Abstract
Study Objective:
To validate the ApneaLINK (AL) as an accurate tool for determining the presence of obstructive sleep apnea (OSA) in an at-risk sleep clinic population in a home test environment.
Methods:
Consecutive participants referred with the suspicion of OSA were evaluated in the home with the AL portable monitor (AL Home), followed by simultaneous data collection with diagnostic polysomnography (PSG) and AL in the sleep laboratory (AL Lab). Prevalence, sensitivity, specificity, and ROC curves were calculated for PSG vs. AL Lab, PSG vs. AL Home, and AL Lab vs. AL Home test. Pearson correlations and Bland-Altman plots were constructed.
Results:
Fifty-three (55% female) participants completed the entire study. The mean age of the population was 45.1 ± 11.3 years, and body mass index was 35.9 ± 9.1 kg/m2. The prevalence of an apnea hypopnea index (AHI) ≥ 15 in the cohort was 35.9%. The results demonstrated a high sensitivity and specificity of the AL respiratory disturbance index (RDI-AL) compared with the AHI from the PSG. The AL Lab had the highest sensitivity and specificity at RDI-AL values ≥ 20 events/h (sensitivity 100%, specificity 92.5%). The AL Home was most sensitive and specific at an RDI-AL ≥ 20 events/h (sensitivity 76.9%, specificity 92.5%). The Pearson correlations for PSG vs. AL Lab and PSG vs. AL Home were ρ = 0.88 and ρ = 0.82, respectively. The Bland-Altman Plots demonstrated good agreement between the methodologies.
Conclusion:
The AL home test is an accurate alternative to PSG in sleep clinic populations at risk for moderate and severe OSA.
Trial Registration:
clinicaltrials.gov ID: NCT00354614.
Citation:
Oktay B; Rice TB; Atwood CW; Passero M; Gupta N; Givelber R; Drumheller OJ; Houck P; Gordon N; Strollo PJ. Evaluation of a single-channel portable monitor for the diagnosis of obstructive sleep apnea. J Clin Sleep Med 2011;7(4):384-390.
Keywords: Obstructive sleep apnea, portable monitoring, diagnosis
Obstructive sleep apnea (OSA) is a major public health problem due to its association with co-morbid conditions such as heart failure,1,2 hypertension,3,4 cardiovascular disease,5,6 cerebrovascular disease,7 insulin resistance,8,9 and increased rates of traffic accidents.10 The main consequences of this syndrome are increased morbidity, mortality, and quality of life impairment.11–13 The prevalence of OSA is estimated to be 2% and 4% for adult middle-aged women and men, respectively.10 Although there is effective treatment for OSA, a remarkable number of patients remain undiagnosed and untreated.14,15 The “reference standard” for OSA diagnosis is the in-laboratory sleep study using full polysomnography (PSG) and the manual scoring criteria set by the American Academy of Sleep Medicine.16 This test has limitations related to cost and access. The high prevalence of OSA and geographical variation related to access and expense has prompted the search for diagnostic alternatives.17,18
Many practitioners use patient questionnaires as a screening tool to evaluate for suspected OSA. These questionnaires are subjective assessment tools that can produce erroneous results and lack sufficient sensitivity and specificity compared to PSG.19 ApneaLINK (AL) is a single-channel device that measures nasal pressure. It was developed to meet the need for a simple, cost-effective, accurate, and reliable tool to identify patients with suspected OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: A large proportion of sleep apnea patients are undiagnosed and more tools to aid in the diagnosis of sleep apnea, particularly in the home, are needed. We investigated the performance of a single-channel portable sleep apnea monitor used in a home setting, to make a diagnosis of sleep apnea in a high pre-test probability sleep clinic population.
Study Impact: The accuracy of the ApneaLINK in the home diagnosis of moderate and severe sleep apnea makes in an attractive alternative to full in-lab polysomnography. Interpretation of the results of home testing will require careful appraisal of pre-test probability by the sleep specialist.
