Abstract
During tissue morphogenesis and homeostasis, cells experience various signals in their environments, including gradients of physical and chemical cues. Spatial and temporal gradients regulate various cell behaviours such as proliferation, migration, and differentiation during development, inflammation, wound healing, and cancer. One of the goals of functional tissue engineering is to create microenvironments that mimic the cellular and tissue complexity found in vivo by incorporating physical, chemical, temporal, and spatial gradients within engineered three-dimensional (3D) scaffolds. Hydrogels are ideal materials for 3D tissue scaffolds that mimic the extracellular matrix (ECM). Various techniques from material science, microscale engineering, and microfluidics are used to synthesise biomimetic hydrogels with encapsulated cells and tailored microenvironments. In particular, a host of methods exist to incorporate micrometer to centimetre scale chemical and physical gradients within hydrogels to mimic the cellular cues found in vivo. In this review, we draw on specific biological examples to motivate hydrogel gradients as tools for studying cell–material interactions. We provide a brief overview of techniques to generate gradient hydrogels and showcase their use to study particular cell behaviours in two-dimensional (2D) and 3D environments. We conclude by summarizing the current and future trends in gradient hydrogels and cell–material interactions in context with the long-term goals of tissue engineering.
Keywords: biomaterials, gradient hydrogels, tissue engineering
INTRODUCTION
The broad goal of tissue engineering and regenerative medicine is to create functional human tissue equivalents for organ repair and replacement. To achieve this goal, engineered tissues must recreate the physical, chemical, and mechanical properties of in vivo tissues and replicate the complex interactions between cells and their microenvironments which regulate tissue morphogenesis, function, and regeneration (Lutolf and Hubbell, 2005; Freytes et al., 2009). Cellular microenvironments consist of the extracellular matrix (ECM), neighbouring cells, and surrounding soluble factors. The ECM provides mechanical support and spatiotemporally regulated biochemical signals to cells to guide their proliferation, differentiation, migration, and apoptosis. Cells, in turn, can interact and remodel the surrounding ECM. This coupled evolution of the ECM, the cytoskeleton, and the nucleus plays an important role in tissue development, homeostasis, and disease progression (Gjorevski and Nelson, 2010).
The ECM contains a plethora of physical, chemical, and mechanical cues to guide a host of cellular processes such as cell–cell interaction, proliferation, differentiation, and migration (Figure 1). The ECM is a highly hydrated, viscoelastic three-dimensional (3D) network containing various proteoglycans, fibrillar proteins, and glycoproteins. Protein fibrils and fibres provide biophysical contact cues to guide cell migration. The ECM also contains soluble macromolecules such as growth factors, chemokines, and cytokines (Figure 1C). Molecular concentration gradients of such factors play an important role in biological phenomena such as chemotaxis (Jeon et al., 2002; Shamloo et al., 2008), morphogenesis, and wound healing (Chung et al., 2005; Pihl et al., 2005; Khademhosseini et al., 2006). The ECM’s mechanical properties also signal cells. For example, mechanical changes in the ECM surrounding a cell can induce structural rearrangements of the cytoskeleton and immobilised proteins, which in turn can generate a cellular response called mechanotransduction (Vogel and Sheetz, 2006). In addition, gradients in the properties of the ECM and the surrounding cell concentration can connect mechanically mismatched tissues such as bone–cartilage interfaces and dentino-enamel junctions (Miserez et al., 2008; Yang and Temenoff, 2009). Ideally, tissue engineering approaches should recreate the salient features of natural ECM in biomaterial scaffolds in vitro.
Figure 1.
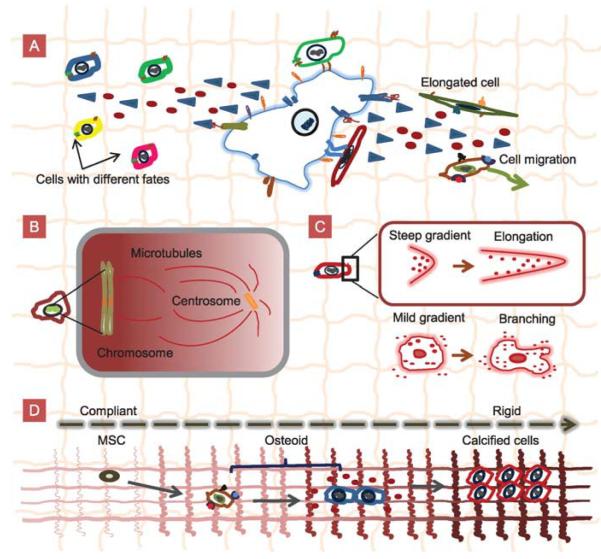
Schematics of cell–cell contacts, cell–ECM interactions, and physicochemical gradients in vivo. A: Cell–cell contact and cell–ECM interactions generate chemical gradients that affect cell behaviours such as cell migration, cell elongation, and cell differentiation. B: Chromosomes generatea Ran-GTP gradient that organises the mitotic spindle during cell division. C: A steep gradient seen by one end of the cell results in elongation whereas a mild gradient seen by the whole cell results in branching. D: Mesenchymal stem cells (MSCs) sequentially differentiate into osteoids and calcified bone cells in response to the graded mechanical signals in the ECM.
Over the past few decades, various technologies have been developed to create spatiotemporal gradients and complex biomaterials incorporating such gradients. Thorough reviews exist of the various methods to create chemical gradients (Keenan and Folch, 2008) and surface-bound gradients (Genzer and Bhat, 2008). Gradient materials have been used to rapidly screen cell–biomaterial interaction (Simon et al., 2009) and to study cellular processes such as migration and angiogenesis in vitro (Chung et al., 2010). Gradient materials have also found widespread use in drug delivery (Peppas and Khare, 1993; Lee et al., 2001) and tissue engineering (Singh et al., 2008).
In this review, we focus on the physical and chemical gradients in hydrogel scaffolds, termed gradient hydrogels, used in tissue engineering. The term “gradient hydrogel” is broadly defined as a hydrogel possessing a gradual spatiotemporal change in at least one property (Genzer and Bhat, 2008). Hydrogels are important for tissue engineering due to their structural and compositional similarities to natural ECM. Hydrogels typically imbibe 95–99% water and possess ECM-like viscoelastic and diffusive transport characteristics (Lutolf, 2009; Slaughter et al., 2009). Due to advances in materials chemistry, a wide range of hydrogels have been synthesised with tunable physical, chemical, and functional properties. Since hydrogels mimic the ECM and their chemistry, cross-linking density and response to environmental stimuli (e.g., heat, light, electrical potential, chemicals, and biological agents) may be manipulated, they are ideal for producing tailored 3D cellular microenvironments. Various studies have linked the mechanical and chemical properties of hydrogels to cell behaviour (He et al., 2010b; Marklein and Burdick, 2010). Currently, most studies on cell–material interaction use a microarray format in which cell behaviour is tested on a finite number of hydrogels, each with spatially homogeneous properties. In contrast, gradient hydrogels exhibit a continuous spatial change in a given property and allow a continuum of these property values to be tested on a single biological sample. Therefore, gradient hydrogels enable high-throughput screening of cell–material interactions and enhance traditional tissue engineering techniques. Gradient hydrogels could also replicate in vivo physical and chemical gradients in vitro for tissue-engineered constructs.
In this review, we will highlight specific biological examples of gradients during tissue morphogenesis and other cellular processes. Synthesis protocols for 2D and 3D gradient hydrogels are then reviewed, followed by the interaction of cells with hydrogels incorporating chemical and physical gradients. Finally, the review concludes with existing challenges and future prospects.
GRADIENTS IN BIOLOGY
Advances in developmental biology have shown that chemical and physical gradients are common in vivo. Such gradients affect a host of cell behaviours such as motility, migration, signalling, and differentiation. Cells in microscale gradients experience different concentrations around their bodies (Makarenkova et al., 2009), while those in gradients spanning larger length scales experience more uniform local concentrations that change spatially (Figure 1C). Long range gradients enable the efficient function of organs such as bones (Phillips et al., 2008) and the heart, and body-wide systems. In particular, the endocrine system uses soluble gradients to signal and direct various functions in the body (Crock et al., 1988; Hess et al., 1997).
Chemical Gradients
Chemical gradients are defined here as gradients of morphogens such as transcription factors, chemokines, and cytokines. Morphogens are signalling molecules that can induce distinct cellular responses in a concentration-dependent manner. The spatial distribution of proteins provides the biochemical cues to direct the organised formation of a tissue (Swartz, 2003). The importance of protein gradients during embryogenesis, capillary sprouting, and wound healing is well documented. The patterning of mammalian embryos is regulated by gradients of morphogens, including hedgehog (Hh), bone morphogenetic protein (BMP), transforming growth factor-β (TGF-β), Wingless Int (Wnts), and fibroblast growth factors (FGFs) (Makarenkova et al., 2009).
