Abstract
Sarkar, S. (Massachusetts Institute of Technology, Cambridge). Properties and regulation of the β-d-galactosidase in Shigella dysenteriae and in Escherichia coli-Shigella dysenteriae hybrids. J. Bacteriol. 91:1477–1488. 1966.—Shigella dysenteriae strain 60 has a β-d-galactosidase related to that of Escherichia coli but more heat-sensitive and with a turnover number about 10 times lower. Hybridization by transduction produces strains with enzymes of intermediate properties by recombination within the z gene. Both E. coli and S. dysenteriae have a regulatory i+ gene. Recombination between i− mutants of the two organisms leads to restoration of the i+ genotype. In S. dysenteriae 60, most of the i− mutants are subject to genetic suppression by suppressor mutations at unlinked loci. The effect of these suppressors on the products of the suppressed i− genes is discussed.
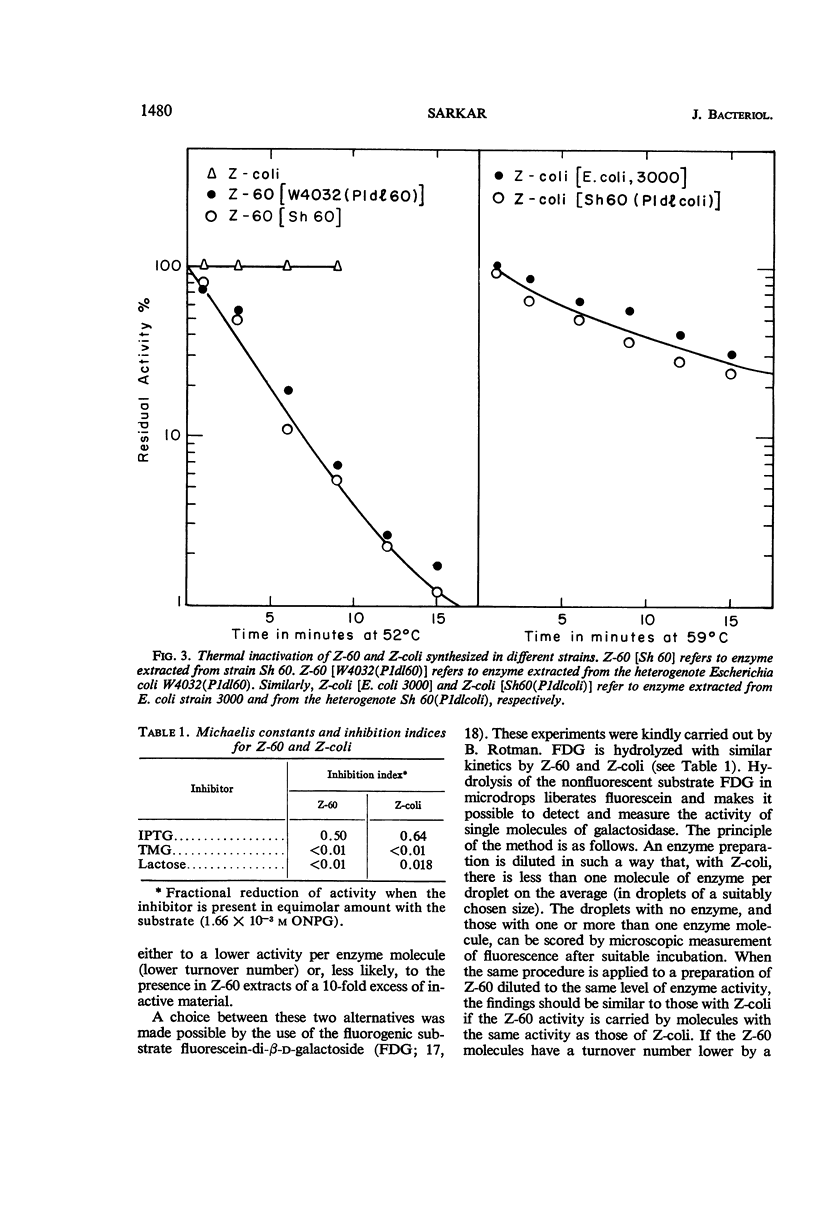
Full text
PDF

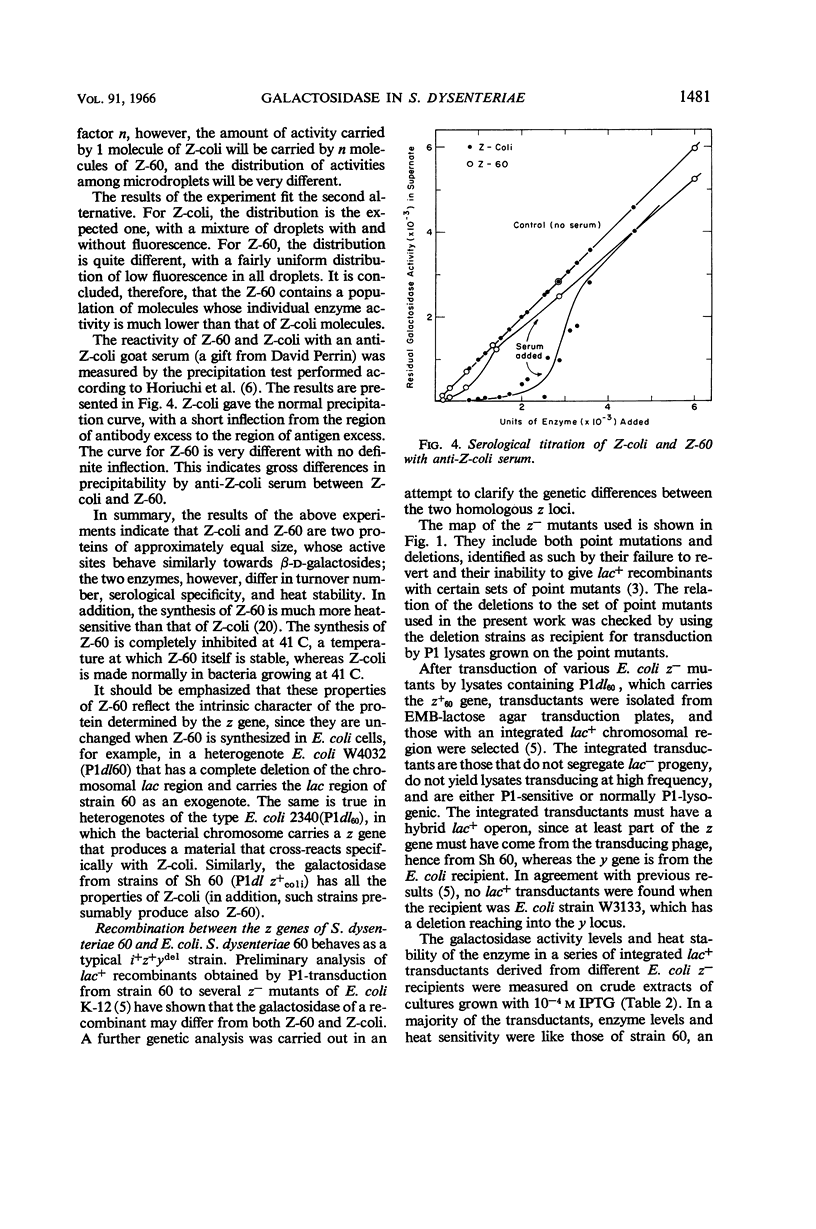



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., LENNOX E., SPIEGELMAN S. On the behavior of the E. coli Pz-"beta-galactoside system" introduced into Shigella dysenteriae. Biochim Biophys Acta. 1960 Apr 8;39:255–266. doi: 10.1016/0006-3002(60)90162-1. [DOI] [PubMed] [Google Scholar]
- COOK A., LEDERBERG J. Recombination studies of lactose nonfermenting mutants of Escherichia coli K-12. Genetics. 1962 Oct;47:1335–1353. doi: 10.1093/genetics/47.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN N. C., HOWARD B. D. DOUBLE HETEROGENOTES FORMED BY P1-MEDIATED TRANSDUCTION OF LAC GENES IN ESCHERICHIA COLI. Virology. 1965 Jan;25:98–110. doi: 10.1016/0042-6822(65)90257-6. [DOI] [PubMed] [Google Scholar]