The primary objective of this study was to validate the AL as an accurate tool for determining the presence of OSA in a sleep laboratory population in a home test environment.
METHODS
Participants
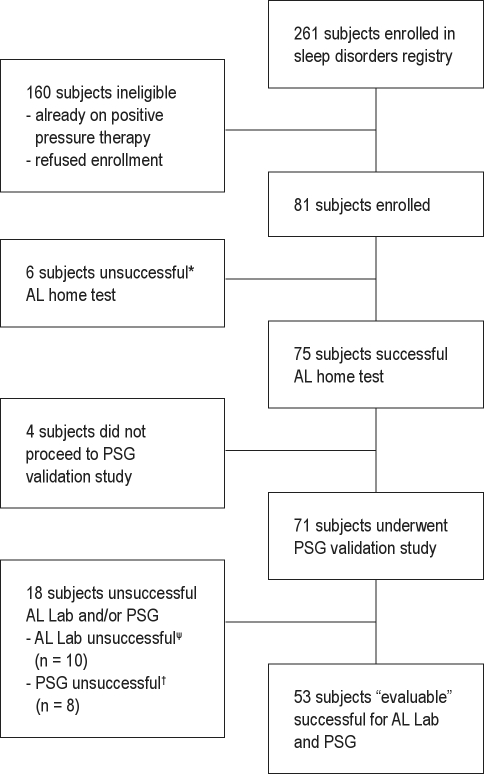
Participants with clinically suspected OSA were recruited from the University of Pittsburgh Sleep Medicine Center. Study enrollment commenced in June 2006 and closed in July 2007. Clinic participants were asked to enroll in our Sleep Disorders Registry by their sleep physician. Participants enrolled in the registry and scheduled for a diagnostic PSG with a diagnosis of suspected OSA were approached by a study coordinator for enrollment. Eligibility criteria included adult participants (≥ 18 years of age) of either gender or any race, suspected sleep disordered breathing, scheduled for diagnostic PSG, willingness to give written informed consent and ability to adhere to all visit schedules, willingness to use AL at home within 2 days of receipt and instruction on the AL, willingness to use AL in the laboratory and undergo PSG within 16 weeks of completing the at-home AL test. Exclusion criteria for participation in the study included a history of sleep apnea, use of positive pressure therapy, use of oxygen therapy, use of medications that could affect sleep, and presence of any serious respiratory or cardiac medical condition that the investigator determined could affect the subject's ability to participate in the study. Consecutive participants providing consent were asked to undergo both the AL home test (AL Home) and the diagnostic in-lab PSG study with simultaneous AL data collection (AL Lab). An “evaluable” participant was defined as someone who (1) completed both the AL home test and the diagnostic PSG study with simultaneous AL lab testing, and (2) had an evaluation time on the AL report ≥ 4 h during both the home test and the diagnostic PSG study. A total of 81 participants met the entry criteria and were enrolled. The first time failure rate for the AL Home test was 17.3% (n = 14). Seventy-five of these 81 participants successfully completed the at-home study. Of the 75 participants completing the at-home study, 53 participants completed the PSG study (with simultaneous AL) with ≥ 4 h evaluation time. Four participants never underwent PSG. Eight participants had < 4 h of sleep time during the PSG. Ten participants had < 4 h of signal duration on the AL lab testing due to signal loss during the study or insufficient signal duration. These 22 participants were considered to be not evaluable and are not included in the study results (See Consort diagram, Figure 1).
Figure 1.
Consort diagram
*Evaluation time on AL home test < 4 hours. ψEvaluation time on AL lab test < 4 hours. †Evaluation time on PSG < 4 hours.
The study was approved by the New England Institutional Review Board (NEIRB) and UPMC Clinical Trials Office (CTO). All participants provided informed consent prior to participation in the study. The study was registered as a NIH clinical trial (ClinicalTrials.gov Identifier NCT00354614).