A classic example of in vivo chemical gradients is the class of protein gradients that regulate mitosis (Figure 1B; Caudron et al., 2005; Clarke, 2005; Bastiaens et al., 2006; Fuller, 2010). During mitosis, chromosomes generate a gradient of the chemical Ran-GTP–importin-β that organises the mitotic spindle during cell division. The concentration of Ran-GTP is thought to decrease away from chromosomes to provide a positional signal that causes changes in microtubule dynamics and organises spindles around chromosomes. Microtubule nucleation occurs only within a region close to the chromosomes, whereas microtubule stabilisation occurs at greater distances from the chromosomes and responds linearly to the gradient (Caudron et al., 2005; Clarke, 2005). Other examples of chemical gradients include the morphogen gradients which control differentiation during embryogenesis (Swartz, 2003; Ashe and Briscoe, 2006) and the chemical gradients that guide axonal growth in nervous tissue.
Chemical gradients are also involved in chemotaxis, in which cells migrate along the concentration gradient of chemoattractants. For example, leukocytes migrate toward sites of inflammation and infection, neurons send projections to specific regions of the brain to find their synaptic partners, and fibroblasts move into the wound space (Wang, 2009). In each case, chemoattractant-induced activation of spatially localised cellular signals causes cells to polarise and move toward the highest concentration of chemoattractant.
Research suggests that in some cases, spatial concentration gradients are generated when morphogens bind differentially to ECM components and receptors. For instance, the differential binding of FGF7 and FGF10 to the ECM component, heparan sulphate (HS) leads to growth factor gradients of different lengths (Makarenkova et al., 2009). The strong affinity of FGF10 for HS keeps FGF10 near its source, forming a relatively steep gradient. In contrast, FGF7 has a lower affinity for HS and diffuses more freely through the ECM creating a longer gradient. The differences in gradient length are integral to the different roles the growth factors play during branching morphogenesis (Makarenkova et al., 2009). The steep gradient of FGF10 near the tip of the cell promotes cell elongation, whereas the longer mild gradient of FGF7 promotes branching of salivary glands (Figure 1C; Makarenkova et al., 2009).
Temporal changes in cellular microenvironments also regulate cell behaviours such as gene expression. The activation of different morphogen target genes occurs at different times. Studies are underway to understand how gradient signals are converted into dynamic spatial gene expression patterns, for example, in neural tube patterning (Kutejova et al., 2009). Incorporating temporal gradients into synthetic ECM could enhance gene expression, cellular migration, and cellular recruitment and therefore functional tissue generation in vitro.
Physical Gradients
Physical gradients are defined here as the gradual change of a physical property such as material stiffness, porosity, and topology. Physical gradients occur naturally in the body and are present at the boundaries between different tissues (Mikos et al., 2006). For instance, articular cartilage is organised in layers containing different protein expressions and collagen fibre alignments. A tissue gradient exists at the ligament–bone interface where the ligament transitions to fibro-cartilage which in turn transitions to bone (Phillips et al., 2008). Teeth contain gradients in composition and mineral density, which give rise to gradients in mechanical properties (Ho et al., 2007). Physical gradients are inherent in bone structure. Bone composition varies from compact (porosity 5–30%) to spongy (porosity 30–90%) (Hall, 2007). The former accounts for 80% of bone mass, whereas the latter accounts for the remaining 20%. However, spongy bone has nearly ten times the surface area of compact bone (Hall, 2007).
The ECM in the heart contains a gradient in perimysial collagen fibre orientation across the heart ventricular wall from the epicardium to the endocardium (Pope et al., 2008). In addition, perimysial collagen in the heart forms three distinct constructs: meshwork for laminar surfaces, convoluted fibres connecting adjacent layers, and longitudinal cords (Pope et al., 2008). The transition from longitudinal cords to laminar surfaces from the epicardium to the midwall occurs gradually. The gradient in the perimysial collagen formation is thought to play an important role in the large wall thickness changes that occur during contraction cycles.
Advancing the study of gradients in tissue morphogenesis and tissue regeneration requires the creation of biomimetic materials with controlled spatial and temporal features and the subsequent study of their effects on cell behaviour. In the next section, we briefly review methods to generate gradients of biologically relevant properties in 2D and 3D hydrogel scaffolds.
METHODS OF GRADIENT GENERATION
Various methods have been developed to create chemical and material gradients on 2D surfaces and in 3D. Extensive reviews exist for surface gradients (Genzer and Bhat, 2008; Keenan and Folch, 2008); here we focus on methods to generate gradient hydrogels, due to their relevance to tissue engineering. Gradient hydrogels are generally formed by a two-step approach. Concentration gradients of prepolymer solutions are first formed, and then stabilised by the appropriate cross-linking method. Exceptions are gradient photo-cross-linking methods, discussed later, microfluidic channels embedded in hydrogels to create gradients in soluble factors (Choi et al., 2007), and drug delivery systems (Lee, 1984; Lee and Kim, 1991; Peppas and Khare, 1993). In drug delivery systems, the hydrogels are first formed and then drugs diffuse through the gels, often simultaneously with hydrogel swelling. The time dependence of the release rate may be controlled by adjusting the shape of the initial concentration distribution (Lee, 1984; Lee and Kim, 1991).
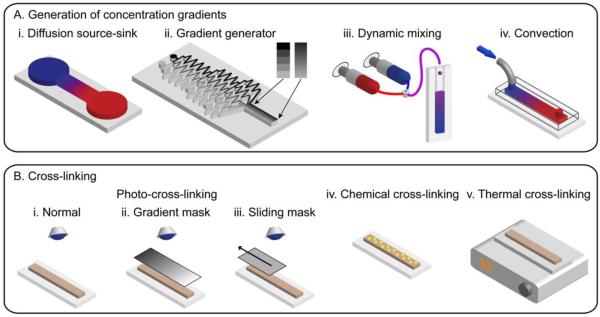
A plethora of methods exist to generate concentration gradients (Figure 2). The majority of the methods considered here produce a gradient in the relative concentration between two solutions containing either different concentrations of the same species, concentrations of different species, or both. The simplest methods employ molecular diffusion to generate gradients between chemical sources and sinks (Figure 2A,i; Abhyankar et al., 2006; Lo et al., 2008). Over the long diffusive timescale of L2/π2D, where D is the molecular diffusivity, the species in the source diffuses through a channel toward the sink to form a gradient of length L. Given sufficient source/sink volumes, stable gradients may be maintained for days. A diffusion source-sink palette device exists that generates 2D gradients (Atencia et al., 2009). Though classic, methods relying solely on diffusion are generally too time consuming for creating gradient hydrogels for cell–material interaction studies, which require cross-linking and often involve cell encapsulation. Methods incorporating fluidic flows to expedite gradient generation are preferred.
Figure 2.
Methods for generating gradient hydrogels. A: Gradient generation by (i) a source-sink diffusion device, (ii) a tree-like microfluidic gradient generator, (iii) mixing the input streams from syringe pumps, and (iv) microfluidic convection. B: Cross-linking by photopolymerisation using (i) a normal setup, (ii) a gradient mask, (iii) a sliding mask, and cross-linking by (iv) chemicals, and (v) heat.
The classic tree-like gradient generator employs serial dilution at successive stages and diffusive mixing to form gradients of hundreds of microns in length in about a minute (Figure 2A,ii; Dertinger et al., 2001; Burdick et al., 2004; Zaari et al., 2004). The gradient shape is controlled by the design of the microchannel network, which can also produce overlapping gradients composed of different species. Since the gradient is positioned laterally across the channel, its length is limited to 1–2 mm. Moreover, the gradient is stable only while the flow is on; once the flow is turned off, diffusion acts to equilibrate the concentration. A second strategy for gradient generation is to use two or more syringe pumps in tandem to pump different solutions at controllable flow rates into a mixer and to output the mixed solution to a mould for further use and stabilisation (Figure 2A,iii). By altering the ratio of the constituents in real-time, multiple gradients with controllable (and programmable) shapes may be superposed. A commercially available gradient maker that employs this mechanism has been used to create gradient hydrogels (DeLong et al., 2005; Nemir et al., 2009). Though flow-based gradient protocols involve shear stress, the flow is generally turned off following gradient generation and stabilisation. Thus, flow-based gradient methods are generally both rapid and compatible with biological samples sensitive to shear stress.
