Abstract
The precise mechanisms by which autophagy participates in the control of intracellular infection have only lately begun to emerge. In a recent issue of Science, Wild et al demonstrate how innate immunity and autophagy cooperate in the clearance of cytosolic Salmonella, thereby shedding new light on the molecular regulation of xenophagy.
Macroautophagy (hereby referred to as autophagy) is a finely tuned, evolutionary conserved pathway whereby intracellular components are sequestered within double-membraned organelles (autophagosomes) and delivered to lysosomes for bulk degradation. Although autophagy may occasionally facilitate cell death (Kroemer et al, 2007), most often autophagy functions as a stress-inducible cytoprotective mechanism (Kroemer et al, 2010). Moreover, baseline levels of autophagy contribute to cellular homeostasis (thus exerting anti-ageing and oncosuppressive functions) by limiting the accumulation of aggregate-prone proteins or damaged organelles (Green et al, 2011). Of note, whereas starvation-induced autophagy occurs in a rather unselective manner, cells can respond to specific types of stress by activating highly selective autophagic pathways including mitophagy and xenophagy, which target mitochondria and invading pathogens, respectively (Kraft et al, 2010). Although the underlying molecular mechanisms have only recently begun to emerge, selective autophagy appears to rely on a set of cytoplasmic receptors that link specific cargoes to autophagosomes. In a recent issue of Science, Wild et al (2011) have shed new light on this issue by describing how optineurin (OPTN), an ubiquitin-binding protein implicated in the pathogenesis of glaucoma, can function as an autophagic receptor at the crossroad between innate immunity and xenophagy.
Xenophagy participates in the first-line defence against viral, bacterial and parasitic infections, and defects in autophagy/xenophagy reportedly result in increased susceptibility to infectious diseases. In line with this notion, invading pathogens have evolved multiple mechanisms for avoiding xenophagic elimination including autophagy-inhibitory proteins and strategies for escaping autophagosomes and lysosomes (Deretic, 2011). However, how invading pathogens would be selectively recognized and targeted to degradation by the xenophagic machinery has remained largely obscure until recently, when it was discovered that components of the innate immune system physically interact with proteins from the autophagic machinery, thus directing the formation of autophagosomes to bacterial entry sites or targeting ubiquitinated bacteria to autophagic degradation (Thurston et al, 2009; Galluzzi et al, 2010; Travassos et al, 2010).
Now, Ivan Dikic's group has added one important piece to this puzzle by outlining how Toll-like receptor 4 (TLR4), a pattern recognition receptor involved in innate immunity, can elicit an OPTN-dependent signalling cascade for the elimination of Salmonella that escape intracellular vacuoles (Wild et al, 2011). Wild et al discovered that OPTN can bind the essential autophagic protein LC3 via an N-terminal LC3-interacting region, thus physically bridging ubiquitin-coated cytosolic Salmonella to nascent autophagosomes and favouring its xenophagic clearance. Thus, OPTN acts as a novel autophagic receptor. Subsequent in silico studies led Wild et al (2011) to hypothesize that OPTN would be subjected to phosphorylation-dependent regulation by an NF-κB-activating kinase, namely TANK-binding kinase 1 (TBK1).
TBK1 was previously reported to respond to bacterial products such as lipopolysaccharide (LPS) by limiting the replication of cytosolic Salmonella (Radtke et al, 2007). The underlying molecular mechanisms were largely elusive, yet some data pointed the involvement of another autophagic receptor, NDP52 (Thurston et al, 2009). In vitro kinase assays and in cellula SILAC-based mass spectrometry demonstrated that, in response to LPS, TLR4-activated TBK1 phosphorylates OPTN at Ser177, thereby increasing its affinity for LC3. In line with these observations, a phospho-mimicking version of OPTN (in which 5 Ser residues were mutated to Asp) bound to LC3 with a higher affinity than its wild-type counterpart, while a non-phosphorylatable version of the protein (in which 5 Ser were substituted by Ala) was strongly impaired in its LC3-binding ability. Of note, OPTN co-localized with TBK1 and NDP52 (but not with yet another autophagic receptor, p62SQSTM1) on the surface of cytosolic Salmonella, suggesting that there are multiple partially overlapping mechanisms by which ubiquitinated bacteria are targeted to xenophagic degradation. The knockdown of OPTN in HeLa cervical cancer cells resulted in a striking increase in cytosolic Salmonella proliferation, further underscoring the functional relevance of OPTN-mediated xenophagy (Wild et al, 2011).
In conclusion, Wild et al have provided novel insights into the crosstalk between components of the innate immune system such as TLR4 and the molecular machinery for xenophagy (Figure 1), as they identified a novel autophagic cargo receptor, OPTN. Moreover, they elucidated one potential mechanism by which autophagic receptors can be regulated during cargo-specific autophagy. Future studies will have to elucidate whether and how such autophagic receptors can be pharmacologically manipulated for the treatment of infectious diseases or other autophagy-related pathologies.
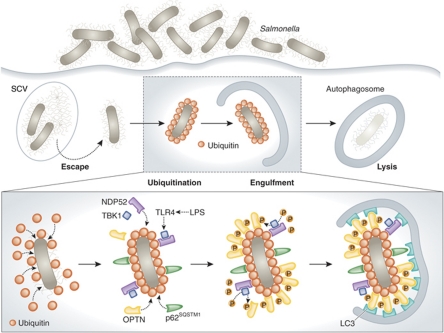
Figure 1.
TBK1-phosphorylated optineurin targets intracellular bacteria to xenophagic degradation. Upon infection, most Salmonella are sequestered into Salmonella-containing vacuoles (SCVs), but some can escape from SCVs and proliferate in the cytosol. To prevent this, cytosolic bacteria are rapidly ubiquitinated, leading to the recruitment of several autophagic receptors including NDP52 and p62SQSTM1 as well as that of the TANK-binding kinase 1 (TBK1). Until recently, the mechanism by which TBK1 would limit bacterial proliferation was not fully understood. Now, it has been discovered that, in response to bacterial products such as lipopolysaccharide (LPS), the pattern recognition receptor Toll-like receptor 4 (TLR4) activates TBK1 leading to the phosphorylation of another autophagic receptor, optineurin (OPTN), on Ser177. Phosphorylated OPTN has a high affinity for the autophagic protein LC3, thereby guiding (together with NDP52 and p62SQSTM1) ubiquitinated bacteria to the autophagic machinery and allowing for their elimination by xenophagy.
Footnotes
The authors declare that they have no conflict of interest.
References
- Deretic V (2011) Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev 240: 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Zitvogel L, Kroemer G (2010) Bacterial invasion: linking autophagy and innate immunity. Curr Biol 20: R106–R108 [DOI] [PubMed] [Google Scholar]
- Green D, Galluzzi L, Kroemer G (2011) Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C, Peter M, Hofmann K (2010) Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol 12: 836–841 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke AL, Delbridge LM, Balachandran S, Barber GN, O’Riordan MX (2007) TBK1 protects vacuolar integrity during intracellular bacterial infection. PLoS Pathog 3: e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F (2009) The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol 10: 1215–1221 [DOI] [PubMed] [Google Scholar]
- Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nunez G, Girardin SE, Philpott DJ (2010) Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol 11: 55–62 [DOI] [PubMed] [Google Scholar]
- Wild P, Farhan H, McEwan DG, Wagner S, Rogov VV, Brady NR, Richter B, Korac J, Waidmann O, Choudhary C, Dotsch V, Bumann D, Dikic I (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333: 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]