Abstract
The McMurdo Dry Valleys of Antarctica harbor numerous permanently ice-covered lakes, which provide a year-round oasis for microbial life. Microbial eukaryotes in these lakes occupy a variety of trophic levels within the simple aquatic food web ranging from primary producers to tertiary predators. Here, we report the first molecular study to describe the vertical distribution of the eukaryotic community residing in the photic zone of the east lobe (ELB) and west lobe (WLB) of the chemically stratified Lake Bonney. The 18S ribosomal RNA (rRNA) libraries revealed vertically stratified populations dominated by photosynthetic protists, with a cryptophyte dominating shallow populations (ELB–6 m; WLB–10 m), a haptophyte occupying mid-depths (both lobes 13 m) and chlorophytes residing in the deepest layers (ELB–18 and 20 m; WLB–15 and 20 m) of the photic zone. A previously undetected stramenopile occurred throughout the water column of both lobes. Temporal variation in the eukaryotic populations was examined during the transition from Antarctic summer (24-h sunlight) to polar night (complete dark). Protist diversity was similar between the two lobes of Lake Bonney due to exchange between the photic zones of the two basins via a narrow bedrock sill. However, vertical and temporal variation in protist distribution occurred, indicating the influence of the unique water chemistry on the biology of the two dry valley watersheds.
Keywords: ice-covered lake, McMurdo Dry Valleys, microbial eukaryotes, polar night, protists
The McMurdo Dry Valleys are the largest ice-free area in Antarctica (∼4000 km2) and represents one of the coldest and driest terrestrial environments on earth. Numerous permanently ice-covered lakes exist within these valleys and are the site of a National Science Foundation (NSF)-funded Long Term Ecological Research Project (LTER; http://www.mcmlter.org/). These lakes, along with those found in other ice-free areas in Antarctica, represent the only year-round liquid water reservoirs on the continent and differ drastically in their physical, chemical and biological characteristics (Priscu, 1997; Lyons et al., 2000). Each lake is isolated from the outside environment by a permanent 3- to 7-m thick ice cover that prevents wind-driven turbulence and yields highly stable water columns that are often chemically stratified. Gas exchange, allochthonous sediment deposition and light penetration are severely limited year round, although the formation of lake moats during the short summer provides some minimal input into the water column (Howard-Williams et al., 1998; Fritsen and Priscu, 1999). Last, owing to low yearly precipitation rates (<10-cm water equivalent per year), water input is derived from glacial melt and is seasonally variable (Lyons et al., 2000).
With the exception of a few reports of microscopic crustaceans (Priscu et al., 1999; Roberts et al., 2004; Laybourn-Parry and Pearce, 2007), the dry valley aquatic food web is extremely simplified and entirely dominated by the microbial loop. Single-celled microbial eukaryotes are photosynthetic and heterotrophic protists, and have important roles in carbon and nutrient cycling at all trophic levels in the dry valley lake food chain, including fixing the majority of the inorganic carbon (Priscu et al., 1999). Molecular surveys in lower latitude aquatic systems have revealed high diversity of 18S ribosomal RNA (rRNA) sequences that are unrelated to existing cultured protists (reviewed in: Caron et al., 2009). To date, there are no reports on the phylogenetic diversity of the dry valley lake protist population: all studies have been based on light microscopy or pigment signatures (reviewed in: Morgan-Kiss et al., 2006).
In an effort to understand biodiversity and ecosystem function during prolonged exposure to low temperatures and darkness, we conducted the first molecular study on protist distribution in the dry valley Lake Bonney that encompassed the transition from 24-h sunlight to the polar night (February to April 2008). Lake Bonney is a chemically stratified McMurdo Dry Valley lake (77°00′S, 162°52′E) that is separated into two lobes (east lobe, ELB; west lobe, WLB). The lobes are separated by a 13-m bedrock sill, which allows exchange between the upper, fresh water layers of the lobes (above 20 m), whereas deeper saline waters of each basin are isolated from each other and are chemically distinct (Priscu, 1997; Priscu et al., 2008). Physical and chemical profiles in Lake Bonney reveal a highly stratified vertical structure of the water columns within each basin, with the chemocline occurring at 18 m in ELB and 15 m in WLB (Supplementary Figures SI 1A, B). Both lobes of Lake Bonney exhibited a distinct chlorophyll-a that is maximum at 13.5 m (Supplementary Figures SI 1C, D), which matches the location of maximum rates of primary production in Lake Bonney (Lizotte and Priscu, 1992). Despite their close proximity, as well as similar climate and basin geology, the water columns of ELB and WLB exhibit chemical variability and the basins are considered to be two distinct watersheds (Lyons et al., 2000).
