Abstract
The electrical activity of the brain does not only reflect the current level of arousal, ongoing behavior or involvement in a specific task, but is also influenced by what kind of activity, and how much sleep and waking occurred before. The best marker of sleep-wake history is the electroencephalogram (EEG) spectral power in slow frequencies (slow-wave activity, 0.5–4 Hz, SWA) during sleep, which is high after extended wakefulness and low after consolidated sleep. While sleep homeostasis has been well characterized in various species and experimental paradigms, the specific mechanisms underlying homeostatic changes in brain activity or their functional significance remain poorly understood. However, several recent studies in humans, rats and computer simulations shed light on the cortical mechanisms underlying sleep regulation. First, it was found that the homeostatic changes in SWA can be fully accounted for by the variations in amplitude and slope of EEG slow waves, which are in turn determined by the efficacy of cortico-cortical connectivity. Specifically, the slopes of sleep slow waves were steeper in early sleep compared to late sleep. Second, the slope of cortical evoked potentials, which is an established marker of synaptic strength, was steeper after waking and decreased after sleep. Furthermore, cortical long-term potentiation (LTP) was partially occluded if it was induced after a period of waking, but it could again be fully expressed after sleep. Finally, multiunit activity recordings during sleep revealed that cortical neurons fired more synchronously after waking, and less so after a period of consolidated sleep. The decline of all these electrophysiological measures - the slopes of slow waves and evoked potentials and neuronal synchrony – during sleep correlated with the decline of the traditional marker of sleep homeostasis, EEG SWA. Taken together, these data suggest that homeostatic changes in sleep EEG are the result of altered neuronal firing and synchrony, which in turn arise from changes in functional neuronal connectivity.
Keywords: sleep homeostasis, synaptic homeostasis, multiunit activity, neurons, cortex
Behavior and brain activity in waking and sleep
In all species carefully studied so far, waking and sleep alternate on a regular basis and neither wake nor sleep last spontaneously for more than several hours (individual rat: Fig 1A) (Tobler, 2005; Cirelli and Tononi, 2008). This observation led to the notion that wake and sleep should be associated with opposite processes, as manifested in increased sleep drive during wake and its dissipation during sleep (Borbely, 1982). A fundamental difference between wakefulness and sleep is the extent to which the brain is engaged in the acquisition and processing of information. During wakefulness, animals face environmental challenges that require an adequate behavioral response, and continuous behavioral adjustments necessarily involve learning, associated with neuronal plasticity (Whitlock et al., 2006). The cortical activity in awake animals is generated not only by ascending influences from specific wake-promoting areas (Villablanca, 2004; Jones, 2005) and intracortical and cortico-subcortical interactions (Boutrel and Koob, 2004; Miller and O’Callaghan, 2006), but also by behavior (Petersen et al., 2003; Vyazovskiy et al., 2006; Gentet et al.) and processing of incoming external stimuli (Sporns et al., 2000). In contrast, in non-rapid eye movement (NREM) sleep neocortical activity is spontaneous and organized largely “from inside” (Steriade et al., 1993c; Steriade and Amzica, 1998b; Steriade et al., 2001).
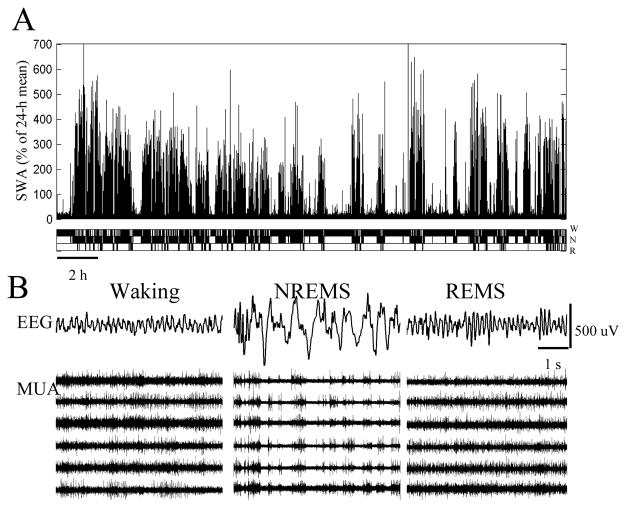
Figure 1. Sleep architecture and vigilance specific cortical activity.
(A) EEG slow-wave activity (SWA, EEG power between 0.5–4 Hz) and corresponding hypnogram during an undisturbed 24-h baseline in one individual rat. Vertical bars depict consecutive 4-s values of SWA. Note that SWA decreases during the 12-h light period and increases after long consolidated waking episodes. (B) From top to bottom: Surface electroencephalogram (EEG) traces from the right barrel cortex during waking, NREM sleep and REM sleep in a representative rat. Raw multiunit activity (MUA) recorded simultaneously in the same rat from a microwire array placed in the left barrel cortex (6 individual channels are shown). Note high frequency tonic firing in waking and REM sleep, and the periods of population neuronal activity and silence in NREM sleep.
Not surprisingly, cortical neuronal firing patterns in wakefulness and another activated state, REM sleep, are profoundly and characteristically different from those in NREM sleep (Verzeano and Negishi, 1960; Murata and Kameda, 1963; Noda and Adey, 1970; Hobson and McCarley, 1971; Desiraju, 1972; Noda and Adey, 1973; Burns et al., 1979; Steriade et al., 2001) (Fig. 1B). At the EEG level, wake in rodents is traditionally distinguished from NREM sleep by the virtual absence of large-amplitude slow waves, and by the presence of pronounced ~7–9 Hz activity presumably arising as a result of physical spread of theta activity from the hippocampus (Green and Arduini, 1954; Petsche and Stumpf, 1960; Whishaw and Vanderwolf, 1973; Robinson, 1980; Leung and Borst, 1987; Sirota et al., 2008). Hippocampal theta activity has been related to voluntary activity, arousal, attention, the representation of spatial position, learning and other behaviors or functions (Green and Arduini, 1954; Vanderwolf, 1969; Feder and Ranck, 1973; Kemp and Kaada, 1975; O’Keefe and Recce, 1993; Buzsaki, 2002). Based on phase-analysis and pharmacological studies it has been postulated that there is more than one generator and more than one type of theta activity in the hippocampus (Kramis et al., 1975; Robinson, 1980). The functional significance of hippocampal theta activity is still unclear, but it can be highly relevant for various aspects of behavior and cognition given the complex interactions between the cortex and hippocampus during sleep and waking (Buzsaki, 1998; Sirota et al., 2003; Tierney et al., 2004; Sirota and Buzsaki, 2005; Born et al., 2006; Isomura et al., 2006; Molle et al., 2006; Tononi et al., 2006; Clemens et al., 2007; Hahn et al., 2007; Ji and Wilson, 2007; Sirota et al., 2008; Wierzynski et al., 2009). Apart from the EEG, the activated pattern of brain activity during waking is also apparent at the level of firing of cortical neurons. Overall, neuronal discharge in waking is largely fast and irregular (Fig. 1B), although it is determined strongly by behavior and involvement in specific tasks.
During NREM sleep, instead, the neocortex is functionally disconnected from the surrounding, and the most distinctive feature of the EEG is the near-synchronous occurrence of slow waves in all or most cortical areas (Sejnowski and Destexhe, 2000; Massimini et al., 2004). The fundamental cellular phenomenon underlying the sleep slow waves is the slow oscillation, which consists of an UP state, characterized by sustained neuronal depolarization and irregular firing, followed by a hyperpolarized DOWN state, during which every cortical cell ceases firing (Steriade et al., 1993c; Amzica and Steriade, 1998; Destexhe et al., 1999; Steriade et al., 2001). In vivo, in vitro and in computo evidence indicates that the DOWN state of the slow oscillation is the result of disfacilitation (i.e. a lack of synaptic input), rather than of active inhibition (Steriade et al., 1993c; Timofeev et al., 2001; Hill and Tononi, 2005). Slow oscillations (<1 Hz) have also been observed in the EEG under anesthesia and in naturally sleeping cats and humans (Achermann and Borbely, 1997; Steriade and Amzica, 1998a). As a result, the firing pattern of cortical neurons in NREM sleep is dominated by periods of elevated population activity (ON periods), lasting several hundreds of milliseconds, alternated with shorter periods of generalized silence, corresponding to the negative phase of EEG slow waves (Fig. 1B). It is currently unclear what determines the regularity in the occurrence of prolonged intracellular UP and DOWN states and extracellular synchronous ON and OFF periods that ultimately give rise to the large number of high-amplitude slow waves typical of deep sleep. A bistability between ON and OFF periods can be brought about by potassium leak channels and Ih currents, whose voltage-dependent properties depend on arousal-promoting neuromodulation (McCormick, 1992a; Steriade, 1993; Steriade et al., 1993a; McCormick and Bal, 1997; Steriade et al., 2001). The altered pattern of neuronal firing during sleep can in turn lead to reduced responsiveness to sensory stimuli, and can be expected to affect brain metabolism.
