Abstract
Osteoporosis is a multifactorial skeletal disorder characterized by decreased bone mass and deteriorated microarchitecture that lead to increased risk of fracture. The disuse osteoporosis refers to bone mass decrements under conditions of decreased mechanical loading, including decreased ground force reaction, muscular contraction, and microgravity-related bone loss in astronauts after space flights. Although there are many effective treatments available for primary osteoporosis, there is a lack of effective treatments for disuse osteoporosis. This is because that the aetiology, pathophysiology, and resultant pathology of disuse osteoporosis differ from those of primary osteoporosis. The objective of this paper is to examine the unique pathology and underlying pathophysiology of disuse osteoporosis.
1. Introduction
Osteoporosis is the condition in which a low bone mass and altered microarchitecture of the bone leads to increased risk of fracture. Traditionally, osteoporosis has been classified into primary and secondary osteoporosis. Primary osteoporosis refers to osteoporotic conditions which are not related to other chronic illnesses and is usually associated with aging and decreased gonadal function, such as decreased level of estrogen, whereas secondary osteoporosis is the type of osteoporosis caused by other health problems. Disuse is one of the many reasons inducing bone loss and resulting in secondary osteoporosis [1]. Disuse osteoporosis has been shown to be a regional phenomenon in the areas with tremendous decrease in weight bearing like lower limbs. Bones of lower limbs are subjected to mechanical stimulations during daily life provided by static gravity-related weight-bearing, ground reaction forces, and dynamic loading generated by muscle contractions during locomotion. Physical exercise is essential for increasing or maintaining bone mass and strength [2]. Milliken et al. [3] have investigated the effect of 1-year supervised weight training exercise on bone mineral density (BMD) in postmenopausal women. The result showed higher BMDs of trochanter and femoral neck in women with weight training exercise than in those lacking exercise. Chan et al. [4] have studied the effect of Tai-Chi excercise on bone quality in postmenopausal women. Postmenopausal women were randomly assigned to an exercise group or a control group. Subjects in the exercise group performed 5 sessions of 45 minutes Tai-Chi per week. After 1 year of Tai-Chi exercise, a greater percentage loss in bone density was observed in the control group when compared to the exercise group, suggesting that performing Tai-Chi exercise could decline bone loss in postmenopausal women. Besides, Feskanich et al. [5] have studied prospectively a cohort on the relationship between walking, leisure time activity, and the risk of hip fracture, showing that physical activity was inversely associated with the risk of hip fracture and that the effect was dose dependent.
Although there are many effective treatments available for primary osteoporosis, there is a lack of effective treatments for disuse osteoporosis. This is because of the fact that the aetiology, pathophysiology, and resultant pathology of disuse osteoporosis differ from those of primary osteoporosis. The objective of this paper is to examine the unique pathology and underlying pathophysiology of disuse osteoporosis.
2. Pathology of Disuse Osteoporosis
Disuse bone loss in general is a reduction of bone mass in relation to bone volume, while the ratio of bone mineral to collagen remains unchanged. The loss of trabecular bone is more rapid and dramatic, while the cortical loss continues for a longer period [6]. However, bones of lower limbs are subjected to three categories of mechanical loadings during daily life, namely, static gravity-related weight bearing, ground reaction forces, and dynamic loading generated by muscle contractions during locomotion. Different health problem associates with absence or decrease in one or more of these mechanical stimulations and will result in bone loss differently in anatomical location, quantity, velocity, and through different mechanisms.
Long-term bed rest results in the absence of ground force reaction and reduction of muscle contractions. Rittweger et al. [7] have carried out a 35 days bed rest trial and assessed bone density 2 weeks after the bed rest. They reported reduction of bone mass in the cancellous bone-rich areas, 1% at distal femur, 3% at patella, and 2% at distal tibia while no changes in distal radius. The same group has observed that bone mass in distal radius remained unchanged after 56 days and 90 days bed rest, while bone mass in distal tibia declined 3.6% and 6% correspondingly [8, 9]. The decreases of cortical bone thickness and density were below 2% after as long as 90 days bed rest. These results suggest that long-term bed rest does not affect balance of bone metabolism very much.
