecht T, Zhang C, Shokat KM, Kops GJ (2010) Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One 5: e10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Chan GK, Yen TJ (2001) Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol 154: 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H (2001) Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev Cell 1: 227–237 [DOI] [PubMed] [Google Scholar]
- Tighe A, Staples O, Taylor S (2008) Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J Cell Biol 181: 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L (2006) Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci USA 103: 10660–10665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Simonetta M, Transidico P, Ferrari K, Mapelli M, De Antoni A, Massimiliano L, Ciliberto A, Faretta M, Salmon ED, Musacchio A (2006) In vitro FRAP identifies the minimal requirements for Mad2 kinetochore dynamics. Curr Biol 16: 755–766 [DOI] [PubMed] [Google Scholar]
- Xia G, Luo X, Habu T, Rizo J, Matsumoto T, Yu H (2004) Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J 23: 3133–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li B, Liu CJ, Tomchick DR, Machius M, Rizo J, Yu H, Luo X (2008) Insights into mad2 regulation in the spindle checkpoint revealed by the crystal structure of the symmetric mad2 dimer. PLoS Biol 6: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Li B, Tomchick DR, Machius M, Rizo J, Yu H, Luo X (2007) p31comet blocks Mad2 activation through structural mimicry. Cell 131: 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.