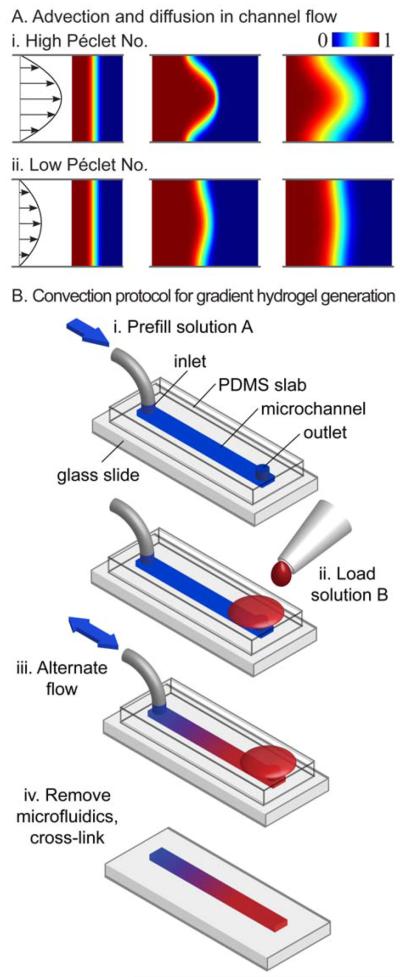
Based on convective spreading in a microchannel (Ajdari et al., 2006; Goulpeau et al., 2007), we have developed a simple rapid method for generating hydrogels with centimetre long gradients of molecules, microbeads, and even cells, in seconds to minutes (Figures 2A,iv and 3; Du et al., 2010; He et al., 2010a). Suspended particles near the channel wall travel more slowly than those near the centre. An initially steep front in concentration across the channel therefore spreads longitudinally as the front flows through the channel. In pure convection, the rate of spreading is proportional to the average flow speed. Counter to intuition, diffusion actually suppresses convection-driven spreading. Particles moving quickly near the centre diffuse toward the wall and slow down, and vice versa, reducing the variation in particle speeds and the resulting spreading. The Péclet number Pe = UH/D quantifies the relative magnitudes of axial rates of transport by convection and diffusion, where U is the average flow speed, H the channel height, and D the molecular diffusivity. Flows with larger Péclet numbers create longer gradients faster (Figure 3A; Du et al., 2010). The following general protocol has been used to generate concentration gradients of molecules, microbeads, and cells in straight rectangular microchannels. First, the channel was prefilled with a solution A (Figure 3B,i). Second, a solution B was loaded at one port and drawn into the channel at a high flow speed (Figure 3B,ii). For diffusible species, pumping fluid back and forth sequentially lengthened the gradient (Figure 3B,iii; Du et al., 2010). The microfluidic system could then be removed for further sample processing (e.g., cross-linking) and analysis (Figure 3B,iv). For non-diffusible species, the process was completed once the gradient was pumped to the opposite end of the channel; reversing the flow would undo the hydrodynamic stretching and collapse the gradient. Computer simulations have been used to select the optimal flow program, given tradeoffs in generation time, gradient length, and cross-sectional uniformity (Du et al., 2010; He et al., 2010a). Various fluidic actuation mechanisms drove the convective spreading in our microchannels. A passive pumping mechanism driven by the difference in curvature pressure between the inlet and outlet droplets produced centimetre scale gradients without the need for additional equipment (Du et al., 2009; He et al., 2010b). The high flow rates and programmable flow control offered by syringe pumps produced longer and more laterally uniform concentration profiles (Du et al., 2010). For gradients involving microscale particles denser than the surrounding fluid, rapid gradient generation is key: once the particles settle to the channel bottom, they all travel at the same slow speed whereupon gradient growth ceases (Du et al., 2010). Therefore, due to its speed, the convection-driven gradient method may be preferable for creating gradients with microscale particles.
Figure 3.
Convection-driven gradient generation. A: Convection and diffusion in microchannel flows at (i) high and (ii) low Péclet numbers. B: Protocol for generating gradient hydrogels with convection. i: The channel is prefilled with solution A. ii: Solution B is loaded into the opposing port. iii: The flow is pumped back and forth in the channel until the gradient is the desired length. iv: The microfluidic system is removed and the gradient in prepolymer is cross-linked (Du et al., 2010).
Hydrogel gradients are formed when concentration gradients of their prepolymer constituents are cross-linked by chemicals, ultra-violet light, or temperature (Figure 2B; Peppas et al., 2006). Cross-linking by photopolymerisation allows for spatial and temporal control of the polymerisation as well as the formation of complex shapes (Nemir et al., 2009; Kloxin et al., 2010; Marklein and Burdick, 2010). With the appropriate photoinitiator and sufficiently mild exposure to ultra-violet light, photo-cross-linking can be rendered biocompatible and can encapsulate living cells within the polymer matrix (Peppas et al., 2006; Nemir et al., 2009; Slaughter et al., 2009). For example, poly(ethylene glycol) (PEG) may be cross-linked by substituting its terminal hydroxyl groups with acrylates to form PEG diacrylate (PEGDA; Nguyen and West, 2002). Collagen may be thermally cross-linked. Rather than creating gradients in the prepolymer solutions, the cross-linking method itself may be used to create gradients. Exposing prepolymer solutions to variable amounts of UV using sliding or gradient greyscale masks produced hydrogels with elastic modulus gradients (Figure 2B,ii,iii). For example, the greyscale mask technique created elastic modulus gradients in polyacrylamide gels ranging from ~2.5 to ~11 kPa over 18 mm (Wong et al., 2003). The sliding mask technique created ~1 cm long elastic modulus gradients in methacrylated hyaluronic acid (HA) (Johnson et al., 2005; Marklein and Burdick, 2010) and PEGDA (Kloxin et al., 2010).
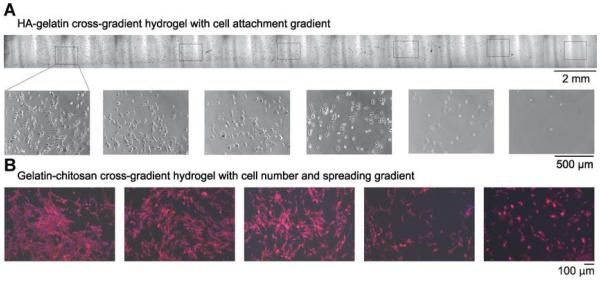
By appropriate choice of input solutions and cross-linking method, the protocols outlined above can create gradients of soluble factors, proteins, beads, and even cells within hydrogels with constant concentrations of other species. In addition, combining the concentration gradient protocols with cross-linking gradient protocols can produce gradient hydrogels with superposed chemical and physical gradients. Applications include toxin, protein, or chemoattractant gradients within gels encapsulating uniform concentrations of cells (Burdick et al., 2004; Du et al., 2009) or a gradient in cell concentration within a 3D gel with a constant nutrient source. Various gradient hydrogels have been synthesised: concentration gradients in the cell-adhesion ligand Arg-Gly-Asp-Ser (RGDS) within a PEGDA hydrogel (Burdick et al., 2004; He et al., 2010b); polyacrylamide hydrogels with gradients in elastic modulus or pore size (Zaari et al., 2004; Lo et al., 2008); a molecular chain length gradient (from 3.4 and 20 kDa) photo-cross-linked to form a PEGDA hydrogel with a gradient in elastic modulus (Nemir et al., 2009); a collagen fibril density gradient (Du et al., 2010); and a concentration gradient in fibronectin fragment FN III9-10 captured on a PEG hydrogel via covalently bound NeutrAvidin (Cosson et al., 2009). Since the advection and diffusion of a species are linear processes, two gradients of non-reacting species may be superposed in the same or opposite direction by adding different species to the two input solutions (Du et al., 2010). Particular examples from our laboratory include a gelatin–HA cross-gradient (Figure 4A; Du et al., 2010) and a composite chitosan–gelatin cross-gradient porous scaffold (Figure 4B; He et al., 2010a).
Figure 4.
A: Phase images (top: lower magnification; bottom: higher magnification) of smooth muscle cells (SMCs) cultured on a HA–gelatin cross-gradient hydrogel (Du et al., 2010). B: Effect of porous gelatin/chitosan cross-gradient on SMC behaviour. SMCs were cultured on the gradient hydrogel for 3 days. Fluorescence microscope images of SMCs show cytoskeletal organisation by F-actin staining (red) and nuclei by DAPI staining (blue) (He et al., 2010a).
