Abstract
Redox-dependent migration and proliferation of vascular smooth muscle cells (SMCs) are central events in the development of vascular proliferative diseases; however, the underlying intracellular signaling mechanisms are not fully understood. We tested the hypothesis that activation of Nox1 NADPH oxidase modulates intracellular calcium levels ([Ca2+]i). Using cultured SMCs from wild type (WT) and Nox1 null (Nox1−/y) mice, we confirmed that thrombin-dependent generation of ROS requires Nox1. Thrombin rapidly increased [Ca2+]i, as measured by fura-2 fluorescence ratio imaging, in WT but not Nox1 null SMCs. The increase in [Ca2+]i in WT SMCs was inhibited by antisense to Nox1 and restored by expression of Nox1 in Nox1 null SMCs. Investigation into potential mechanisms by which Nox1 modulates [Ca2+]i showed that thrombin-induced inositol triphosphate generation and thapsigargin-induced intracellular calcium mobilization were similar in WT and Nox1 null SMCs. To examine the effects of Nox1 on Ca2+ entry, cells were either bathed in Ca2+-free media or exposed to dihydropyridines to block L-type Ca2+ channel activity. Treatment with nifedipine or removal of extracellular Ca2+ reduced the thrombin-mediated increase of [Ca2+]i in WT SMCs, whereas the response in Nox1 null SMCs was unchanged. Sodium vanadate, an inhibitor of protein tyrosine phosphatases, restored the thrombin-induced increase of [Ca2+]i in Nox1 null SMCs. Migration of SMCs was impaired with deficiency of Nox1 and restored with expression of Nox1 or addition of sodium vanadate. In summary, we conclude that Nox1 NADPH oxidase modulates Ca2+ mobilization in SMCs, in part through regulation of Ca2+ influx, to thereby promote cell migration.
Keywords: calcium influx, NADPH oxidase, migration, vascular disease
Introduction
NADPH oxidases contribute to vascular proliferative diseases 1, 2. Nox1 is the inducible catalytic subunit of NADPH oxidase in smooth muscle cells (SMCs) and is responsible for cell activation. Nox1 mediates redox-dependent signaling via regulation of gene transcription, resulting in SMC migration and proliferation 3, 4. The mechanisms by which Nox1-derived ROS activate cellular signaling are complex and not completely defined. Recent studies have provided evidence linking ROS and Ca2+ signaling in the vasculature 5-9. For example, in isolated arterioles, either global inhibition of NADPH oxidase or treatment with ROS scavengers prevents angiotensin II(AngII)-dependent increases in [Ca2+]i 10. ROS control of Ca2+ signaling can occur through multiple mechanisms, including activation of Ca2+ release from intracellular stores, extracellular Ca2+ entry, or inhibition of Ca2+ reuptake 6, 8. In vascular cells ROS increases sensitivity of IP3R to IP3 and promotes Ca2+ entry via activation of voltage-gated Ca2+ channels 11, 12.
In this study, we used a combination of pharmacological inhibitors and genetic manipulation of Nox1 expression to examine the role of Nox1 activation in modulating [Ca2+]i. Our data demonstrate that activation of Nox1 increases [Ca2+]i, in part via influx of extracellular Ca2+ involving activation of L-type Ca2+ channel. In addition, the effect of Nox1 on Ca2+ mobilization is required for SMC migration. Redox control of Ca2+ handling is a novel mechanism by which Nox1 can modulate SMC signaling and function.
Materials and Methods
Vascular Smooth Muscle Cell Culture
Thoracic aortas from male Nox1 null (Nox1−/y) 13 and control littermate WT (Nox1+/y) mice were obtained and SMCs were isolated and cultured as previously described 14. The cells were maintained in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 humidified incubator. Experiments were performed using cells between passage 4-10, and serum-deprived conditions were obtained by incubating 24 h in DMEM containing 0.1% FBS. Studies conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the University of Iowa Institutional Animal Care and Use Committee.
Adenovirus-mediated Gene Transfer
Experiments utilized the E1-deleted replication deficient recombinant adenoviral vectors encoding Nox1 (AdNox1)15, antisense Nox1 (AdNox1-AS)16, green fluorescent protein (AdGFP), or empty vector (AdEmpty). Adenovirus was mixed with the cationic polymer poly-L-lysine (250 molecules/virus particle)17 and added to SMCs in serum-free DMEM18. After 4 hrs, media was replaced with DMEM containing 10% FBS for 48 hrs.
