Abstract
Astrocytes provide structural and metabolic support for neuronal networks, but direct evidence demonstrating their active role in complex behaviors is limited. Central respiratory chemosensitivity is an essential mechanism which, via regulation of breathing, maintains constant levels of blood and brain PCO2/pH. We found that astrocytes of the brainstem chemoreceptor areas are highly chemosensitive. They responded to physiological decreases in pH with vigorous elevations in intracellular Ca2+ and release of ATP. ATP propagated astrocytic Ca2+ excitation, activated chemoreceptor neurons, and induced adaptive increases in breathing. Mimicking pH-evoked Ca2+ responses by optogenetic stimulation of astrocytes expressing channelrhodopsin-2 activated chemoreceptor neurons via ATP-dependent mechanism and triggered robust respiratory responses in vivo. This demonstrates a potentially crucial role for brain glial cells in mediating a fundamental physiological reflex.
The role of astrocytes in the brain is by no means limited to just providing structural and metabolic support to neurons. Astrocytes are closely associated with cerebral blood vessels and are thought to regulate cerebrovascular tone adjusting blood supply to match local metabolic demands(1-6). A single astrocyte may enwrap several neuronal somata(7) and make contact with thousands of synapses(8), potentially regulating synaptic strength and information processing(4,9-14). However, direct evidence demonstrating the functional role of astrocytes in complex behaviors is only starting to emerge(15).
Astrocytes provide a vascular-neuronal interface and are in a position to quickly relay blood-borne stimuli to the activities of neuronal networks. Does this have a functional significance for the detection of the relevant stimuli by brain chemosensors which monitor key homeostatic parameters including glucose concentration, pH and PCO2? Here we tested the hypothesis that astrocytes which reside within the respiratory chemoreceptor areas of the brainstem are functional respiratory pH sensors (online text 2.1-2.2).
Because astrocytes are electrically non-excitable, but display Ca2+ excitability (reactive increases in cytosolic [Ca2+]i concentration), we studied their behavior using genetically encoded Ca2+ indicator – Case12(16) (online text 1.3). Case12 was expressed in astrocytes residing at and near the classical chemosensitive area(17) of the ventral surface of the medulla oblongata (VS) of rats using an adenoviral vector with enhanced shortened glial fibrillatory acidic protein (GFAP) promoter (18)(fig. S1, S2; online text 1.3)
In vivo, a 0.2 pH unit decrease on the VS of anesthetized and artificially ventilated rats (n=7) evoked an immediate increase in [Ca2+]i across the field of astrocytes transduced with Case12 (Fig. 1a; movie S1). Note that intracellular acidification reduces Case12 fluorescence and may, therefore, mask the late phases of the response (online text 1.3 and 2.5). Prolonged and sustained astrocytic [Ca2+]i responses were observed more laterally, at the level of the chemosensitive retrotrapezoid nucleus (RTN, Fig 1a). Subsequent histological examination of the chemoresponsive areas confirmed contacts of transduced astrocytes with pia mater and penetrating arterioles (fig. S3).
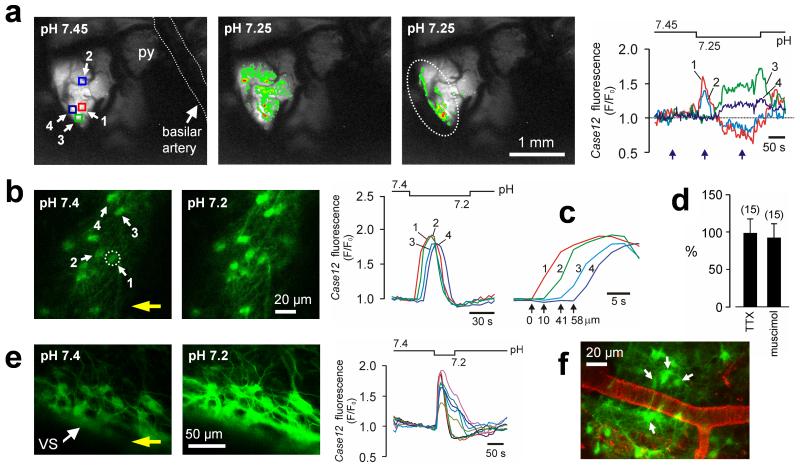
Fig. 1. Astrocytes residing near the VS are exquisitely pH-sensitive.