In addition to chemical and stiffness gradients, gradients in porosity and pore size, which affect cell affinity and viability, have also been generated. Based on mass transport, the ideal pore network design minimises dead space, unconnected pores, and tortuosity (Botchwey et al., 2003). Tubular scaffolds with radial gradients in pore size and porosity have been created by spinning a two-material system of collagen and glycosaminoglycan (Harley et al., 2006). A pore size gradient ranging from 45 to 260 μm with a corresponding increase in porosity was made in an agarose/gelatin system (Tripathi et al., 2009). The agarose was self-gelated at −12°C and the gelatin was simultaneously cross-linked by glutaraldehyde (Tripathi et al., 2009). In another example, cone-like porous structures with a gradient in pore size from 20–30 μm to 330 μm were formed during the cryogenic cross-linking of gelatin scaffolds with embedded parallel channels (Dubruel et al., 2007). Ongoing work aims to improve control over hydrogel pore characteristics (Annabi et al., 2010).
Controlling the shape of gradient profiles may be important for producing tailored cell signals and for creating biomaterials with spatially tuned mechanical properties. Sophisticated devices exist to produce spatially and temporally varying gradient profiles. Given any desired monotonic decreasing profile, coflowing input solutions may be partitioned by successive rows of dividers in a channel to produce the gradient profile; increasing the number of rows of dividers increases the resolution (Irimia et al., 2006). Variants of the gradient generator have produced preprogrammed arbitrary profiles (Lee et al., 2009), complex and overlapping profiles (Dertinger et al., 2001), and 2D overlapping orthogonal gradients (Cosson et al., 2009). Hybrid devices consisting of multiple syringe pumps inputting solutions into gradient generators produced controllable and temporally changing gradient profiles (Lin et al., 2004; Amarie et al., 2007; Cooksey et al., 2009) and also 2D profiles (Cooksey et al., 2009). Recently, a modular device has been developed to produce arbitrary gradient profiles that can be updated to other arbitrary profiles in real-time (Frisk et al., 2008). Another microfluidic gradient device has been developed to produce high-resolution gradients of virtually any form, including those with wavy features (Galas et al., 2009). Further shape control may be achieved with discrete gradients. Stepwise stiffness gradients and patterned interlocking blocks of different stiffnesses have been produced in hydrogels (Cheung et al., 2009). Step widths as small as 27 μm were fabricated with microfluidic-based lithography, varied polymer chain lengths, and concentration differences (Cheung et al., 2009).
Though most of the methods presented here have generated 3D gradient hydrogels, few studies exist on the behaviour of encapsulated cells in such 3D microenvironments. One such study used a diffusion source-sink device to create gradients of controlled slopes about cells encapsulated in hydrogels (Frisk et al., 2008). Following exposure to chemical and physical gradients, biological samples are generally analysed to measure their response. The analysis is often complicated for samples encapsulated in 3D matrices which may obscure the sample. The use of reversible gels could allow encapsulated cells to be washed out for further analysis following the experiment.
Hydrogels with temporal gradients enable time-dependent signals to be delivered to cells in vitro (Choi et al., 2007; Peret and Murphy, 2008; Wang et al., 2009). Temporal and spatial gradients of small and large soluble solutes were created and maintained in alginate scaffolds by embedded microchannels (Choi et al., 2007). Proteins or biodegradable protein-loaded microspheres were distributed in PEG hydrogels to create soluble protein gradients of different controlled slopes and temporal dependence (Peret and Murphy, 2008).
New photodegradable hydrogels make possible the real-time manipulation of material properties and gradients in cellular microenvironments. Recently, a photodegradable PEG hydrogel was synthesised with a network backbone degradable with UV, visible light, and two-photon irradiation (Kloxin et al., 2009). The macroscopic characteristics of gel stiffness, diffusivity, and water content could be altered in real-time and the hydrogel itself could be fully eroded, all while maintaining biocompatible conditions. In addition, 3D regions could be selectively eroded with a two-photon laser-scanning microscope (Kloxin et al., 2009). The synergy between these new material synthesis technologies and gradient methods should produce more biologically relevant microenvironments.
GRADIENTS IN TISSUE ENGINEERING
The gradient generation methods outlined in the previous section are now being used to recreate cellular microenvironments to answer fundamental questions regarding cell behaviour and to control certain behaviours to direct tissue regeneration. In particular, chemical and physical gradients are being embedded into hydrogels to study and control a range of cellular phenomena. Studies involving cells seeded on the surface of hydrogels are classified as 2D, whereas those involving cells encapsulated within hydrogels are classified as 3D.
Chemical Gradients in Hydrogels
A host of studies have linked the effects of chemical gradients to cell behaviour. Cell responses depend on both the absolute concentration and the slope of the concentration gradient. Therefore, the source concentration, concentration range, and gradient slope (or equivalently, length) are important in the design of chemical gradients for in vitro cell-based experiments (Peret and Murphy, 2008). Moreover, since hydrogels provide unique 3D cellular microenvironments mimicking the ECM, researchers have incorporated chemical gradients into hydrogels. As described in the Chemical Gradients Section, chemicals such as growth factors and adhesion peptides are bound to the ECM with different affinities via electrostatic interactions with HS. Therefore, chemical gradients are divided into two categories: immobilised gradients, where molecules are tightly bound to the ECM and gradients of diffusible soluble factors not strongly bound to the ECM.
Immobilised gradients
Various studies have immobilised proteins such as growth factors and adhesion peptides in hydrogel networks (Moore et al., 2006; Musoke-Zawedde and Shoichet, 2006; Vepari and Kaplan, 2006) to study the resulting cell behaviour including cell attachment (Burdick et al., 2004; He et al., 2010b), alignment, and migration (DeLong et al., 2005; Cosson et al., 2009) as well as neurite extension (Dodla and Bellamkonda, 2006) and axonal guidance (Kapur and Shoichet, 2004). Immobilisation is useful for either mimicking a similar process in the body or to maintain a stable gradient over a long time period. For example, smooth muscle cells (SMCs) aligned and migrated in the direction of increasing concentration of bFGF covalently immobilised on photo-cross-linkable PEG hydrogels (DeLong et al., 2005). In another example, PEGDA hydrogels incorporating a gradient of the cell adhesion ligand peptide RGDS exhibited a cell attachment gradient (He et al., 2010b).
Multiple immobilised growth factor gradients have been superposed in hydrogel networks (Moore et al., 2006; Cosson et al., 2009). Superposed concentration gradients of nerve growth factor (NGF) and neurotrophin-3 (NT-3) were immobilised in poly(2-hydroxyethylmethacrylate) (pHEMA) hydrogel to study their effects on chick dorsal root ganglia (DRG) (Moore et al., 2006). In these experiments, both of the receptors for NGF and NT-3 were colocalised on the same DRG neurons, indicating that the dual gradients of NGF and NT-3 acted synergistically and not merely additively. In another example, parallel, antiparallel, and overlapping orthogonal gradients of multiple proteins were immobilised on PEG hydrogels to assess the effects of fibronectin gradients on human foreskin fibroblast (HFF) migration (Cosson et al., 2009). The orientation of cell migration, as well as the migration rate of the cells, depended on the concentration of immobilised fibronectin fragments.
To accurately recreate 3D in vivo microenvironments, in vitro cell-based experiments should encapsulate cells in 3D gradient scaffolds. For example, photoimmobilised gradients of laminin-1 (LN-1) of different slopes provided 3D directional cues to neurons encapsulated in 3D agarose scaffolds (Dodla and Bellamkonda, 2006). DRG neurite extension rates were significantly higher in scaffolds with LN-1 concentration gradients than in scaffolds with uniform concentrations of LN-1. Such gradient scaffolds with 3D directional cues represent a new generation of tissue-engineered materials for guided tissue and nerve regeneration.
A number of limitations exist when using covalently immobilised proteins for cell-based studies (Peret and Murphy, 2008). The strength of the covalent bond is protein dependent; thus, not all proteins may be covalently immobilised. Immobilised proteins may not be effectively presented to the cells, compromising their functions. Lastly, covalent linkages can affect the cellular uptake of proteins and cell signalling pathways, which may hinder replication of a natural system. Soluble protein gradients are often used to overcome these shortcomings.
Soluble growth factor gradients
Soluble factor gradients incorporated in hydrogels can mimic natural processes such as chemotaxis and angiogenesis. Chemotaxis was simulated on agarose hydrogels by observing the migration of adherent differentiated HL-60 cells and non-adherent bacterial cells up linear chemoattractant concentration gradients. Angiogenesis was simulated in vitro in 3D collagen scaffolds by measuring endothelial cell (EC) migration in the presence of a gradient of angiogenic vascular endothelial growth factor (VEGF; Vickerman et al., 2008; Chung et al., 2009). Over several days of culture, ECs in the VEGF gradient formed sprouting structures into the collagen scaffold, while those in a control scaffold without a VEGF gradient were more restrained and migrated markedly less. This study not only illustrated the utility of hydrogels for mimicking biological processes in 3D microenvironments, but also demonstrated a 3D scaffold design that promoted vascularisation.