Phylogenetic diversity of protists residing within the photic zone of Lake Bonney was explored. Lake water (1 l) samples for phylogenetic analyses were collected at 6, 13, 18, and 20 m in ELB and 10, 13, 15 and 20 m in WLB, and filtered onto Durapore polyvinylidene fluoride membrane filters. DNA was extracted from filtered lake water and partial 18S rRNA genes (∼1000 bp) were amplified using universal eukaryote primers (Lopez-Garcia et al., 2001) from 21 samples (see Supplementary Information). Clone libraries were constructed, and protist diversity was assessed using a combined restriction fragment length polymorphism/sequencing approach. Unique restriction fragment length polymorphism patterns were defined as operational taxonomic units and each operational taxonomic unit was sequenced at least once for phylogenetic identification. Nucleotide–nucleotide BLAST was used to identify nearest relative sequences (refer to Supplementary Table SI 1 for sequences used in phylogenetic trees). The 18S rRNA genes were deposited in GenBank under accession numbers GU969060 to GU969102.
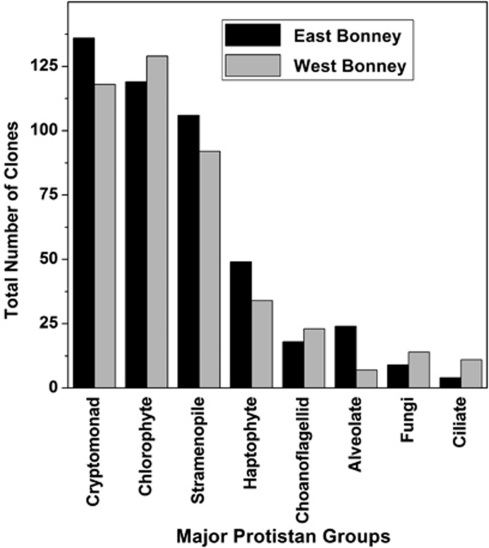
Among >800 clones analyzed, 49 unique eukaryotic phylotypes were identified. More than 90% of the sequences aligned with taxonomically assigned known groups. The remainder of the clones was related to uncultured organisms from a broad range of eukaryotic groups. Clones were organized into eight eukaryotic supergroups: the majority (∼85%) of the clones aligned with known phototrophic microbial eukaryotes. The two most dominant groups, Cryptophyta and Chlorophyta, represented more than half of the total clones (Figure 1). The vast majority of cryptophyte sequences were related to the marine nanoplankton, Geminigera cryophila (Supplementary Figure SI 2A). Although G. cryophila has not been previously reported in the McMurdo Dry Valley lakes, it seems probable that it is the abundant cryptophyte previously identified in several of the dry valley lakes by microscopic methods (Laybourn-Parry et al., 1992, 1997; Lizotte and Priscu, 1992). Most chlorophyte sequences were related to polar Chlamydomonas species (Supplementary Figure SI 2B). We also detected the green alga, Chlamydomonas raudensis UWO 241, the only well-studied psychrophilic phototrophic protist isolated from Lake Bonney (Neale and Priscu, 1995; Priscu, 1995; Morgan-Kiss et al., 2006). Stramenopiles were also prevalent in both lobes, representing 22% of the total clones (Figure 1). The most abundant stramenopile phylotype was related to Nannochloropsis species (Supplementary Figure SI 2C). Nannochloropsis-dominated algal blooms have been detected in other aquatic systems during cold-water periods (Krienitz et al., 2000; Fietz et al., 2005; Fawley and Fawley, 2007), suggesting that they are well adapted to low temperatures. Haptophytes were more abundant in ELB (10%) than WLB (8%). The majority of haptophyte sequences were most closely related to the prymnesiophyte, Isochrysis species (Supplementary Figure SI 2A). The remaining sequences (∼15%) were related to alveolates and ciliates, as well as several phylotypes related to cercozoans and choanoflagellids (Figure 1; Supplementary Figure SI 2A).