(a) In vivo imaging of pH-evoked astrocytic [Ca2+]i responses in the ventrolateral area of the brainstem surface transduced with AVV-sGFAP-Case12 in an anesthetized adult rat. Right traces: changes in VS astrocytic [Ca2+]i in response to a decrease in pH. Pseudocolored images (left) were taken at times indicated by blue arrows. Squares indicate regions of interest. Here and elsewhere the pH bar shows when the solution with lower pH is reaching and starts leaving the preparation. Dashed line outlines approximate boundary of the RTN. py – pyramidal tract. (b) VS astrocytes identified by Case12 fluorescence in a horizontal slice from an adult rat in which the ventral medulla was transduced with AVV-sGFAP-Case12. Acidification induces rapid increases in [Ca2+]i as determined by changes in Case12 fluorescence. Two fluorescent images obtained before and at the peak of [Ca2+]i response. Circle indicates an astrocyte responding first to pH change in the field of view. Yellow arrow shows the direction of the flow in the chamber. (c) Zoomed in Ca2+ transients to emphasize the latency differences between responses of individual astrocytes shown in (b). (d) No effect of TTX or muscimol on acidification-induced [Ca2+]i responses in VS astrocytes expressed as percentage of the peak initial response. Numbers of individual astrocytes sampled from 3-5 separate experiments are given in brackets. (e) Acidification-evoked [Ca2+]i responses in VS astrocytes of organotypic brainstem slice transduced with AVV-sGFAP-Case12. (f) VS vasculature visualized with lectin in a horizontal slice prepared from an AVV-sGFAP-Case12–transduced rat. Arrows point at pH-responsive astrocytes.
Propagating Ca2+ excitation of ventral medullary astrocytes in response to acidification was also observed in different in vitro preparations such as acute horizontal brainstem slices of adult rats (n = 56 slices; Fig. 1b,c; movie S2), organotypic brainstem slice cultures (n = 114 slices; Fig. 1e, fig S4; movie S3) and dissociated VS cell cultures (n = 19 cultures; fig. S5). In brainstem slices of adult rats, in which blood vessels were visualized with lectin, many pH-sensitive astrocytes were found to be located adjacent to the VS vasculature (Fig. 1f).
Acidification-induced Ca2+ excitation of VS astrocytes is unlikely to be secondary to increased activity of local neurons. To minimize neuronal influences, tetrodotoxin (blocker of voltage-activated sodium channels) and muscimol (potent GABAA receptor agonist) were applied. Both drugs were found to silence RTN neurons (the only known type of pH-responsive neurons in this area; see below), but neither drug affected pH-induced [Ca2+]i responses monitored using Case12 or Rhod-2 fluorescence (Fig. 1d; fig. S6). Furthermore, activation of RTN chemoreceptor neurons (n = 11) by current injection failed to trigger [Ca2+]i elevations even in the immediately adjacent astrocytes (fig. S7).
High pH-sensitivity is a distinctive feature of astrocytes residing near the VS. Astrocytes from the cerebral cortex or dorsal brainstem generated no [Ca2+]i signals in response to acidification (fig S8). Moreover, low magnification Rhod-2 imaging throughout the whole brainstem cross-section, revealed that chemosensory stimulation evokes Ca2+ responses originating and propagating only near the VS (n = 8; fig. S9; movie S4).
Propagation of pH-evoked Ca2+ excitation among ventral medullary astrocytes is largely mediated by the actions of ATP (online text 2.3). A decrease in pH from 7.4 to 7.2 (at constant [HCO3−] and nominal PCO2) triggers sustained ATP release (peak increase 1.0 ± 0.3 μM, n = 8) from the VS in horizontal slices of young adult rats (Fig. 2a), confirming our previous findings(19). Blockade of ATP signaling dramatically diminishes pH-evoked astrocytic responses – in cultured and acute brainstem slices, pH-evoked astrocytic Ca2+ responses were abolished in the presence of ATP hydrolyzing enzyme apyrase (25 U ml−1) (Fig. 2b, 2e). Furthermore, ATP receptor antagonists MRS2179 (3 μM), PPADS (5 μM) or TNP-ATP (20 nM) reduced acidification-induced astrocytic [Ca2+]i signals by 82% (p = 0.005; Fig. 2c), 80% (p = 0.005), and 83% (p = 0.048), respectively (Fig. 2e). In line with the absence of acidification-induced [Ca2+]i responses in Ca2+-free medium (Fig. 2e; fig. S10), this pharmacological profile suggests the involvement of ionotropic P2X receptors (online text 2.4).
