Abstract
Spindle pole bodies (SPBs), like nuclear pore complexes, are embedded in the nuclear envelope (NE) at sites of fusion of the inner and outer nuclear membranes. A network of interacting proteins is required to insert a cytoplasmic SPB precursor into the NE. A central player of this network is Nbp1 that interacts with the conserved integral membrane protein Ndc1. Here, we establish that Nbp1 is a monotopic membrane protein that is essential for SPB insertion at the inner face of the NE. In vitro and in vivo studies identified an N-terminal amphipathic α-helix of Nbp1 as a membrane-binding element, with crucial functions in SPB duplication. The karyopherin Kap123 binds to a nuclear localization sequence next to this amphipathic α-helix and prevents unspecific tethering of Nbp1 to membranes. After transport into the nucleus, Nbp1 binds to the inner nuclear membrane. These data define the targeting pathway of a SPB component and suggest that the amphipathic α-helix of Nbp1 is important for SPB insertion into the NE from within the nucleus.
Keywords: amphipathic helix, in-plane membrane anchor, Kap123, Nbp1, spindle pole body duplication
Introduction
The yeast spindle pole body (SPB) is the functional equivalent of the mammalian centrosome (Jaspersen and Winey, 2004). Like the centrosome, the SPB organizes microtubules and duplicates only once during the cell cycle. However, the necessity for the nucleation of both cytoplasmic and nuclear microtubules within a closed mitosis requires that the budding yeast SPB is embedded within the nuclear envelope (NE) in much the same way as the nuclear pore complex (NPC) (Byers and Goetsch, 1975).
SPB duplication is initiated at a specialized substructure, named the half bridge (Byers and Goetsch, 1975; Adams and Kilmartin, 1999). The half bridge extends from one side of the central plaque on top of the nuclear and cytoplasmic sides of the NE. The first step in SPB duplication is the elongation of the half bridge.
Next, in early G1 phase of the cell cycle, a miniature version of the cytoplasmic SPB domain, known as the satellite, assembles at the distal end of the elongated half bridge. After cells transit the cell cycle commitment point START, the satellite expands into a duplication plaque that is then inserted into the NE from the cytoplasmic side of the NE. This insertion step facilitates the assembly of the nuclear part of the SPB (Byers and Goetsch, 1975; Adams and Kilmartin, 1999; Jaspersen and Winey, 2004).
Genetic studies have identified an SPB-associated network of four proteins that is essential for NE insertion of the duplication plaque. This network contains the integral membrane protein Mps2, the Mps2 interacting protein Bbp1, the integral membrane protein Ndc1 and Nbp1. Nbp1 interacts with Ndc1 and the Mps2–Bbp1 complex (Winey et al, 1991, 1993; Schramm et al, 2000; Araki et al, 2006), making it to a key player of the SPB insertion machinery. Malfunction of any of these proteins causes a defect in duplication plaque insertion. In all such mutants, the duplication plaque accumulates as a ‘dead pole’ on the cytoplasmic surface of the NE. Such ‘dead poles’ organize cytoplasmic microtubules but the absence of the nuclear, inner plaque, means that these structures are unable to assemble nuclear microtubules. The ‘dead pole’ therefore fails to participate in chromosome segregation.
The molecular mechanisms by which Nbp1, Mps2–Bbp1 and Ndc1 drive the insertion of the duplication plaque into the double membrane of the NE are not understood. However, it is important to note, that Ndc1 also is required for NPC assembly where it acts together with the integral membrane proteins Pom34 and Pom152 to form membrane rings, which anchor the NPC to the pore membrane (Lau et al, 2004; Alber et al, 2007; Onischenko et al, 2009; Doucet and Hetzer, 2010). This dual function for Ndc1 suggests that the mechanistic principals for NE insertion and anchoring of SPBs are similar to those for NPCs.
NPCs and SPBs are embedded in the NE at sites of fusion between the inner and outer nuclear membranes. Membrane bending may facilitate this fusion of the two nuclear membranes (Blumenthal et al, 2003; Farsad and De Camilli, 2003; Antonin, 2009). Recently, reticulons, membrane-bending proteins of the ER (Voeltz et al, 2006), were found to be required for the assembly of NPCs (Antonin, 2009; Dawson et al, 2009; Doucet and Hetzer, 2010; Shibata et al, 2010).
Proteins that sense and generate membrane curvature often contain membrane-active amphipathic α-helices (AH). For example, five of the scaffold nucleoporins (Nup133, Nup120, Nup85, Nup170 and Nup188) contain an AH with an ALPS (ArfGAP1 lipid packing sensor; Drin et al, 2007) motif that targets curved membranes and may help to anchor NPCs to the NE (Alber et al, 2007; Doucet and Hetzer, 2010; Drin and Antonny, 2010). Other proteins such as Sar1, epsin and Arf1-GTP contain AH that are involved in membrane curvature initiation and membrane remodelling (Ford et al, 2002; Lee et al, 2005; Beck et al, 2008).
Here, we have assessed the presence of a membrane inserting AH in proteins that are required for SPB insertion. We show that Nbp1 contains an N-terminal AH adjacent to a nuclear localization sequence (NLS). Binding of Kap123 to this Nbp1 NLS inhibits the membrane-binding activity of the AH. Our data suggest that Nbp1 assists insertion of the cytoplasmic SPB intermediate into the NE from within the nucleus possibly by modulating membrane behaviour through its AH.
Results
Analysis of the primary structure predicts an in-plane membrane anchor and NLS in Nbp1
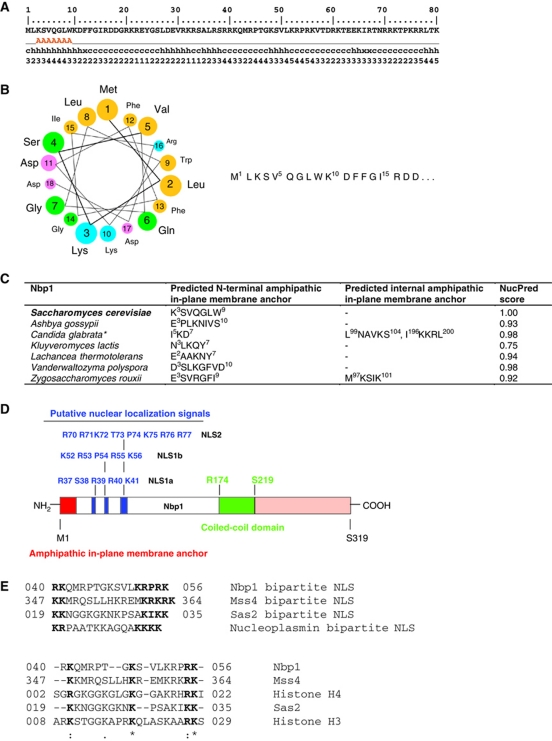
The SPB is embedded within the NE at sites of highly curved membrane at which the inner and outer nuclear membranes fuse (Byers and Goetsch, 1975). To find out whether proteins of the SPB insertion network have a membrane-active AH, which may stabilize or generate curved fusion sites, we scanned Nbp1, Ndc1, Bbp1 and Mps2 with the AmphipaSeeK program (Sapay et al, 2006). This program predicts amino-acid sequences with a composition similar to known AH functioning as in-plane membrane (IPM) anchors in monotopic membrane proteins. IPM anchors are more hydrophilic than transmembrane helices and more hydrophobic than amphipathic helices from soluble proteins (Sapay et al, 2006). The AmphipaSeeK program predicted an IPM anchor at the N-terminus of Nbp1 (Figure 1A) but none in either Bbp1 or Ndc1. For Mps2 three residues at the N-terminus were predicted to function as IPM, but the score was only just above the threshold level. The helical wheel projection confirmed that an α-helix of Nbp1 comprising of amino acids 1–18 would be amphipathic (Figure 1B). Interestingly, we found this N-terminal IPM in all Nbp1 orthologues (family of Saccharomycetaceae) (Figure 1C), despite low amino-acid sequence identity of the N-terminal sequences. Further analysis of the primary structure revealed, that the IPM anchor is probably the only membrane interacting motif of Nbp1, because neither a N-myristoylation pattern nor prenylation motif were predicted (PSORTII Server).
Figure 1.
IPM anchor and domain structure of Nbp1. (A) AmphipaSeeK output for Nbp1: first line (only the first 80 N-terminal amino-acid residues are shown): sequence position and protein sequence, second line: AmphipaSeeK prediction (parameters: high specificity/low sensitivity/training set=30 monotopic proteins; prediction smoothing used (window size=7, smoothing factor=0.20), A=IPM anchor; third line: predicted secondary structure; fourth line: amphipathy (from 0 low to 5 high amphipathy based on the sequence-average μH). (B) Helical wheel projection of the first 18 N-terminal residues of Nbp1 (helical wheel applet: http://cti.itc.virginia.edu/~cmg/Demo/wheel/wheelApp.html). Colouring: dark yellow—non-polar; green—polar, uncharged; magenta—acidic; light blue—basic. (C) AmphipaSeeK prediction of IPM anchors of Nbp1 proteins from budding yeasts and NucPred score. Some Nbp1 homologues contain a second predicted IPM anchor. *Prediction smoothing not used for Candida glabrata Nbp1. (D) Domain organization of Nbp1 and putative NLS. (E) Comparison of bipartite NLS sequences in Nbp1, Mss4 (phosphoinositide PI4P 5-kinase), Sas2 (histone acetyltransferase subunit of SAS-I) with the classical bipartite NLS of nucleoplasmin (upper part) and ClustalW2 sequence alignment of putative NLSs bound by Kap123 (lower part).