Indeed, it is well known that cerebral metabolic rates are largely determined by the levels of neuronal activity (Attwell and Laughlin, 2001; Attwell and Gibb, 2005). For example, barbiturate anesthesia, which is associated with inhibition of catecholamine release (Hirota et al., 2000) and marked suppression of neuronal activity (Antkowiak, 1999), is characterized by lowered cerebral blood flow (Hendrich et al., 2001; Lowry and Fillenz, 2001) and reduced 2-DG utilization (Sokoloff et al., 1977). Given the pronounced differences in cortical firing between sleep and waking, one could expect also vigilance-state specific differences in brain metabolism. Indeed, compared to waking, the uptake of 2-deoxyglucose during NREM sleep is reduced in animals (Kennedy et al., 1982; Ramm and Frost, 1983, 1986), while a global deactivation of the neocortex and thalamus has been demonstrated with PET studies in humans (reviewed in (Maquet, 2000)). Moreover, there is evidence that mRNA and protein levels of genes involved in cerebral energy metabolism (e.g. mitochondrial enzymes and glucose transporters) are lower during sleep than during waking (Petit et al., 2002; Cirelli et al., 2004; Nikonova et al., 2005), and several key components of the mitochondrial electron transport chain are upregulated after prolonged waking (Nikonova et al., 2010). During NREM sleep metabolic activity is inversely correlated with slow EEG activity (Hofle et al., 1997; Czisch et al., 2004; Dang-Vu et al., 2005), presumably because larger and more frequent slow waves are associated with longer and more frequent periods of neuronal silence (Calvet et al., 1973; Esser et al., 2007; Vyazovskiy et al., 2009b).
Thus, behavior, cortical and subcortical neuronal activity and brain metabolism differ significantly between vigilance states. This notion suggests that in physiological conditions brain state can be maintained and regulated at different levels of activity. However, the question arises whether brain activity is not only different depending on whether the animal is awake or asleep, but also depending on for how long it has been awake or asleep before, within the same vigilance state.
Global and local regulation of sleep
It is well known that sleep is homeostatically regulated (Borbely, 1982; Daan et al., 1984). In most species, sleep pressure increases as a function of time spent awake and decreases in the course of sleep. There are several markers of sleep pressure (Borbely, 2000; Cirelli and Tononi, 2008). For example, after sleep deprivation sleep time increases, sleep episodes become longer, and the number of brief awakenings decreases (Franken et al., 1991; Huber et al., 2000b; Vyazovskiy et al., 2002; Tobler, 2005; Vyazovskiy et al., 2007b). However, the best characterized physiological indicator of the sleep-wake history is the level of EEG slow-wave activity (SWA, EEG power between 0.5 and 4.0 Hz) during NREMS ((Borbely, 1982; Daan et al., 1984), reviewed in (Borbely, 2000)) (Fig. 1A, 2A). In mammals, sleep SWA is high in early sleep, when sleep pressure is increased physiologically, and decreases progressively to reach low levels in late sleep (Rosenberg et al., 1976; Tobler and Borbely, 1986; Franken et al., 1991; Vyazovskiy et al., 2007b). Moreover, sleep SWA increases further with sleep deprivation, and is reduced by naps (Borbely, 2000; Tobler, 2005). Sleep pressure builds up especially after extended wake periods (Borbely et al., 1984; Tobler and Borbely, 1986) and even during relatively short spontaneous waking episodes (Werth et al., 1996b; Huber et al., 2000b; Vyazovskiy et al., 2006), and, and several studies showed that the changes in sleep need unlikely arise from stress or changes in brain temperature incurred during preceding waking (Tobler et al., 1983; Franken et al., 1991).
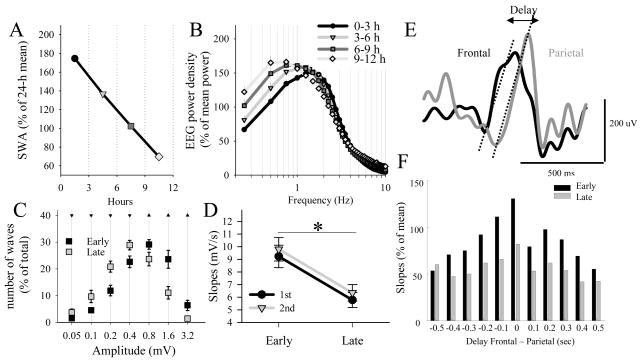
Figure 2. Homeostatic regulation of SWA and slow wave parameters.
(A) Time course of SWA in NREM sleep during the light period. Mean values (n=15 rats, SEM) are plotted for consecutive 3-h intervals. (B) Time course of EEG power spectra in NREM sleep during the light period (same data as in (A). Mean spectra are plotted for consecutive 3-h intervals. Note a progressive shift of the spectral peak towards slower frequencies in the course of the light period. (C) Distribution of the amplitude of slow waves during early and late sleep. Mean values (SEM, n = 15 rats) are plotted as percentage of the total number of waves. Triangles: amplitude ranges where slow wave incidence was higher during early sleep (triangles up) or higher during late sleep (triangles down, p<0.05, Sidak test). (D) Slopes of the 1st and 2nd segment of EEG slow waves in early sleep and late sleep. Mean values, n=15 rats. Asterisk: p<0.05, paired t-test. (E) Near-simultaneously occurring LFP slow waves recorded from layer V of the frontal and parietal cortical areas. Note that the parietal slow wave is delayed relative to the frontal wave. Dotted lines depict slopes of the slow waves. (F) Relationship between the delay between slow waves occurring in the frontal and in the parietal derivation and the corresponding slow wave slopes. One individual rat is shown. Note that slow waves have steepest slopes when they occur synchronously in the two derivations, especially in early sleep.
While the homeostatic regulation of SWA is a precise, ubiquitous and basic feature of sleep in mammals and birds (Tobler, 2005; Cirelli and Tononi, 2008), its underlying mechanisms remain unknown. However, an important insight came from the observation that sleep is not only a global process but has a local use-dependent component manifested in regional differences in SWA (Krueger and Obal, 1993; Kattler et al., 1994; Krueger et al., 2008). Over the last two decades multiple studies showed that during spontaneous sleep SWA is not uniform across the cortical surface, but topographically organized. In both humans and animals, SWA is more intense in the frontal derivations, especially in early sleep or after sleep deprivation (Werth et al., 1996a; Cajochen et al., 1999; Huber et al., 2000a; Finelli et al., 2001; Vyazovskiy et al., 2002; Zavada et al., 2009). Frontal predominance of sleep SWA may arise from cortico-cortical connectivity (Vyazovskiy and Tobler, 2005), cognitive load (Anderson and Horne, 2003b, a), topographic differences in cortical metabolism (Drummond and Brown, 2001; Vyazovskiy et al., 2004b) or preceding motor activity (Leemburg et al.; Vyazovskiy et al., 2006; Vyazovskiy et al., 2007a). An intriguing possibility is that the anterior-posterior gradient in SWA is related to a “preferred origin” of sleep slow waves in frontal regions, from where slow waves would propagate across cortical areas. Such a possibility is suggested in various species and preparations, e.g. in vitro, in anesthetized animals, and in sleeping rats and humans (Leemburg et al.; Sanchez-Vives and McCormick, 2000; Timofeev et al., 2000; MacLean et al., 2005; Richardson et al., 2005; Massimini et al., 2007; Rigas and Castro-Alamancos, 2007; Murphy et al., 2009; Vyazovskiy et al., 2009a), and may have a functional role.
For example, more recently, a number of studies addressed the question of whether regional differences in SWA are activity- or use-dependent. It was found that peripheral experimentally-induced stimulation or the spontaneous use of circumscribed cortical areas leads to more intense local slow waves (Kattler et al., 1994; Vyazovskiy et al., 2000; Vyazovskiy et al., 2004b). Moreover, local, topographically distinct enhancement of slow waves was associated with learning of a motor task (Huber et al., 2004; Vyazovskiy and Tobler, 2008; Hanlon et al., 2009), while unilateral arm-immobilization led to a local decrease in slow waves (Huber et al., 2006). Such observations suggested that waking activities could in a selective manner affect intensity of subsequent sleep. Indeed, it is well known that staying awake inevitably leads to decreased neurobehavioral performance (Dijk et al., 1992; Czeisler, 2009) and altered brain metabolism (Everson et al., 1994; Wu et al., 2006), suggesting that being awake leads to changes in cortical networks, which are manifested in enhanced SWA during subsequent sleep. It has recently been proposed that waking, which is associated with a variety of plastic processes, is associated with net synaptic potentiation, whereas sleep results in synaptic downscaling (Tononi and Cirelli, 2003, 2006). The experimental evidence for this hypothesis has been provided by several studies in humans, rodents and flies (Vyazovskiy et al., 2000; Cirelli et al., 2004; Huber et al., 2004; Huber et al., 2006; Huber et al., 2007b; Huber et al., 2007a; Bellina et al., 2008; De Gennaro et al., 2008; Vyazovskiy et al., 2008; Gilestro et al., 2009; Hanlon et al., 2009), as well as in rats and mice in vitro (Liu et al., 2010). Most importantly, such regulation of cortical plasticity would ensure the restoration of the brain’s ability to learn by preventing synaptic saturation, and would maintain an overall balance of space and energy resources.