Figure 1.
Relative contribution of major eukaryotic groups from Eukarya 18S rRNA partial sequences in the photic zone of Lake Bonney. Lake samples were collected throughout the water column of ELB (black bars) and WLB (gray bars) at various time points between February and April 2008. Data represent relative abundance of phylotypes from 860 clones screened by restriction fragment length polymorphism (RFLP). Unique RFLPs were confirmed by sequencing. Clones were organized into eight eukaryotic supergroups.
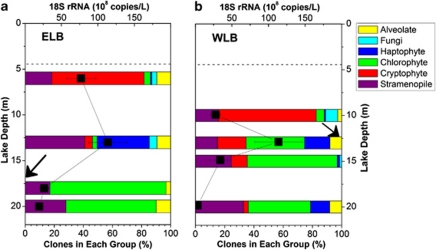
Vertical stratification of the eukaryotic community was examined in both basins of Lake Bonney (Figure 2). Cryptophytes represented >60% of all sequences in the shallow water samples, whereas haptophytes were dominant in mid-depths (13-m) of both lobes. Chlorophyte sequences dominated the libraries generated from 18- and 20-m lake samples of ELB (up to 94% total clones), and 13, 15 and 20 m (up to 62% total clones) in WLB. The vast majority of these protists were related to flagellated strains, which fits well with earlier observations that the dry valley lakes are dominated by flagellated phytoplankton that actively maintain their position in the water column (Laybourn-Parry, 2009). In contrast, stramenopile sequences related to Nannochloropsis were distributed throughout the photic zone of both lobes, which suggests that this non-flagellated protist does not maintain its position in the water column. In addition, a quantitative PCR approach targeting 18S rRNA was used to determine the variation in rRNA transcript abundance through the photic zone of both basins. Maximum 18S rRNA levels occurred at a depth of 13 m in both lobes (Figure 2), which corresponded with both chlorophyll-a (Supplementary Figure SI 1) and primary productivity rate maxima (Lizotte and Priscu, 1992). Last, species diversity, estimated as Shannon–Wiener index (H), and species evenness (E) varied between sampling depths (Supplementary Table SI 2). Eukaryotic populations residing at 13 m in both lobes exhibited the highest species diversity and evenness. ELB 13-m populations exhibited a higher species diversity (H=2.18) compared with WLB 13 m (H=1.84), whereas both sites exhibited comparable species evenness (E=0.73–0.74). Deeper layers of both lobes exhibited low species diversity (H=0.69–0.99 in ELB; H=1.2–1.45 in WLB) relative to shallower depths. The diversity indices in Lake Bonney were comparable with diversity indices reported for marine Antarctic microbial eukaryote community (Piquet et al., 2008).
Figure 2.
Depth distribution of 18S rRNA clones and 18S rRNA abundance in ELB (a) and WLB (b) of Lake Bonney. Samples were collected at four sampling depths throughout the photic zone of both basins various times at various time points between February and April 2008. Data represent the combined set of sequences across all time points. Unique phylotypes were identified by a restriction fragment length polymorphism/sequencing approach. The 18S rRNA transcript levels were quantified using quantitative PCR as described in the methods (n=4). Position of ice-cover is shown (dashed line). Arrows denote the location of the chemocline.