Design
This was a prospective cohort design study. Participants were trained on use of the AL home test and provided the device for a one-night study. They returned the device to the clinical site using a prepaid mailer. If the AL use was < 4 h, the at-home test was repeated. If the repeated AL test was ≥ 4 h, the participant continued and scheduled the diagnostic PSG. If the retest was < 4 h, the participant exited from the study. Participants were not informed of their study results until they completed the study. Following the successful AL home test, the participant underwent a diagnostic PSG study. The diagnostic PSG study was performed within 16 weeks of the participant completing the AL home test. There was no attempt to ensure weight was stable between studies. All participants had simultaneous diagnostic PSG and AL lab testing. The simultaneous diagnostic PSG and AL lab testing were accomplished by using a bifurcating nasal cannula to capture the appropriate signal from both the PSG and AL devices. Comparison of AL Home and AL Lab RDI-AL was also performed. The results of the AL and diagnostic PSG were compared.
Medical and sleep history were recorded by research nurses at the screening visit. Sleep history domains assessed included the presence of snoring, apneas, and daytime sleepiness. Participants completed the Epworth Sleepiness Scale at the time of polysomnography.
ApneaLINK Device
The ApneaLINK device is a single-channel diagnostic tool for OSA (Figure 2). The device consists of a nasal cannula attached to a small case that houses a pressure transducer. The device is held in place by a belt worn around the user's chest. Three simple steps are required by the patients: (1) apply the nasal cannula; (2) strap the recorder to the chest; and (3) press the button (the flashing LED confirms correct operation for successful recording). The RDI-AL used for analysis was automatically analyzed by the AL software and was calculated by adding the total number of apneas and hypopneas per hour of recording time. An apnea was defined as a decrease in airflow ≥ 80% of baseline for ≥ 10 sec. The AL maximum apnea duration was set at 80 sec. A hypopnea was defined as a decrease in airflow by 50% to 80% of baseline for ≥ 10 sec; AL maximum hypopnea duration was set at 100 sec. The AL firmware version 2.97 and the scoring software version 5.13 were used. The AL device operates on battery power (2 AA batteries), has a sampling rate of 100 Hz, and has a 16-bit signal processor. The internal memory storage is 15 MB, which allows for approximately 10 h of data collection. Visual inspection of all AL studies was done by the primary investigator to ensure signal fidelity. The AL software analyzes data generated by the flow signal without manual editing, producing a 1-page report.20
Figure 2.
Picture of portable monitoring device
Polysomnography
Full PSG was performed using the SomnoStar Pro system (Viasys Healthcare). Channels monitored and recorded with surface electrodes included electroencephalogram, electrooculogram, and submental electromyogram. Arterial oxygen saturation was recorded by digital pulse oximetry. Chest and abdominal effort were recorded using inductance plethysmography. Airflow was recorded via nasal cannula with a pressure transducer and a thermocouple. Apnea was defined as complete cessation of airflow ≥ 10 sec; hypopnea was defined as a 50% decrease in airflow accompanied by ≥ 4% drop in oxygen saturation. American Academy of Sleep Medicine (AASM) sleep scoring criteria were used for scoring the diagnostic PSG.16 The AHI derived from PSG was based on sleep time, whereas the RDI-AL was based on total recording time.
Statistical Analysis
Baseline demographic data were summarized descriptively, using mean, standard deviation, and range for continuous variables and proportions for categorical variables. The AL tests were compared to the reference standard PSG. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated according to the definition of OSA for AHI values of 5, 10, 15, 20, and 30 events/hour. Accuracy was calculated by total true positives and true negatives, and dividing by the total number. Significance was determined by the McNemar test for matched pairs.
ROC curves were constructed to graphically represent the tradeoff between the false negative and false positive rates for various cutoffs. Bland-Altman plots were used as a graphical representation of the observed differences between the paired measurements to examine relationship between the magnitude and degree of variation in the AHI measurements (RDI-AL Lab)-AHI (PSG)). The correlations between the AHI from the PSG and the RDI-AL were determined using the Pearson correlation coefficient.