By combining PredictNLS online, NUCDISC (PSORTII Server) and NucPred, we identified two potential nuclear localization sequence (NLSs) close to the IPM anchor: a bipartite (NLS1) consisting of R37–S38–R39–R40–K41 (NLS1a) and K52–R53–P54–R55–K56 (NLS1b) and a monopartite NLS2 (R70–R71–K72–T73–P74–K75–R76–R77) (Figure 1D). The bipartite NLS of Nbp1 resembled that of nucleoplasmin, which is characterized by two positively charged amino acids separated by a linker of 10 amino-acid residues from four basic residues (Figure 1E; Robbins et al, 1991).
The central domain of Nbp1 comprising of amino acids R174–S219 was predicted to be a coiled-coil domain (COILS Server/Lupas's algorithm; Lupas et al, 1991).
Nbp1 localizes to the inner membrane of the NE via an N-terminal IPM anchor
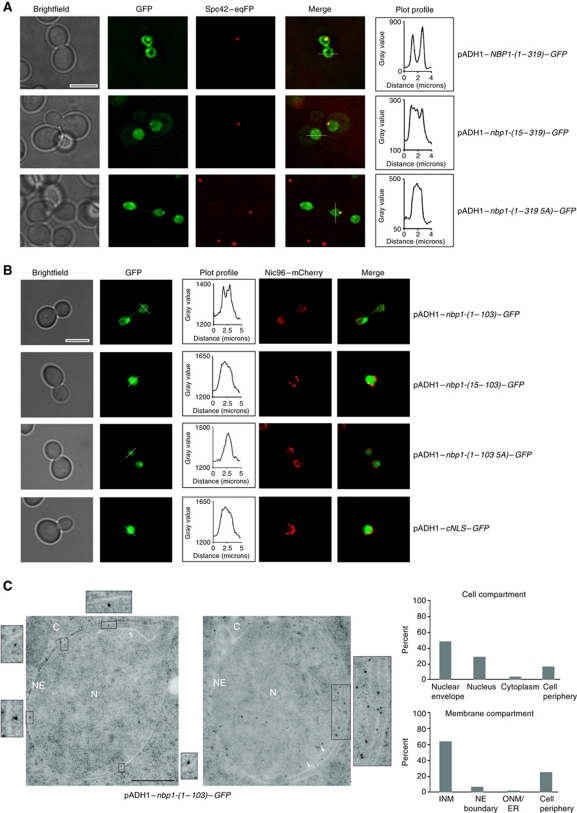
Nbp1 specifically localizes at the SPB and immunoelectron microscopy (EM) data have shown that Nbp1 is associated mainly with the central plaque periphery that contacts the NE (Araki et al, 2006). Thus, it is unclear whether Nbp1 binds to SPBs from the cytoplasmic or the nuclear side. To analyse the targeting route of Nbp1, we fused sequences encoding either full-length NBP1-(1–319) or nbp1-(1–103) that codes for the IPM and the putative NLSs with two GFP sequences (Stade et al, 1997). These gene fusions were expressed from the pADH1 promoter. Nbp1-(1–319)–GFP localized to the SPB and to the NE (Figure 2A), whereas Nbp1-(1–103)–GFP only associated with the NE (Figure 2B). These data indicate that the N-terminal residues 1–103 of Nbp1 are sufficient to target the protein to the NE.
Figure 2.
Targeting of overproduced Nbp1–GFP to the NE depends on the N-terminal IPM anchor of Nbp1. (A, B) The subcellular localization of the indicated Nbp1–GFP (A, full-length; B, N-terminal domain of Nbp1) and cNLS–GFP (B) constructs was analysed at 30°C by fluorescence microscopy. Spc42–eqFP (A) and Nic96–mCherry (B) were used as marker proteins for SPB and the NE, respectively. The GFP fluorescence intensity in the nucleus and the NE (white line) is shown in the plot profile. Bar, 5 μm. (C) Immuno-EM of yeast nbp1-(1–103)–GFP cells. Localization of Nbp1-(1–103)–GFP was analysed with anti-GFP antibody. Three-fold (left) and two-fold (right) enlargements of gold particles close to the inner nuclear membrane are shown. White arrows indicate additional membrane structures forming inside the nucleus (enlargement on the right). The cellular distribution of the Nbp1-(1–103)–GFP signal was quantified as percentage of gold particles in the indicated cell compartment and membrane compartment. Ten distinct sections for a total of 150 gold particles were analysed. C, cytoplasm, INM, inner nuclear membrane; N, nucleus; NE, nuclear envelope; ONM, outer nuclear membrane. Bar, 0.5 μm.
To analyse the importance of Nbp1's N-terminal IPM anchor for membrane localization, we deleted amino-acid residues 1–14 in the full-length (Nbp1-(15–319)) and in the truncated Nbp1–GFP fusion proteins (Nbp1-(15–103)). In an alternative approach, we exchanged the hydrophobic residues Leu2, Val5, Trp9, Phe12, Phe13 within the N-terminal helix of both fusion proteins with Ala (5A mutants), thereby drastically decreasing the hydrophobicity on the hydrophobic face of the helix. The AmphipaSeeK program no longer predicted an IPM anchor in the Nbp1-5A mutant protein. Fluorescence microscopy showed that although Nbp1-(1–319 5A)–GFP, Nbp1-(15–319)–GFP, Nbp1-(1–103 5A)–GFP and Nbp1-(15–103)–GFP accumulated within the nucleus, they were not enriched at the NE and showed the same distribution as cNLS–GFP (Figure 2A and B). This indicates that the N-terminal AH is the major membrane localization signal within Nbp1.
We next analysed the integrity of the Nbp1–GFP fusion proteins in cell extracts by immunoblotting. In all cases, one protein band of the calculated molecular weight was detected with anti-GFP antibodies, excluding proteolytic degradation of the fusion proteins (Supplementary Figure S1).
Our analyses suggest that the overproduced Nbp1 localizes to the NE. To test whether Nbp1-(1–103)–GFP is enriched at one side, the other or both sides of the NE, we performed immuno-EM. The majority of the gold label was detected in close proximity to the inner nuclear membrane (INM; Figure 2C, see enlargements). The EM analysis also showed that the overproduced Nbp1-(1–103)–GFP induced formation of intra-nuclear membranes (Figure 2C, enlargement on the right). Induction of similar membranous structures has previously been described for Nup53 and other proteins with an AH (Marelli et al, 2001).
To further investigate the role of the IPM anchor of Nbp1 in INM targeting, we fused nbp1-(1–20) to the reporter cNLS–GFP (Supplementary Figure S2A) and expressed this gene fusion in yeast cells carrying NIC96–mCherry to mark the NE. cNLS–GFP resided predominantly in the nucleoplasm (Stade et al, 1997; Supplementary Figure S2B), whereas Nbp1-(1–20)–cNLS–GFP was targeted to the nuclear rim (Supplementary Figure S2B). We therefore concluded that the presence of an N-terminal IPM anchor and a NLS is sufficient for NE targeting.
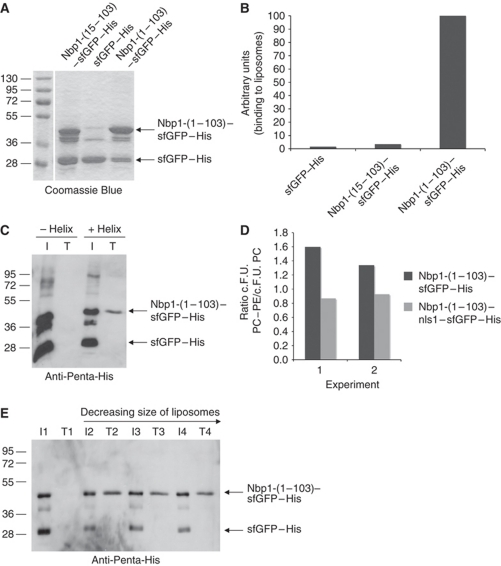
Nbp1 interacts with phosphatidylcholine liposomes in a manner that is dependent upon its IPM motif
To exclude the possibility that binding of Nbp1 to membranes is indirect and occurs via an interaction with other membrane proteins rather than a direct association with membrane lipids, we performed two types of liposome-binding assays. With the first assay, we monitored whether the GFP-labelled proteins bind to liposomes in a flow cytometric assay (FACS; Temmerman and Nickel, 2009). In the second, we used a flotation assay to enable us to assess affinity of proteins for liposomes as their buoyancy promoted migration through a density gradient (Bigay and Antonny, 2005). We purified recombinant Nbp1-(1–103)–sfGFP–His and Nbp1-(15–103)–sfGFP–His proteins from Escherichia coli (Figure 3A) and tested their binding to phosphatidylcholine (PC) containing liposomes. To aid visualization, these liposomes had been supplemented with 1 mol% rhodamine-labelled phosphatidylethanolamine (PE). In this experiment, superfolder GFP (sfGFP) was used to tag Nbp1 and its derivatives because it folds regardless of the solubility of the fusion partner (Pedelacq et al, 2006). In both, the flow cytometric analysis (Figure 3B) and flotation assays (Figure 3C), Nbp1-(1–103)–sfGFP–His bound to liposomes. Importantly, deletion of the N-terminal AH in Nbp1 (Nbp1-(15–103)–sfGFP–His) led to a drastic reduction in liposome association (Figure 3B and C), suggesting that the IPM anchor was responsible for the association between the protein and lipids. Both FACS and immunoblot analysis of samples of the flotation assay established that sfGFP–His, which was present as a contaminating protein in Nbp1–sfGFP–His preparations, did not bind to the liposomes (Figure 3B and C).
Figure 3.