Disuse osteoporosis includes the reduction of bone mass after spinal cord injury (SCI) and other brain neurologic conditions as well. SCI leads to substantial reduction in ground force reaction and muscle contraction in the lower limbs resulting in dramatic reduction in bone mass. Result of a cross-sectional study carried by Garland et al. [10] demonstrated more than 20% bone loss at distal femur 3 months after injury in posttraumatic paraplegic and quadriplegic SCI patients. The researchers reported also that the rate of bone loss below pelvis was rapid and linear in the acute stages. In another cross-sectional study with larger sample size, Kiratli et al. [11] found reduction of bone mineral density by 27%, 25%, and 43% in femoral neck, mid-shaft, and distal femur, respectively, compared with the controls. Beside of SCI, lower limb amputation, acquired brain injury, and other neurologic conditions can also lead to disabilities which, in turn, result in disuse osteoporosis. A recent cross-sectional study by Smith et al. [12] showed that 42.4% and 23.5% of disabled patients after neurologic traumas, such as SCI or other conditions for at least 3 months, had developed osteopenia and osteoporosis, respectively. The researchers suggested further that ambulatory status and duration of disability were independent predictors of bone mineral densities at femoral neck and total proximal femur.
Exposure to microgravity would lead to reduced weight-bearing and ground reaction forces that result in reduction in bone mass. Several studies on effects of microgravity on skeleton focused on the impacts on skeletons of astronauts after spaceflights. Collet et al. [13] analyzed the BMD and biochemical parameters of 2 astronauts who stayed 1 and 6 months, respectively, in space. A slight decrease in trabecular bone mass in distal tibia metaphysis was observed at the end of the first month of spaceflight, whereas remarkable bone losses in both trabecular and cortical bones was observed after 6 months of spaceflight. After 6 month of recovery, the trabecular bone mass was still significantly lower than normal, whereas no difference could be seen in cortical bone. However, the impacts of microgravity on human skeletons are highly varied. Another study on 11 astronauts by Vico et al. [14] showed greater bone losses occurred in cancellous bone compared to cortical bone. The mean decrease in cancellous BMD of the 11 astronauts was 5.4% after 6 months of spaceflight but the range of reduction varied from 0.4% to 23.4%. The astronaut who spent the longest time in space did not have the greatest bone loss.
In comparison of the three causes of disuse osteoporosis, that is, long-term bed rest, paralysis, and microgravity, all of them involve the reduction of ground reaction forces and weight-bearing activities. However, patients with long-term bed rest or paralysis are further subjected to reduced or even absence of muscular contraction. This situation is different in the case of astronauts whose muscular contractions are not restricted, and this may be a possible reason account for the great variations of bone loss in previous findings. Furthermore, muscular contraction can be the most important force out of the 3 categories of mechanical loading for keeping bone mass.
3. Pathophysiology of Disuse Osteoporosis
Disuse osteoporosis can be resulted from failure for the bone to achieve the optimal peak bone mass and strength, if disuse occurred during the period of bone mass accumulation. On the other hand, disuse osteoporosis can be the result of an accelerated rate of bone resorption and slower bone formation in adults [15].
Ralston [16] indicated that peak bone mass and strength could be determined by genetic factors which affect the level of BMD, biochemical markers of bone turnover, and mechanical properties of bone. Results of association studies have suggested that polymorphisms of components in the gene-signaling pathway of genes such as COL1A1, ESR1, and LRP5 were associated with bone mass level and fracture risk. The influence of genetic factors on skeletal development is most pronounced in young people. The impact of genetic factors diminishes with age because of the increasing impact of environmental and nutritional factors.