Detection of ROS
Thrombin-induced changes in ROS levels in Nox1 null and WT SMCs were detected by Amplex Red. SMCs were incubated with Amplex Red (20 μM) and HRP (0.2 U/ml) for 30 min, after which the fluorescence intensity of the media was determined (excitation and emission wavelengths of 545 and 590 nm, respectively) and normalized to cell number.
Intracellular Calcium Measurement
Thrombin-stimulated changes in [Ca2+]i were assessed by Fura-2 fluorescence ratio imaging using a microscopic digital imaging system (Photon Technology International), as described previously 19, 20. Briefly, WT or Nox1 null SMCs grown on 25 mm coverslips were loaded with the Ca2+-specific dye Fura-2AM (1 μM, Molecular Probes/Invitrogen) for 30 minutes at 37° C. After washing with Hank’s balanced salt solution (HBSS), cells were incubated for 20 minutes at 37° C in HBSS to allow complete hydrolysis of Fura-2AM to Fura-2. Real-time shifts in Fura-2 ratio fluorescence, indicating changes in [Ca2+]i, were recorded before, during, and after stimulating SMCs with thrombin (1 U/mL) or H2O2 (100 μM). To examine the role of NADPH oxidase, WT SMCs were pretreated with the NADPH oxidase inhibitor diphenylene iodonium (DPI, 10 μM, Sigma-Aldrich) for 1 hour prior to thrombin stimulation. In other studies, [Ca2+]i was examined in WT SMCs expressing antisense against Nox1 (AdNox1-AS) or Nox1 null SMCs expressing Nox1 (AdNox1). The patency of intracellular Ca2+ stores in SMCs was determined by treating cells with thapsigargin (5 μg/mL, Sigma-Aldrich). The contribution of extracellular Ca2+ influx on thrombin-mediated increases in [Ca2+]i was examined by bathing SMCs in Ca2+-free HBSS or treating with nifedipine (1 μM) during thrombin stimulation. Summary data represent the average difference in the basal and peak increase in [Ca2+]i, except for the dihydropyridine experiments in which the change in [Ca2+]i was determined at all timepoints, and the lowest value was subtracted from the highest value.
Inositol triphosphate (IP3) Levels
Cells were grown in 6-well plates to 80-90% confluency, washed with the assay media (inositol-free DMEM containing 20 mM HEPES, 2 mM glutamine, 10 μg/mL streptomycin, 10 U/mL penicillin, and 0.1% BSA) and then incubated in the assay media containing 4 μCi/ml [2-3H]myo-inositol (NEN Life Science Products) for 18-24 h at 37°C. At the end of the labeling period, the cells were incubated with assay media containing 20 mM LiCl for 15 min at 37°C followed by addition of thrombin (1 U/mL) for 5 min. Cells were placed on ice and the media was quickly aspirated and replaced with equal volumes of cold 1.5 N perchloric acid (PCA) and 0.5 M HCIO4. After a 30-min incubation on ice, the extracts were collected, centrifuged, and the supernatants were neutralized by the addition of 0.72 M KOH/0.6 M KHC03. The precipitated KClO4 salt was removed by centrifugation, and the supernatants were mixed with 100 mM inositol and water. These samples were then used for the assay of inositol phosphates. An ion-exchange resin AG-1-X8 (200-400 mesh, formate, Bio-Rad) was rehydrated with water, poured onto 0.5 × 3.0-cm chromatography columns and washed once with water and twice with 10 mM myo-inositol. The cell extracts were then applied to the columns, followed by several washing steps, including 10 mM myo-inositol and 5 mM sodium borate/60 mM sodium formate solution. The last elution step was performed with 0.1 M formic acid/1.0 M ammonium formate solution. This fraction contains inositol phosphates. Samples were counted in a liquid scintillation counter.
Cell Migration
The migration of SMCs was determined by scratch wound assay and by modified Boyden chamber method. Where indicated, cells were infected with AdNox1-AS, AdNox1, or AdGFP for 24 hrs followed by serum starvation for an additional 24 hrs. For scratch wound assays, the serum-starved SMC monolayer was disrupted with a sterile cell scraper to create a cell-free zone. Cells were then washed with medium and treated with or without thrombin (1 U/mL) in DMEM containing 0.1% FBS and images were taken 24 hrs after injury, using a microscope equipped with a digital camera. For the modified Boyden chamber method, SMC migration was determined in Transwell cell-culture chambers with collagen polycarbonate membrane with 8-μm pores. SMCs were grown to ~80% confluence and then made quiescent in 0.1% serum for 48 hrs before migration. SMCs (106 cells/mL) were added to the upper chamber of the transwell and allowed to attach to the membrane for 30 min. Chambers contained media with 0.1% serum. Migration of SMCs was induced by the addition of thrombin (1 U/ml) in the presence or absence of nifedipine (1 μM, Sigma Aldrich) or sodium vanadate (200 μM, Sigma-Aldrich) to the lower compartment. After 6 hrs, nonmigrated cells were removed from the upper chamber. SMCs migrating to the lower surface of the membrane were fluorescently stained with DAPI and quantitated microscopically.