It is reasonable to assume that an increase in cortical synaptic strength, engendered by prolonged waking, would lead to specific changes in spontaneous and evoked cortical activity, both at the level of the EEG and cortical neurons. In the next section, we will discuss the changes in EEG waves during sleep incurred as a function of preceding sleep/wake history and their functional significance.
Homeostatic sleep pressure is reflected in the amplitude and slopes of sleep slow waves
Traditionally, sleep homeostasis is measured with EEG power spectral analysis, which shows larger SWA at sleep onset after sleep deprivation relative to baseline. However, we reasoned that if sleep is associated with a generalized decrease in cortical synaptic strength, the latter should be most apparent in the temporal domain of the EEG. Therefore, in a recent series of studies in vivo, complemented by computer simulations (Esser et al., 2007; Riedner et al., 2007; Vyazovskiy et al., 2007c) we set out to investigate how changes in the EEG signal per se account for the homeostatic changes in the corresponding power spectra.
First, apart from the well-known overall decline of spectral power in the SWA range with decreasing sleep pressure, we observed a redistribution of power towards lower frequencies, resulting in a shift of the spectral peak (Fig 2B). The mechanisms underlying this shift had not been investigated before, although several previous studies had reported a dissociation between the homeostatic behavior of frequencies <1 Hz and the remaining SWA frequencies (Dijk et al., 1987; Achermann and Borbely, 1997, 1998; Campbell et al., 2006).
To disentangle the mechanisms underlying the decrease of SWA from the shift of the spectral peak, we first quantified individual slow wave events and compared the relationship between their amplitude and incidence between early and late sleep. Invariably, in both rats and humans we found that, while the total number of slow waves during sleep remained roughly constant, the proportion of high-amplitude slow waves decreased from early to late sleep (Riedner et al., 2007; Vyazovskiy et al., 2007c) (Fig 2C). Computer simulations revealed that such decrease in slow wave incidence could fully account for an overall decline of SWA (Vyazovskiy et al., 2007c). Thus, we concluded that the overall homeostatic decline of spectral power in SWA range occurs largely due to lower incidence of high amplitude slow waves.
Next, we reasoned that slowing of the peak of the spectrum could arise from the change in the shape of slow waves, as suggested by previous studies employing period-amplitude analysis, which showed an increase in slow wave period with decreasing sleep pressure (Feinberg et al., 1978; Bergmann et al., 1987; Mistlberger et al., 1987). Indeed, when we performed additional simulations where slow wave amplitude and incidence were kept constant, but the slope of slow waves was varied, a shift of the spectral power was observed, as we found in vivo (Vyazovskiy et al., 2007c). Consistently, in both animals and humans we found a clear-cut decrease in the slope of slow waves with decreasing homeostatic sleep pressure (Riedner et al., 2007; Vyazovskiy et al., 2007c; Bersagliere and Achermann, 2009) (Fig 2D). Thus, while decreased incidence of slow waves may account for the overall lower SWA values, the changes in the slopes may lead to the slowing of the spectral peak.
In order to address the mechanisms underlying the decrease in slow wave slopes we performed further computer simulations (Esser et al., 2007), which revealed that changes in slow wave slope can be accounted for by the speed of recruitment/decruitment of neurons in the population slow oscillation (the steeper the slope, the quicker the recruitment), which was determined by the efficacy of cortical connections. Additional experiments performed in vivo also suggested that the slopes of cortical sleep slow waves reflect the level of synchrony between cortical regions, especially in early sleep (Fig 2E,F). Thus, these results suggested that stronger crosstalk between neuronal populations after increased sleep pressure is reflected in steeper slopes of slow waves. A similar link between sleep homeostasis and cortical synchrony was also reported in mice, in which interhemispheric coherence of slow EEG frequencies increased after sleep deprivation and declined during recovery (Vyazovskiy et al., 2004a).
Thus, altogether these data suggested that high levels of EEG SWA in early sleep are brought about by i) more frequent occurrence of high amplitude slow waves and ii) more synchronous cortical activity, as measured by slow wave slopes. We suggest that slow wave slope may be an even more sensitive marker of sleep homeostasis than SWA, since their slope was steeper in early sleep compared to late sleep even after the two conditions were matched for amplitude and SWA (Riedner et al., 2007; Vyazovskiy et al., 2007c). As traveling slow waves in the developing neocortex may be important for maturation of cortical circuits (Conhaim et al.), spatial neuronal synchrony during sleep may be important for some aspects of plasticity and ultimately to the function of sleep. On the other hand, such increased sensitivity would be expected if the slope of the slow waves reflects some basic cellular and network phenomena, for example the efficacy of cortico-cortical connectivity. Therefore, next we asked whether cortical net synaptic strength indeed increases during wake and decreases during sleep.
Homeostatic sleep pressure is reflected in slopes of the early and late components of electrically evoked cortical responses
If wake and sleep are associated with a net increase and decrease of synaptic strength respectively, then sleep-wake history should be reflected in the parameters of cortical evoked potentials. This hypothesis is based on the notion that the amplitude or the slope of excitatory postsynaptic potential (EPSP) or field potentials increased as a result of high-frequency tetanization of afferent pathways in hippocampal slices (Alger and Teyler, 1976), cortical slices (Lee et al., 1991; Aizenman et al., 1996; Artola et al., 1996; Rioult-Pedotti et al., 1998), in the hippocampus in vivo (Bliss and Lomo, 1973; Abraham et al., 1985; Bliss and Collingridge, 1993) and in the cortex in vivo (Iriki et al., 1991; Beiko and Cain, 1998; Glazewski et al., 1998). Empirical data and computational experiments show that the time derivative, i.e., the slope, of EPSP is proportional to the amplitude of transmembrane currents (Araki and Terzuolo, 1962; Rall, 1967). It has been postulated that neuronal plasticity is manifested in long-term potentiation (LTP) and long-term depression (LTD) of synaptic transmission, which involve rapid adjustments in the strengths of individual synapses in response to specific patterns of correlated synaptic activity (Stellwagen and Malenka, 2006), or in vivo experience (Rioult-Pedotti et al., 2000; Whitlock et al., 2006). Since active wake is likely associated with learning, and most learning occurs through potentiation, it can be expected that wake would lead to potentiation of cortical circuits (Tononi and Cirelli, 2003, 2006). The hypothesis that waking, which is characterized by increased norepinephrine modulation (Armstrong-James and Fox, 1983; Jones, 1991; McCormick et al., 1991; Berridge and Waterhouse, 2003) is associated with synaptic potentiation (Tononi and Cirelli, 2006) was supported by the observation that cortical catecholaminergic depletion disrupts plasticity (Bear and Singer, 1986), and affects waking-related gene expression (Cirelli et al., 1996; Cirelli et al., 2004), and subsequent increase in sleep SWA (Cirelli et al., 2005). Consistently, it has recently been shown that a wake-dependent increase in another well-known arousal-related neuromodulator histamine (McCormick, 1992a; Jones, 2005) promoted hippocampal LTP in freely moving rats (Luo and Leung).
To evaluate changes in synaptic efficacy as a function of preceding wake and sleep, we implanted two groups of rats with bipolar concentric electrodes for electrical stimulation and chronic intracortical LFP recordings (Vyazovskiy et al., 2008). We recorded LFPs from the frontal cortex on one side of the brain after electrical stimulation of the opposite side in quietly awake rats (Vyazovskiy et al., 2008), and measured the slope of the first negative component of this transcallosal evoked response after several hours of continuous waking and sleep. We hypothesized that, in analogy with LTP induction, increased slopes of the evoked responses after wake would reflect stronger cortico-cortical synapses. Indeed, we found that the slope of the first LFP component increased on average by 22% from the beginning to the end of the continuous waking period (Vyazovskiy et al., 2008) – an increase of magnitude similar to that recently found in the hippocampus in vivo after physiological learning (Whitlock et al., 2006). By contrast, in rats that had been almost continuously asleep during the first 4 hours of the light phase, the slope of the first LFP component decreased by almost 20% between the two recording sessions. Thus, an electrophysiological indicator of synaptic efficacy - the slope of the early component of cortical evoked potentials – was high after spontaneous wakefulness and low after spontaneous sleep. Moreover, the increase in slope correlated positively with preceding waking duration, whereas the magnitude of its decline was determined by the duration of preceding NREM sleep (Vyazovskiy et al., 2008). Crucially, the increase in the slope of the LFP response after a period of waking positively correlated with SWA at the beginning of the subsequent sleep period, while the decrease in the slope correlated positively with the decrease in SWA and slow wave slopes during sleep (Vyazovskiy et al., 2008). Thus, the changes in synaptic efficacy could be revealed both with electrically evoked and spontaneously generated volleys (Vyazovskiy et al., 2008).