Recombinant Nbp1-(1–103)–sfGFP–His binds to liposomes. (A) SDS–PAGE analysis of purified Nbp1 proteins used for liposome-binding assays. (B) Liposome binding analysed by using FACS. Fluorescence-labelled liposomes were incubated with 11 μg recombinant Nbp1 protein in 100 μl buffer, sorted via flow cytometry and GFP fluorescence was detected. The relative GFP fluorescence of Nbp1-(1–103)–sfGFP–His bound to liposomes was set to 100 (importance of AH for liposome binding was confirmed in three independent FACS experiments). (C) Binding of Nbp1-(15–103)–sfGFP–His (−helix) and Nbp1-(1–103)–sfGFP–His (+helix) to liposomes as analysed by the flotation assay. About 7.5 μg of each protein was incubated with liposomes extruded through a polycarbonate filter of 400 nm pore size. Lipid-bound proteins from the top fraction T of the sucrose gradient were analysed by immunoblotting with an anti-Penta-His antibody (15% of each of the top fractions was used) in comparison with the input (I, 3.75% of each assay was used). (D) Binding of Nbp1-(1–103)–sfGFP–His to liposomes depends on the lipid composition. In contrast to Nbp1-(1–103)–nls1–sfGFP–His, Nbp1-(1–103)–sfGFP–His bound better to PC (89 mol%)/PE (10 mol%)/PE-rhodamine (1 mol%) liposomes than to PC liposomes. Two independent FACS experiments are shown; c.F.U. (corrected fluorescence units; Temmerman and Nickel, 2009). (E) Binding of Nbp1-(1–103)–sfGFP–His protein to liposomes of different curvature. Nbp1-(1–103)–sfGFP–His (about 5 μg) was incubated with PC-liposomes extruded through polycarbonate filters of decreasing pore size (2: 400 nm, 3: 100 nm, 4: 50 nm) or without liposomes (assay 1). Lipid-bound proteins from the top fraction (T, 15% of each of the top fraction was set in) of the sucrose gradient were analysed by immunoblotting with an anti-Penta-His antibody in comparison with the input (I, 1.875% of each assay was used).
To analyse if liposome binding of Nbp1 depends on the lipid composition, we used PC liposomes supplemented with 10 mol% PE. PE is next to PC, the most abundant phospholipid of the yeast nuclear membrane (Blagovic et al, 2001). Binding of Nbp1-(1–103)–sfGFP to PC/PE-liposomes was increased by about 50% compared with PC-liposomes. However, if the positively charged residues of the putative nuclear localization signals NLS-1a and NLS-1b were exchanged for Asn/Gln (nls1 mutations), no increase in liposome binding was observed (Figure 3D), indicating that positive charges besides the IPM anchor can serve as secondary membrane-binding element.
We also asked whether Nbp1-(1–103)–sfGFP–His binding showed a preference for liposomes of a particular size. However, equal binding efficiencies were observed in the floatation assay for a range of size classes of liposomes, suggesting that the affinity of the IPM anchor of Nbp1 for lipids, under our experimental conditions, is not sensitive to the degree of membrane curvature (Figure 3E).
Taken together, these data indicate that the IMP anchor of Nbp1 directly interacts with membranes.
Impairment of the IPM anchor of Nbp1 causes defects in SPB duplication
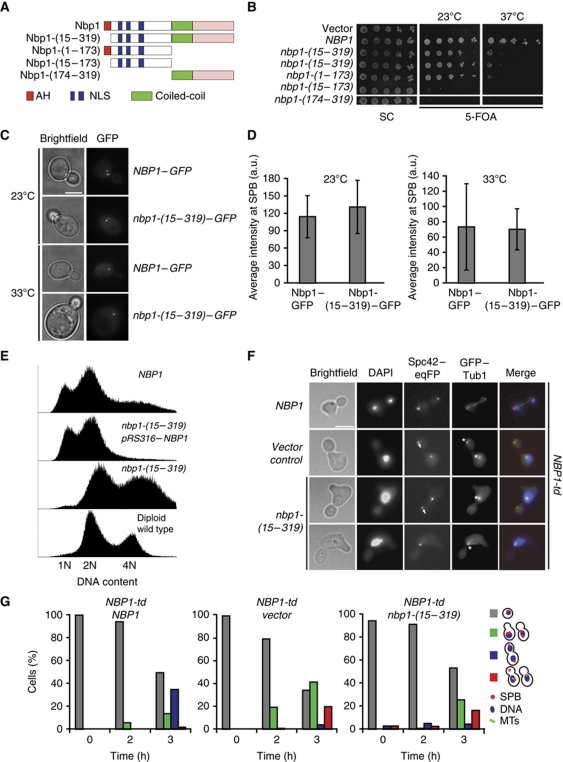
NBP1 is an essential gene and temperature induced degradation of an Nbp1-degron construct (Nbp1-td) leads to defects in the insertion of the newly assembled SPB into the NE and genetic instability (Araki et al, 2006). Therefore, if the AH domain is important for the essential function of Nbp1 in SPB insertion, we expected to see defects in cell growth, maintenance of ploidy and SPB duplication in the NBP1 mutants that lacked the functional IPM anchor (Araki et al, 2006).
We addressed this issue, by using chromosomally integrated NBP1 deletion constructs transcribed under the control of endogenous promoter. We first analysed the requirement for different regions of Nbp1 for the provision of Nbp1's essential function. Nbp1-(1–173) lacking both, the central coiled-coil (codons 174–219) and the C-terminal domain (codons 220–319), allowed growth of cells at 23°C (Figure 4A and B). In contrast, the C-terminal portion of the molecule Nbp1-(174–319), which was present at lower protein levels than Nbp1 (Supplementary Figure S3A), did not provide essential Nbp1 functions (Figure 4A and B). Importantly, inactivation of the IPM anchor in the Nbp1-(1–173) construct by either deletion of the entire motif (Nbp1-(15–173)) (Figure 4A and B) or point mutations within the motif (Nbp1-(1–173 5A)) (Supplementary Figure S3B) impaired the essential function of Nbp1. In contrast, impairment of the IPM anchor in the full-length Nbp1 (Nbp1-(15–319) and Nbp1-(1–319 5A)) caused a conditional lethal growth defect of cells at 37°C (Figure 4A and B; Supplementary Figure S3B). Immunoblot analysis showed that Nbp1-(1–319), Nbp1-(15–319) and Nbp1-(1–319 5A) were expressed to similar levels (Supplementary Figure S3A). These data establish that the IPM anchor is important to fulfil an essential function of Nbp1.
Figure 4.
The AH of Nbp1 is essential for insertion of the SPB. (A) Schematic representation of Nbp1 domains and deletion constructs. Red: AH, amphipathic α-helix; blue: NLS1 and NLS2; green: coil-coiled; pink: C-terminal domain of NBP1. (B) Test of functionality of NBP1 constructs of (A). Five-fold serial dilutions of nbp1Δ pRS316–NBP1 cells with the indicated NBP1 integration plasmids were incubated at 23 and 37°C on 5-FOA and SC plates. (C) SPB localization of the indicated GFP constructs in cells. (D) Quantification of SPB signal of NBP1–GFP and nbp1-(15–319)–GFP cells. Ten SPBs in six different cells were analysed. (E) DNA content of the indicated cells grown at 23°C as determined by FACS analysis. (F) NBP1-td cells with NBP1, vector or nbp1-(15–319) were synchronized with α-factor at 23°C and released from G1-arrest in YPR medium containing galactose at 37°C. Samples were taken every hour, fixed, stained with DAPI and analysed for spindle formation and the occurrence of a ‘dead pole’. Pictures taken after 3 h at 37°C are shown. Arrows and asterisks mark ‘dead pole’ and monopolar spindle, respectively. (G) Quantification of cells of (F). Cells were categorized according to DAPI signal as indicated in the cartoons on the right (red dot, SPB; blue circle, DNA; green lines, microtubules). Red bars in the histogram represent the percentage of cells with monopolar spindle or ‘dead pole’. Grey bar, non-budded cells; green bar, budded cells with one DAPI signal; blue bar, large-budded cells with two DAPI signals and two functional SPBs. N⩾100 cells were analysed per time point.
A comparison of the efficiency of SPB targeting of wild-type Nbp1–GFP with that of the Nbp1-(15–319)–GFP mutant that lacks the AH revealed a similar binding efficiency of each protein for SPBs (Figure 4C and D) at 23 and 33°C (the restrictive temperature of nbp1-(15–319)–GFP cells (Supplementary Figure S3C)). Thus, the AH anchor is not important for the association of Nbp1 with the SPB. Importantly, Nbp1-(1–173)–GFP also stained the nuclear rim confirming that the N-terminal portion of Nbp1 has an intrinsic NE targeting activity (Supplementary Figure S3D).
nbp1-(15–319) cells were larger than NBP1 wild-type control cells (Supplementary Figure S3E). This could indicate a change in ploidy, a phenotype that is frequently associated with SPB duplication mutants (Chial et al, 1999). Indeed, analysis of several independently constructed nbp1-(15–319) cells showed that their ploidy exceeded that of wild-type diploids even when cells were constantly maintained at 23°C (Figure 4E). We suggest that this is an intrinsic phenotype arising as a consequence of the deletion of the IPM anchor of Nbp1. By altering gene dosage, cells may be able to accumulate a suppressing gene that then partially compensates for the loss of the IPM anchor of Nbp1 (Torres et al, 2010).
We assessed spindle formation in α-factor synchronized NBP1 and nbp1-(15–319) cells carrying SPC42–mCherry and GFP–TUB1 to visualize SPBs and microtubules. nbp1-(15–319) cells arrested in cell-cycle progression as large-budded cells with a defective spindle at 37°C. As has been reported for NBP1-td cells (Araki et al, 2006), several cells displayed a phenotype in which only one SPB was functional and associated with nuclear microtubules, while the second SPB was either absent (Supplementary Figure S3E, lower panel) or present as a ‘dead pole’, nucleating only cytoplasmic and not nuclear microtubules (Supplementary Figure S3E, middle panel). The frequency of the ‘dead pole’ was difficult to quantify because of the distorted and enlarged morphology of nbp1-(15–319) cells. α-Factor synchronized NBP1 and nbp1-(15–319) cells were also characterized by EM confirming that nbp1-(15–319) cells at 37°C had defects in SPB insertion into the NE (Supplementary Figure S4).