Cell proliferation
Cell proliferation was determined by measuring [3H]-thymidine incorporation into SMCs infected with either AdNox1-AS or AdGFP as described previously 21.
Statistics
Data are expressed as mean ± SEM and analyzed by the Student t test when comparing only 2 groups, and by ANOVA followed by Newman-Keuls correction for multiple comparisons when comparing more than 2 groups. A value of p<0.05 was defined statistical significance.
Results
Thrombin-Stimulated Increase in [Ca2+]i is Dependent on Nox1
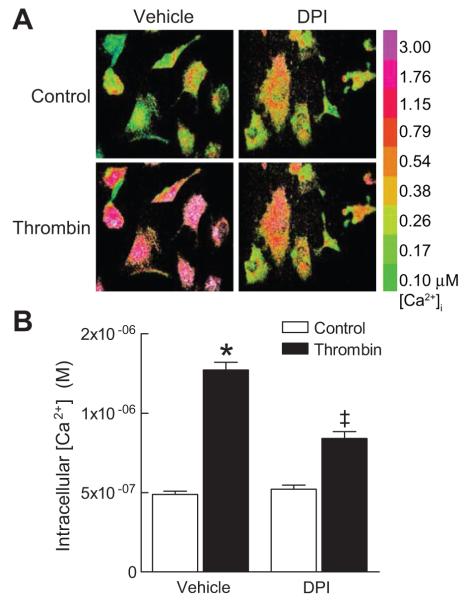
Although NADPH oxidase-derived ROS have been implicated in thrombin-mediated SMCs proliferation and migration 22-24, little is known regarding the second messengers involved in ROS signaling. Therefore, we examined the role of NADPH oxidases in modulating intracellular Ca2+ in response to thrombin. In WT SMCs, thrombin caused a rapid increase in [Ca2+]i that was markedly attenuated when cells were pretreated with DPI (Fig 1A, B), an inhibitor of thrombin-induced ROS (Supplemental Fig. S1). Nox1 is the inducible isoform of NADPH oxidase in SMCs 25, and we have previously shown that thrombin activates Nox1 15(Supplemental Fig S1, available in online Data Supplement, please see http://hyper.ahajournals.org). Therefore, we next examined whether Nox1 is responsible for the thrombin-induced increases in [Ca2+]i. Thrombin caused a rapid and transient increase in [Ca2+]i, peaking 25 to 30 seconds after stimulation (Fig 2B). Expression of an antisense targeting Nox1 inhibited thrombin-mediated increase in both ROS levels and in [Ca2+]i in WT SMCs (Fig 2). Similarly, the peak increase in [Ca2+]i in Nox1 null SMCs was markedly reduced as compared to WT cells. Heterologous expression of Nox1 in Nox1 null SMCs restored the thrombin-mediated increase in ROS and in [Ca2+]i (Fig 2). The effects we observe are specific to the absence of Nox1-derived ROS because addition of H2O2 to WT and Nox1 null SMCs resulted in a similar increase in [Ca2+]i (WT: 32±12%; Nox1 null: 40±13%; n=6). Taken together, these data indicate that Nox1 initiates intracellular Ca2+ mobilization in response to thrombin in SMCs.
Figure 1.
Thrombin-induced increase in [Ca2+]i is dependent on NADPH oxidase. (A) Representative Fura-2 ratiometric images of [Ca2+]i showing inhibitory effects of DPI on thrombin-induced increase in [Ca2+]i in WT SMCs. (B) Summary data of thrombin-induced Ca2+ response in WT SMCs pretreated with vehicle (n=150 cells) or DPI (n=129 cells) followed by stimulation with thrombin. * P<0.05 vs. control; ‡ P<0.05 vs. vehicle + thrombin.
Figure 2.