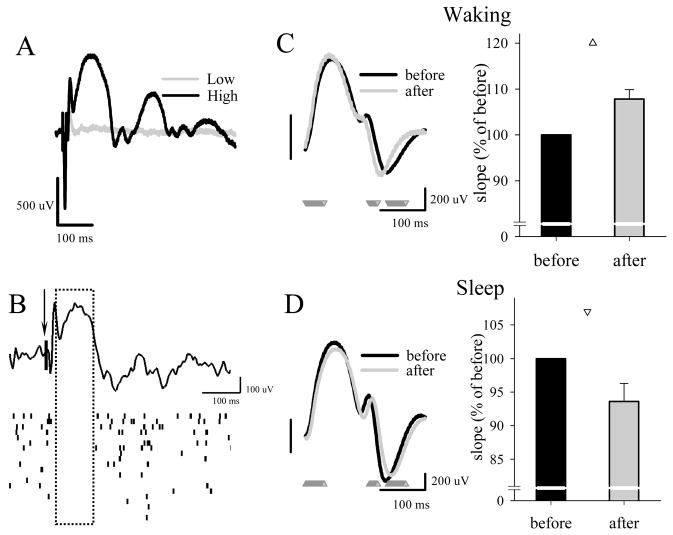
We noticed that if the intensity of electrical stimuli is sufficiently high, the early, presumably monosynaptic, negative potentials are followed by a large, ~ 150 ms-long depth-positive waves (Fig 3A), similar to previously described late potentials evoked by electrical stimulation in slices or under anesthesia (Chang, 1953; McCormick, 1992b; Contreras and Steriade, 1995). The late cortical potential is known to be strongly attenuated in active waking, is related to the suppression of multiunit activity (Vanderwolf et al., 1987) (Fig 3B), and is considered a population inhibitory postsynaptic potential, occurring as a result of polysynaptic activation of cortical interneurons (McCormick, 1992b). A similar cortical potential was also recorded after thalamic stimulation that evokes hyperpolarization of neurons in the deep cortical layers (Ohsaki and Iwama, 1961). Intriguingly, we found that the slope of the late potential was consistently steeper after a period of wakefulness and decreased after sleep (Fig 3C,D), just like the slope of sleep slow waves (Vyazovskiy et al., 2007c) or the slope of early monosynaptic potentials (Vyazovskiy et al., 2008).
Figure 3. Sleep-wake history affects the late component of transcallosal evoked response.
(A) Individual representative evoked responses after stimulation at low and high intensity. (B) Representative individual transcallosal evoked LFP response and the corresponding neuronal activity (raster below the LFP trace, each bar is a spike). Note that all neurons remain silent for about 100 ms after the stimulus (arrow), during the positive LFP wave (boxed). C. Left: Average late component of the evoked response before and after a 4-h period of waking. Mean values (n=15 rats). Triangle depicts significant difference (paired t-test). Right: Slopes of the late component before and after 4 hours of waking. Values are mean + SEM (n=15) expressed as % of the “before” condition (=100%). D. Left: Average late component of the evoked response before and after a 4-h period of consolidated sleep. Mean values (n=24 rats). Triangles depict significant differences (paired t-test). Right: Slopes of the late component before and after 4 hours of sleep. Values are mean + SEM (n=24) expressed as % of the “before” condition (=100%).
While specific cellular and network mechanisms underlying the slopes of the early and late component in waking and the slope of slow waves during sleep are presumably different, their consistent changes in relation to the sleep/wake history unequivocally indicate that the state of cortical networks is altered following waking and sleep in an opposite manner. Intracortical stimulation appears a promising tool to investigate dynamic changes in the responsiveness of cortical networks as well as the mechanisms of synaptic interactions after sleep and waking. The question then arises: what are the consequences of increased synaptic strength after waking on brain functioning?
Increased homeostatic sleep pressure affects cortical plasticity
An important property of the potentiation of cortical synapses is saturation, which is manifested in a relative inability to further enhance the amplitude of synaptic currents in the response to the stimulus of increasing intensity or frequency (Heynen and Bear, 2001). Saturation of both LTP and LTD after repeated electrical or pharmacological stimulation was found in different species and preparations (Frey et al., 1995; Doyere et al., 1997; Moser et al., 1998; Heynen and Bear, 2001; Lante et al., 2006). We hypothesized therefore that if wake is associated with a physiological increase in synaptic strength, then the experimentally induced LTP should be, at least partially, blocked after waking and, on the contrary, facilitated again after sleep.
While mechanisms underlying satiability of LTP are not yet clear, it appears to be a crucial property for establishing a functional link between LTP-like processes and learning. It was predicted that an experimental treatment that results in LTP saturation should produce deficits in learning of a task that requires an involvement of the corresponding neuronal circuit (Moser and Moser, 1999). Indeed, there is compelling evidence that by triggering LTP-like mechanisms, learning can strengthen synapses to near the maximum of their modification range, impairing the further induction of LTP (Foster et al., 1996; Rioult-Pedotti et al., 2000; Sacchetti et al., 2002). Several studies have shown conclusively that LTP or LTD are strongly affected by learning and vice versa (Castro et al., 1989; Barnes et al., 1994; Moser et al., 1998; Rioult-Pedotti et al., 1998; Moser and Moser, 1999; Rioult-Pedotti et al., 2000; Ziemann, 2004; Stefan et al., 2006; Whitlock et al., 2006; Makhracheva-Stepochkina et al., 2008). There is also indirect evidence suggesting that LTP-like plasticity may be partially saturated after wakefulness. For example, several in vitro studies in the hippocampus showed that insufficient or fragmented sleep impairs the induction of LTP but favors the induction of LTD (e.g. (Campbell et al., 2002; McDermott et al., 2003; Kopp et al., 2006; Tartar et al., 2006)).
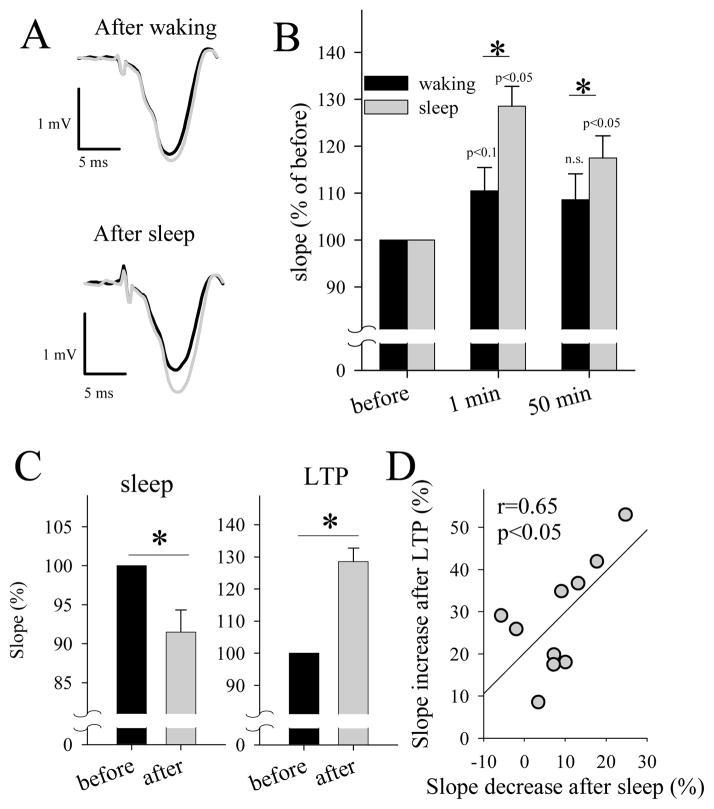
The occlusion of electrically-induced LTP after wake is therefore a key test to prove that staying awake shares the same mechanisms underlying LTP. To address this possibility, we used an LTP induction paradigm as in (Racine et al., 1995; Vyazovskiy et al., 2008). First, we found that after a period of waking the magnitude of LTP induction was smaller compared to that seen after the period of sleep (Fig 4A,B). Next, to prove that this effect was indeed sleep-related, we correlated the magnitude of the slope decline after sleep with the magnitude of its subsequent increase after LTP, and found that they were positively correlated (Fig 4C,D). In other words, the more synaptic strength declined during sleep, the larger was the capacity for its subsequent increase after LTP. This result suggested that sleep might restore the ability to undergo further cortical potentiation. Finally, we observed that NREM SWA was also increased after LTP by ~12% as compared to pre-LTP levels (p<0.05), suggesting that local strengthening of synapses as a consequence of the LTP induction could have resulted in a more intense local sleep.