The increased ploidy of nbp1-(15–319) cells may partly suppress the SPB insertion defect, thus helping the cells to survive in the presence of the mutation. To prevent adaptation, we integrated NBP1, nbp1-(15–319) and the empty plasmid into the genome of NBP1-td cells, in which Nbp1-td can be degraded upon induction of Gal1–UBR1 at 37°C (Araki et al, 2006), allowing us to analyse the phenotype of the nbp1 gene integrated as single copy into the LEU2 locus. NBP1-td cells carrying either the empty integration plasmid or NBP1-td nbp1-(15–319) gave rise to large-budded cells with a monopolar spindle and a ‘dead pole’ (Figure 4F, indicated with asterisks and arrows, respectively; Figure 4G). The frequency of this defect (20% for NBP1-td empty plasmid; 16% for NBP1-td nbp1-(15–319)) was in the range reported for NBP1-td cells (30%; Araki et al, 2006). In contrast, a ‘dead pole’ phenotype was rarely observed in NBP1-td NBP1 cells (Figure 4G). The ‘dead pole’ phenotype was analysed in greater detail in an additional experiment using NBP1-td SPC42–eqFP SPC110–GFP cells. Spc110 only becomes incorporated into the SPB upon duplication plaque insertion, while Spc42 is part of the duplication plaque (Jaspersen and Winey, 2004). Thus, a ‘dead pole’ contains Spc42-eqFP but no Spc110-GFP. In nbp1-(15–319) cells Spc110–GFP was frequently not associated with the newly assembled SPB, indicating an SPB insertion defect (Supplementary Figure S5).
Together, these data show that the IPM anchor in Nbp1 is essential for the insertion of the duplication plaque into the SPB but is not required for the targeting of the protein to the SPB.
The SPB protein Nbp1 interacts with the karyopherin Kap123
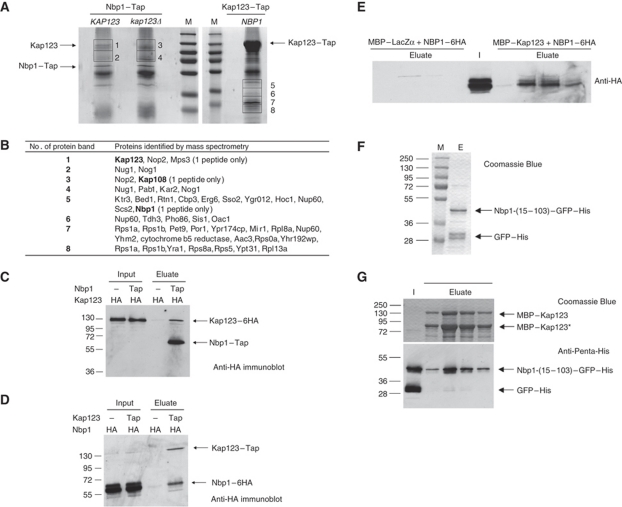
To get a further understanding of the function of Nbp1 in SPB membrane insertion, we purified Nbp1 interacting proteins using Nbp1–Tap as bait. Mass spectrometry (MS) of these precipitates identified the karyopherin Kap123 (Rout et al, 1997; Schlenstedt et al, 1997) as a major Nbp1-binding partner (Figure 5A and B). Conversely, Nbp1 was identified in Kap123–Tap purifications. Repetition of the Tap purification using yeast cells in which the predicted prey protein (either Kap123 or Nbp1) was fused with six copies of the HA-epitope tag confirmed interaction between Nbp1 and Kap123 (Figure 5C and D).
Figure 5.
Nbp1 interacts with Kap123. (A) Purification of Nbp1–Tap and Kap123–Tap proteins. Nbp1–Tap was either purified from NBP1–Tap KAP123 cells or from NBP1–Tap kap123Δ cells and separated by SDS–PAGE. Lane M, molecular weight marker (from top to bottom: 250, 130, 95, 72, 55, 36 and 28 kDa). Copurified proteins in the molecular weight range of ∼70–140 kDa (gel slices 1–4) were analysed by MS. In case of Kap123–Tap, proteins between 20 and 45 kDa (gel slices 5–8) were analysed by MS. (B) Identification of proteins that copurified with Nbp1–Tap and Kap123–Tap by MS. Only proteins above a Mascot threshold score of 100 are listed. Heat shock proteins and translation elongation factors were omitted. Kap123 was identified by 21 peptide matches (coverage of 17.7%; result was confirmed in a second independent Nbp1–Tap purification) in slice 1 (NBP1–Tap KAP123), but is missing in slice 3 (NBP1–Tap kap123Δ). In gel slice 5, Nbp1 (37.4 kDa) was identified with one peptide, which is explained by the low copy number of Nbp1. (C) Anti-HA (12CA5) immunoblot of Nbp1–Tap purification from KAP123–6HA NBP1 cells (negative control) and KAP123–6HA NBP1–Tap cells. Input, total soluble proteins after extraction with 1% Triton X-100; eluate, proteins eluted from washed IgG-dynabeads. The antibody also binds to the protein A tag of the Tap-tag. (D) Immunoblot analysis of Kap123–Tap purification from KAP123 NBP1–6HA cells (negative control) and KAP123–TAP NBP1–6HA cells using an anti-HA antibody. Input: total soluble proteins after extraction with 1% Triton X-100. Eluate: proteins eluted from washed IgG-dynabeads. (E) Nbp1-(1–319)–6HA interacts with MBP–Kap123 but not with MBP–LacZα. Total soluble proteins after extraction with 1% Triton X-100 from NBP1–6HA cells (I, input) were applied either to amylose–MBP–LacZα material or to amylose–MBP–Kap123. After washing, proteins were eluted in four fractions with 10 mM maltose (eluate) and analysed by immunoblotting with an anti-HA antibody. (F) Purification of Nbp1-(15–103)–GFP–His from E. coli. M, molecular weight standards; E, eluate from the NiNTA column. Nbp1-(15–103)–GFP–His was partially degraded to GFP–His. (G) Nbp1-(15–103)–GFP–His binds to MBP–Kap123. Purified Nbp1-(15–103)–GFP–His was applied to an amylose–MBP–Kap123 column. Proteins co-eluted with MBP–Kap123 (SDS–PAGE) were analysed by immunoblotting using an anti-Penta-His antibody. I, input; eluate (elution fractions 1–4).
Interestingly, other proteins that copurified with Kap123–Tap also posses when analysed with the AmphipaSeeK program putative IPM anchors: the NPC protein Nup60 (binds to Kap123; Krogan et al, 2006), the ribosomal proteins Rps1a and 1b (interact with Kap123; Gavin et al, 2006) and the Hsp40 co-chaperon Sis1. Whether Kap123 binds preferentially to proteins with an NLS next to an AH requires further experimentation.
Using an in vitro binding assay, we asked whether Nbp1 bound directly to Kap123. We did not succeed in purifying full-length Nbp1-(1–319) protein due to its insolubility. However, we demonstrated that Nbp1-(1–319)–6HA of yeast cell extracts bound to immobilized MBP–Kap123 but not to MBP–LacZα (Figure 5E). Since nuclear localization signals are only predicted within the N-terminal domain of Nbp1 (Figure 1D), we used the purified Nbp1-(15–103)–GFP–His for the analysis of direct binding between Nbp1 and Kap123 (Figure 5F). Recombinant MBP–Kap123 that was partially degraded to a shorter fragment (MBP–Kap123*) was tested as the binding partner. After washing the beads, MBP–Kap123 and Nbp1-(15–103)–GFP–His co-eluted, confirming the interaction between the two proteins (Figure 5G). In contrast, GFP–His did not bind to MBP–Kap123. Together, these data suggest that Kap123 binds directly to Nbp1.
Kap123 binds the bipartite NLS1 of Nbp1
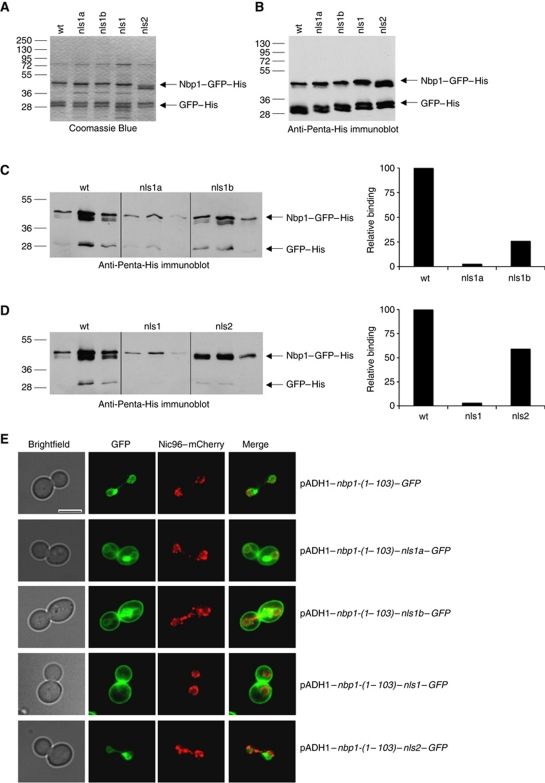
As outlined above (Figure 1D and E), Nbp1 has two putative NLS motifs, a bipartite NLS1 consisting of NLS1a and NLS1b and a monopartite NLS2. We mutated all of the Lys residues in these three sequences to Asn and all Arg residues to Gln (nls1a, R37Q–S38–R39Q–R40Q–K41N; nls1b, K52N–R53Q–P54–R55Q–K56N; nls1, R37Q–S38–R39Q–R40Q–K41N.–.K52N–R53Q–P54–R55Q–K56N; nls2, R70Q–R71Q–K72N–T73–P74–K75N–R76Q–R77Q). We then analysed the affinity of these Nbp1–NLS mutants for Kap123 in the in vitro binding assay and characterized the localization and functionality of the mutant proteins.