Nox1 is required for the thrombin-induced increase of [Ca2+]i in SMCs. (A) WT and Nox1 null SMCs were infected with adenoviruses expressing Nox1-AS or Nox1, respectively, and thrombin-induced changes in ROS levels were detected by Amplex Red and normalized to cell number (n=8). (B) Representative Fura-2 ratiometric Ca2+ tracings from WT or Nox1 null SMCs infected with AdEmpty, AdGFP, AdNox1-AS, or AdNox1. Arrows indicate time of thrombin stimulation. (C) Summary data from WT cells infected with AdEmpty (n=141 cells) or AdNox1-AS (n=60 cells); or Nox1 null cells infected with AdGFP (n=57 cells) or AdNox1 (n=62 cells). Unless otherwise indicated, AdGFP was used to control for the bicistronic GFP expressed in the AdNox1 and AdNox1-AS vectors.* P<0.05 vs. WT control; ‡ P<0.05 vs. WT + thrombin; # P<0.05 vs. Nox1 null + thrombin.
Nox1 Mediates Influx of Extracellular Ca2+ in Thrombin-stimulated SMCs
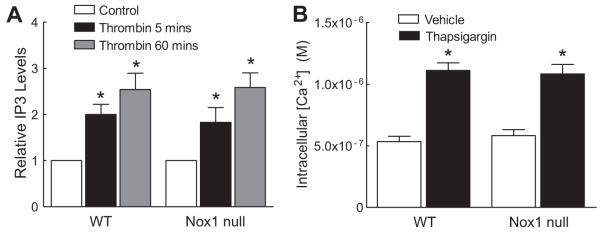
It has previously been shown that the thrombin-mediated increase in [Ca2+]i is secondary to the activation of phospholipase C with a subsequent increase in inositol 1,4,5-trisphosphate (IP3) 26. We assessed whether the observed differences in [Ca2+]i between WT and Nox1 null SMCs in response to thrombin resulted from differences in IP3 generation. WT and Nox1 null SMCs demonstrated a similar increase of IP3 levels at 5 and 60 minutes after thrombin (Fig 3A), indicating that IP3 levels are independent of Nox1-derived ROS. Furthermore, in response to thapsigargin, an inhibitor of the sarco-endoplasmic reticulum Ca2+-ATPases (SERCAs) 27, [Ca2+]i increased to similar levels in WT and Nox1 null cells (Fig 3B), suggesting no difference in IP3-sensitive Ca2+ pools.
Figure 3.
IP3 levels and intracellular Ca2+ release are normal in Nox1 null SMCs after thrombin stimulation. (A) Summary data of IP3 levels in WT and Nox1 null SMCs at 5 minutes and 60 minutes following thrombin stimulation (n=3). * P<0.05 vs. WT control. (B) Summary data showing the effect of thapsigargin on [Ca2+]i in WT (n=103 cells) and Nox1 null SMCs (n=63 cells). * P<0.05 vs. WT vehicle.
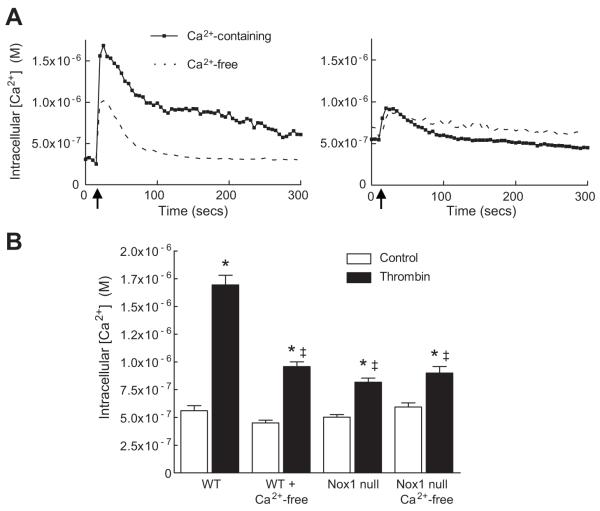
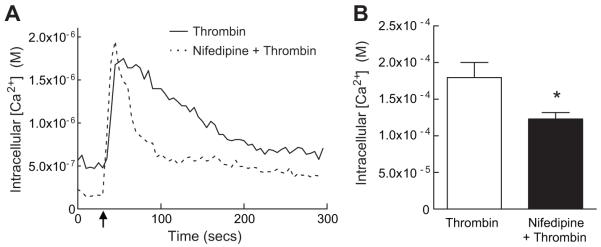
Based on these observations, we next tested whether Nox1 mediates the influx of extracellular Ca2+ in response to thrombin. When WT cells were bathed in Ca2+-free media, the magnitude of increase in [Ca2+]i following thrombin was significantly inhibited (Fig 4A), confirming influx of extracellular Ca2+ after stimulation with thrombin. In contrast to WT cells, the absence of extracellular Ca2+ had no effect on the thrombin-mediated increase in [Ca2+]i in Nox1 null cells (Fig 4B). These data suggest that Nox1-derived ROS modulate the influx of extracellular Ca2+ in response to thrombin. To extend these findings, we examined the contribution of L-type Ca2+ channels since activity of voltage-gated Ca2+ channels are known to be regulated by redox modification of cysteine and methionine residues 7. Treatment with the dihydropyridine nifedipine prevented the thrombin-stimulated increase in [Ca2+]i (Fig 5). Similar results were obtained with nitrendipine (Supplemental Fig S2). These findings implicate L-type Ca2+ channel activity as the source of extracellular Ca2+ influx in response to thrombin and a potential target of Nox1-derived ROS.