RESULTS
The study population had 55% female participants. The mean age was 45.1 years, and the average BMI was 35.9 kg/m2. Patient demographics and medical and sleep history are shown in Table 1. Frequent snoring, excessive sleepiness, apneas, and unrefreshing sleep were present in the majority of the participants. The mean Epworth Sleepiness Scale score was 10 (± 4.9). The prevalence of an AHI ≥ 15 in the cohort was 35.9%.
Table 1.
Demographic characteristics, comorbidities and sleep history of 53 participants in the study
| Demographic Characteristics | Results |
|---|---|
| Age, y | 45.1 ± 11.3 (23-70) |
| BMI (kg/m2) | 35.9 ± 9.1 (19.6-54.5) |
| Sex | |
| Men | 24 (45) |
| Women | 29 (55) |
| Comorbidities | |
| Impaired cognition | 17 (32) |
| Mood disorders | 21 (40) |
| Insomnia | 17 (32) |
| Arrhythmias | 6 (11) |
| Hypertension | 23 (43) |
| Asthma | 14 (26) |
| Bronchitis | 5 (9) |
| Diabetes type 1 | 3 (6) |
| Diabetes type 2 | 6 (11) |
| Sleep History | |
| Frequent snoring | 47 (89) |
| Excessive sleepiness | 36 (68) |
| Breathless at night | 17 (32) |
| Stop breathing at night | 29 (55) |
| Unrefreshed after sleeping | 44 (83) |
Data are represented as number (%) except age and BMI, which are mean ± SD (range).
Table 2 presents the prevalence rates for OSA based on PSG, AL lab test, and AL home test. The results were similar across the 3 methods for the various cutoff points. There was, however, a statistically significant difference between AL Home and PSG prevalence for AHI ≥ 5 (56.6% vs. 75.5%, p = 0.02).
Table 2.
Prevalence rates for PSG, AL Lab, and AL Home test at different AHI/RDI-AL cutoffs
| AHI/RDI-AL | PSG | AL Lab | AL Home | P-value |
|---|---|---|---|---|
| ≥ 5 | 40 (75.5) | 39 (73.6) | 30 (56.6) | 0.02* |
| ≥ 10 | 28 (52.8) | 28 (52.8) | 23 (43.4) | n.s. |
| ≥ 15 | 19 (35.9) | 19 (35.9) | 19 (35.9) | n.s. |
| ≥ 20 | 13 (24.5) | 16 (30.2) | 13 (24.5) | n.s. |
| ≥ 30 | 9 (17.0 | 8 (15.1) | 7 (13.2) | n.s. |
Data are presented as number (%).
Difference between AL Home and PSG, based on McNemar test for matched pairs.
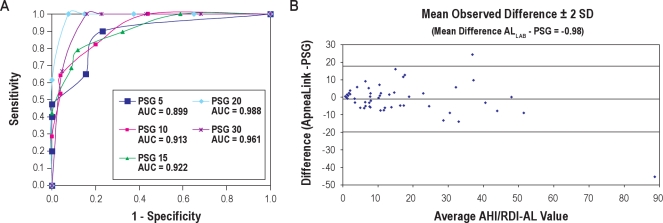
Table 3 shows sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios of the AL Lab compared simultaneously to PSG at the same AHI cut-off for both devices (e.g., if OSA is diagnosed on the basis of AHI ≥ 5 when measured by PSG, this is compared to a cutoff of RDI-AL ≥ 5 for OSA when measured by AL Lab). At AHI ≥ 15, sensitivity for the AL Lab was 79%, while specificity remained high at 88%. The AL Lab test had the highest sensitivity and specificity at AHI values ≥ 20 (sensitivity 100%, specificity 92.5%). Sensitivity-specificity comparisons were plotted graphically using ROC curve analysis. The ROC curve plots sensitivity against 1 – specificity for various RDI-AL cutoffs, yielding a graphical representation of the tradeoff between false negative and false positive rates. Figure 3A represents the ROC curves for AL lab test vs. PSG. The area under the curve (AUC) data demonstrate values ≥ 0.899 for all RDI-AL values, showing high agreement with the PSG. An RDI-AL cutoff of 20 provided the best results. The accuracy for determining this degree of OSA was 88.7%.