Figure 4. LTP is partially occluded after waking.
(A) Representative average evoked LFPs from the right frontal cortex induced by transcallosal stimulation of the left frontal cortex in a quietly awake rat before (black) and after (grey) LTP induction. Note a minor change in the response when LTP induction was attempted after waking (top), and a substantial increase when LTP was induced after a period of sleep (bottom). (B) Slopes of evoked potentials immediately before and after LTP induction (1 min, 50 min) attempted following a period of waking or sleep. Values are mean ± SEM (n=10) expressed as % of the corresponding “before” condition (=100%). (C) Left: Slopes of evoked LFPs before and after a consolidated 4-h period of sleep. Right: Slopes of evoked LFPs before and after LTP induction (mean values, SEM, n=10). Asterisks: p < 0.05; two-tailed paired t-test. (D) Relationship between the decrease in LFP slope after sleep and the following increase after LTP, shown in (C). Each circle is an individual rat (n=10).
Caution is warranted, however, in interpreting changes of cortical evoked field potentials, as they might be affected by behavior, brain temperature, and background activity. Moreover, they reflect the summed activity of a relatively large heterogeneous population of neurons and may be confounded by antidromic or polysynaptic components. Thus, a recent study (Liu et al., 2010) used a direct measure of synaptic efficacy, i.e. measured the effects of sleep/wake on miniature excitatory postsynaptic currents (mEPSCs). This is because the analysis of mEPSCs amplitude and frequency is one of the best established methods to directly measure synaptic strength: changes in mEPSCs frequency are thought to result from modification of the presynaptic component of synaptic transmission, while amplitude changes indicate alterations in the postsynaptic component. The study used coronal cortical slices from the frontal cortex of rats, and compared mEPSCs after wake (at night) vs. sleep (during the day), after sleep vs. sleep deprivation (both during the day), and after sleep deprivation vs. recovery sleep (both during the day). In all cases it was found that frequency and amplitude of mEPSCs recorded from layer II/III cortical neurons increased after wake and sleep deprivation as compared to sleep, and another experiment confirmed these results in sleep deprived mice compared to sleeping mice (Liu et al., 2010).
Thus, this collective evidence, encompassing spontaneous and evoked cortical activity at the level of sleep slow waves, the early and late components of LFPs as well as of spontaneously occurring mEPSCs, suggests that physiological waking is associated with net synaptic potentiation of cortical synapses. It is well known that EEG and LFP slow waves as well as evoked field potentials are large-scale phenomena, which reflect concerted, mainly postsynaptic activity of relatively large populations of cortical neurons. The question remains, how does the spiking activity of individual cortical neurons change in relation to the sleep-wake history?
Homeostatic sleep pressure is reflected in synchronization of cortical neurons
It is well known that cortical activity consists of ongoing changes of membrane potentials and spiking of individual neurons that are tightly linked to vigilance state, movement, behavior and the presence or absence of sensory stimuli (Steriade et al., 2001; Petersen et al., 2003; MacLean et al., 2005; Sakata and Harris, 2009; Vyazovskiy et al., 2009b). The fluctuations in neuronal activity are not only caused by external sensory stimuli that reach the neocortex via thalamocortical projections (Steriade et al., 1993b; Crunelli and Hughes, 2009; Sakata and Harris, 2009) but can arise also spontaneously from local intracortical networks (Petersen et al., 2003). What are the factors that determine spontaneous firing patterns of cortical neurons? This question is relevant since changes of the internal brain state affect markedly cortical responsiveness (Petersen et al., 2003; Hasenstaub et al., 2007; Watson et al., 2008; Vyazovskiy et al., 2009a). Moreover, periodic synchronous increases of neuronal activity can recruit large distributed cortical areas (Diesmann et al., 1999; Ikegaya et al., 2004; Thiagarajan et al., 2010), while decreases in firing can contribute significantly to energy savings (Sokoloff et al., 1977; Attwell and Laughlin, 2001).
During NREM sleep large populations of neurons transition quasi-synchronously between periods of elevated activity (ON-periods) and neuronal silence (OFF-periods) (Sirota and Buzsaki, 2005; Ji and Wilson, 2007; Vyazovskiy et al., 2009b). There is a close temporal relationship between ON or OFF periods, the underlying cortical slow oscillation, and simultaneously recorded slow waves (Contreras and Steriade, 1995; Amzica and Steriade, 1998). Specifically, the surface negativity in the EEG signal (or depth positivity in the local field potential, LFP) corresponds to the DOWN state of cortical neurons as recorded intracellularly, and to the suppression of spiking activity as recorded extracellularly, suggesting that EEG or LFP slow waves are a reflection of near-synchronous transitions between UP and DOWN states in large populations of cortical neurons (Murata and Kameda, 1963; Calvet et al., 1973; Noda and Adey, 1973; Burns et al., 1979; Steriade et al., 1993b; Contreras and Steriade, 1995; Steriade et al., 2001; Molle et al., 2006; Mukovski et al., 2006; Ji and Wilson, 2007; Luczak et al., 2007). However, until recently it was unknown whether cortical neurons fire differently depending on how long the brain has been awake or asleep.
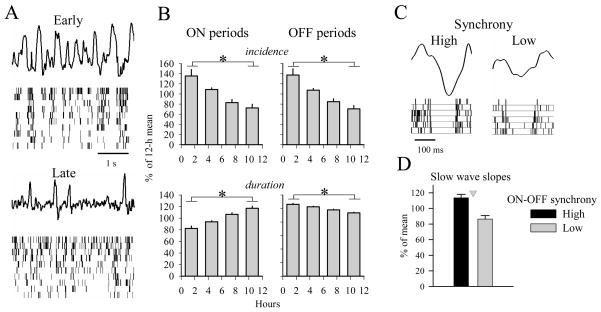
In order to address this question, we implanted chronic microwire arrays in the frontal and parietal cortex and recorded cortical unit activity along with the EEG and LFPs during spontaneous sleep and wake in freely behaving rats (Vyazovskiy et al., 2009b). In NREM sleep, a striking difference was apparent between early and late sleep: during early sleep, when large slow waves predominate, short ON periods alternated frequently with relatively long OFF periods, whereas in late sleep, when large slow waves are rare, ON periods were longer and only occasionally interrupted by short OFF periods (Fig 5A). Quantitative analysis revealed that both the duration of population ON and OFF periods changed as a function of preceding sleep and waking. During early sleep, when most neurons were active or silent synchronously, ON periods were short and frequent. During late sleep, the periods of activity and inactivity of individual neurons became progressively less synchronized (Fig 5B). Notably, the changes in the number and duration of ON and OFF periods were correlated with the decrease in SWA in the course of sleep (Vyazovskiy et al., 2009b), suggesting that homeostatic changes in sleep SWA arise from an altered pattern of cortical neuronal activity.
Figure 5. Sleep-wake history affects cortical neuronal firing patterns.
(A) 4-s frontal LFP records in early and late NREM sleep. Corresponding raster plots of spike activity (10 units are shown, each vertical line is a spike) are shown below the LFP traces. Note the close temporal relationship between silent (OFF) periods and the positive phases of LFP slow waves in NREM sleep. (B) Mean values (n = 6 rats) of incidence and duration of the ON and OFF periods shown for consecutive 3-h intervals during the light period expressed as percentage of the corresponding mean 12-h value. (C) Representative examples of a high-amplitude slow EEG wave with steep slopes typical of early sleep (left) and low-amplitude slow wave typical of late sleep (right), and the corresponding OFF periods (raster plots, each bar is a spike). Note that neurons are more synchronous at the ON-OFF and OFF-ON transitions during the high-amplitude slow wave. (D) Mean slopes of slow waves corresponding to synchronous ON-OFF transition (high: top 50%) and asynchronous ON-OFF transitions (low: bottom 50%). Neuronal synchrony was computed as the average latency between the last spikes of individual neurons to the onset of the population OFF period. Note that high neuronal synchrony is associated with steeper slow wave slopes (triangle, p<0.01). Mean values, n=6 rats.