Figure 4.
Nox 1 activation mediates influx of extracellular Ca2+ in SMCs. (A) Representative Fura-2 ratiometric tracings of thrombin-induced increase in [Ca2+]i in WT (left panel) and Nox1 null (right panel) SMCs. Arrow indicates time of thrombin addition. (B) Summary data of thrombin-mediated increase in [Ca2+]i in WT SMCs bathed in Ca2+-containing (n=89 cells) or Ca2+-free media (n=179 cells) and Nox1 null SMCs bathed in Ca2+-containing (n=88 cells) or Ca2+-free media (n=89 cells). * P<0.05 vs. WT control; ‡ P<0.05 vs. WT + thrombin in Ca2+-containing media.
Figure 5.
Thrombin-stimulated extracellular calcium influx is mediated by L-type Ca2+ channel activity. (A) Representative Fura-2 radiometric tracings of [Ca2+]i in WT SMCs after treatment with thrombin in the presence or absence of nifedipine. Arrow indicates time of thrombin addition. (B) Summary data showing the effect of nifedipine on thrombin-stimulated [Ca2+]i (thrombin, n=14 cells; thrombin + nifedipine, n=30 cells). * P<0.05 vs. thrombin.
Inhibition of protein tyrosine phosphatase 1B (PTP1B) by ROS has been implicated in modulating activity of Ca2+ entry channels 28, and cellular ROS are known to inactivate PTPs by the reversible oxidation of cysteine residues in the active site 29, thereby modifying the magnitude and duration of signaling events. Furthermore, it has been shown that PTP inactivation in response to AngII requires Nox1-derived ROS30. Treatment of Nox1 null SMCs with sodium vanadate, an inhibitor of PTPs, partially restored the thrombin-mediated increase in [Ca2+]i (vehicle: 0.51±0.02 μM; thrombin: 0.77±0.03 μM; sodium vanadate 0.58±0.04 μM; sodium vanadate + thrombin 1.02±0.07 μM; n=26 cells; P<0.05), identifying inactivation of PTPs as an additional potential mechanism by which Nox1 can influence Ca2+ signaling.
Thrombin-induced SMCs Migration and Proliferation is Mediated by Nox1
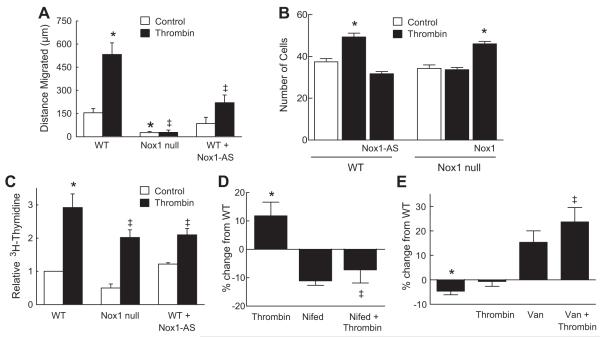
Previous investigators have shown thrombin mediates proliferation and migration of SMCs 31-33. It has been also shown that NADPH oxidase-derived ROS control thrombin-induced SMC migration 22, 23. We examined how the Nox1 regulation of Ca2+ influx affects thrombin-induced migration of SMCs. As compared to control, thrombin increased the distance that WT SMCs migrated; however, thrombin-induced migration was abolished in Nox1 null SMCs and reduced in WT SMCs treated with antisense to Nox1 (Fig 6A). This approach to measure migration cannot differentiate between the changes in cell growth versus migration. Therefore, we next used the modified Boyden chamber method to further assess the role of Nox1 in migration (Fig 6B). Thrombin increased the transmigration of WT SMCs. In contrast, Nox1 null SMCs did not display a similar increase in migration to thrombin. The importance of Nox1 in this response was further verified by the loss of transmigration in WT cells pretreated with Nox1 antisense and the rescue of migration in Nox1 null cells following exogenous expression of Nox1. With regards to proliferation, thrombin-induced DNA synthesis was increased three-fold in WT SMCs as compared with untreated WT cells, but was reduced in SMCs deficient in Nox1 and in WT SMCs expressing Nox1 antisense (Fig 6C). These findings are similar to recent reports identifying a role for Nox1 in activation of SMCs by PDGF 3 and basic fibroblast growth factor 4. We next investigated the role of Ca2+ in Nox1-dependent SMC migration. Consistent with the effects on Ca2+ influx, treatment of WT SMCs with nifedipine prevented transmigration to thrombin (Fig 6D). Since sodium vanadate restored the thrombin-mediated increase in [Ca2+]i in Nox1 null SMCs, we tested the hypothesis that inhibition of PTPs would restore migration of Nox1 null SMCs. There was a trend for sodium vanadate to increase migration of Nox1 null SMCs in the absence of thrombin (Fig 6E). The addition of thrombin did not further increase migration of Nox1 null SMCs pretreated with sodium vanadate. These data suggest that PTPs may be downstream effectors of Nox1-mediated redox signaling.