Soluble factor gradients embedded in hydrogels have been used to engineer bone–cartilage tissue interfaces, referred to as osteochondral tissue engineering. Gradient distributions of microspheres with recombinant human bone morphogenic protein 2 (rhBMP-2) and insulin-like growth factor (rhIGF-I) in alginate and silk scaffolds have been used to study the osteochondrogenic differentiation of mesenchymal stem cells (hMSCs; Wang et al., 2009). hMSCs cultured on silk scaffolds exhibited osteogenic (bone-like) and chondrogenic (cartilage-like) differentiation along concentration gradients of rhBMP-2 and cross-gradients of rhBMP-2/rhIGF-I. hMSC differentiation did not follow the gradients of rhBMP-2 and rhIGF-I in the alginate scaffolds, likely because the range and slope of the gradients were too small or the rapid diffusion of growth factors degraded the gradients too quickly. This novel technique offers control over growth factor distribution and temporal release within hydrogels.
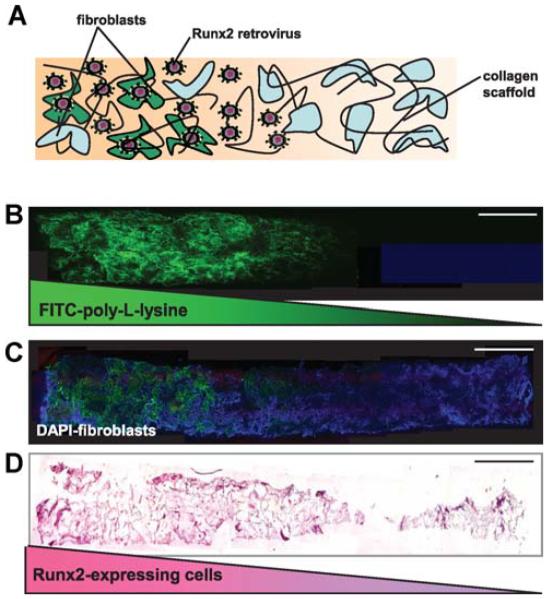
Gradient hydrogels are also being tested as transition zones at interfaces in engineered tissue grafts. A recent approach exploited spatially regulated gene transfer to create a continuous tissue gradient in a bone–soft tissue interface model (Phillips et al., 2008). Fibroblasts were encapsulated in collagen hydrogels with 3D immobilised gradients of retrovirus encoding the osteogenic transcription factor Runx2/Cbfa1. The gene delivery induced a gradient in differentiation of fibroblasts into osteoblasts as well as a gradient of matrix components. The resulting patterned distribution of fibroblasts, osteoblasts, and matrix components was reminiscent of natural interfacial tissue zones (Figure 5). Although this strategy has not yet recreated the intermediate fibro-cartilage zone typically present within the bone–ligament enthesis, it serves as the first step toward engineered complex grafting templates that mimic the cellular and structural characteristics of native tissue.
Figure 5.
Spatially regulated gene modification of fibroblasts within 3D collagen scaffolds. A: Schematic representation of fibroblast-seeded scaffolds containing spatial patterns of the Runx2 retrovirus (R2RV). The gradient was created by partially coating the proximal portion (left side) of the collagen scaffolds with poly-L-lysine (PLL) at a dipping speed of 170 μm/s. The scaffolds were then incubated in retroviral supernatant and seeded with cells. B: Confocal microscopy images of a graded distribution of FITC-labelled PLL (green). C: Confocal microscopy images of FITC-labelled PLL gradient colocalised with uniformly distributed cell nuclei (DAPI, blue). Scale bar: 2 mm. D: Immuno-histochemical staining for eGFP (pink) counterstained with haematoxylin (blue) revealed a gradient of Runx2-expressing cells. Scale bar: 2 mm (Phillips et al., 2008).
Hydrogels with chemical gradients are in widespread use as rapid screening platforms for studying various cellular processes. These gradient hydrogels are now being integrated with tools from developmental biology and genetics to engineer more complex and biologically relevant tissues.
Physical Gradients in Hydrogels
Physical gradients regulate cellular behaviours such as motility, migration, signalling, differentiation, and proliferation as well as processes such as spreading and cytoskeletal organisation. Physical gradients are divided into two classes: stiffness gradients and pore size/porosity gradients.
Stiffness gradients
To remain viable, many types of normal tissue cells must adhere to a solid substrate such as natural ECM or an engineered scaffold (Discher et al., 2005). The parameter characterising the stiffness of a solid is the elastic modulus. The elastic modulus is measured by applying a stress (force per unit area) to the material and measuring the resulting change in length (or strain) (Hartsuijker and Welleman, 2006). The slope of the linear portion of the stress–strain curve is the elastic modulus for the material (Hartsuijker and Welleman, 2006). For biological tissue, the stress–strain curve is typically linear for strains up to 10–20% of the maximum strain. Cells sense the stiffness of a substrate by exerting forces on it through adhesion complexes and their actin–myosin cytoskeleton. More generally, the elastic modulus of a substrate is likely to play a major role in cellular differentiation, development, regeneration, and disease (Discher et al., 2005). Hydrogels with gradients in elastic modulus have been created to study the effect of material stiffness on cells.
Material stiffness can affect cell migration in a process called durotaxis (Lo et al., 2000) or mechanotaxis (Gray et al., 2003). Cell migration is an essential part of morphogenesis (Juliano and Haskill, 1993), inflammation (Parente et al., 1979a,b), wound healing (Martin, 1997), and tumour metastasis (Berstein and Liotta, 1994). One of the first examples of the effect of material stiffness on cell migration was shown on a hydrogel formed by a gradient of acrylamide and bis-acrylamide exhibiting a gradient in elastic modulus ranging from 140 to 300 kdyn/cm2 (Lo et al., 2000). Fibroblasts seeded on these gels moved toward or stayed on the stiffer regions of the gels, demonstrating durotaxis (Lo et al., 2000). Elastic modulus gradients in polyacrylamide (PAAM) hydrogels have also demonstrated durotaxis. Vascular smooth muscle cells (VSMCs) seeded on PAAM hydrogels with ~1 cm long gradients in elastic modulus ranging from 5 to 35 kPa migrated to the stiffer regions of the hydrogels 24 h after seeding (Wong et al., 2003). Cell migration also depends on the absolute value of the elastic modulus. In one study, fibroblasts moved in random directions on a 100-μm long styrenated gelatin gradient with elastic modulus ranging from 200 to 400 kPa, but preferentially migrated toward regions of increasing elastic modulus for gradients in the ranges 10–80 kPa or 50–300 kPa (Kidoaki and Matsuda, 2008). Durotaxis to stiffer regions was also observed when macrophages were seeded on a ~5-cm long PEGDA gradient with elastic modulus ~3 to 100 kPa (Nemir et al., 2009).
Material stiffness can affect cell spreading and proliferation. Previous research has shown that as the substrate stiffness increases, so do the linkages between the cell surface receptors and the substrate (Choquet et al., 1997). To expedite testing, hydrogels with elastic modulus gradients have been used to test a continuum of stiffnesses on a single biological sample. In one example, VSMCs were seeded on a PAAM hydrogel with a 2.8-mm long elastic modulus gradient ranging from 3 to 40 kPa (Zaari et al., 2004). After 18 h of culture, cell spreading dramatically increased on the regions of the gradient scaffold with elastic modulus above ~30 kPa. In another experiment, hMSCs were seeded on a methacrylated HA scaffold with a 15-mm long gradient in elastic modulus ranging from ~ 3 to ~90 kPa (Marklein and Burdick, 2010). For this case, the elastic modulus for optimal spreading and proliferation ranged from ~25 to ~90 kPa. Similar results were found for HFFs plated on hydrogels with discrete stiffness gradients ranging from 8 to 50 kPa (Cheung et al., 2009).
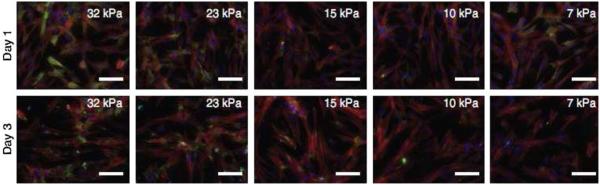
New photodegradable hydrogels make possible the real-time manipulation of the cellular microenvironment which influences cytoskeletal organisation, differentiation, cell signalling, and process extension, leading to dynamic studies on cell connectivity, migration, and cell–matrix interaction. In one experiment, valvular interstitial cells (VICs) were cultured on such photodegradable PEG hydrogels exhibiting elasticity gradients in the range of 7–32 kPa (Kloxin et al., 2010). By the third day of culture, pronounced smooth muscle actin (αSMA) stress fibres were observed indicating significant myofibroblast differentiation of the seeded VICs in the direction of higher elasticity modulus. The hydrogel sheet was then irradiated in situ to reduce its elastic modulus to 7 kPa. By the fifth day of culture the VICs no longer exhibited detectable αSMA in their cytoskeleton, indicating cytoskeletal reorganisation and VIC deactivation from the myofibroblast phenotype (Figure 6; Kloxin et al., 2010). Such studies have highlighted the importance of the elastic modulus in dictating various cell behaviours.