Skeletal growth and repair occur through bone-remodeling which is a tightly regulated process. The normal bone mass is maintained during remodeling, based on the balance between bone formation and bone resorption. The bone-remodeling cycle begins with the resorption phase during which osteoclasts are recruited to the remodeling site on the bone surface, and these osteoclasts will then excavate the bone surface in the subsequent 2 to 4 weeks. After the resorption phase, the osteoclasts move away from the site, and osteoblast precursors move to the site and differentiate to become osteoblasts. During the subsequent 2 to 4 months of formation phase, mature osteoblasts deposit an organ matrix, which will then be mineralized [17]. However, because of certain events such as hormonal changes at menopause, the balance between bone formation and resorption is disturbed, and resorption occurs at a higher rate than that of formation leading to osteoporosis.
Osteoclasts are multinucleated cells derived from monocyte/macrophage lineage (Figure 1) [19] and are the only type of cells capable of resorbing bone [20]. The rate of bone resorption is determined by the number and activity of osteoclasts. During bone resorption, osteoclasts adhere to the bone matrix forming a deeply folded membrane and secrete protons and hydrolytic enzymes to the lacuna. The lacuna is then demineralized by the acidic environment due to proton secretion, leading to the exposure of organic components of the bone, such as collagen, to the hydrolytic enzymes, resulting in degradation of the organic components [21]. Bone resorption is an important physiological process for bone modeling and remodeling. However, increased rate of bone resorption may result in the depletion of bone mass and to disruption of skeletal microarchitecture leading to skeletal fragility. Receptor activator of nuclear factor kappaB ligand (RANKL) is a cytokine that belongs to the TNF family; it is essential for osteoclast formation and function. RANKL is found on the surface of osteoblasts and the interaction between RANKL and its receptors RANK on osteoclast precursors triggers the maturation of osteoclasts, thus inducing bone resorption. The RANKL-RANK interaction is prevented by the natural RANKL inhibitor, osteoprotegerin (OPG). OPG is also a TNF family member that binds to RANKL and, hence, inhibits the binding of RANKL to RANK. Therefore, the activity of osteoclasts is partially dependent on the balance between RANKL and OPG (Figure 2) [22]. In addition to RANKL and OPG, there are many other cytokines such as IL-1, TNF-α and prostaglandin E2 that have been identified as regulators for osteoclastic activity [23, 24]. Furthermore, Hughes et al. [25] showed that estrogen was able to negatively regulate the formation and function of osteoclast by reducing the lifespan of the cells by promoting apoptosis. This finding provides an insight on the cause of postmenopausal osteoporosis. Osteoclastic bone resorption is a highly regulated process. However, excessive osteoclastic activity without the complementary actions by osteoblasts will result in skeletal fragility. Based on previous studies, it is likely that bone resorption is influenced by complex interactions between different factors with osteoclasts, and also among the different factors.
Figure 1.
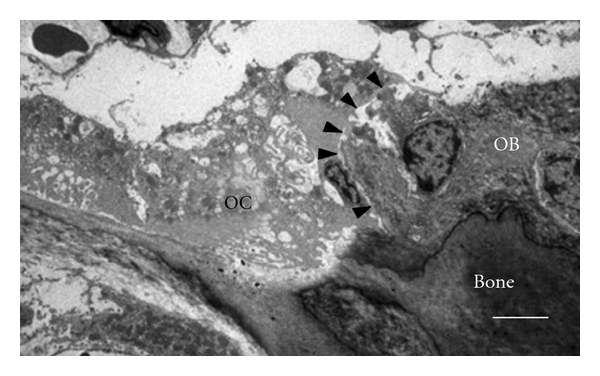
Transition electron micrograph of osteoclast-osteoblast contact in mouse tibial bone marrow (14-week-old male). Arrowheads indicate a contact surface between osteoclasts (OC) and osteoblasts (OB). Scale bar, 5 μm [18].
Figure 2.