- FRANKLIN N. C., LURIA S. E. Transduction by bacteriophage P-1 and the properties of the lac genetic region in E. coli and S. dysenteriae. Virology. 1961 Nov;15:299–311. doi: 10.1016/0042-6822(61)90362-2. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
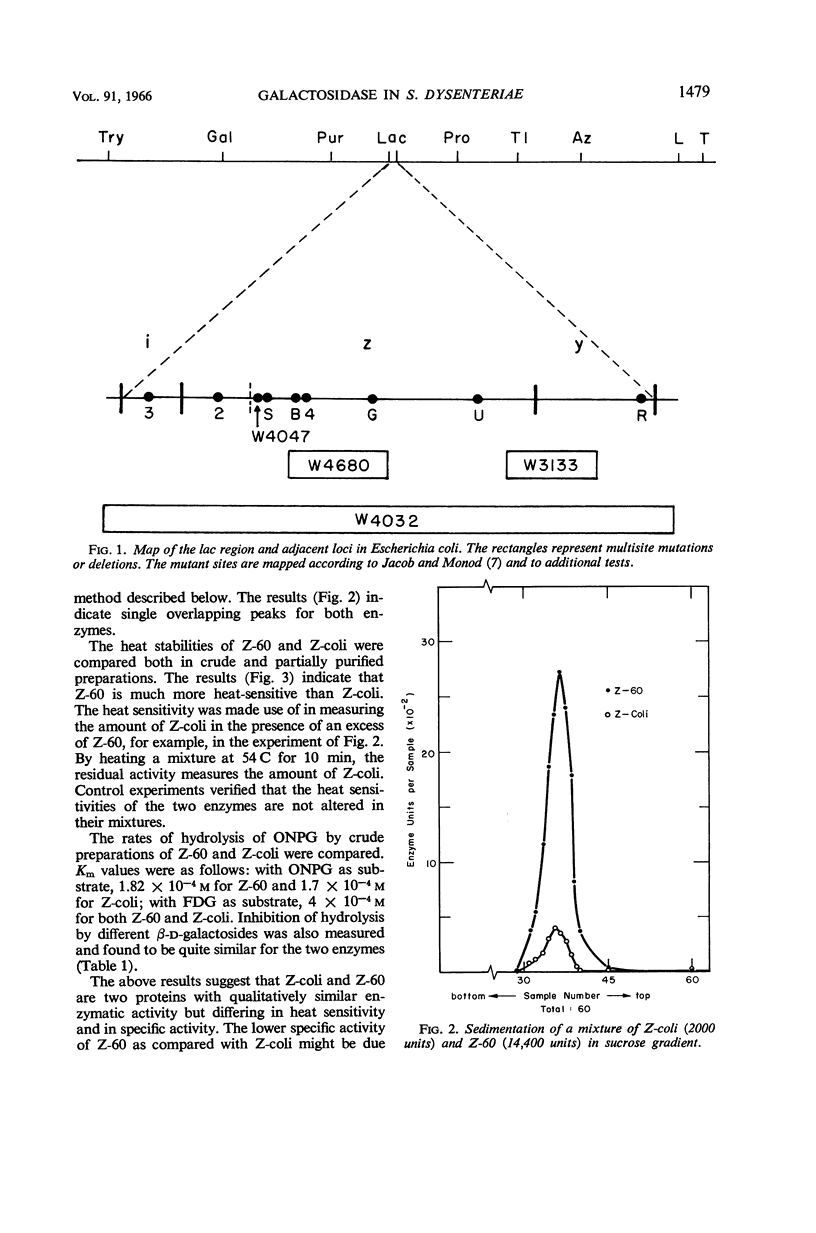
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
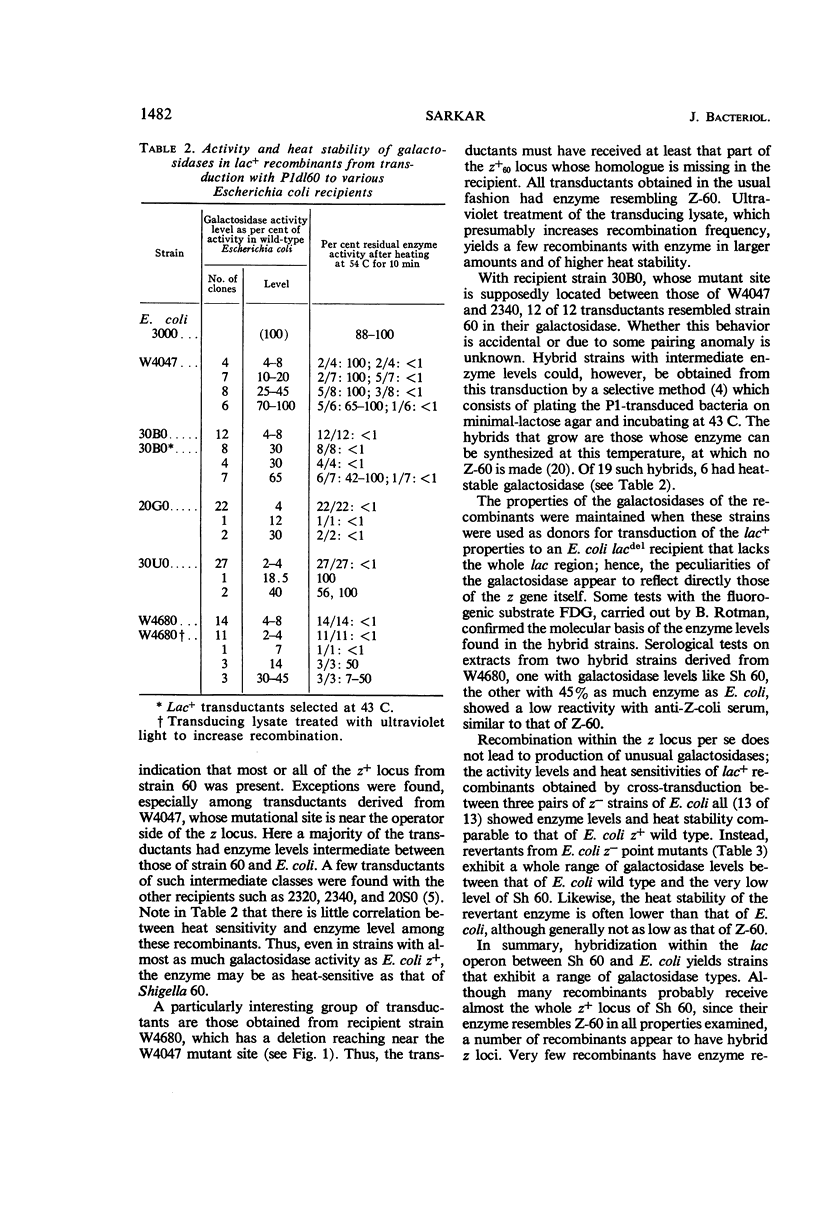
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LI K., BARKSDALE L., GARMISE L. Phenotypic alterations associated with the bacteriophage carrier state of Shigella dysenteriae. J Gen Microbiol. 1961 Mar;24:355–367. doi: 10.1099/00221287-24-3-355. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- RICKENBERG H. V. Occurrence of beta-galactosidase in the genus Shigella. J Bacteriol. 1960 Sep;80:421–422. doi: 10.1128/jb.80.3.421-422.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B. Measurement of activity of single molecules of beta-D-galactosidase. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B., ZDERIC J. A., EDELSTEIN M. Fluorogenic substrates for beta-D-galactosidases and phosphatases derived from flurescein (3,6-dihydroxyfluoran) and its monomethylether. Proc Natl Acad Sci U S A. 1963 Jul;50:1–6. doi: 10.1073/pnas.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADLER J. R., NOVICK A. THE PROPERTIES OF REPRESSOR AND THE KINETICS OF ITS ACTION. J Mol Biol. 1965 Jun;12:305–327. doi: 10.1016/s0022-2836(65)80255-8. [DOI] [PubMed] [Google Scholar]
- SARKAR S., LURIA S. E. Regulation of biosynthesis of a heat-sensititive beta-D-galactosidase in Shigella dysenteriae. Biochim Biophys Acta. 1963 Mar 26;68:505–508. doi: 10.1016/0006-3002(63)90178-1. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LAWRENCE P. S. Gene action. Annu Rev Microbiol. 1960;14:311–340. doi: 10.1146/annurev.mi.14.100160.001523. [DOI] [PubMed] [Google Scholar]