Figure 6.
Thrombin-induced SMC migration and proliferation are Nox1-dependent. (A) Summary data of the distance that SMCs migrated after scratch wound in the presence of thrombin. (B) Summary data of SMC migration measured using the modified Boyden chamber method. (C) SMC proliferation was measured by [3H] thymidine incorporation. Data are normalized to control WT cells. (D) Effect of nifedipine on migration of WT SMCs was measured as in (B). Data are reported as % change from WT control. (E) Effect of sodium vanadate on migration of Nox1 null SMCs was measured as in (B). Data are reported as % change from WT control. For A-D, * P< 0.05 vs. WT control; ‡ P< 0.05 vs. WT + thrombin. For E, * P<0.05 vs. WT control; ‡ P< 0.05 vs. Nox1 null + thrombin. Data represent means ± SEM of 3-4 experiments in duplicate.
Discussion
Previous studies have identified NADPH oxidase-derived ROS as critical signaling intermediates in SMC migration and proliferation 3, 4, 22. Herein we show that activation of the Nox1-based NAPDH oxidase by thrombin modulates intracellular Ca2+ levels in SMCs in part via influx of extracellular Ca2+. In addition, we show that SMC migration involves Nox1-dependent increases in [Ca2+]i. These effects involve activation of L-type Ca2+ channels and inactivation of PTPs. These are the first data to identify redox control of Ca2+ influx as a mechanism by which Nox1 alters SMC function.
Several lines of evidence suggest that Nox1-derived ROS are critical to development of vascular disease. We have recently shown that in a model of hypercholesterolemia, deficiency of Nox1 reduces atherosclerotic lesion area in aorta 34. Within days of arterial balloon injury, Nox1 expression and ROS levels are increased in neointimal SMCs 35, and following vascular injury, neointimal formation 3 and proliferation 34 are reduced in Nox1 null mice. Genetic manipulation of Nox1 confirms the role of Nox1-derived ROS in cell proliferation 36, 37 and SMC migration 3,4. Although these studies provide strong evidence for Nox1 in the pathogenesis of vascular disease, the mechanisms by which Nox1-derived ROS activate SMC is not completely understood. Our data contribute to the field by defining a mechanism by which Nox1 modulates intracellular Ca2+ levels to induce cellular processes.
The activation of SMCs by thrombin involves activation of Nox1 15 and Ca2+-sensitive signaling pathways 26, 38, 39; however, the relationship between ROS and Ca2+ signaling in this context remains unclear. The multiple mechanisms by which ROS regulate intracellular calcium homeostasis, such as activation of Ca2+ release and entry channels and inhibition of Ca2+ reuptake have been extensively reviewed. Voltage gated Ca2+ channels contain many cysteine and methionine residues susceptible to redox modification that could influence channel function 7. In addition to direct effects of ROS on calcium channels, oxidation of regulatory proteins such as calmodulin may also affect channel function. Changes in the cellular redox state can increase [Ca2+]i by inducing Ca2+ influx through voltage-dependent Ca2+ channels, by stimulating IP3-mediated Ca2+ mobilization from intracellular stores, by stimulating ryanodine receptors (RyR), and by inhibiting activity of sarcoplasmic reticulum endoplasmic reticulum calcium ATPase (SERCA) or plasma membrane Ca2+ ATPase 5-8.