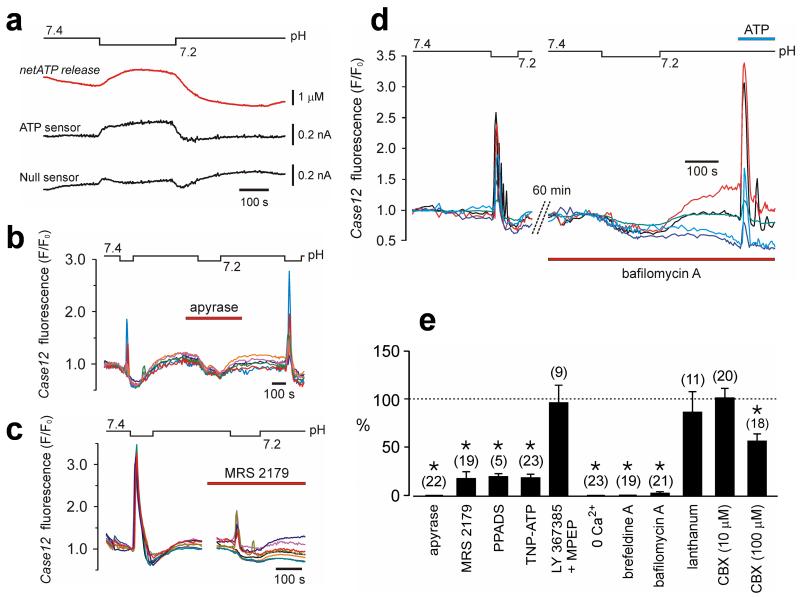
Fig. 2. Exocytotic release of ATP propagates pH-induced Ca2+ excitation among VS astrocytes.
(a) A 0.2 unit decrease in pH induces sustained ATP release from the VS as detected with biosensors placed on the pia mater in horizontal slices prepared from adult rats. “net ATP” trace represents the difference in signal between ATP and null (control) sensor currents. (b) Apyrase abolishes pH-evoked [Ca2+]i responses in VS astrocytes. Traces illustrate the effects of apyrase on pH-induced changes in Case12 fluorescence of six individual astrocytes (adult rat slice preparation). Note the dip in fluorescence due to acidification-induced quenching of Case12 fluorescence. (c) The effect of MRS2179 on acidification-induced [Ca2+]i responses of eight individual VS astrocytes (organotypic brainstem slice). (d) Bafilomycin A abolishes pH-evoked Ca2+ excitation of VS astrocytes (five individual astrocytes in slice preparation of an adult rat). (e) The effects of apyrase, P2 receptor antagonists, mGlu1a and mGlu5 receptor antagonists (LY367385 and MPEP, 100 μM each), blockers of pannexin/connexin hemichannels and gap junctions – lanthanum (100 μM) and carbenoxolone (CBX), or inhibitors of exocytotic mechanisms on acidification-induced [Ca2+]i responses in VS astrocytes expressed as the percentage of the initial response. Numbers of individual astrocytes sampled from 3-5 separate experiments are given in brackets (*p < 0.05).
In response to a decrease in pH, the VS astrocytes spread Ca2+ excitation partially via gap junctions and predominantly by exocytotic release of ATP (online text 2.3). Gap junction blocker carbenoxolone at high concentration (100 μ) was only partially effective in reducing astrocytic [Ca2+]i responses (by 43%; p = 0.001; Fig. 2e and fig. S11). In contrast, brefeldine A (vesicular trafficking inhibitor; 50 μM) or bafilomycin A (vesicular H+-ATPase inhibitor; 2 μM) both effectively abolished acidification-induced Ca2+ excitation of VS astrocytes (Fig 2d and 2e). Neither brefeldine A nor bafilomycin A prevented responses of VS astrocytes to applied ATP (Fig. 2d), indicating that astrocytic reactivity to ATP was not affected by these compounds.
Released ATP also links astrocytic excitation to the increased activity of medullary chemoreceptor neurons. The RTN, adjacent to the VS, has been advocated to play a key role in central respiratory chemosensitivity(20) (online text 2.7). RTN neurons respond to changes in pH, reside within the marginal VS glial layer or have extensive dendritic projections to it, project to the respiratory network and stimulate breathing upon activation (20, 21).