For the in vitro experiments, the four different mutant Nbp1-(15–103)–GFP–His proteins were purified from E. coli (Figure 6A and B) and their ability to bind immobilized MBP–Kap123 was compared with wild-type Nbp1-(15–103)–GFP–His. Nbp1–nls2 association with MBP–Kap123 was about 60% of that exhibited by the wild-type protein, whereas the binding of Nbp1–nls1a and Nbp1–nls1b was reduced by 97 and 74%, respectively (Figure 6C and D). The Nbp1–nls1 double mutant exhibited similar behaviour to Nbp1–nls1a (Figure 6C and D). In summary, the in vitro binding experiments suggested that the direct association between Nbp1 and Kap123 occurs mainly via NLS1.
Figure 6.
Identification of a bipartite NLS in Nbp1. (A, B) Purified recombinant mutant Nbp1-(15–103)–GFP–His proteins were analysed by SDS–PAGE (A) and by immunoblotting using anti-Penta-His antibodies (B): wt, Nbp1-(15–103)–GFP–His; nls1a, Nbp1-(15–103)–nls1a–GFP–His; nls1b, Nbp1-(15–103)–nls1b–GFP–His; nls1, Nbp1-(15–103)–nls1–GFP–His; nls2, Nbp1-(15–103)–nls2–GFP–His. (C) Binding of wt, and mutant Nbp1-(15–103)–GFP–His proteins (nls1a and nls1b) to MBP–Kap123 (for experimental details see Supplementary data). Proteins were eluted with 10 mM maltose from amylose–MBP–Kap123 beads in three fractions. Proteins were analysed by immunoblotting with anti-Penta-His antibodies (left part). The amount of eluted Nbp1–GFP–His proteins was quantified from a shorter exposure of the blot (right part). (D) Binding of wt and mutant (nls1 and nls2) Nbp1-(15–103)–GFP–His proteins to MBP–Kap123 was analysed as shown in (C). Comparison of input proteins (A, B) with proteins eluted from amylose–MBP–Kap123 columns shows that there is only minor unspecific binding of sfGFP–His. (E) NE targeting of Nbp1-(1–103)–GFP depends on a bipartite NLS. The subcellular localization of the indicated Nbp1–GFP proteins was analysed at 30°C by fluorescence microscopy. Bar, 5 μm.
Using pADH1–NBP1–GFP reporter constructs, we confirmed the importance of NLS1 for the nuclear import of Nbp1. Mutations in NLS2 of Nbp1 did not alter NE targeting of either Nbp1-(1–103)–nls2–GFP (Figure 6E) or Nbp1-(1–319)–nls2–GFP (Supplementary Figure S6). However, NLS1a or NLS1b mutants were partially mislocalized to the cell periphery. When both NLS1a/b motifs were mutated simultaneously, Nbp1-(1–103)–nls1–GFP was mainly present at the cell periphery but hardly detectable at the NE (Figure 6E).
When NLS1a and NLS1b were mutated in full-length Nbp1, the overproduced protein Nbp1-(1–319)–nls1–GFP was no longer at the NE but resided at the SPB and punctuate signals in the cytoplasm and at the cell periphery (Supplementary Figure S6). Thus, localization studies of the Nbp1–nls mutants emphasises the importance of NLS1 for NE targeting of Nbp1.
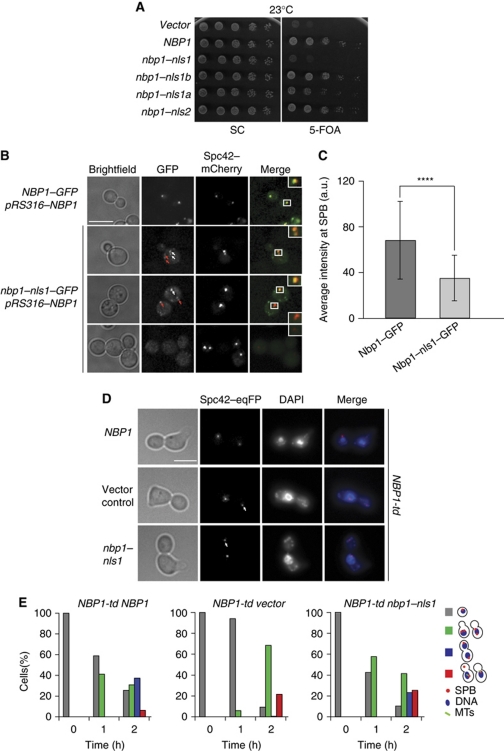
NLS1 of NBP1 is essential for viability
We next asked whether the bipartite NLS1 (NLS1a and NLS1b) and the monopartite NLS2 of Nbp1 are important for the protein to fulfil its essential function. Mutations in only NLS1b or NLS2 of NBP1 did not affect cell growth (Figure 7A). In contrast, nbp1–nls1a conferred a weak slow growth phenotype, while nbp1–nls1 cells (mutations in NLS1a and NLS1b) were inviable (Figure 7A). We also compared the localization of Nbp1–GFP and Nbp1–nls1–GFP. Because nbp1–nls1 is non-functional, we assessed the distribution of Nbp1–nls1–GFP in the presence of wild-type Nbp1. Both, Nbp1–GFP and Nbp1–nls1–GFP, were recruited to SPBs (Figure 7B, white arrows). However, the SPB signal of Nbp1–nls1–GFP was lower than that of Nbp1–GFP (Figure 7C). Moreover, while Nbp1 only localized at SPBs, Nbp1–nls1 also gave additional punctuate staining, mostly at the cell periphery (Figure 7B, red arrows). Immunoblot analysis showed that Nbp1-(1–319)–nls1 and Nbp1-(1–319) were expressed to similar levels (Supplementary Figure S3A). We conclude that the bipartite NLS1 of Nbp1 is essential for function and proper localization of the protein.
Figure 7.
The NLS1 of Nbp1 is essential for the NE insertion of the newly assembled SPB. (A) Test of functionality of nbp1–nls1, nbp1–nls1a, nbp1–nls1b and nbp1–nls2. All constructs are expressed as C-terminal GFP fusions. nbp1Δ pRS316–NBP1 was transformed with the indicated NBP1 integration plasmids. Five-fold serial dilutions of cells were incubated at 23°C on 5-FOA and SC plates. (B) SPB localization of the indicated GFP constructs. NBP1–GFP/NBP1 and nbp1–nls1–GFP/NBP1 cells were analysed for the localization of the GFP-tagged Nbp1. Spc42–mCherry was used as SPB marker. White arrows indicate Nbp1–GFP or Nbp1–nls1–GFP that co-localizes with the SPB. Red arrows highlight GFP signals near the cell cortex. The insets on the right are two-fold magnifications of the boxed signals. Bar, 5 μm. (C) Quantification of the Nbp1–GFP and Nbp1–nls1–GFP SPB signals of (B). N=44 cells. ****P⩽10−4. (D) α-Factor synchronized NBP1-td SPC42–eqFP cells with NBP1–GFP, vector or nbp1–nls1–GFP were analysed as described in Figure 4F. The pictures of fixed cells were taken after 2 h at 37°C. The white arrows indicate ‘dead poles’. Bar, 5 μm. (E) Quantification of the experiment of (D). Cells were categorized as indicated in the cartoons on the right (see Figure 4G for description).
To understand whether Nbp1–nls1 is defective in SPB insertion, we analysed the phenotype of α-factor synchronized NBP1-td (degron) cells transformed with NBP1–GFP, nbp1–nls1–GFP and the empty plasmid upon induction of Gal1–UBR1 at 37°C (to induce destruction of the wild-type molecule). NBP1-td nbp1–nls1–GFP and NBP1-td harbouring the empty plasmid accumulated a similar number of large-budded cells with a ‘dead pole’ (Figure 7D, white arrows; and Figure 7E; Supplementary Figure S5). In contrast, only a minor fraction of the NBP1-td NBP1–GFP cells exhibited this phenotype. Thus, the NLS1 of Nbp1 is important for the insertion of the duplication plaque into the NE.