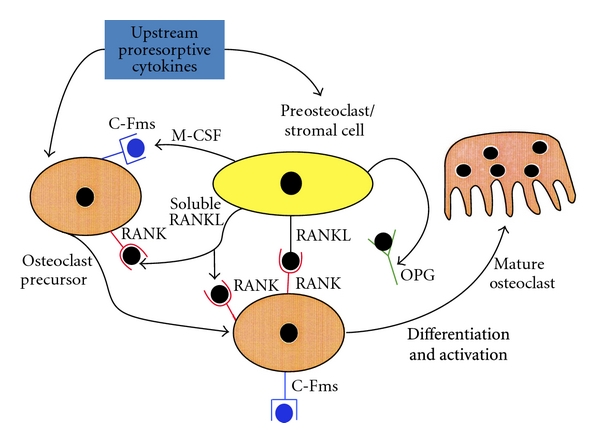
Regulatory mechanisms of osteoclastogenesis [22].
Skeletal remodeling is the result of both bone resorption and bone formation. In cases in which the rate of bone resorption is increased, there could be no apparent bone loss if the rate of bone formation is matched, since the bone removed will be replaced by new bone formation. Osteoporosis is, thus, an imbalance condition where the rate of bone resorption is higher than the one of bone formation, resulting in continuous loss of bone and deterioration of skeletal microarchitecture. Hence, it is important to determine what factors will affect the rate of bone formation as well as the coupling process between bone resorption and formation.
Osteoblasts are mononucleate cells which are responsible for bone formation. During ossification, or bone forming, osteoblasts produce an unmineralized, organic portion of the bone matrix known as osteoid. The osteoids produced will then mineralize, with minerals such as calcium and zinc, to form new bone tissue. Osteoblasts arise from mesenchymal progenitor cells, which possess the master gene of osteoblast differentiation, Runx2 (previously known as Cbfa1, Aml3, or Pebp2a1). In 1997, Komori and coworkers demonstrated that intramembranous and endochondral ossification were completely blocked in mice with Cbfa1 gene mutated, where Cbfa1 was a transcription factor belonging to the Runt-domain gene family [26]. The same year, Otto et al. showed that homozygous Cbfa1-deficient mice died of respiratory failure shortly after birth. In that study, they found that there was neither osteoblast nor bone in the skeletons of the homozygous Cbfa1-deficient mice. Also, heterozygous mutants exhibited specific skeletal abnormalities that were characteristic of a human heritable skeletal disorder, cleidocranial dysplasia [27]. These findings suggested that the Cbfa1 (Runx2) gene is essential for osteoblast differentiation and bone formation.
The differentiation of osteoblasts from mesenchymal progenitor cells is influenced by growth factors such as fibroblasts growth factor (FGF), transforming growth factor beta (TGF-β), and bone morphogenetic proteins (BMPs) (Figure 3). D'Ippolito et al. [28] pointed out that the number of osteoblasts tended to decrease as people reach old age. This leads to the decreased rate of bone formation and results in osteoporosis. According to a review by Rosen [29], the insulin-like growth factor (IGF) system is linked to the process of skeletal acquisition, and IGF-1 is essential for normal bone formation. Previous study by Sakata et al. [30] showed that skeletal unloading led to resistant to the anabolic actions of IGF-I on bone, and this was associated with the reduction in integrin expression. This result suggests that IGF-I may be involved in the reaction mechanism of disuse osteoporosis. Beside of IGF-I, the levels of various factors and hormones such as BMPs and PTH have been demonstrated to be changed in response to skeletal unloading [31]. However, the exact mechanisms of how these factors respond to changes in mechanical loading are still unclear.
Figure 3.