RTN neurons characteristically express transcription factor Phox2b and were fluorescently labeled with EGFP in organotypic slices using an adenoviral vector with PRSx8 promoter (Phox2b-activated promoter(22)) (Fig. 3a). A decrease in pH led to a reversible depolarization of all recorded RTN neurons (n = 8; Fig. 3a,3b). Caudally located catecholaminergic C1 neurons were insensitive to pH (fig. S12). MRS2179 (10 μM; n = 8; Fig. 3a,3b) or apyrase (25 U ml−1; n = 3, fig. S13) had no effect on resting membrane potential but markedly reduced pH-evoked depolarizations of RTN neurons (p = 0.014 and p = 0.04, respectively). In a separate experiment, [Ca2+]i responses of RTN neurons were visualized using a genetically encoded Ca2+ sensor TN-XXL(23) expressed under PRSx8 promoter control (Fig. 3c). Again, acidification-induced [Ca2+]i elevations in RTN neurons were suppressed by MRS2179 (3 μM, n=9, p=0.008; Fig. 3c, 3d), confirming that their pH-sensitivity is largely mediated by prior release of ATP (online text 2.7).
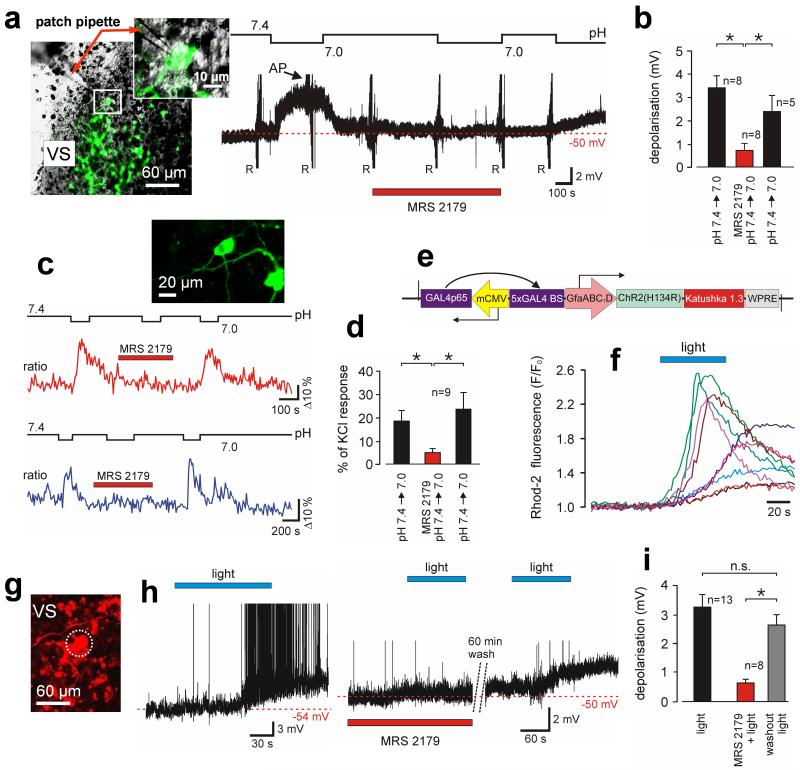
Fig. 3. ATP mediates responses of chemoreceptor neurons to decreases in pH or following selective light-induced Ca2+ excitation of adjacent astrocytes.
(a) Left: image of the ventral aspect of an organotypic brainstem slice showing EGFP labeled Phox2b-expressing RTN neurons one of which is patch clamped. Right: time-condensed record of the membrane potential of an RTN neuron responding to acidification in the absence and presence of MRS2179. AP - action potentials (truncated). R – resistance tests using current pulses. (b) Summary of MRS2179 effect on pH-evoked depolarizations in RTN neurons. (c) Effect of MRS2179 on acidification-induced [Ca2+]i responses of RTN neurons from two different experiments (ratiometric imaging using TN-XXL). Inset: RTN neurons expressing TN-XXL. (d) Summary data showing significant effect of MRS2179 on pH-evoked [Ca2+]i responses of RTN neurons. (e) Layout of AVV-sGFAP-ChR2(H134R)-Katushka1.3. (f) Primary astrocytes displaying increases in [Ca2+]i in response to 470 nm light. (g) Ventral aspect of the organotypic slice showing a recorded DsRed2-labeled RTN neuron surrounded by ChR2(H134R)-Katushka1.3-expressing astrocytes. (h) Membrane potential of two different RTN neurons illustrating their responses to light activation of adjacent ChR2(H134R)-expressing astrocytes in the absence (left), presence (middle) or after washout (right) of MRS2179. (i) Effects of MRS2179 on depolarizations of RTN neurones evoked by optogenetic activation of neighboring astrocytes (*p<0.05).