Nbp1 is mislocalized in both kap123Δ cells and mutants that disrupt the RanGTP cycle but not in kap108Δ or nup170Δ cells
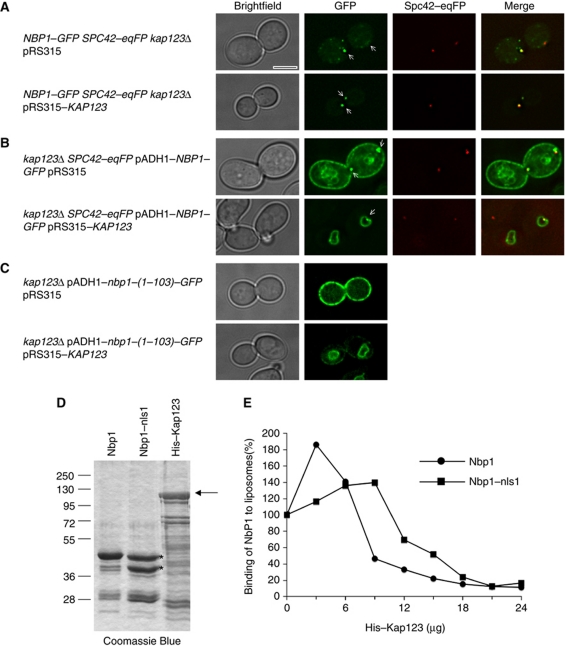
To investigate whether the observed interaction between Nbp1–Kap123 is important for NE and SPB targeting, we assessed whether endogenous Nbp1–GFP and overproduced Nbp1–GFP proteins are mislocalized in kap123Δ cells. In kap123Δ cells, Nbp1–GFP and overexpressed Nbp1-(1–319)–GFP localized at the cell periphery and at the SPB (Figure 8A and B; Supplementary Figure S7). Similarly, the shorter Nbp1-(1–103)–GFP protein, that in KAP123 wild-type cells bound to the NE (Figure 2B), was mislocalized to the cell periphery in kap123Δ cells (Figure 8C; Supplementary Figure S8). In all cases, the kap123Δ phenotype was complemented by plasmid pRS315–KAP123 (Figure 8A–C). Recently, it has been shown that kap123Δ cells diploidize at an increased frequency and that the frequency of large mononucleated cells is enhanced in kap123Δ cultures (Ptak et al, 2009). We observed a similar increase in kap123Δ cell size, especially when full-length Nbp1 was overproduced (Figure 8A–C).
Figure 8.
Mislocalization of Nbp1–GFP in kap123Δ cells and blocking of liposome binding of Nbp1 by Kap123. (A–C) The subcellular localization of chromosomally GFP-tagged Nbp1 (A) or the indicated overproduced Nbp1–GFP proteins (B, C) was analysed in kap123Δ SPC42–eqFP cells with pRS315 (upper panel) or pRS315–KAP123 (lower panel) by fluorescence microscopy at 30°C. Arrows mark the SPB signal. Note, only one Z-plane is shown, so that not all SPBs are visible. Bar, 5 μm. (D) SDS–PAGE analysis of purified Nbp1-(1–103)–sfGFP–His, Nbp1-(1–103)–nls1–sfGFP–His and His–Kap123 proteins (arrow). (E) Binding of Nbp1-(1–103)–sfGFP–His to liposomes is impaired in the presence of His–Kap123. Nbp1-(1–103)–sfGFP–His and Nbp1-(1–103)–nls1–sfGFP–His were incubated with fluorescence-labelled liposomes (extruded through a 400-nm filter) in a total volume of 100 μl in the presence of increasing amounts of purified His–Kap123 protein (0–24 μg; 18 μg of His–Kap123 correspond to a molar ratio of about 1 for Nbp1-(1–103)–sfGFP–His). Nbp1-(1–103)–nls1–sfGFP–His concentration was adjusted in such a way that the sum of the amounts of both prominent protein bands (marked with asterisks; lower band is probably a degradation product lacking the AH and additional N-terminal residues) corresponds to the amount of Nbp1-(1–103)–sfGFP–His (about 6 μg). Thus, the used molar ratio of Kap123/Nbp1 is at least equal for both proteins but most likely higher for Nbp1–nls1 than for Nbp1. The corrected GFP fluorescences (c.F.U. units) of Nbp1-(1–103)–sfGFP–His and Nbp1-(1–103)–nls1–sfGFP–His bound to liposomes in the absence of His–Kap123 were set to 100.
Evidence for nuclear import in the absence of Kap123 was obtained using Nbp1-(15–103)–GFP. The Nbp1-(15–103)–GFP mutant, which lacks the N-terminal IPM anchor motif, predominantly accumulated in the nuclei of wild-type and kap123Δ cells (Supplementary Figure S8), while Nbp1-(1–103)–GFP accumulated at the cell cortex (Figure 8C). These data suggest that Nbp1 binds to other karyopherins, but there is a specific role for Kap123 in both preventing unspecific membrane association of Nbp1 and targeting it to the INM. A candidate for such an additional karyopherin is Kap108 because it was also found in the Nbp1 purification (Figure 5B). However, Nbp1–GFP distribution was unaffected by removal of Kap108 (Supplementary Figure S7). In addition, Nbp1–GFP was also correctly localized in nup170Δ cells (Supplementary Figure S7). Nup170 is important in targeting of the integral membrane proteins Heh1 and Heh2 to the inner NE (King et al, 2006).
To confirm that transport of Nbp1 into the nucleus occurs via the Ran-dependent nuclear import pathway (reviewed in Wente and Rout, 2010), we analysed the localization of the Nbp1-(1–103)–GFP reporter protein in conditional lethal rna1-1 (encoding RanGAP), prp20-1 and prp20-G282S (RanGEF) cells. In the W303 wild-type cells incubated at 34°C, the restrictive temperature for the RAN cycle mutants, most of the reporter resided at the NE. In contrast, in all mutant cells, the Nbp1-(1–103)–GFP signal associated with the cell periphery (Supplementary Figure S9). The RAN GTP cycle is essential for proper localization of Nbp1-(1–103)–GFP.
Kap123 inhibits binding of Nbp1-(1–103)–sfGFP–His to liposomes
By binding to Nbp1, Kap123 may prevent the interaction of the IPM anchor with non-nuclear membranes. In this model, release of Nbp1 from Kap123 by RanGTP in the nucleus would target Nbp1 to the INM. To support this model, we analysed, whether Kap123 reduced the binding of Nbp1 to liposomes in vitro. Increasing His–Kap123 concentrations strongly impaired the association of Nbp1–(1–103)–sfGFP–His with liposomes (Figure 8D and E). To address the specificity of the Kap123 effect on Nbp1 binding to liposomes, we incubated the liposomes with the mutant protein Nbp1-(1–103)–nls1–sfGFP–His, which has decreased binding affinity for Kap123 (Figure 6). Clearly, higher concentrations of Kap123 were necessary to obtain reduction in liposome binding for Nbp1-(1–103)–nls1–GFP (Figure 8E). Interestingly, this analysis also indicated that Kap123 changed the shape index of the liposomes (Supplementary Figure S10; Temmerman and Nickel, 2009). We conclude that Kap123 attenuates the membrane-binding properties of Nbp1 by blocking membrane-binding elements.
Discussion
The Nbp1–Ndc1–Mps2–Bbp1 protein network is needed for insertion of the large cytoplasmic SPB precursor into the NE. How and from which side of the nucleus this insertion machine works is unclear. The data described in this manuscript unravel the targeting mechanism for the INM protein Nbp1 by the karyopherin Kap123. In addition, we provide first insights into the mechanism by which Nbp1 promotes SPB insertion.
Targeting of Nbp1 to the inner NE by a Kap123 driven pathway
Nbp1 is a SPB-associated protein that by immuno-EM was identified as residing mainly at the periphery of the SPB central plaque (Araki et al, 2006), the site at which the inner and outer membranes of the NE fuse (Byers and Goetsch, 1975). This localization raises the question as to whether Nbp1 functions from the cytoplasmic, the nuclear or from both sides of the NE. A nuclear function would demand a nuclear routing mechanism for Nbp1.
Bioinformatic analysis of proteins of the SPB insertion network predicted an N-terminal IPM anchor next to two putative NLS sequences in the N-terminus of Nbp1 (Figure 1). We now propose a model in which the shielding of the IPM anchor sequence and possibly other membrane-active Nbp1 elements by the karyopherin Kap123 hinders the ability of this IPM to associate with membranes until the association between Kap123 and Nbp1 is disrupted by the RAN system following nuclear import. In this model, Kap123 binds to the bipartite NLS1 immediately after ribosomal biosynthesis of Nbp1. As NLS1 sits right next to the IPM anchor (Figure 1), this docking of Kap123 blocks the ability of the anchor to directly insert into membranes. In vivo and vitro experiments support that Kap123 has the ability to block binding of Nbp1 to membrane structures (Figure 8). In summary, the NE association of Nbp1 relies on the presence of Kap123, the RAN system and the IPM anchor and NLS1 of Nbp1.
Integral membrane proteins are synthesized at the ER and diffuse freely between ER and outer nuclear membrane. The import of integral INM proteins can then occur either by lateral diffusion or by a Kap60/Kap95-dependent pathway through the peripheral channels of the NPC (Zuleger et al, 2008). The Kap123–Nbp1 pathway is different from the Kap60/Kap95-dependent import of the integral INM proteins Heh1 and Heh2 (King et al, 2006; Lusk et al, 2007). This pathway requires the NPC proteins Nup2 and Nup170. An alternative pathway is defined by the integral INM protein Doa10. Doa10 has no obvious NLS and trafficking to the INM depends on Nup188 and Pom152 but not Nup2 (Deng and Hochstrasser, 2006). However, nuclear import of Nbp1 requires an NLS and is independent on NUP170 (Figure 6; Supplementary Figure S7).
The two-signal nature of the Kap123–Nbp1 pathway bears similarity with the targeting of other peripheral INM proteins (Holtz et al, 1989; Resh, 1996; Hofemeister et al, 2000; Lai et al, 2009). In lamins, an NLS is combined with a peptide motif that is subjected to lipidation and in Trm1, an NLS is accompanied by an ∼20 amino acids motif (residues 133–151) that contains the INM targeting information (Lai et al, 2009); using the AmphipaSeeK prediction program, we failed to identify an IPM anchor in this region of Trm1). Nup53 and Pct1 are additional examples for nuclear membrane-associated proteins; as Nbp1 they contain an amphipathic helical domain that confers membrane association and an NLS for Kap121 (Nup53) or Kap60/Kap95 (Pct1)-dependent nuclear import (Marelli et al, 2001; Lusk et al, 2002; MacKinnon et al, 2009; Dennis et al, 2011).