Table 3.
Sensitivity and specificity of the AL Lab RDI-AL compared to polysomnography AHI during simultaneous testing
| AHI/RDI-AL | Sensitivity | Specificity | PPV | NPV | LR (+) | LR (-) |
|---|---|---|---|---|---|---|
| ≥ 5 | 90.0 | 76.9 | 93.2 | 71.4 | 3.90 | 0.13 |
| ≥ 10 | 82.1 | 80.0 | 82.1 | 80.0 | 4.11 | 0.22 |
| ≥ 15 | 79.0 | 88.2 | 79.0 | 88.2 | 6.69 | 0.24 |
| ≥ 20 | 100.0 | 92.5 | 81.3 | 100.0 | 13.33 | 0.00 |
| ≥ 30 | 66.7 | 95.5 | 75.0 | 93.3 | 14.82 | 0.35 |
PPV, positive predictive value; NPV, negative predictive value; LR (+), positive likelihood ratio; LR (-), negative likelihood ratio. AHI/RDI-AL are presented as events/hour. Sensitivity, specificity, PPV, and NPV are presented as percent.
Figure 3.
(A)Receiver-operator characteristic curves AL Lab RDI-AL and polysomnography AHI. (B) Bland-Altman Plot of AL Lab RDI-AL and Polysomnography AHI during simsltaneous testing. PSG, polysomnography; AUC, area under the curve.
The Bland-Altman plot is a graphical representation of the observed differences between paired measurements. Using this method, the differences between the 2 techniques (AL Lab and PSG) were plotted against the averages of the 2 techniques. A horizontal line was drawn at the mean difference (−0.98), and at the mean difference ± 2 times the standard deviation of the differences. The mean difference provides an estimate of whether the 2 methods, on average, return similar results. Figure 3B presents the Bland-Altman Plot of RDI-AL/AHI: AL Lab vs. PSG. This plot shows very good agreement between AL lab test and PSG, with a mean difference of −0.98. Pearson correlation was also performed. The AL lab test demonstrated a correlation coefficient of ρ = 0.88 against PSG. The result demonstrates good correlation between the methodologies.
Table 4 shows the sensitivity, specificity, positive predictive value, negative predictive value, and positive and negative likelihood ratios of the AL home test compared with PSG and AL lab test, respectively. For AL Home vs. PSG, the AL Home was most sensitive and specific at an AHI ≥ 20. The AL Home sensitivity was 76.9% and specificity is 92.5%.
Table 4.
| A | ||||||
| AHI/RDI-AL | Sensitivity | Specificity | PPV | NPV | LR (+) | LR (-) |
| ≥ 5 | 67.5 | 76.9 | 90.0 | 43.5 | 2.92 | 0.42 |
| ≥ 10 | 75.0 | 92.0 | 91.3 | 76.7 | 9.38 | 0.27 |
| ≥ 15 | 73.7 | 85.3 | 73.7 | 85.3 | 5.01 | 0.31 |
| ≥ 20 | 76.9 | 92.5 | 76.9 | 92.5 | 10.25 | 0.25 |
| ≥ 30 | 55.6 | 95.5 | 71.4 | 91.3 | 12.36 | 0.46 |
| B | ||||||
| RDI-AL | Sensitivity | Specificity | PPV | NPV | LR (+) | LR (-) |
| ≥ 5 | 66.7 | 71.4 | 86.7 | 43.5 | 2.33 | 0.47 |
| ≥ 10 | 75.0 | 92.0 | 91.3 | 76.7 | 9.38 | 0.27 |
| ≥ 15 | 79.0 | 88.2 | 79.0 | 88.2 | 6.69 | 0.24 |
| ≥ 20 | 75.0 | 97.3 | 92.3 | 90.0 | 27.28 | 0.26 |
| ≥ 30 | 50.0 | 93.3 | 57.1 | 91.3 | 7.46 | 0.54 |
(A) Sensitivity and specificity of the AL Home Test RDI-AL compared to polysomnography AHI. (B) Sensitivity and specificity of the AL Home Test RDI-AL compared to AL Lab Test RDI-AL.