It was apparent that in early sleep, following prolonged bouts of wakefulness, individual neurons stopped or resumed firing in near synchrony with the rest of the population. By contrast, during late sleep, following prolonged bouts of NREM sleep, the time of entry into ON and OFF periods was much less synchronous (Vyazovskiy et al., 2009b). The changes in neuronal synchrony at the ON-OFF and OFF-ON transitions appeared to be related to the morphology of slow waves. Specifically, we found that firing with high synchrony was associated with steep slopes of simultaneously occurring EEG slow waves, whereas low synchrony was associated with decreased slopes of the corresponding slow waves (Fig 5C,D). Finally, we found that the changes in neuronal synchrony during sleep correlated with the changes in both slow wave slopes and SWA (Vyazovskiy et al., 2009b). These observations supported the results of computer simulations (Esser et al., 2007) and provided direct in vivo evidence that EEG slow wave slopes, as well as their changes as a function of the sleep/wake history, are determined by the rate of recruitment and decruitment of cortical neurons into the slow oscillation. Thus, the sleep-wake history, that is associated with plastic processes in cortical networks, is reflected also in neuronal synchrony.
Concluding remarks
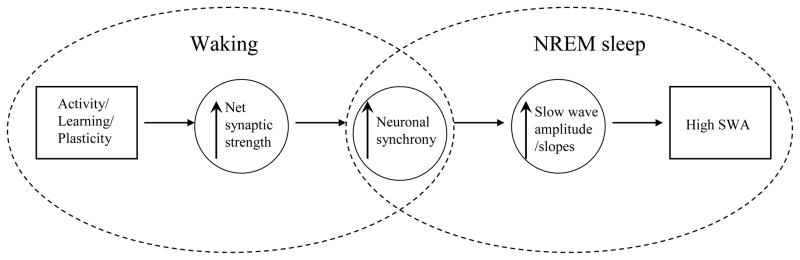
Homeostatic sleep regulation is manifested in predictable changes of sleep EEG SWA. A crucial question is how the changes in SWA arise from cortical networks, and why SWA is affected by the sleep-wake history. Recent studies began to elucidate cellular and network mechanisms underlying the changes in EEG slow waves that bring about well-known homeostatic changes of SWA. In this review we presented and discussed electrophysiological correlates of sleep homeostasis at three levels: sleep EEG/LFP slow waves, evoked cortical activity and spontaneous neuronal firing patterns. The principal observations presented here can be summarized as follows: physiological or prolonged waking leads to i) increased incidence and steeper slopes of EEG slow waves; ii) increased slopes of the early and late components of evoked field potentials; iii) partial LTP occlusion and iv) increased synchrony among cortical neurons. Crucially, all these changes were reversed by sleep and correlated with changes of the traditional marker of sleep homeostasis – SWA. How can all these changes at different levels be integrated in one coherent picture? One possibility is that increased net cortical synaptic strength during waking (Vyazovskiy et al., 2000; Cirelli et al., 2004; Huber et al., 2004; Huber et al., 2006; Huber et al., 2007b; Huber et al., 2007a; Bellina et al., 2008; De Gennaro et al., 2008; Vyazovskiy et al., 2008; Gilestro et al., 2009; Hanlon et al., 2009; Liu et al., 2010) is the mechanism directly underlying increased neuronal synchrony, which then translates in more frequent incidence of high amplitude slow waves in subsequent sleep, leading in turn to homeostatically increased sleep SWA (Fig. 6).
Figure 6. Waking activities result in a homeostatic increase in NREM sleep SWA.
Waking activity is associated with learning/plasticity that lead to increased cortical net synaptic strength. More efficient neuronal connectivity results in more efficient neuronal synchronization, which is manifested in faster rates of decruitment and recruitment of neurons into the slow oscillation in NREM sleep. The occurrence of frequent prolonged periods of neuronal population silence is manifested in the EEG signal as frequent incidence of high amplitude slow waves with steep slopes. High amplitude slow waves in turn account for high spectral power in SWA band.
The essential steps could be the initiation of the UP state at the level of individual cortical neurons and its subsequent propagation across large cortical assemblies. It has been shown that the onset of the UP state is caused by a gradual membrane depolarization that builds up due to the summation of subthreshold events, which occur as a result of spontaneous release of neurotransmitter (Timofeev et al., 2000; Chauvette et al., 2010). Once a subset of neurons enters an UP state, it might be capable of recruiting other neurons, primarily those that, as suggested before, receive most dense or strongest projections from other neurons (Timofeev et al., 2000; Chauvette et al., 2010). Similarly, in humans and rats, a volley of locally applied electrical or magnetic activity that is sufficiently strong to excite and recruit a large cortical neuronal population is capable of inducing full-fledged EEG slow waves during natural sleep (Massimini et al., 2007; Vyazovskiy et al., 2009a). Subsequently, activation can spread through local cortical networks in a sequential order (Luczak et al., 2007), presumably along the gradient of strongest connections within a network. It can be expected that the more frequent occurrence of higher-amplitude mEPSCs after prolonged waking (Liu et al., 2010) would likely lead to a more frequent occurrence of neuronal UP states, and if the connections between neurons are strong, this would result in faster rates of their recruitment in the population ON periods (Esser et al., 2007; Riedner et al., 2007; Vyazovskiy et al., 2007c; Vyazovskiy et al., 2009a; Vyazovskiy et al., 2009b), and in their efficient propagation across large cortical areas (Massimini et al., 2004; Vyazovskiy et al., 2009a). Thus, more frequent EEG slow waves with higher amplitude and steeper slopes would occur in the EEG signals, resulting in higher SWA.
Acknowledgments
Supported by NIMH P20 MH077967 (CC), NIH Director’s Pioneer award (GT), AFOSR FA9550-08-1-0244 (GT) and Swiss National Science Foundation grant PBZHB-106264 (VVV). We thank Ugo Faraguna, Aaron Nelson, Michael Dash, Erin C. Hanlon, Brady Riedner, Eric C. Landsness, Umberto Olcese, and Steve Esser for help with the experiments and data analysis.
References
- Abraham WC, Bliss TV, Goddard GV. Heterosynaptic changes accompany long-term but not short-term potentiation of the perforant path in the anaesthetized rat. J Physiol. 1985;363:335–349. doi: 10.1113/jphysiol.1985.sp015714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achermann P, Borbely AA. Low-frequency (< 1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. doi: 10.1016/s0306-4522(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Achermann P, Borbely AA. Temporal evolution of coherence and power in the human sleep electroencephalogram. J Sleep Res. 1998;7(Suppl 1):36–41. doi: 10.1046/j.1365-2869.7.s1.6.x. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Kirkwood A, Bear MF. A current source density analysis of evoked responses in slices of adult rat visual cortex: implications for the regulation of long-term potentiation. Cereb Cortex. 1996;6:751–758. doi: 10.1093/cercor/6.6.751. [DOI] [PubMed] [Google Scholar]
- Alger BE, Teyler TJ. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003a;40:349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Anderson C, Horne JA. Electroencephalographic activities during wakefulness and sleep in the frontal cortex of healthy older people: links with “thinking”. Sleep. 2003b;26:968–972. [PubMed] [Google Scholar]
- Antkowiak B. Different actions of general anesthetics on the firing patterns of neocortical neurons mediated by the GABA(A) receptor. Anesthesiology. 1999;91:500–511. doi: 10.1097/00000542-199908000-00025. [DOI] [PubMed] [Google Scholar]
- Araki T, Terzuolo CA. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K. Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol. 1983;335:427–447. doi: 10.1113/jphysiol.1983.sp014542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Hensch T, Singer W. Calcium-induced long-term depression in the visual cortex of the rat in vitro. J Neurophysiol. 1996;76:984–994. doi: 10.1152/jn.1996.76.2.984. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Beiko J, Cain DP. The effect of water maze spatial training on posterior parietal cortex transcallosal evoked field potentials in the rat. Cereb Cortex. 1998;8:407–414. doi: 10.1093/cercor/8.5.407. [DOI] [PubMed] [Google Scholar]
- Bellina V, Huber R, Rosanova M, Mariotti M, Tononi G, Massimini M. Cortical excitability and sleep homeostasis in humans: a TMS/hd-EEG study. Journal of Sleep Research. 2008;17:39. [Google Scholar]
- Bergmann BM, Mistlberger RE, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: stage and diurnal variations and effects of suprachiasmatic nuclei lesions. Sleep. 1987;10:523–536. [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bersagliere A, Achermann P. Slow oscillations in human non-rapid eye movement sleep electroencephalogram: effects of increased sleep pressure. J Sleep Res. 2009 doi: 10.1111/j.1365-2869.2009.00775.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbely AA, Tobler I, Hanagasioglu M. Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res. 1984;14:171–182. doi: 10.1016/0166-4328(84)90186-4. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Achermann P. Homeostasis of human sleep and models of sleep regulation. 3. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- Burns BD, Stean JP, Webb AC. The effects of sleep on neurons in isolated cerebral cortex. Proc R Soc Lond B Biol Sci. 1979;206:281–291. doi: 10.1098/rspb.1979.0105. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–69. [PubMed] [Google Scholar]