Figure 6.
Differentiation of fibroblasts into myofibroblasts mediated by elasticity gradients in PEG hydrogels. Valvular interstitial cells (VICs) cultured on elasticity gradient hydrogels were immunostained for αSMA (green), an indicator of differentiation to myofibroblasts, as well as F-actin (red) and nuclei (blue). Images were taken at different representative positions along the gradient (elastic moduli noted on image). By day 3, a higher myofibroblastic differentiation was observed in cells on the high modulus side of the gradient (Kloxin et al., 2010).
Pore size and porosity gradients
Pore size and porosity affect cell affinity and viability by influencing cell binding, movement, intercellular signalling, and the transport of nutrients and metabolites (Oh et al., 2007). An agarose/gelatin scaffold has been synthesised that is anisotropic, viscoelastic, soft, tissue-like, and biocompatible (Tripathi et al., 2009). Initial results on its effects on in vitro fibroblasts make it a possible candidate for cartilage scaffolds. In another experiment, porous gelatin scaffolds with embedded parallel channels, conical pores, and a gradient in pore size were seeded with human endothelial, epithelial, fibroblast, glial, or osteoblast cells (Dubruel et al., 2007). Cell behaviour was compared to that on a gelatin scaffold with a constant spherical pore size of 135 μm. After 4 weeks of culture, comparisons between the observed cell behaviours on the two scaffolds suggested that pore size and geometry did not affect cell adhesion, proliferation, or spreading over the pore sizes and geometries in the study (Dubruel et al., 2007).
Biomimetic bone scaffolds have been fabricated with pore size and porosity gradients (Harley et al., 2006; Dubruel et al., 2007; Oh et al., 2007; Tripathi et al., 2009; Annabi et al., 2010). For example, osteoblasts, the cells responsible for bone formation, grow faster within a scaffold with pores ranging in size from 380 to 405 μm, while actual bone formation occurs most rapidly in pores of size 290–310 μm (Oh et al., 2007). Gradients are incorporated into biomimetic tissue-like materials to enhance their development and function.
In addition to cell scaffolds, gels with pore size gradients have been used in microfluidic electrophoresis applications such as on-chip protein sizing (Lo et al., 2008). Both linear and nonlinear pore size gradients were generated using microfluidics, diffusion, and photolithography. A compact protein sizing electrophoresis device with a ~0.3-cm long pore size gradient analysed the molecular weights of a wide range of proteins (Lo et al., 2008). The device required only 89 V to operate compared to the ~1 kV required by conventional devices using longer gradients. Using pore size gradients to induce different cell behaviours is still an active area of research.
Recently, a versatile method was developed to combine chemical and physical gradients in PEG hydrogels (Park et al., 2009). Fibroblasts seeded on such combined physico-chemical gradient hydrogels aligned, migrated, and attached in response to the material stiffness, and proliferated in response to the chemical nutrient gradient. Further work in this direction will enhance the level of complexity of engineered tissues.
CHALLENGES AND FUTURE PROSPECTS
Gradient hydrogels provide 2D and 3D microenvironments for studying cell behaviour and rapidly screening optimal chemical and physical properties. Microfluidic and materials synthesis methods have progressed sufficiently to produce hydrogels with gradients in soluble factors and physical properties. To study cellular processes such as adhesion, migration, differentiation, and angiogenesis, cells may be seeded on 2D hydrogel surfaces or encapsulated in 3D hydrogel matrices with gradients of soluble factors (e.g., toxins, chemoattractants, growth factors), or immobilised proteins (e.g., adhesion ligands). Hydrogels have also been created incorporating gradients of complex shapes or multiple superposed gradients, including a combined chemical and physical gradient (Park et al., 2009). Most of the existing literature on cell–material interactions pertains to 2D surfaces of gradient hydrogels. Studies are beginning to appear on the behaviour of cells encapsulated in 3D matrices, more closely mimicking the situation in vivo. 3D scaffolds are somewhat more complex to fabricate, characterise, and culture with encapsulated cells. More importantly, measuring the response of cells encapsulated in 3D matrices is also more challenging than for those seeded on 2D surfaces.
Ongoing and future research is tasked with improving gradient generation methods and integrating them with material synthesis, genetics, and other biomedical fields to recreate the complexity of in vivo tissues and organs. Improved methods of gradient shape control and stability must be developed and integrated into tissue engineering applications. Techniques to combine multiple chemical and physical gradients must also be developed. Improved and standardised methods are also required to properly correlate cell response to the applied gradient and biological cues. Moreover, all such techniques must be made more user-friendly and accessible to a broad range of researchers. Soon, material synthesis techniques should be sufficiently advanced to create physiologically relevant gradient materials to study complex spatiotemporal phenomena such as tissue morphogenesis. Smart biomaterials incorporating multiple gradient cues inside scaffolds could then be created for the regeneration of complex, high-order grafting templates that mimic the cellular and structural characteristics of native tissue.
ACKNOWLEDGEMENTS
This research was funded by the US Army Engineer Research and Development Center, the Institute for Soldier Nanotechnology, the NIH (HL092836, DE019024, EB007249), Office of Naval Research Young Investigator Program, and the National Science Foundation CAREER award (A.K.). S.S. acknowledges a postdoctoral fellowship awarded by Fonds de Recherche sur la Nature et les Technologies (FQRNT), Québec, Canada. We thank Jiankang He for the unpublished images in Figure 4B. We also thank Dr. Andrés J. Garcia, Georgia Institute of Technology for providing the original images for Figure 5.
REFERENCES
- Abhyankar VV, Lokuta MA, Huttenlocher A, Beebe DJ. Characterization of a Membrane-Based Gradient Generator for Use in Cell-Signaling Studies. Lab Chip. 2006;6:389. doi: 10.1039/b514133h. [DOI] [PubMed] [Google Scholar]
- Ajdari A, Bontoux N, Stone HA. Hydrodynamic Dispersion in Shallow Microchannels: The Effect of Cross-Sectional Shape. Anal. Chem. 2006;78:387–392. doi: 10.1021/ac0508651. [DOI] [PubMed] [Google Scholar]
- Amarie D, Glazier JA, Jacobson SC. Compact Microfluidic Structures for Generating Spatial and Temporal Gradients. Anal. Chem. 2007;79:9471–9477. doi: 10.1021/ac0714967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, Dehghani F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Pt. B Rev. 2010;16:371–383. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe HL, Briscoe J. The Interpretation of Morphogen Gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- Atencia J, Morrow J, Locascio LE. The Microfluidic Palette: A Diffusive Gradient Generator With Spatio-Temporal Control. Lab Chip. 2009;9:2707–2714. doi: 10.1039/b902113b. [DOI] [PubMed] [Google Scholar]
- Bastiaens P, Caudron M, Niethammer P, Karsenti E. Gradients in the Self-Organization of the Mitotic Spindle. Trends Cell Biol. 2006;16:125–134. doi: 10.1016/j.tcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Berstein LR, Liotta LA. Molecular Mediators of Interactions With Extracellular Matrix Components in Metastasis and Angiogenesis. Curr. Opin. Oncol. 1994;6:106–113. doi: 10.1097/00001622-199401000-00015. [DOI] [PubMed] [Google Scholar]