PPV, positive predictive value; NPV, negative predictive value; LR (+), positive likelihood ratio; LR (-), negative likelihood ratio. AHI/RDI-AL are presented as events/hour. Sensitivity, specificity, PPV and NPV are presented as percent.
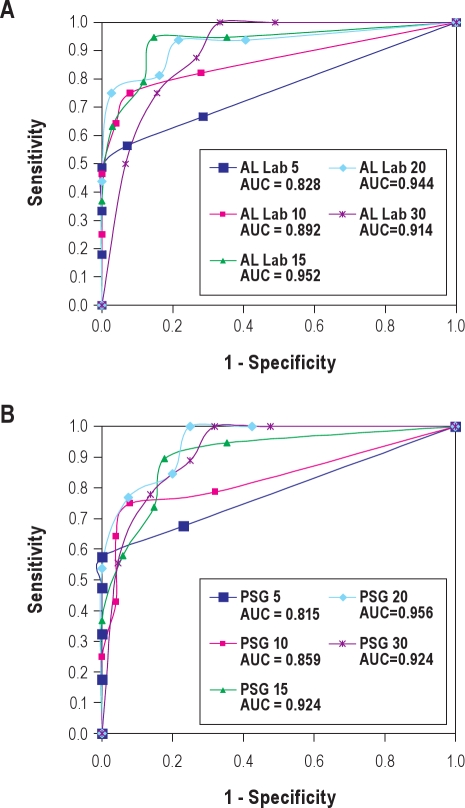
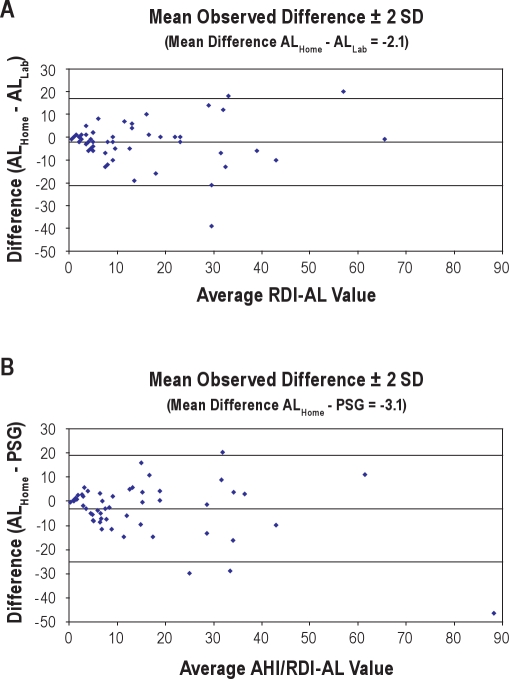
Figure 4A shows the ROC curves and AUC of AL home test vs. AL lab test at all RDI-AL values. In this curve, RDI-AL ≥ 15 provided the best results. AUC analysis demonstrated very good agreement at RDI-AL ≥ 15 and ≥ 20. ROC curves for AL Home versus PSG provided the best results at RDI-AL ≥ 20 (Figure 4B). AUC data also showed very good agreement at RDI-AL ≥ 15. The Bland-Altman Plots of AL Home vs. AL Lab and AL Home vs. PSG had values of −2.1 and −3.1 as the mean differences, respectively. There was good agreement between AL home test and AL lab test and that of PSG (Figures 5A and 5B). The Pearson correlation coefficients of AL home test with PSG and AL lab test were ρ = 0.82 and ρ = 0.81, respectively.