In order to mimic Ca2+ excitation of astrocytes we generated an adenoviral vector where a mutant of the light-sensitive channelrhodopsin-2 (ChR2-H134R(24)) is fused to a far red-shifted fluorescent protein Katushka1.3 (25) and expressed using enhanced GFAP promoter (18) (Fig. 3e, fig. S14). In primary cultures and in brainstem slices of adult rats astrocytes transduced with this construct displayed robust increases in [Ca2+]i in response to 470 nm light (Fig. 3f, fig. S15-S17).
Optogenetic activation of VS astrocytes transduced with AVV-sGFAP-ChR2(H134R)-Katushka1.3 in organotypic brainstem slices triggered immediate ATP release (fig. S18) and evoked long-lasting (28 ± 9 min) depolarizations of all recorded DsRed-labeled RTN neurons (n = 13; Fig. 3g-i). These depolarizations were reversibly prevented in the presence of MRS2179 (10 μM) (p = 0.002; Fig. 3h, 3i).
To determine the functional significance of Ca2+ excitation of VS astrocytes, we conducted experiments in anaesthetized, vagotomized and artificially ventilated rats transduced with AVV-sGFAP-ChR2(H134R)-Katushka1.3 in the ventral areas of the brainstem. The VS was exposed and phrenic nerve activity was recorded to monitor central respiratory drive. Unilateral illumination (445 nm) of the transduced side of the VS triggered robust respiratory activity from hypocapnic apnea in all 8 animals tested (Fig. 4a, movie S5). An increase in phrenic nerve amplitude was also observed following optogenetic activation of VS astrocytes in 6 animals breathing normally (p<0.01; Fig. 4b). MRS2179 prevented the respiratory effects of optogenetic stimulation of astrocytes (n = 7, p=0.006; Fig. 4c, 4d). No responses were induced following illumination of the brainstem side not expressing the transgene. Histological analysis of the areas stimulation of which evoked increases in breathing (Fig. 4e) revealed close association of transduced astrocytes with the VS and Phox2b-expressing neurons (Fig. 4f).
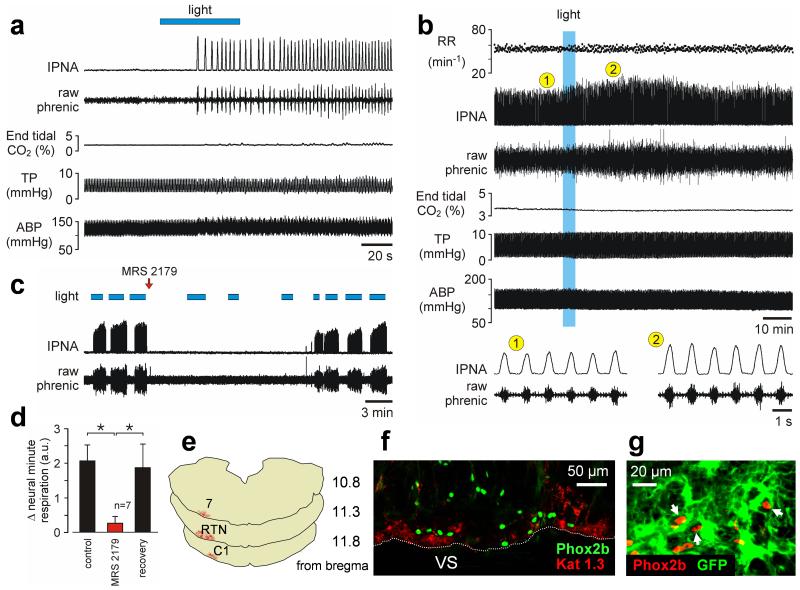
Fig. 4. Optogenetic activation of VS astrocytes stimulates breathing in vivo.
(a) Unilateral photostimulation of VS astrocytes expressing ChR2(H134R)-Katushka1.3 is sufficient to trigger respiratory activity from hypocapnic apnea in an anesthetized rat. Hypocapnic apnea was induced by mechanical hyperventilation to reduce arterial levels of PCO2/[H+] below the apneic threshold. IPNA – integrated phrenic nerve activity. TP – tracheal pressure. ABP – arterial blood pressure. (b) Lasting effect of light activation of VS astrocytes in an animal breathing normally. RR – respiratory rate. (c) Time-condensed record illustrating effects of repeated stimulations of VS astrocytes on phrenic nerve activity before and after a single application of MRS2179 (100 μM, 20 μl) on the VS. Note spontaneous recovery of the response over time. (d) Summary data of MRS2179 effect on the increases in neural minute respiration (the product of phrenic frequency and amplitude) evoked by light-activation of VS astrocytes (*p < 0.05). (e) Rostro-caudal distribution of astrocytes expressing ChR2(H134R)-Katushka1.3 in the brainstem of the rat from the experiment shown in (a). 7 – facial nucleus. RTN – retrotrapezoid nucleus. C1 - catecholaminergic cell group. (f) ChR2(H134R)-Katushka1.3 (Kat 1.3) expression in astrocytes is identified by red fluorescence distributed near the VS in close association with Phox2b-immunoreactive neurons (green nuclei). Coronal brainstem section. (g) Phox2b-expressing chemoreceptor RTN neurons (red nuclei) embedded in the astrocytic network (astrocytes are transduced with Case12 in this example to reveal their morphology).