- Botchwey EA, Dupree MA, Pollack SR, Levine EM, Laurencin CT. Tissue Engineered Bone: Measurement of Nutrient Transport in Three-Dimensional Matrices. J. Biomed. Mater. Res. A. 2003;67:357–367. doi: 10.1002/jbm.a.10111. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Khademhosseini A, Langer R. Fabrication of Gradient Hydrogels Using a Microfluidics/Photopolymerization Process. Langmuir. 2004;20:5153–5156. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- Caudron M, Bunt G, Bastiaens P, Karsenti E. Spatial Coordination of Spindle Assembly by Chromosome-Mediated Signaling Gradients. Science. 2005;309:1373. doi: 10.1126/science.1115964. [DOI] [PubMed] [Google Scholar]
- Cheung YK, Azeloglu EU, Shiovitz DA, Costa KD, Seliktar D, Sia SK. Microscale Control of Stiffness in a Cell-Adhesive Substrate Using Microfluidics-Based Lithography. Angew. Chem. Int. Ed. Engl. 2009;48:7188–7192. doi: 10.1002/anie.200900807. [DOI] [PubMed] [Google Scholar]
- Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Stroock AD. Microfluidic Scaffolds for Tissue Engineering. Nat. Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular Matrix Rigidity Causes Strengthening of Integrin–Cytoskeleton Linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Human Neural Stem Cell Growth and Differentiation in a Gradient-Generating Microfluidic Device. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell Migration Into Scaffolds Under Co-Culture Conditions in a Microfluidic Platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- Chung S, Sudo R, Vickerman V, Zervantonakis IK, Kamm RD. Microfluidic Platforms for Studies of Angiogenesis, Cell Migration, and Cell–Cell Interactions. Ann. Biomed. Eng. 2010;38:1164–1177. doi: 10.1007/s10439-010-9899-3. [DOI] [PubMed] [Google Scholar]
- Clarke PR. Cell Biology. A Gradient Signal Orchestrates the Mitotic Spindle. Science. 2005;309:1334–1335. doi: 10.1126/science.1117842. [DOI] [PubMed] [Google Scholar]
- Cooksey GA, Sip CG, Folch A. A Multi-Purpose Microfluidic Perfusion System With Combinatorial Choice of Inputs, Mixtures, Gradient Patterns, and Flow Rates. Lab Chip. 2009;9:417–426. doi: 10.1039/b806803h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson S, Kobel SA, Lutolf MP. Capturing Complex Protein Gradients on Biomimetic Hydrogels for Cell-Based Assays. Adv. Funct. Mater. 2009;19:3411–3419. [Google Scholar]
- Crock PA, Pestell RG, Calenti AJ, Gilford EJ, Henderson JK, Best JD, Alford FP. Multiple Pituitary Hormone Gradients From Inferior Petrosal Sinus Sampling in Cushing’s Disease. Acta Endocrinol. 1988;119:75–80. doi: 10.1530/acta.0.1190075. [DOI] [PubMed] [Google Scholar]
- DeLong SA, Moon JJ, West JL. Covalently Immobilized Gradients of bFGF on Hydrogel Scaffolds for Directed Cell Migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Dertinger SKW, Chiu DT, Jeon NL, Whitesides GM. Generation of Gradients Having Complex Shapes Using Microfluidic Networks. Anal. Chem. 2001;73:1240–1246. [Google Scholar]
- Discher DE, Janmey P, Wang Y-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science. 2005;301:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dodla MC, Bellamkonda RV. Anisotropic Scaffolds Facilitate Enhanced Neurite Extension In Vitro. J. Biomed. Mater. Res. A. 2006;78A:213–221. doi: 10.1002/jbm.a.30747. [DOI] [PubMed] [Google Scholar]
- Du Y, Shim J, Vidula M, Hancock MJ, Lo E, Chung BG, Borenstein JT, Khabiry M, Cropek DM, Khademhosseini A. Rapid Generation of Spatially and Temporally Controllable Long-Range Concentration Gradients in a Microfluidic Device. Lab Chip. 2009;9:761–767. doi: 10.1039/b815990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Hancock MJ, He J, Villa-Uribe JL, Wang B, Cropek DM, Khademhosseini A. Convection-Driven Generation of Long-Range Material Gradients. Biomaterials. 2010;31:2686–2694. doi: 10.1016/j.biomaterials.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubruel P, Unger R, Vlierberghe SV, Cnudde V, Jacobs PJ, Schacht E, Kirkpatrick CJ. Porous Gelatin Hydrogels: 2. In Vitro Cell Interaction Study. Biomacromolecules. 2007;8:338–344. doi: 10.1021/bm0606869. [DOI] [PubMed] [Google Scholar]
- Freytes DO, Wan LQ, Vunjak-Novakovic G. Geometry and Force Control of Cell Function. J. Cell. Biochem. 2009;108:1047–1058. doi: 10.1002/jcb.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk ML, Berthier E, Tepp WH, Johnson EA, Beebe DJ. Bead-Based Microfluidic Toxin Sensor Integrating Evaporative Signal Amplification. Lab Chip. 2008;8:1793–1800. doi: 10.1039/b811075a. [DOI] [PubMed] [Google Scholar]
- Fuller B. Self-Organization of Intracellular Gradients During Mitosis. Cell Division. 2010;5:5. doi: 10.1186/1747-1028-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas J-C, Bartolo D, Studer V. A Microfluidic Concentration Profile Generator. microTAS 2009, 13th Intl. Conf. Miniaturized Systems for Chemistry and Life Sciences; Jeju, Korea. 2009. [Google Scholar]
- Genzer J, Bhat RR. Surface-Bound Soft Matter Gradients. Langmuir. 2008;24:2294–2317. doi: 10.1021/la7033164. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Bidirectional Extracellular Matrix Signaling During Tissue Morphogenesis. Cytokine Growth Factor Rev. 2009;20:459–465. doi: 10.1016/j.cytogfr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulpeau J, Lonetti B, Trouchet D, Ajdari A, Tabeling P. Building Up Longitudinal Concentration Gradients in Shallow Microchannels. Lab Chip. 2007;7:1154–1161. doi: 10.1039/b706340g. [DOI] [PubMed] [Google Scholar]
- Gray DS, Tien J, Chen CS. Repositioning of Cells by Mechanotaxis on Surfaces With Micropatterned Young’s Modulus. J. Biomed. Mater. Res. A. 2003;66:605–614. doi: 10.1002/jbm.a.10585. [DOI] [PubMed] [Google Scholar]
- Hall SJ. Basic Biomechanics. McGraw-Hill; Boston, MA: 2007. [Google Scholar]
- Harley BA, Hastings AZ, Yannas IV, Sannino A. Fabricating Tubular Scaffolds With a Radial Pore Size Gradient by a Spinning Technique. Biomaterials. 2006;27:866–874. doi: 10.1016/j.biomaterials.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Hartsuijker C, Welleman JW. Engineering Mechanics. Springer; Dordrecht: 2006. [Google Scholar]
- He J, Du Y, Guo Y, Hancock MJ, Wang B, Shin H, Wu J, Li D, Khademhosseini A. Microfluidic Synthesis of Composite Cross-Gradient Materials for Investigating Cell–Biomaterial Interactions. Biotechnol. Bioeng. 2010a doi: 10.1002/bit.22901. In press. DOI: 10.1002/bit.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Du Y, Villa-Uribe JL, Hwang C, Li D, Khademhosseini A. Rapid Generation of Biologically Relevant Hydrogels Containing Long-Range Chemical Gradients. Adv. Funct. Mater. 2010b;20:131–137. doi: 10.1002/adfm.200901311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A Role for Oestrogens in the Male Reproductive System. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SP, Marshall SJ, Ryder MI, Marshall GW. The Tooth Attachment Mechanism Defined by Structure, Chemical Composition and Mechanical Properties of Collagen Fibers in the Periodontium. Biomaterials. 2007;28:5238–5245. doi: 10.1016/j.biomaterials.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia D, Geba DA, Toner M. Universal Microfluidic Gradient Generator. Anal. Chem. 2006;78:3472–3477. doi: 10.1021/ac0518710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon NL, Baskaran H, Dertinger SKW, Whitesides GM, Van De Water L, Toner M. Neutrophil Chemotaxis in Linear and Complex Gradients of Interleukin-8 Formed in a Microfabricated Device. Nat. Biotechnol. 2002;20:826–830. doi: 10.1038/nbt712. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Reynolds TB, Stansbury JW, Bowman CN. High Throughput Kinetic Analysis of Photopolymer Conversion Using Composition and Exposure Time Gradients. Polymer. 2005;46:3300–3306. [Google Scholar]
- Juliano RL, Haskill S. Signal Transduction From the Extracellular Matrix. J. Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur TA, Shoichet MS. Immobilized Concentration Gradients of Nerve Growth Factor Guide Neurite Outgrowth. J. Biomed. Mater. Res. A. 2004;68A:235–243. doi: 10.1002/jbm.a.10168. [DOI] [PubMed] [Google Scholar]