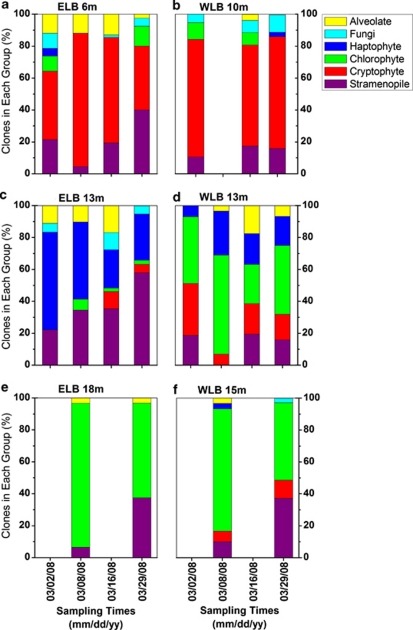
The transition from 24-h full sunlight to complete darkness during the summer–winter transition represents one of the most marked climatic events that the microbial communities are exposed to in the dry valley lakes. Photosynthetically available radiation declined gradually throughout the polar night transition, and at time points after 29 March, surface photosynthetically available radiation was <1 μmol photons per m2 s−1 (Supplementary Figure SI 3). Alterations in protist distribution during the polar night transition were monitored (Figure 3). Cryptophytes were prevalent throughout the polar transition in shallow depths of both lobes (Figures 3a and b). Mixotrophy (combined photosynthesis with phagotrophic uptake of particulate food) appears to be a major adaptive strategy in Antarctic lakes and probably allows cryptophyte populations to switch from phototrophy to heterotrophy when available light is extremely limited (Laybourn-Parry, 2002). Cryptophyte populations may also rely on mixotrophy to supplement nutrient acquisition in the oligotrophic shallow waters of Lake Bonney, which are limited for phosphorus (Priscu, 1995). Haptophytes made up 61% of the clones in 13-m ELB early in the polar transition but were replaced by stramenopiles during the polar transition (Figure 3c). In contrast, stramenopile abundance remained low in 13-m samples of WLB because of the high abundance of chlorophytes at this depth (Figure 3d). Last, clone libraries from 18-m ELB and 15-m WLB were dominated by chlorophytes early in the transition, but were replaced by stramenopiles as photosynthetically available radiation declined (Figures 3e and f). Thus, mixotrophic ability could be important not only under light-limiting conditions during the decline of polar summer but also for supplementation of N and P in the nutrient-poor shallow depths of Lake Bonney. Conversely, nutrient limitations may restrict obligate photoautotrophs such as the chlorophyte C. raudensis to the deep photic zone (⩽18 m) of ELB, whereas chlorophytes residing in WLB are able to occupy mid-depths because of the shallower chemocline (Figure 2; Supplementary Figures SI 1E, F).
Figure 3.
Variation in the distribution of major protist groups in response to the transition from summer to polar winter in Lake Bonney. Samples were collected at three sampling depths from ELB (a, c and e) and WLB (b, d and f) of Lake Bonney at various time points between February and April 2008. Unique phylotypes were identified by a restriction fragment length polymorphism/sequencing approach.
The Lake Bonney chlorophyte C. raudensis possesses a unique photochemical apparatus that is well adapted to efficiently capture light under extreme shade conditions (Morgan et al., 1998; Morgan-Kiss et al., 2005). However, it is unable to utilize a variety of organic carbon sources as an alternative energy source (Morgan-Kiss et al., 2006). The ability to efficiently capture light under extreme shade is an important adaptive strategy contributing to the dominance of chlorophytes in the deep layers of Lake Bonney. Furthermore, lower photosynthetically available radiation levels in WLB versus ELB could also influence the observed variability in the chlorophyte distribution between the two lobes (Figures 2 and 3; Supplementary Figure SI 3). Thus, we propose that the cryptophyte and chlorophyte populations exhibit differential adaptive strategies that impact their location in the water column as well as their response to polar winter. We suggest that cryptophyte populations supplement phototrophy with heterotrophy (that is, phagotrophy) in the nutrient-deficient shallow waters and may remain metabolically active during the polar winter by switching to heterotrophy. In contrast, chlorophytes rely on a modified light-harvesting apparatus to capture light energy in the deeper layers of the water column during the summer, but rely on alternative strategies such as breakdown of starch reserves when light is absent in the winter months (Morgan-Kiss et al., 2005). The extent of trophic versatility (that is, mixotrophic ability) in the other major Lake Bonney protist groups, such as Nannochloropsis and Isochrysis, is currently not known.