Figure 4.
Receiver-operator characteristic curves
(A) RDI-AL Home versus RDI-AL from Lab study. (B) RDI-AL Home versus AHI from PSG. PSG, polysomnography; AUC, area under the curve.
Figure 5.
Bland-Altman Plots
(A) RDI-AL Home versus RDI-AL Lab. (B) RDI-AL Home versus polysomnography AHI. PSG, polysomnography.
DISCUSSION
In the present study, the diagnostic accuracy of the AL device for use in patients with suspected OSA was investigated in a population referred to a sleep center for assessment of sleep apnea. The study demonstrates that the AL can be used with confidence in clinical practice as an effective diagnostic device for OSA in a population with high pretest probability for moderate or severe OSA.
The AL home test had the highest specificity and sensitivity at an RDI-AL ≥ 20 events per hour. ROC curves for AL Home versus PSG provided the best results at an RDI-AL of 20 events per hour. AUC analysis for the AL home test showed excellent agreement at an RDI-AL ≥ 10 versus AHI from the PSG. The device was able to identify that level of sleep apnea with a sensitivity of 75%. The specificity of the device at a level of RDI-AL ≥ 10 was 92%. This yielded discriminative positive and negative likelihood ratios of 9.38 and 0.27, respectively.
Results obtained from AL lab test versus PSG demonstrated very good agreement at almost all levels of RDI-AL. The sensitivity and specificity for AL lab test at RDI-AL levels of 20 events per hour were excellent (sensitivity 100%, specificity 93%). All these results are consistent with the results of previous validation studies that demonstrated good sensitivity and specificity for the AL device at an RDI-AL of 10 or more and that of 15 or more, when compared with the AHI obtained from PSG.21,22 Recently, a published guideline for portable monitoring (PM) recommended that “PM may be used as an alternative to polysomnography for the diagnosis of OSA in patients with a high pretest probability of moderate to severe OSA.”23 Our data are in support of this recommendation with a sensitivity and specificity at an RDI-AL level of 15 or more, similar to the two other validation studies.21,22 We would therefore recommend initiating positive pressure therapy with a result of 15 or more on a home study.
Within the USA and internationally, there is considerable variation to access for diagnostic PSG.15 In addition to the access issue, PSG is a relatively expensive test requiring highly trained personnel and limited to sleep centers.19 The simplicity of this device, ease of use, and the ability to perform diagnostic testing outside the laboratory for a low cost (approximately $10 including the cannula, batteries, and mailer), make it an attractive alternative to establish a diagnosis of OSA in moderate to high risk populations (such as sleep clinic patients).22
The potential limitations of the current study should be considered. The AL device provides information about airflow via nasal pressure alone. Data related to oxygen desaturation, sleep stage, and respiratory effort were not collected. The AL does not discriminate obstructive from central events. The location of the study (home versus sleep lab) may have had an effect on the results.21,22 The device is also less sensitive for OSA at the lowest levels of the AHI (5-14). This means that in someone with high pretest probability for OSA, a negative AL home study should raise suspicion as a false negative. Repeat home testing with the AL or other limited channel cardiopulmonary monitors for sleep apnea diagnosis should be considered in that case. Further, full PSG in the sleep lab should also be considered in those whose home testing is equivocal or technically limited. Due to the nature of the sleep clinic population, there were few participants with a PSG AHI < 5. This limits the ability to test the specificity (negative in the absence of disease) of the device. The AL lab test had higher sensitivity and specificity than AL home tests. The correlation between PSG and AL lab test was stronger than that of between PSG and AL home test, although both demonstrated a high correlation. Explanations for this finding could include night to night variability in the AHI, differences due to the unattended nature of the home study, and unmeasured differences in sleep quality in the home versus laboratory setting.