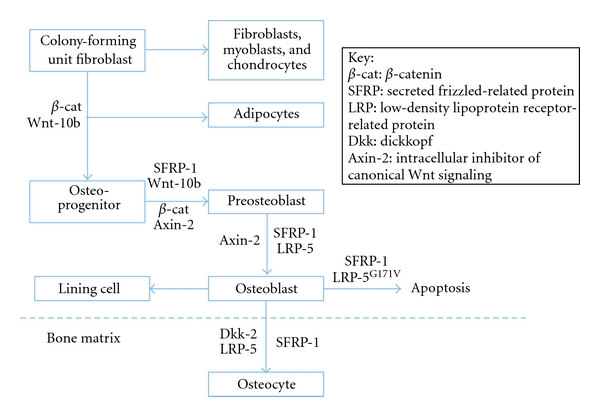
Role of canonical Wnt signaling in the control of osteoblastogenesis.
Sclerostin is the product of the SOST gene that has been found to bind to LRP5/6 receptors. This binding inhibits the Wnt signaling pathway and is antagonistic to bone formation [32]. A recent study by Robling showed that mechanical stimulation decreased sclerostin expression, whereas significant increase in SOST expression in tibias was observed in hindlimb unloaded animals. Thus, the level of sclerostin, and hence bone formation, appears to be affected by mechanical stimulation [33]. A recent study by Lin et al. [34] further suggested that the responses of bone to mechanical unloading were mediated via sclerostin, probably by antagonizing Wnt/β-catenin signaling. In the study, wild-type and SOST knockout mice were unloaded for 2 weeks. Decreased Wnt/β-catenin signaling in association with increased expression of SOST was observed in wild-type mice upon unloading. However, in the absence of sclerostin in SOST −/− mice, decrease in Wnt/β-catenin signaling and inhibition of osteoblast activity upon unloading were diminished. The results showed that bone masses of SOST −/− mice in both loaded and unloaded groups were significantly higher than those of wild-type groups. More importantly, unloading-induced bone loss was prevented in SOST −/− mice, and Wnt/β-catenin signaling, which was shown to be involved in response to mechanical unloading, was not altered in unloaded SOST −/− mice. The findings suggest that sclerostin play an important role in development of disuse osteoporosis via its action in Wnt/β-catenin signaling.
4. Conclusion
Osteoporosis is a multifactorial skeletal disorder that can be related to various risk factors. Physical disability, advancing age and/or exposure to microgravity increase the risk of suffering from osteoporosis, and such disuse osteoporosis is associated with huge economic and health burden. On the other hand, it is an obstacle for space technology advancement. Understanding the pathology and the underlying mechanisms of disuse osteoporosis is important for the development of new strategies on pharmaceuticals or treatment protocols for preventing or reducing disuse osteoporosis. In this paper, the effects of long-term bed rest, paralysis, and exposure to microgravity on skeletons were outlined. Also, recent research works on underlying mechanisms of disuse osteoporosis were described. Certainly, the entire picture of the pathophysiology of disuse osteoporosis is still unclear, but further investigations on action mechanism of hormones such as IGF-I and antagonists of sclerostin would provide insights on prospective research works.