Although molecular analyses on the bacterial communities residing in Antarctic lakes has begun to describe the phylogeny and functionality of the prokaryotic communities (Karr et al., 2003, 2006; Glatz et al., 2006), our understanding of the diversity and ecological roles the eukaryotic microorganisms remains limited. Our study provides the first phylogenetic data set on 18S rRNA diversity in a McMurdo Dry Valley lake and revealed that microbial eukaryotes are a diverse group within Lake Bonney. Differences in trophic versatility and the ability of some species possess to switch from photoautotrophy to heterotrophy during the transition from 24-h sunlight to the darkness of the polar night is likely a key strategy for surviving in these and other Antarctic lakes.
Acknowledgments
We thank the McMurdo LTER and Ratheon Inc. personnel for logistical support in Antarctica. This work was supported by the NSF Office of Polar Programs grants OPP-0631659 to RMK and OPP-0631494, OPP-432595 and MCB-0237335 to JCP. This paper is dedicated to the memory of our colleague Dr John Wilson Hawes (Department of Chemistry and Biochemistry & Center for Bioinformatics, Miami University).
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Caron DA, Worden AZ, Countway PD, Demir E, Heidelberg KB. Protists are microbes too: a perspective. ISME J. 2009;3:4–12. doi: 10.1038/ismej.2008.101. [DOI] [PubMed] [Google Scholar]
- Fawley KP, Fawley MW. Observations on the diversity and ecology of freshwater Nannochloropsis (Eustigmatophyceae), with descriptions of new taxa. Protist. 2007;158:325–336. doi: 10.1016/j.protis.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Fietz A, Bleib W, Hepperle D, Koppitz H, Krienitz L, Nicklisch A. First record of Nannochloropsis limnetica (Eustigmatophyceae) in the autotrophic picoplankton from Lake Baikal. J Phycol. 2005;35:780–790. [Google Scholar]
- Fritsen CH, Priscu JC. Seasonal change in the optical properties of the permanent ice cover on Lake Bonney, Antarctica: consequences for lake productivity and dynamics. Limnol Oceanogr. 1999;44:447–454. [Google Scholar]
- Glatz RE, Lepp PW, Ward BB, Francis CA. Planktonic microbial community composition across steep physical/chemical gradients in permanently ice-covered Lake Bonney, Antarctica. Geobiol. 2006;4:53–67. [Google Scholar]
- Howard-Williams CA, Schwarz A-M, Hawes I, Priscu JC.1998Optical properties of lakes of the McMurdo Dry ValleyIn: Priscu JC (ed).Ecosystem Dynamics in a Polar Desert: The McMurdo Dry Valleys, Antarctica American Geophysical Union: Washington, DC [Google Scholar]
- Karr EA, Ng JM, Belchik SM, Sattley WM, Madigan MT, Achenbach LA. Biodiversity of methanogenic and other archaea in the permanently frozen Lake Fryxell, Antarctica. App Env Microbiol. 2006;72:1663–1666. doi: 10.1128/AEM.72.2.1663-1666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr EA, Sattley WM, Jung DO, Madigan MT, Achenbach LA. Remarkable diversity of phototrophic purple bacteria in a permanently frozen Antarctic Lake. Appl Environ Microbiol. 2003;69:4910–4914. doi: 10.1128/AEM.69.8.4910-4914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienitz L, Hepperle D, Stich HB, Weiler W. Nannochloropsis limnetica (Eustigmatophyceae), a new species of picoplankton from freshwater. Phycologia. 2000;35:219–227. [Google Scholar]
- Laybourn-Parry J. Survival mechanisms in Antarctic Lakes. Philos Trans R Soc Lond B Biol Sci. 2002;357:863–869. doi: 10.1098/rstb.2002.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn-Parry J. No place too cold. Science. 2009;324:1521–1522. doi: 10.1126/science.1173645. [DOI] [PubMed] [Google Scholar]