- Calvet J, Fourment A, Thiefry M. Electrical activity in neocortical projection and association areas during slow wave sleep. Brain Res. 1973;52:173–187. doi: 10.1016/0006-8993(73)90657-4. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Higgins LM, Darchia N, Feinberg I. Homeostatic behavior of fast Fourier transform power in very low frequency non-rapid eye movement human electroencephalogram. Neuroscience. 2006;140:1395–1399. doi: 10.1016/j.neuroscience.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Castro CA, Silbert LH, McNaughton BL, Barnes CA. Recovery of spatial learning deficits after decay of electrically induced synaptic enhancement in the hippocampus. Nature. 1989;342:545–548. doi: 10.1038/342545a0. [DOI] [PubMed] [Google Scholar]
- Chang HT. Cortical response to activity of callosal neurons. J Neurophysiol. 1953;16:117–131. doi: 10.1152/jn.1953.16.2.117. [DOI] [PubMed] [Google Scholar]
- Chauvette S, Volgushev M, Timofeev I. Origin of Active States in Local Neocortical Networks during Slow Sleep Oscillation. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Molle M, Eross L, Barsi P, Halasz P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- Conhaim J, Cedarbaum ER, Barahimi M, Moore JG, Becker MI, Gleiss H, Kohl C, Moody WJ. Bimodal septal and cortical triggering and complex propagation patterns of spontaneous waves of activity in the developing mouse cerebral cortex. Dev Neurobiol. 2010 doi: 10.1002/dneu.20797. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2009;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA. Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession. Trans Am Clin Climatol Assoc. 2009;120:249–285. [PMC free article] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Pollmacher T, Auer DP. Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur J Neurosci. 2004;20:566–574. doi: 10.1111/j.1460-9568.2004.03518.x. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, Maquet P, Peigneux P. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Fratello F, Marzano C, Moroni F, Curcio G, Tempesta D, Pellicciari MC, Pirulli C, Ferrara M, Rossini PM. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS ONE. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiraju T. Discharge properties of neurons of the parietal association cortex during states of sleep and wakefulness in the monkey. Brain Res. 1972;47:69–75. doi: 10.1016/0006-8993(72)90252-1. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesmann M, Gewaltig MO, Aertsen A. Stable propagation of synchronous spiking in cortical neural networks. Nature. 1999;402:529–533. doi: 10.1038/990101. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biol Rhythms. 1987;2:207–219. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Doyere V, Srebro B, Laroche S. Heterosynaptic LTD and depotentiation in the medial perforant path of the dentate gyrus in the freely moving rat. J Neurophysiol. 1997;77:571–578. doi: 10.1152/jn.1997.77.2.571. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology. 2001;25:S68–73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G. Sleep homeostasis and cortical synchronization: I. Modeling the effects of synaptic strength on sleep slow waves. Sleep. 2007;30:1617–1630. doi: 10.1093/sleep/30.12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson CA, Smith CB, Sokoloff L. Effects of prolonged sleep deprivation on local rates of cerebral energy metabolism in freely moving rats. J Neurosci. 1994;14:6769–6778. doi: 10.1523/JNEUROSCI.14-11-06769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder R, Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. II. Hippocampal slow waves and theta cell firing during bar pressing and other behaviors. Exp Neurol. 1973;41:532–555. doi: 10.1016/0014-4886(73)90291-4. [DOI] [PubMed] [Google Scholar]
- Feinberg I, March JD, Fein G, Floyd TC, Walker JM, Price L. Period and amplitude analysis of 0.5-3 c/sec activity in NREM sleep of young adults. Electroencephalogr Clin Neurophysiol. 1978;44:202–213. doi: 10.1016/0013-4694(78)90266-3. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–2290. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res. 1996;736:243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Frey U, Schollmeier K, Reymann KG, Seidenbecher T. Asymptotic hippocampal long-term potentiation in rats does not preclude additional potentiation at later phases. Neuroscience. 1995;67:799–807. doi: 10.1016/0306-4522(95)00117-2. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Avermann M, Matyas F, Staiger JF, Petersen CC. Membrane Potential Dynamics of GABAergic Neurons in the Barrel Cortex of Behaving Mice. Neuron. 2010;65:422–435. doi: 10.1016/j.neuron.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Herman C, McKenna M, Chapman PF, Fox K. Long-term potentiation in vivo in layers II/III of rat barrel cortex. Neuropharmacology. 1998;37:581–592. doi: 10.1016/s0028-3908(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc Natl Acad Sci U S A. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–729. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich KS, Kochanek PM, Melick JA, Schiding JK, Statler KD, Williams DS, Marion DW, Ho C. Cerebral perfusion during anesthesia with fentanyl, isoflurane, or pentobarbital in normal rats studied by arterial spin-labeled MRI. Magn Reson Med. 2001;46:202–206. doi: 10.1002/mrm.1178. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21:9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- Hirota K, Kudo M, Kudo T, Kitayama M, Kushikata T, Lambert DG, Matsuki A. Barbiturates inhibit K(+)-evoked noradrenaline and dopamine release from rat striatal slices--involvement of voltage sensitive Ca(2+) channels. Neurosci Lett. 2000;291:175–178. doi: 10.1016/s0304-3940(00)01408-7. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Deboer T, Tobler I. Topography of EEG dynamics after sleep deprivation in mice. J Neurophysiol. 2000a;84:1888–1893. doi: 10.1152/jn.2000.84.4.1888. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000b;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- Huber R, Tononi G, Cirelli C. Exploratory behavior, cortical BDNF expression, and sleep homeostasis. Sleep. 2007a;30:129–139. doi: 10.1093/sleep/30.2.129. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007b;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, Yuste R. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation of thalamic input to the motor cortex induced by coactivation of thalamocortical and corticocortical afferents. J Neurophysiol. 1991;65:1435–1441. doi: 10.1152/jn.1991.65.6.1435. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G. Integration and Segregation of Activity in Entorhinal-Hippocampal Subregions by Neocortical Slow Oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Jones BE. The role of noradrenergic locus coeruleus neurons and neighboring cholinergic neurons of the pontomesencephalic tegmentum in sleep-wake states. Prog Brain Res. 1991;88:533–543. doi: 10.1016/s0079-6123(08)63832-7. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Kemp IR, Kaada BR. The relation of hippocampal theta activity to arousal, attentive behaviour and somato-motor movements in unrestrained cats. Brain Res. 1975;95:323–342. doi: 10.1016/0006-8993(75)90110-9. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Gillin JC, Mendelson W, Suda S, Miyaoka M, Ito M, Nakamura RK, Storch FI, Pettigrew K, Mishkin M, Sokoloff L. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature. 1982;297:325–327. doi: 10.1038/297325a0. [DOI] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramis R, Vanderwolf CH, Bland BH. Two types of hippocampal rhythmical slow activity in both the rabbit and the rat: relations to behavior and effects of atropine, diethyl ether, urethane, and pentobarbital. Exp Neurol. 1975;49:58–85. doi: 10.1016/0014-4886(75)90195-8. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lante F, Cavalier M, Cohen-Solal C, Guiramand J, Vignes M. Developmental switch from LTD to LTP in low frequency-induced plasticity. Hippocampus. 2006;16:981–989. doi: 10.1002/hipo.20228. [DOI] [PubMed] [Google Scholar]
- Lee SM, Weisskopf MG, Ebner FF. Horizontal long-term potentiation of responses in rat somatosensory cortex. Brain Res. 1991;544:303–310. doi: 10.1016/0006-8993(91)90069-8. [DOI] [PubMed] [Google Scholar]
- Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LW, Borst JG. Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behavior. Brain Res. 1987;407:68–80. doi: 10.1016/0006-8993(87)91220-0. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry JP, Fillenz M. Real-time monitoring of brain energy metabolism in vivo using microelectrochemical sensors: the effects of anesthesia. Bioelectrochemistry. 2001;54:39–47. doi: 10.1016/s1567-5394(01)00109-8. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Leung LS. Endogenous histamine facilitates long-term potentiation in the hippocampus during walking. J Neurosci. 2010;30:7845–7852. doi: 10.1523/JNEUROSCI.1127-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Makhracheva-Stepochkina D, Frey S, Frey JU, Korz V. Spatial learning in the holeboard impairs an early phase of long-term potentiation in the rat hippocampal CA1-region. Neurobiol Learn Mem. 2008;89:545–551. doi: 10.1016/j.nlm.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9:207–231. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol. 1992a;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992b;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. The pharmacology of wakefulness. Metabolism. 2006;55:S13–19. doi: 10.1016/j.metabol.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Mistlberger R, Bergmann B, Rechtschaffen A. Period-amplitude analysis of rat electroencephalogram: effects of sleep deprivation and exercise. Sleep. 1987;10:508–522. [PubMed] [Google Scholar]
- Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006;96:62–70. doi: 10.1152/jn.00014.2006. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. Is learning blocked by saturation of synaptic weights in the hippocampus? Neurosci Biobehav Rev. 1999;23:661–672. doi: 10.1016/s0149-7634(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Mukovski M, Chauvette S, Timofeev I, Volgushev M. Detection of Active and Silent States in Neocortical Neurons from the Field Potential Signal during Slow-Wave Sleep. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj157. [DOI] [PubMed] [Google Scholar]
- Murata K, Kameda K. The Activity of Single Cortical Neurones of Unrestrained Cats During Sleep and Wakefulness. Arch Ital Biol. 1963;101:306–331. [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonova EV, Vijayasarathy C, Zhang L, Cater JR, Galante RJ, Ward SE, Avadhani NG, Pack AI. Differences in activity of cytochrome C oxidase in brain between sleep and wakefulness. Sleep. 2005;28:21–27. doi: 10.1093/sleep/28.1.21. [DOI] [PubMed] [Google Scholar]
- Nikonova EV, Naidoo N, Zhang L, Romer M, Cater JR, Scharf MT, Galante RJ, Pack AI. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Adey WR. Firing of neuron pairs in cat association cortex during sleep and wakefulness. J Neurophysiol. 1970;33:672–684. doi: 10.1152/jn.1970.33.5.672. [DOI] [PubMed] [Google Scholar]
- Noda H, Adey WR. Neuronal activity in the association cortex of the cat during sleep, wakefulness and anesthesia. Brain Res. 1973;54:243–259. doi: 10.1016/0006-8993(73)90047-4. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Ohsaki K, Iwama K. Prolonged slow negative wave in primary sensory evoked potentials of dog cerebral cortex. Tohoku J Exp Med. 1961;74:137–148. doi: 10.1620/tjem.74.137. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, Tobler I, Allaman I, Borbely AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16:1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C. Topographic and toposcopic study of origin and spread of the regular synchronized arousal pattern in the rabbit. Electroencephalogr Clin Neurophysiol. 1960;12:589–600. doi: 10.1016/0013-4694(60)90101-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Chapman CA, Trepel C, Teskey GC, Milgram NW. Post-activation potentiation in the neocortex. IV. Multiple sessions required for induction of long-term potentiation in the chronic preparation. Brain Res. 1995;702:87–93. doi: 10.1016/0006-8993(95)01025-0. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Ramm P, Frost BJ. Regional metabolic activity in the rat brain during sleep-wake activity. Sleep. 1983;6:196–216. doi: 10.1093/sleep/6.3.196. [DOI] [PubMed] [Google Scholar]
- Ramm P, Frost BJ. Cerebral and local cerebral metabolism in the cat during slow wave and REM sleep. Brain Res. 1986;365:112–124. doi: 10.1016/0006-8993(86)90728-6. [DOI] [PubMed] [Google Scholar]
- Richardson KA, Schiff SJ, Gluckman BJ. Control of traveling waves in the Mammalian cortex. Phys Rev Lett. 2005;94:028103. doi: 10.1103/PhysRevLett.94.028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Hippocampal rhythmic slow activity (RSA; theta): a critical analysis of selected studies and discussion of possible species-differences. Brain Res. 1980;203:69–101. doi: 10.1016/0165-0173(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg RS, Bergmann BM, Rechtschaffen A. Variations in slow wave activity during sleep in the rat. Physiol Behav. 1976;17:931–938. doi: 10.1016/0031-9384(76)90011-1. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Bucherelli C, Roberto M, Tassoni G, Brunelli M. Time-dependent inhibition of hippocampal LTP in vitro following contextual fear conditioning in the rat. Eur J Neurosci. 2002;15:143–150. doi: 10.1046/j.0953-816x.2001.01844.x. [DOI] [PubMed] [Google Scholar]
- Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- Sirota A, Buzsaki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3:245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Netw. 2000;13:909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex. 2006;16:376–385. doi: 10.1093/cercor/bhi116. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Steriade M. Cholinergic blockage of network- and intrinsically generated slow oscillations promotes waking and REM sleep activity patterns in thalamic and cortical neurons. Prog Brain Res. 1993;98:345–355. doi: 10.1016/s0079-6123(08)62418-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Slow sleep oscillation, rhythmic K-complexes, and their paroxysmal developments. J Sleep Res. 1998a;7(Suppl 1):30–35. doi: 10.1046/j.1365-2869.7.s1.4.x. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F. Coalescence of sleep rhythms and their chronology in corticothalamic networks. Sleep Res Online. 1998b;1:1–10. [PubMed] [Google Scholar]
- Steriade M, Amzica F, Nunez A. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993a;70:1385–1400. doi: 10.1152/jn.1993.70.4.1385. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993b;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993c;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lebedev MA, Nicolelis MA, Plenz D. Coherence potentials: loss-less, all-or-none network events in the cortex. PLoS Biol. 2010;8:e1000278. doi: 10.1371/journal.pbio.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney PL, Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur J Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc Natl Acad Sci U S A. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Tobler I. Phylogeny of sleep regulation. In: MHK, TR, WCD, editors. Principles and Practice of Sleep Medicine. Philadelphia: W. B. Saunders; 2005. [Google Scholar]
- Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- Tobler I, Murison R, Ursin R, Ursin H, Borbely AA. The effect of sleep deprivation and recovery sleep on plasma corticosterone in the rat. Neurosci Lett. 1983;35:297–300. doi: 10.1016/0304-3940(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tononi G, Massimini M, Riedner BA. Sleepy dialogues between cortex and hippocampus: who talks to whom? Neuron. 2006;52:748–749. doi: 10.1016/j.neuron.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH, Harvey GC, Leung LW. Transcallosal evoked potentials in relation to behavior in the rat: effects of atropine, p-chlorophenylalanine, reserpine, scopolamine and trifluoperazine. Behav Brain Res. 1987;25:31–48. doi: 10.1016/0166-4328(87)90043-x. [DOI] [PubMed] [Google Scholar]
- Verzeano M, Negishi K. Neuronal activity in cortical and thalamic networks. J Gen Physiol. 1960;43(6)(Suppl):177–195. doi: 10.1085/jgp.43.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca JR. Counterpointing the functional role of the forebrain and of the brainstem in the control of the sleep-waking system. J Sleep Res. 2004;13:179–208. doi: 10.1111/j.1365-2869.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Achermann P, Borbely AA, Tobler I. Interhemispheric coherence of the sleep electroencephalogram in mice with congenital callosal dysgenesis. Neuroscience. 2004a;124:481–488. doi: 10.1016/j.neuroscience.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis. J Sleep Res. 2005;14:299–304. doi: 10.1111/j.1365-2869.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–975. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Borbely AA, Tobler I. Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J Neurophysiol. 2002;88:2280–2286. doi: 10.1152/jn.00304.2002. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I, Winsky-Sommerer R. Alteration of behavior in mice by muscimol is associated with regional electroencephalogram synchronization. Neuroscience. 2007a;147:833–841. doi: 10.1016/j.neuroscience.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull. 2007b;74:37–44. doi: 10.1016/j.brainresbull.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004b;20:1363–1370. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Ruijgrok G, Deboer T, Tobler I. Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb Cortex. 2006;16:328–336. doi: 10.1093/cercor/bhi110. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007c;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol. 2009a;101:1921–1931. doi: 10.1152/jn.91157.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009b;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BO, MacLean JN, Yuste R. UP states protect ongoing cortical activity from thalamic inputs. PLoS ONE. 2008;3:e3971. doi: 10.1371/journal.pone.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth E, Achermann P, Borbely AA. Brain topography of the human sleep EEG: antero-posterior shifts of spectral power. Neuroreport. 1996a;8:123–127. doi: 10.1097/00001756-199612200-00025. [DOI] [PubMed] [Google Scholar]
- Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996b;271:R501–510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Vanderwolf CH. Hippocampal EEG and behavior: changes in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol. 1973;8:461–484. doi: 10.1016/s0091-6773(73)80041-0. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- Zavada A, Strijkstra AM, Boerema AS, Daan S, Beersma DG. Evidence for differential human slow-wave activity regulation across the brain. J Sleep Res. 2009;18:3–10. doi: 10.1111/j.1365-2869.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- Ziemann U. LTP-like plasticity in human motor cortex. Suppl Clin Neurophysiol. 2004;57:702–707. doi: 10.1016/s1567-424x(09)70410-6. [DOI] [PubMed] [Google Scholar]