Multiple integrated mechanisms regulate intracellular Ca2+ levels. Our data demonstrate that thrombin elicited an acute transient increase in [Ca2+]i followed by sustained phase of elevated [Ca2+]i. However, in Ca2+-free media, the peak increase in [Ca2+]i was significantly inhibited and the sustained plateau phase was virtually absent, suggesting that thrombin induces an influx of extracellular Ca2+. The transient changes in [Ca2+]i in Nox-1 null SMCs in the presence of Ca2+ mimicked that of WT SMCs in either Ca2+-free media or after treatment with nifedipine. These data suggest that Nox1-derived ROS contribute to the thrombin-mediated increase in [Ca2+]i through influx of extracellular Ca2+. ROS have been shown to increase the sensitivity of IP3R to promote IP3-mediated Ca2+ release 11. Our observation of no difference in total IP3 levels in WT vs. Nox1 null SMCs does not preclude the possibility that Nox1 activation alters the sensitivity of IP3R to IP3. It has also been demonstrated that ROS sustains Ca2+ influx via inhibition of PTPs, presumably by maintaining phosphorylation of Ca2+ entry channels 28. In our study, pharmacologic inhibition of PTPs in Nox1 null SMCs partially restored thrombin-induced increases in [Ca2+]i. These findings are consistent with another study using a rat model of hypertension that found that Nox1-derived ROS is required for AngII-dependent inactivation of the PTP SHP-2 30.
Similar to our results with thrombin, a recent study has shown that deficiency of Nox1in SMCs reduces [Ca2+]i in response to AngII 9. These findings were attributed to abnormalities in trafficking of the AngII receptor AT1R to the plasma membrane. In our study, activation of IP3 was similar in WT and Nox1 null cells, indicating that, in contrast to the response to AngII, the blunted increase in [Ca2+]i in Nox1 null cells to thrombin is not due to dysregulation of thrombin receptor signaling or trafficking.
Nox1 has been shown to be integral for migration of SMCs. SMC migration involves a complicated and coordinated series of steps and is an important component of vascular remodeling. Transient changes in [Ca2+]i are likely to be a key regulating signal for migration of SMCs 40. Our data demonstrate that Nox1 is required for migration of SMCs. We extend these findings to implicate a role for Ca2+ influx in Nox1-mediated migration. Similarly, the inhibition of PTPs increased SMC migration in Nox1-deficient cells. This observation of increased migration in the absence of an agonist is consistent with thrombin causing Nox1-mediated inactivation of PTPs.
Perspectives
Increasing evidence defines a role for NADPH oxidases in the pathogenesis of vascular disease. In this study, we demonstrate that Nox1-derived ROS in SMCs are critical for changes in [Ca2+]i via a mechanism that involves influx of extracellular Ca2+. These data provide direct evidence that Nox1 contributes to Ca2+ homeostasis in SMCs and identifies potential redox-sensitive mechanisms of SMC activation, which is important in regulation of vascular tone. Although treatment with antioxidants showed promise in experimental models of hypertension and restenosis, results from large clinical trials have been disappointing. Future strategies that focus on Nox1 as a potential target have the potential to reduce the morbidity and mortality associated with cardiovascular disease.
Supplementary Material
Acknowledgements
The authors wish to thank associates of the University of Iowa Roy J. and Lucille A. Carver College of Medicine Central Microscopy Research Facility and the Gene Transfer Vector Core Facility of the University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases (supported by NIH/NIDDK P30 DK 54759). We thank Kristina W. Thiel for assistance in manuscript preparation.
Sources of Funding
This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs (FJM) and by NIH grant HL081750 (FJM) and HL14388 (R.C.B).