- Keenan TM, Folch A. Biomolecular Gradients in Cell Culture Systems. Lab Chip. 2008;8:34–57. doi: 10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale Technologies for Tissue Engineering and Biology. Proc. Natl. Acad. Sci. USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidoaki S, Matsuda T. Microelastic Gradient Gelatinous Gels to Induce Cellular Mechanotaxis. J. Biotechnol. 2008;133:225–230. doi: 10.1016/j.jbiotec.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloxin AM, Benton JA, Anseth KS. In Situ Elasticity Modulation With Dynamic Substrates to Direct Cell Phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutejova E, Briscoe J, Kicheva A. Temporal Dynamics of Patterning by Morphogen Gradients. Curr. Opin. Genet. Dev. 2009;19:315–322. doi: 10.1016/j.gde.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lee PI. Effect of Non-Uniform Initial Drug Concentration Distribution on the Kinetics of Drug Release From Glassy Hydrogel Matrices. Polymer. 1984;25:973–978. [Google Scholar]
- Lee PI, Kim CJ. Probing the Mechanism of Drug Release From Hydrogels. J. Control. Release. 1991;16:229–236. [Google Scholar]
- Lee GB, Hung CI, Ke BJ, Huang GR, Hwei BH, Lai HF. Hydrodynamic Focusing for a Micromachined Flow Cytometer. J. Fluid Eng. 2001;123:672–679. [Google Scholar]
- Lee K, Kim C, Ahn B, Panchapakesan R. Generalized Serial Dilution Module for Monotonic and Arbitrary Microfluidic Gradient Generators. Lab Chip. 2009;9:709. doi: 10.1039/b813582g. [DOI] [PubMed] [Google Scholar]
- Lin F, Saadi W, Rhee SW, Wang SJ, Mittal S, Jeon NL. Generation of Dynamic Temporal and Spatial Concentration Gradients Using Microfluidic Devices. Lab Chip. 2004;4:164–167. doi: 10.1039/b313600k. [DOI] [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang Y. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CT, Throckmorton DJ, Singh AK, Herr AE. Photopolymerized Diffusion-Defined Polyacrylamide Gradient Gels for On-Chip Protein Sizing. Lab Chip. 2008;8:1273–1279. doi: 10.1039/b804485f. [DOI] [PubMed] [Google Scholar]
- Lutolf MP. Integration Column: Artificial ECM: Expanding the Cell Biology Toolbox in 3D. Integr. Biol. 2009;1:235–241. doi: 10.1039/b902243k. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential Interactions of FGFs With Heparan Sulfate Control Gradient Formation and Branching Morphogenesis. Sci. Signal. 2009;2:10. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklein RA, Burdick JA. Spatially Controlled Hydrogel Mechanics to Modulate Stem Cell Interactions. Soft Matter. 2010;6:136–143. [Google Scholar]
- Martin P. Wound Healing—Aiming for Perfect Skin Regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, Van Dyke ME, Kaplan D, Vunjak-Novakovic G. Engineering Complex Tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserez A, Schneberk T, Sun C, Zok FW, Waite JH. The Transition From Stiff to Compliant Materials in Squid Beaks. Science. 2008;319:1816–1819. doi: 10.1126/science.1154117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Macsween M, Shoichet M. Immobilized Concentration Gradients of Neurotrophic Factors Guide Neurite Outgrowth of Primary Neurons in Macroporous Scaffolds. Tissue Eng. 2006;12:267–278. doi: 10.1089/ten.2006.12.267. [DOI] [PubMed] [Google Scholar]
- Musoke-Zawedde P, Shoichet MS. Anisotropic Three-Dimensional Peptide Channels Guide Neurite Outgrowth Within a Biodegradable Hydrogel Matrix. Biomed. Mater. 2006;1:162–169. doi: 10.1088/1748-6041/1/3/011. [DOI] [PubMed] [Google Scholar]
- Nemir S, Hayenga HN, West JL. PEGDA Hydrogels With Patterned Elasticity: Novel Tools for the Study of Cell Response to Substrate Rigidity. Biotechnol. Bioeng. 2009;105:636–644. doi: 10.1002/bit.22574. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, West JL. Photopolymerizable Hydrogels for Tissue Engineering Applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- Oh SH, Park IK, Kim JM, Lee JH. In Vitro and In Vivo Characteristics of PCL Scaffolds With Pore Size Gradient Fabricated by a Centrifugation Method. Biomaterials. 2007;28:1664–1671. doi: 10.1016/j.biomaterials.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Parente L, Koh MS, Willoughby DA, Kitchen A. Studies on Cell Motility in Inflammation. I. The Chemotactic Activity of Experimental, Immunological and Non-Immunological, Inflammatory Exudates. Agents Actions. 1979a;9:190–195. doi: 10.1007/BF02024733. [DOI] [PubMed] [Google Scholar]
- Parente L, Koh MS, Willoughby DA, Kitchen A. Studies on Cell Motility in Inflammation. II. The In Vivo Effect of Anti-Inflammatory and Anti-Rheumatic Drugs on Chemotaxis In Vitro. Agents Actions. 1979b;9:196–200. doi: 10.1007/BF02024734. [DOI] [PubMed] [Google Scholar]
- Park JY, Yoo SJ, Hwang CM, Lee SH. Simultaneous Generation of Chemical Concentration and Mechanical Shear Stress Gradients Using Microfluidic Osmotic Flow Comparable to Interstitial Flow. Lab Chip. 2009;9:2194–2202. doi: 10.1039/b822006a. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Khare AR. Preparation, Structure and Diffusional Behavior of Hydrogels in Controlled Release. Adv. Drug. Deliv. Rev. 1993;11:1–35. [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006;18:1345. [Google Scholar]
- Peret BJ, Murphy WL. Controllable Soluble Protein Concentration Gradients in Hydrogel Networks. Adv. Funct. Mater. 2008;18:3410–3417. doi: 10.1002/adfm.200800218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering Graded Tissue Interfaces. Proc. Natl. Acad. Sci. USA. 2008;105:12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl J, Sinclair J, Sahlin E, Karlsson M, Petterson F, Olofsson J, Orwar O. Microfluidic Gradient-Generating Device for Pharmacological Profiling. Anal. Chem. 2005;77:3897–3903. doi: 10.1021/ac050218+. [DOI] [PubMed] [Google Scholar]
- Pope AJ, Sands GB, Smaill BH, LeGrice IJ. Three-Dimensional Transmural Organization of Perimysial Collagen in the Heart. Am. J. Physiol. Heart C. 2008;295:H1243–H1252. doi: 10.1152/ajpheart.00484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamloo A, Ma N, Poo M, Sohn LL, Heilshorn SC. Endothelial Cell Polarization and Chemotaxis in a Microfluidic Device. Lab Chip. 2008;8:1292–1299. doi: 10.1039/b719788h. [DOI] [PubMed] [Google Scholar]
- Simon CG, Yang YY, Thomas V, Dorsey SM, Morgan AW. Cell Interactions With Biomaterials Gradients and Arrays. Comb. Chem. High Throughput Screen. 2009;12:544–553. doi: 10.2174/138620709788681961. [DOI] [PubMed] [Google Scholar]
- Singh M, Berkland C, Detamore MS. Strategies and Applications for Incorporating Physical and Chemical Signal Gradients in Tissue Engineering. Tissue Eng. Pt. B Rev. 2008;14:341–366. doi: 10.1089/ten.teb.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA. Signaling in Morphogenesis: Transport Cues in Morphogenesis. Curr. Opin. Biotechnol. 2003;14:547–550. doi: 10.1016/j.copbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Kathuria N, Kumar A. Elastic and Macroporous Agarose–Gelatin Cryogels With Isotropic and Anisotropic Porosity for Tissue Engineering. J. Biomed. Mater. Res. A. 2009;90:680–694. doi: 10.1002/jbm.a.32127. [DOI] [PubMed] [Google Scholar]
- Vepari CP, Kaplan DL. Covalently Immobilized Enzyme Gradients Within Three-Dimensional Porous Scaffolds. Biotechnol. Bioeng. 2006;93:1130–1137. doi: 10.1002/bit.20833. [DOI] [PubMed] [Google Scholar]
- Vickerman V, Blundo J, Chung S, Kamm R. Design, Fabrication and Implementation of a Novel Multi-Parameter Control Microfluidic Platform for Three-Dimensional Cell Culture and Real-Time Imaging. Lab Chip. 2008;8:1468–1477. doi: 10.1039/b802395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, Sheetz M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- Wang F. The Signaling Mechanisms Underlying Cell Polarity and Chemotaxis. Cold Spring Harb. Perspect. Biol. 2009;1:a002980. doi: 10.1101/cshperspect.a002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth Factor Gradients via Microsphere Delivery in Biopolymer Scaffolds for Osteochondral Tissue Engineering. J. Control. Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed Movement of Vascular Smooth Muscle Cells on Gradient-Compliant Hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- Yang PJ, Temenoff JS. Engineering Orthopedic Tissue Interfaces. Tissue Eng. Pt. B Rev. 2009;15:127–141. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaari N, Rajagopalan P, Kim SK, Engler AJ, Wong JY. Photopolymerization in Microfluidic Gradient Generators: Microscale Control of Substrate Compliance to Manipulate Cell Response. Adv. Mater. 2004;16:2133–2136. [Google Scholar]