- Laybourn-Parry J, James M, McKnight DM, Priscu JC, Spaulding S, Shiel R. The microbial plankton of Lake Fryxell, Southern Victoria Land, Antarctica. Polar Biol. 1997;17:54–61. [Google Scholar]
- Laybourn-Parry J, James MR, McKnight DM, Priscu J, Spaulding SA, Shiel R. The microbial plankton of Lake Fryxell, southern Victoria Land, Antarctica during the summers of 1992 and 1994. Polar Biol. 1992;17:54–61. [Google Scholar]
- Laybourn-Parry J, Pearce DA. The biodiversity and ecology of Antarctic Lakes: models for evolution. Philos Trans R Soc Lond B Biol Sci. 2007;362:2273–2289. doi: 10.1098/rstb.2006.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte MP, Priscu JP. Algal pigments as markers for stratified phytoplankton populations in Lake Bonney (dry valleys) Antarc J US. 1992;27:259–260. [Google Scholar]
- Lopez-Garcia P, Rodriguez-Valera F, Pedros-Alio C, Moreira D. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Lett Nat. 2001;409:603–607. doi: 10.1038/35054537. [DOI] [PubMed] [Google Scholar]
- Lyons WB, Fountain AG, Doran PT, Priscu J, Neumann K. The importance of landscape position and legacy: the evolution of the Taylor Valley Lake District. Freshwat Biol. 2000;43:355–367. [Google Scholar]
- Morgan RM, Ivanov AG, Priscu JC, Maxwell DP, Hüner NPA. Structure and composition of the photochemical apparatus of the Antarctic green alga, Chlamydomonas subcaudata. Photosyn Res. 1998;56:303–314. [Google Scholar]
- Morgan-Kiss RM, Ivanov AG, Pocock T, Król M, Gudynaite-Savitch L, Hüner NPA. The Antarctic psychrophile, Chlamydomonas raudensis ETTL (UWO241) (chlorophyceae, chlorophyta) exhibits a limited capacity to photoacclimate to red light. J Phycol. 2005;41:791–800. [Google Scholar]
- Morgan-Kiss RM, Priscu JP, Pocock T, Gudynaite-Savitch L, Hüner NPA. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol Molec Biol Rev. 2006;70:222–252. doi: 10.1128/MMBR.70.1.222-252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale PJ, Priscu JC. The photosynthetic apparatus of phytoplankton from a perennially ice-covered Antarctic Lake: acclimation to an extreme shade environment. Plant Cell Physiol. 1995;36:253–263. [Google Scholar]
- Piquet AM, Bolhuis H, Davidson AT, Thomson PG, Buma AG. Diversity and dynamics of Antarctic marine microbial eukaryotes under manipulated environmental UV radiation. FEMS Microbiol Ecol. 2008;66:352–366. doi: 10.1111/j.1574-6941.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- Priscu JC. Phytoplankton nutrient deficiency in lakes of the McMurdo Dry Valleys, Antarctica. Freshw Biol. 1995;34:215–227. [Google Scholar]
- Priscu JC. The biogeochemistry of nitrous oxide in permanently ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Global Change Biol. 1997;3:301–305. [Google Scholar]
- Priscu JC, Christner BC, Dore JE, Westley MB, Popp BN, Casciotti KL, et al. Extremely supersaturated N2O in a perennially ice-covered Antarctic Lake: molecular and stable isotopic evidence for a biogeochemical relict. Limnol Oceanogr. 2008;53:2439–2450. [Google Scholar]
- Priscu JC, Wolf CF, Takacs CD, Fritsen CH, Laybourn-Parry J, Roberts JKM, et al. Carbon transformations in the water column of a perennially ice-covered Antarctic Lake. Biosci. 1999;49:997–1008. [Google Scholar]
- Roberts EC, Priscu JC, Wolf CF, Lyons WB, Laybourn-Parry J. The distribution of microplankton in the McMurdo Dry Valley Lakes, Antarctica: response to ecosystem legacy or present day climatic controls. Polar Biol. 2004;27:238–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.