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Lee MY, Martin AS, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause K-H, Banfi B, Lambeth JD, Lassegue B, Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder K, Helmcke I, Palfi K, Krause KH, Busse R, Brandes RP. Nox1 mediates basic fibroblast growth factor-induced migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1736–1743. doi: 10.1161/ATVBAHA.107.142117. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 6.Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal. 2005;7:1302–1314. doi: 10.1089/ars.2005.7.1302. [DOI] [PubMed] [Google Scholar]
- 7.Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9:409–435. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 8.Trebak M, Ginnan R, Singer HA, Jourd’heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal. 2010;12:657–674. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basset O, Deffert C, Foti M, Bedard K, Jaquet V, Ogier-Denis E, Krause KH. NADPH oxidase 1 deficiency alters caveolin phosphorylation and angiotensin II-receptor localization in vascular smooth muscle. Antioxid Redox Signal. 2009;11:2371–2384. doi: 10.1089/ars.2009.2584. [DOI] [PubMed] [Google Scholar]
- 10.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2005;289:F1012–1019. doi: 10.1152/ajprenal.00144.2005. [DOI] [PubMed] [Google Scholar]
- 11.Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1, 4, 5-trisphosphate in human endothelial cells. J Biol Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 12.Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential Calcium Regulation by Hydrogen Peroxide and Superoxide in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. J Cardiovasc Pharmacol. 2004;44:200. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Miller FJ, Jr, Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: endothelium-dependent dilation and differential responses to acetylcholine. Cardiovasc Res. 1998;38:744–750. doi: 10.1016/s0008-6363(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 15.Miller FJ, Jr, Chu X, Stanic B, Tian X, Sharma RV, Davisson RL, Lamb FS. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal. 2010;12:583–593. doi: 10.1089/ars.2009.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91 phox homologues in vascular smooth muscle cells. nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 17.Fasbender A, Zabner J, Chillon M, Moninger TO, Puga AP, Davidson BL, Welsh MJ. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. Journal of Biological Chemistry. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 18.Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor-kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 19.Sharma RV, Chapleau MW, Hajduczok G, Wachtel RE, Waite LJ, Bhalla RC, Abboud FM. Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience. 1995;66:433–441. doi: 10.1016/0306-4522(94)00560-r. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 21.Stanic B, Katsuyama M, Miller FJ., Jr. An oxidized extracellular oxidation-reduction state increases Nox1 expression and proliferation in vascular smooth muscle cells via epidermal growth factor receptor activation. Arterioscler Thromb Vasc Biol. 2010;30:2234–2241. doi: 10.1161/ATVBAHA.110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson C, Ruef J, Madamanchi NR, Barry-Lane P, Hu Z, Horaist C, Ballinger CA, Brasier AR, Bode C, Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47 phox may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Castresana MR, Newman WH. Reactive oxygen species-sensitive p38 MAPK controls thrombin-induced migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2004;36:49–56. doi: 10.1016/j.yjmcc.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–1059. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berk BC, Taubman MB, Griendling KK, Cragoe EJ, Jr, Fenton JW, Brock TA. Thrombin-stimulated events in cultured vascular smooth-muscle cells. Biochem J. 1991;274:799. doi: 10.1042/bj2740799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 28.Bogeski I, Bozem M, Sternfeld L, Hofer HW, Schulz I. Inhibition of protein tyrosine phosphatase 1B by reactive oxygen species leads to maintenance of Ca2+ influx following store depletion in HEK 293 cells. Cell Calcium. 2006;40:1–10. doi: 10.1016/j.ceca.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 30.Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res. 2008;103:149–158. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 31.Kalmes A, Vesti BR, Daum G, Abraham JA, Clowes AW. Heparin Blockade of Thrombin-Induced Smooth Muscle Cell Migration Involves Inhibition of Epidermal Growth Factor (EGF) Receptor Transactivation by Heparin-Binding EGF-Like Growth Factor. Circ Res. 2000;87:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 32.Cao H, Dronadula N, Rao GN. Thrombin induces expression of FGF-2 via activation of PI3K-Akt-Fra-1 signaling axis leading to DNA synthesis and motility in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C172–182. doi: 10.1152/ajpcell.00284.2005. [DOI] [PubMed] [Google Scholar]
- 33.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 36.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 37.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc.Natl.Acad.Sci.U.S.A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berk BC, Taubman MB, Cragoe EJ, Fenton JW, Griendling KK. Thrombin signal transduction mechanisms in rat vascular smooth muscle cells. Calcium and protein kinase C-dependent and-independent pathways. J Biol Chem. 1990;265:17334–17340. [PubMed] [Google Scholar]
- 39.Stepien O, Marche P. Amlodipine inhibits thapsigargin-sensitive C a 2+ stores in thrombin-stimulated vascular smooth muscle cells. Am J Physiol - Heart Circ Physiol. 2000;279:1220–1227. doi: 10.1152/ajpheart.2000.279.3.H1220. [DOI] [PubMed] [Google Scholar]
- 40.Scherberich A, Campos-Toimil M, Ronde P, Takeda K, Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci. 2000;113:653–662. doi: 10.1242/jcs.113.4.653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.