References
- 1.Howard A. Coding for bone diseases. For The Record. 2011;23(9):p. 27. [Google Scholar]
- 2.Rutherford O. The role of exercise in prevention of osteoporosis. Physiotherapy. 1990;76:522–526. [Google Scholar]
- 3.Milliken LA, Wilhelmy J, Martin CJ, et al. Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in postmenopausal women exercising for 1 year. Journals of Gerontology A: Biological Sciences and Medical Sciences. 2006;61(5):488–494. doi: 10.1093/gerona/61.5.488. [DOI] [PubMed] [Google Scholar]
- 4.Chan K, Qin L, Lau M, et al. A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Archives of Physical Medicine and Rehabilitation. 2004;85(5):717–722. doi: 10.1016/j.apmr.2003.08.091. [DOI] [PubMed] [Google Scholar]
- 5.Feskanich D, Willett W, Colditz G. Walking and leisure-time activity and risk of hip fracture in postmenopausal women. Journal of the American Medical Association. 2002;288(18):2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annual Review of Biomedical Engineering. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 7.Rittweger J, Simunic B, Bilancio G, et al. Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone. 2009;44(4):612–618. doi: 10.1016/j.bone.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Rittweger J, Frost HM, Schiessl H, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36(6):1019–1029. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Rittweger J, Beller G, Armbrecht G, et al. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone. 2010;46(1):137–147. doi: 10.1016/j.bone.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 10.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. Journal of Orthopaedic Research. 1992;10(3):371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 11.Kiratli BJ, Smith AE, Nauenberg T, Kallfelz CF, Perkash I. Bone mineral and geometric changes through the femur with immobilization due to spinal cord injury. Journal of Rehabilitation Research and Development. 2000;37(2):225–233. [PubMed] [Google Scholar]
- 12.Smith EM, Comiskey CM, Carroll AM. A study of bone mineral density in adults with disability. Archives of Physical Medicine and Rehabilitation. 2009;90(7):1127–1135. doi: 10.1016/j.apmr.2008.09.578. [DOI] [PubMed] [Google Scholar]
- 13.Collet P, Uebelhart D, Vico L, et al. Effects of 1—and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20(6):547–551. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 14.Vico L, Collet P, Guignandon A, et al. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. The Lancet. 2000;355(9215):1607–1611. doi: 10.1016/s0140-6736(00)02217-0. [DOI] [PubMed] [Google Scholar]
- 15.Raisz L, Bilezikian J, Martin T. Principles of Bone Biology. 3rd edition. Vol. 2. Elsevier; 2008. Pathophysiology of osteoporosis; pp. 1635–1647. [Google Scholar]
- 16.Ralston S. Principles of Bone Biology. 3rd edition. Vol. 2. Elsevier; 2008. Genetic determinants of bone mass and osteoporotic fracture; pp. 1611–1634. [Google Scholar]
- 17.Martin T, Rodan G. Osteoporosis. 2nd edition. 2001. Coupling of bone resorption and formation during bone remodeling; pp. 361–371. [Google Scholar]
- 18.Matsuo K, Irie N. Osteoclast-osteoblast communication. Archives of Biochemistry and Biophysics. 2008;473:201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Narducci P, Nicolin V. Differentiation of activated monocytes into osteoclast-like cells on a hydroxyapatite substrate: an in vitro study. Annals of Anatomy. 2009;191(4):349–355. doi: 10.1016/j.aanat.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Baron R, Neff L, Vignery A. Differentiation and functional characteristics of osteoclasts. Bone. 1985;6:p. 414. [Google Scholar]
- 21.Horne W, Duong L, Sanjay A, Baron R. Principles of Bone Biology. 3rd edition. Elsevier; 2008. Regulating bone resorption: targeting integrins, calcitonin receptor, and cathepsin K; pp. 221–236. [Google Scholar]
- 22.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 23.Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136(7):3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- 24.Ammann P, Rizzoli R, Bonjour JP, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. Journal of Clinical Investigation. 1997;99(7):1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes DE, Dai A, Tiffee JC, Li HH, Munoy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nature Medicine. 1996;2(10):1132–1135. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 26.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89(5):755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 27.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 28.D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. Journal of Bone and Mineral Research. 1999;14(7):1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 29.Rosen CJ. Insulin-like growth factor I and bone mineral density: experience from animal models and human observational studies. Best Practice and Research: Clinical Endocrinology & Metabolism. 2004;18(3):423–435. doi: 10.1016/j.beem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. Journal of Bone and Mineral Research. 2004;19(3):436–446. doi: 10.1359/JBMR.0301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikle DD, Sakata T, Halloran BP. The impact of skeletal unloading on bone formation. Gravitational and Space Biology Bulletin. 2003;16(2):45–54. [PubMed] [Google Scholar]
- 32.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. Journal of Biological Chemistry. 2005;280(20):19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- 33.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. Journal of Biological Chemistry. 2008;283(9):5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 34.Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. Journal of Bone and Mineral Research. 2009;24(10):1651–1661. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]