KAP123 is a non-essential gene (Rout et al, 1997). In contrast, nuclear import of Nbp1 is essential for viability (Figure 7). Thus, it is likely that other karyopherins have overlapping functions with Kap123 in Nbp1 binding and INM targeting. It is well established that cargoes for nuclear import are often recognized by several karyopherins (Chook and Süel, 2010).
The identification of Kap123 as an importin for Nbp1 was unexpected, since Kap123 was originally discovered as a karyopherin for ribosomal proteins and histones (Rout et al, 1997; Schlenstedt et al, 1997). The ribosomal proteins and histones do not contain classical bipartite nuclear localization signals, leading to the assumption that Kap123 mediates a distinct import pathway. In the meantime, however, it is clear that several other karyopherins are also involved in nuclear import of histones (Greiner et al, 2004; Chook and Süel, 2010). Furthermore, it has now been shown that nuclear import of proteins with classical bipartite NLS motifs can also be Kap123 dependent. Examples are Sas2 (Schaper et al, 2005) and Mss4 (Audhya and Emr, 2003), although it remains to be determined whether Kap123 directly interacts with the NLS of these proteins. Interestingly, the bipartite NLS of Sas2 shares similarity with a sequence of histones H3 and H4 that could function as an NLS (Figure 1E; Schaper et al, 2005). In this current study, we have demonstrated that the NLS1a and NLS1b motifs of Nbp1 are important for the Nbp1/Kap123 interaction. However, the specific amino acids of NLS1 that mediate this interaction remain to be investigated.
Membrane insertion of the SPB
The observation that nbp1–nls1 cells have similar defects in SPB insertion as NBP1 null cells is consistent with the notion that the nuclear import of Nbp1 is essential for SPB insertion (Figure 7). We therefore suggest that Nbp1 has an essential function in SPB NE insertion from within the nucleus. Nuclear processes also contribute to the de novo assembly of NPCs (D’Angelo et al, 2006; Yavuz et al, 2010).
What is the nuclear function of Nbp1 in the SPB duplication process? In G1 cells, the SPB carries the satellite structure at the distal tip of the bridge. After cells transit START, this satellite expands to form the duplication plaque (Byers and Goetsch, 1975). Importantly, a characteristic bend develops in the bridge at the proximal end of the duplication plaque and the two nuclear membranes fuse followed by the insertion of the duplication plaque (Adams and Kilmartin, 1999, 2000). These events probably require membrane remodelling activities (Antonin, 2009).
We propose that nuclear Nbp1, together with Ndc1 and their interacting partner Mps2 that may also reside on the INM (Araki et al, 2006; Lusk et al, 2007), has membrane remodelling activity. The IPM helix of Nbp1 could facilitate membrane fusion and stabilization of the highly curved fusion site of the two membranes. Consistently, the presence of the membrane-active AH in Nbp1 was found to be important for the insertion of the duplication plaque into the NE.
The liposome-binding assay did not indicate a preference of Nbp1 for curved membranes (Figure 3E), which is in accordance with the observation that the AH of Nbp1 does not contain the Ser/Thr-rich ALPS motif that is found in curvature sensing proteins, such as the Arf1–GAP protein (Bigay et al, 2003, 2005; Drin et al, 2007). However, we cannot exclude that under other experimental conditions (e.g. a different lipid composition) or using Nbp1 proteins containing also the coiled-coil domain, Nbp1 senses curvature. Importantly, the AHs derived from membrane remodelling proteins, such as Sar1, Arf1 or epsin, bind membranes without exhibiting any preference of the curvature but can induce membrane curvature at high protein:lipid ratios by the, so-called, wedge effect and bilayer couple mechanism (reviewed in Drin and Antonny, 2010). The local accumulation of Nbp1 to high levels at SPBs could well suggest that it may use a very similar mechanism to promote membrane curvature. It remains open how Nbp1 becomes enriched at the INM of the SPB insertion site during SPB duplication and how Nbp1 interacts with the other proteins of the SPB insertion network.
Materials and methods
Yeast strains, media and plasmids
Yeast strains and plasmids are listed in Supplementary Tables I and II. Further information is provided in the supplement.
Protein methods
Yeast extracts were prepared using alkaline lysis and TCA precipitation (Janke et al, 2004). Anti-HA (12CA5), anti-Tub2, anti-GFP (Roche), anti-penta-His (Qiagen) and HRP-conjugated goat anti-mouse (Jackson) antibodies were used for protein detection.
Nbp1–sfGFP–His fusion protein was purified under denaturing conditions by NiNTA chromatography and then renatured by dialysis. Nbp1-(15–103)–GFP–His proteins were purified by Ni-NTA chromatography under native conditions. For Tap-tag purification, rabbit-IgG was coupled to epoxy-activated Dynabeads M-270 (Invitrogen). His–Kap123 was purified by NiNTA chromatography, MBP–Kap123 was purified by amylose affinity chromatography. Binding of Nbp1-(1–103)–sfGFP–His to liposomes was analysed by flow cytometry (Temmerman and Nickel, 2009) and by a flotation assay (Bigay and Antonny, 2005). For more details see the supplement.
Fluorescence microscopy
For live-cell imaging, cells were adhered to small glass-bottom Petri dishes (MatTek) by concanavalin A. Alternatively, cells were fixed with 70% EtOH at 4°C and incubated in PBS containing 4′,6-diamidino-2-phenylindole (DAPI) to visualize DNA. Z series of images were acquired with a wide-field epifluorescence microscope (Axio Imager.A1; Carl Zeiss MicroImaging, Inc.) equipped with a plan-Fluar × 100 NA 1.45 oil immersion objective (Carl Zeiss MicroImaging, Inc.), a camera (Cascade:1K; Photometrics) and MetaMorph software (Universal Imaging Corp.) (Figures 2B, 4F, 6E and 7D; Supplementary Figures S3E, S5, S6, S7, S8 and S9). A Deltavision microscope (Applied Precision) equipped with GFP and TRITC filters (Chroma Technology Corp.), a × 100 NA 1.4 oil immersion objective (plan Apo, IX70; Olympus) a CoolSNAP HQ camera (Photometrics) and a SoftWoRx 3.5.0 software (Applied Precision) was used to acquire and process Z series images in Figures 2A, 4C, 7B and 8A–C and Supplementary Figures S2, S3D and S7. Fluorescence intensity at SPB was measured by using the Z-plane with the SPB in focus. Image J was used for the quantification. One Z-plane is shown in Figures 2A and B, 6E and 8A–C and Supplementary Figures S2, S3D, S6, S7, S8 and S9; maximum Z-series projections are shown in Figures 4C–F and 7B–D and Supplementary Figure S5. Deconvolved images are shown in Figures 2A, 7B and 8A–C and Supplementary Figures S2, S3D and S7.
Adobe Photoshop and Image J were used to mount the images and to produce merged colour images. No manipulations other than contrast and brightness adjustments were used.
Immunoelectron microscopy
pADH1–nbp1-(1–103)–GFP and control cells were grown in SC–Ura at 30°C to logarithmic phase and subsequently subjected to high-pressure freezing followed by freeze substituted in 0.1% uranyl acetate, 1% water in acetone and embedded in lowicryl HM20 (Polysciences, Warrington, PA, USA) as previously described (Höög and Antony, 2007; Di Ventura et al, 2008). Thin sections were treated with blocking buffer (1.5% BSA, 0.1% fish skin gelatin in PBS), then incubated with goat anti-GFP antibodies (Rockland), followed by rabbit anti-goat antibodies (Cappel) and protein A-gold conjugates (10 nm, Utrecht University, Utrecht, The Netherlands). Images were collected with a CM120 biotwin electron microscope (FEI, Eindhoven, The Netherlands). Digital acquisitions were made with a Keen View CCD camera (Soft Imaging System, Muenster, Germany).
Supplementary Material
Acknowledgments
The work of ES, FW and WN was supported by grants from the Deutsche Forschungsgemeinschaft SFB638. LDC was supported by GRK1188. Dr K Weis is acknowledged for plasmid pKW431 and Dr JK Locker for the help with the immuno-EM analysis of yeast cells. Yeast mutants were obtained from Drs S Wente and E Hurt. We thank the mass spec facility of the ZMBH and the EM facility of the EMBL for generous support. Dr M Seedorf is acknowledged for helpful discussions. We thank U Jäckle for expert technical assistance and Dr I Hagan for critically reading the manuscript.