CONCLUSION
In sleep clinic populations with high pretest probability for OSA, the AL is an attractive alternative diagnostic tool to in lab PSG. The AL can accurately detect moderate and severe OSA (RDI-AL ≥ 20), whereas sensitivity and specificity are less robust at lower values of the RDI-AL.
DISCLOSURE STATEMENT
This study was funded by a research grant from ResMed Corporation. Dr. Atwood has received research funds from Vapotherm for research not related to this manuscript. He has also received equipment from Respironics, Medcare and Resmed for research studies. He has been a paid consultant to Cephalon and an unpaid advisor to Itamar Medical, Respironics and ResMed. Dr. Drumheller has received research funding from ResMed, the ResMed Foundation and Respironics for work completed with the approval of the University of Pittsburgh Office of Research. Ms. Gordon has been under contract to ResMed for this study and other projects for database design and statistical analysis. Dr Strollo is currently a co-investigator on four NIH grants and two foundation grants (Will Rogers Foundation and ResMed Foundation). He is the principal investigator on one industry grant funded by ResMed, Corp. He has pending support as a principal site investigator for two grants from Philips-Respironics and the Canadian Institutes of Health. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
PJS and CWA were responsible for the general conception and study design. PJS was responsible for the data collection methodology and analysis. BO drafted the manuscript, with input from PJS, CWA, RG, NG, PH, and OD. OD coordinated the study. PH and NG performed the statistical analyses. NG, MP, RG, CWA, and PJS contributed to clinical work related to the study and editing the manuscript. TBR contributed to the preparation and editing of the manuscript. All authors have approved the current version. The authors thank Shari Rogers, CRNP, and Janet Peterson, CRNP, for their assistance with participant recruitment.
REFERENCES
- 1.Javaheri S, Parker T, Liming J, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97:2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 2.Sin D, Fitzgerald F, Parker J, et al. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 3.Robinson G, Stradling J, Davies R. Sleep 6: obstructive sleep apnoea/hypopnoea syndrome and hypertension. Thorax. 2004;59:1089–94. doi: 10.1136/thx.2003.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney C, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton G, Solin P, Naughton M. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–6. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti C, Aldrich M. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Ip M, Lam B, Ng M, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi N, Sorkin J, Katzel L, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 11.Marin J, Carrizo S, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 12.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 13.Fornas C, Ballester E, Arteta E, et al. Measurement of general health status in obstructive sleep apnea hypopnea patients. Sleep. 1995;18:876–9. doi: 10.1093/sleep/18.10.876. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Blustein J, Finn L, et al. Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep. 1997;20:608–13. doi: 10.1093/sleep/20.8.608. [DOI] [PubMed] [Google Scholar]
- 15.Flemons W, Douglas N, Kuna S, et al. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med. 2004;169:668–72. doi: 10.1164/rccm.200308-1124PP. [DOI] [PubMed] [Google Scholar]
- 16.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Ballester E, Solans M, Vila X, et al. Evaluation of a portable respiratory recording device for detecting apnoeas and hypopnoeas in subjects from a general population. Eur Respir J. 2000;16:123–7. doi: 10.1034/j.1399-3003.2000.16a22.x. [DOI] [PubMed] [Google Scholar]
- 18.Dingli K, Coleman E, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 19.Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 20.Nigro C, Dibur E, Aimaretti S, et al. Comparison of the automatic analysis versus the manual scoring from ApneaLink™ device for the diagnosis of obstructive sleep apnoea syndrome. Sleep Breath. 2010 Oct 3; doi: 10.1007/s11325-010-0421-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Teschler T, Weinreich G, et al. [Validation of microMESAM as screening device for sleep disordered breathing] Pneumologie. 2003;57:734–40. doi: 10.1055/s-2003-812423. [DOI] [PubMed] [Google Scholar]
- 22.Erman M, Stewart D, Einhorn D, et al. Validation of the ApneaLink for the screening of sleep apnea: a novel and simple single-channel recording device. J Clin Sleep Med. 2007;3:387–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Collop N, Anderson W, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]