Author contributions: TK, LDC and ES designed the experiments and prepared the manuscript. TK analysed the primary structure of Nbp1 and did the experiments described in Figures 2A and B, 3A, 5, 6 and 8A–D and Supplementary Figures S1, S2 and S6–S9. LDC performed the experiments described in Figures 2C, 4, 7 and Supplementary Figure S3. AN and ES did the experiments described in Supplementary Figures S4 and S5, respectively. Liposome-binding studies (Figures 3, 8E; Supplementary S10) were designed by WN, FW, TK, FA and HMM and performed by TK, FA and HMM. All authors discussed the results and commented on the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams IR, Kilmartin JV (1999) Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J Cell Biol 145: 809–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams IR, Kilmartin JV (2000) Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol 10: 329–335 [DOI] [PubMed] [Google Scholar]
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, Rout MP (2007) The molecular architecture of the nuclear pore complex. Nature 450: 695–701 [DOI] [PubMed] [Google Scholar]
- Antonin W (2009) Nuclear envelope: membrane bending for pore formation? Curr Biol 19: R410–R412 [DOI] [PubMed] [Google Scholar]
- Araki Y, Lau CK, Maekawa H, Jaspersen SL, Giddings TH Jr, Schiebel E, Winey M (2006) The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol Biol Cell 17: 1959–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Emr SD (2003) Regulation of PI4,5P2 synthesis by nuclear-cytoplasmic shuttling of the Mss4 lipid kinase. EMBO J 22: 4223–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R, Sun Z, Adolf F, Rutz C, Bassler J, Wild K, Sinning I, Hurt E, Brügger B, Bethune J, Wieland F (2008) Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc Natl Acad Sci USA 105: 11731–11736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Antonny B (2005) Real-time assays for the assembly-disassembly cycle of COP coats on liposomes of defined size. Methods Enzymol 404: 95–107 [DOI] [PubMed] [Google Scholar]
- Bigay J, Casella JF, Drin G, Mesmin B, Antonny B (2005) ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J 24: 2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigay J, Gounon P, Robineau S, Antonny B (2003) Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426: 563–566 [DOI] [PubMed] [Google Scholar]
- Blagovic B, Rupcic J, Mesaric M, Georgiu K, Maric V (2001) Lipid composition of brewer's yeast. Food Technol Biotechnol 39: 175–181 [Google Scholar]
- Blumenthal R, Clague MJ, Durell SR, Epand RM (2003) Membrane fusion. Chem Rev 103: 53–69 [DOI] [PubMed] [Google Scholar]
- Byers B, Goetsch L (1975) Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol 124: 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chial HJ, Giddings TH Jr, Siewert EA, Hoyt MA, Winey M (1999) Altered dosage of the Saccharomyces cerevisiae spindle pole body duplication gene, NDC1, leads to aneuploidy and polyploidy. Proc Natl Acad Sci USA 96: 10200–10205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chook YM, Süel KE (2010) Nuclear import by karyopherin-betas: recognition and inhibition. Biochim Biophys Acta (doi:10.1016/j.bbamcr.2010.10.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo MA, Anderson DJ, Richard E, Hetzer MW (2006) Nuclear pores form de novo from both sides of the nuclear envelope. Science 312: 440–443 [DOI] [PubMed] [Google Scholar]
- Dawson TR, Lazarus MD, Hetzer MW, Wente SR (2009) ER membrane-bending proteins are necessary for de novo nuclear pore formation. J Cell Biol 184: 659–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Hochstrasser M (2006) Spatially regulated ubiquitin ligation by an ER/nuclear membrane ligase. Nature 443: 827–831 [DOI] [PubMed] [Google Scholar]
- Dennis MK, Taneva SG, Cornell RB (2011) The intrinsically disordered nuclear localization signal and phosphorylation segments distinguish the membrane affinity of two cytidylyltransferase isoforms. J Biol Chem 286: 12349–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ventura B, Funaya C, Antony C, Knop M, Serrano L (2008) Reconstitution of Mdm2-dependent post-translational modifications of p53 in yeast. PLoS One 3: e1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet CM, Hetzer MW (2010) Nuclear pore biogenesis into an intact nuclear envelope. Chromosoma 119: 469–477 [DOI] [PubMed] [Google Scholar]
- Drin G, Antonny B (2010) Amphipathic helices and membrane curvature. FEBS Lett 584: 1840–1847 [DOI] [PubMed] [Google Scholar]
- Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B (2007) A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol 14: 138–146 [DOI] [PubMed] [Google Scholar]
- Farsad K, De Camilli P (2003) Mechanisms of membrane deformation. Curr Opin Cell Biol 15: 372–381 [DOI] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT (2002) Curvature of clathrin-coated pits driven by epsin. Nature 419: 361–366 [DOI] [PubMed] [Google Scholar]
- Gavin AC, Aloy P, Grandi P, Krause R, Boesche M, Marzioch M, Rau C, Jensen LJ, Bastuck S, Dumpelfeld B, Edelmann A, Heurtier MA, Hoffman V, Hoefert C, Klein K, Hudak M, Michon AM, Schelder M, Schirle M, Remor M et al. (2006) Proteome survey reveals modularity of the yeast cell machinery. Nature 440: 631–636 [DOI] [PubMed] [Google Scholar]
- Greiner M, Caesar S, Schlenstedt G (2004) The histones H2A/H2B and H3/H4 are imported into the yeast nucleus by different mechanisms. Eur J Cell Biol 83: 511–520 [DOI] [PubMed] [Google Scholar]
- Hofemeister H, Weber K, Stick R (2000) Association of prenylated proteins with the plasma membrane and the inner nuclear membrane is mediated by the same membrane-targeting motifs. Mol Biol Cell 11: 3233–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz D, Tanaka RA, Hartwig J, McKeon F (1989) The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell 59: 969–977 [DOI] [PubMed] [Google Scholar]
- Höög JL, Antony C (2007) Whole-cell investigation of microtubule cytoskeleton architecture by electron tomography. Meth Cell Biol 79: 145–167 [DOI] [PubMed] [Google Scholar]
- Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, Knop M (2004) A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21: 947–962 [DOI] [PubMed] [Google Scholar]
- Jaspersen SL, Winey M (2004) The budding yeast spindle pole body: structure, duplication, and function. Annu Rev Cell Dev Biol 20: 1–28 [DOI] [PubMed] [Google Scholar]
- King MC, Lusk CP, Blobel G (2006) Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature 442: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, Shales M, Zhang X, Davey M, Robinson MD, Paccanaro A, Bray JE, Sheung A, Beattie B et al. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440: 637–643 [DOI] [PubMed] [Google Scholar]
- Lai TP, Stauffer KA, Murthi A, Shaheen HH, Peng G, Martin NC, Hopper AK (2009) Mechanism and a peptide motif for targeting peripheral proteins to the yeast inner nuclear membrane. Traffic 10: 1243–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CK, Giddings TH. Jr, Winey M (2004) A novel allele of Saccharomyces cerevisiae NDC1 reveals a potential role for the spindle pole body component Ndc1p in nuclear pore assembly. Eukaryot Cell 3: 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MCS, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R (2005) Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122: 605–617 [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Lusk CP, Blobel G, King MC (2007) Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol 8: 414–420 [DOI] [PubMed] [Google Scholar]
- Lusk CP, Makhnevych T, Marelli M, Aitchison JD, Wozniak RW (2002) Karyopherins in nuclear pore biogenesis: a role for Kap121p in the assembly of Nup53p into nuclear pore complexes. J Cell Biol 159: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon MA, Curwin AJ, Gaspard GJ, Suraci AB, Fernandez-Murray JP, McMaster CR (2009) The Kap60-Kap95 karyopherin complex directly regulates phosphatidylcholine synthesis. J Biol Chem 284: 7376–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW (2001) A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Cell Biol 153: 709–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onischenko E, Stanton LH, Madrid AS, Kieselbach T, Weis K (2009) Role of the Ndc1 interaction network in yeast nuclear pore complex assembly and maintenance. J Cell Biol 185: 475–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS (2006) Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24: 79–88 [DOI] [PubMed] [Google Scholar]
- Ptak C, Anderson AM, Scott RJ, Vosse DV, Rogers RS, Sydorskyy Y, Aitchison JD, Wozniak RW (2009) A role for the karyopherin Kap123p in microtubule stability. Traffic 10: 1619–1634 [DOI] [PubMed] [Google Scholar]
- Resh MD (1996) Regulation of cellular signalling by fatty acid acylation and prenylation of signal transduction proteins. Cell Signal 8: 403–412 [DOI] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C (1991) Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell 64: 615–623 [DOI] [PubMed] [Google Scholar]
- Rout MP, Blobel G, Aitchison JD (1997) A distinct nuclear import pathway used by ribosomal proteins. Cell 89: 715–725 [DOI] [PubMed] [Google Scholar]
- Sapay N, Guermeur Y, Deleage G (2006) Prediction of amphipathic in-plane membrane anchors in monotopic proteins using a SVM classifier. BMC Bioinformatics 7: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper S, Franke J, Meijsing SH, Ehrenhofer-Murray AE (2005) Nuclear import of the histone acetyltransferase complex SAS-I in Saccharomyces cerevisiae. J Cell Sci 118: 1473–1484 [DOI] [PubMed] [Google Scholar]
- Schlenstedt G, Smirnova E, Deane R, Solsbacher J, Kutay U, Görlich D, Ponstingl H, Bischoff FR (1997) Yrb4p, a yeast ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J 16: 6237–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm C, Elliott S, Shevchenko A, Schiebel E (2000) The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. EMBO J 19: 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Shemesh T, Prinz WA, Palazzo AF, Kozlov MM, Rapoport TA (2010) Mechanisms determining the morphology of the peripheral ER. Cell 143: 774–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K (1997) Exportin 1 (Crm1p) is an essential nuclear export factor. Cell 90: 1041–1050 [DOI] [PubMed] [Google Scholar]
- Temmerman K, Nickel W (2009) A novel flow cytometric assay to quantify interactions between proteins and membrane lipids. J Lipid Res 50: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres EM, Dephoure N, Panneerselvam A, Tucker CM, Whittaker CA, Gygi SP, Dunham MJ, Amon A (2010) Identification of aneuploidy-tolerating mutations. Cell 143: 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124: 573–586 [DOI] [PubMed] [Google Scholar]
- Wente SR, Rout MP (2010) The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol 2: a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Goetsch L, Baum P, Byers B (1991) MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol 114: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Hoyt MA, Chan C, Goetsch L, Botstein D, Byers B (1993) NDC1: a nuclear periphery component required for yeast spindle pole body duplication. J Cell Biol 122: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz S, Santarella-Mellwig R, Koch B, Jaedicke A, Mattaj IW, Antonin W (2010) NLS-mediated NPC functions of the nucleoporin Pom121. FEBS Lett 584: 3292–3298 [DOI] [PubMed] [Google Scholar]
- Zuleger N, Korfali N, Schirmer EC (2008) Inner nuclear membrane protein transport is mediated by multiple mechanisms. Biochem Soc Trans 36: 1373–1377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.