Although previous reports suggested that astrocytes could be potentially important for chemoreception(26,27), it was generally believed that central respiratory chemosensory function is a property of one or several highly specialized neuronal populations located in the medulla oblongata and pons. While our data do not exclude the existence of such neurons, we demonstrate that astrocytes may fulfill an equivalent role. Indeed, astrocytes are intimately associated with blood vessels supplying the lower brainstem – surface pial arteries rest on glia limitans while penetrating arterioles and capillaries are enwrapped by astrocytic end-feet (fig. S3). Therefore, anatomically, astrocytes are ideally positioned to sense the composition of the arterial blood entering the brain and integrate this information with PCO2/[H+] levels of brain parenchyma. They have the unique ability to sense physiological changes in PCO2/[H+] and then impart these changes to respiratory neuronal network to modify breathing patterns and adjust lung ventilation accordingly (although, the initial chemosensory event linking a decrease in pH with VS astrocytic [Ca2+]i response as yet remains unknown, online text 2.6). This identifies astroglia as an important component of one of the most fundamental mammalian homeostatic reflexes and provides direct evidence for an active role of astrocytes in functionally relevant information processing in the central nervous system.
Supplementary Material
Acknowledgments
We are grateful to The Wellcome Trust (grant no. 079040) and British Heart Foundation for financial support.
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Materials and Methods
Supporting Text: Extended Discussion
Figs. S1 to S26
Supporting Table S1
Supporting References S1 to S51
Supporting Movies S1 to S5
References and Notes
- 1.Fields RD, Stevens-Graham B. Science. 2002;298:556. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zonta M, et al. Nat. Neurosci. 2003;6:43. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 3.Takano T, et al. Nat. Neurosci. 2006;9:260. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 4.Haydon PG, Carmignoto G. Physiol Reviews. 2006;86:1009. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C, Nedergaard M. Nat. Neurosci. 2007;10:1369. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 6.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Nature. 2008;456:745. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. J. Neurosci. 2007;27:6473. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushong EA, Martone ME, Jones YZ, Ellisman MH. J. Neurosci. 2002;22:183. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J, Jiang L, Goldman SA, Nedergaard M. Nat. Neurosci. 1998;1:683. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 10.Pascual O, et al. Science. 2005;310:113. [Google Scholar]
- 11.Wang X, et al. Nat. Neurosci. 2006;9:816. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 12.Jourdain P, et al. Nat. Neurosci. 2007;10:331. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 13.Schummers J, Yu H, Sur M. Science. 2008;320:1638. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 14.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Nature. 2010;463:232. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halassa MM, et al. Neuron. 2009;61:213. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souslova EA, et al. BMC Biotechnology. 2007;7:37. doi: 10.1186/1472-6750-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeschcke HH. J. Physiol. 1982;332:1. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B, Paton JF, Kasparov S. BMC. Biotechnol. 2008;8:49. doi: 10.1186/1472-6750-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gourine AV, Llaudet E, Dale N, Spyer KM. Nature. 2005;436:108. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- 20.Mulkey DK, et al. Nat. Neurosci. 2004;7:1360. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 21.Abbott SB, et al. J. Neurosci. 2009;29:5806. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang DY, Carlezon WA, Jr., Isacson O, Kim KS. Human Gene Therapy. 2001;12:1731. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- 23.Mank M, et al. Nat. Methods. 2008;5:805. doi: 10.1038/nmeth.1243. [DOI] [PubMed] [Google Scholar]
- 24.Gradinaru V, et al. J. Neurosci. 2007;27:14231. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shcherbo D, et al. Biochem. J. 2009;418:567. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Pflugers Archive. 1978;376:229. doi: 10.1007/BF00584955. [DOI] [PubMed] [Google Scholar]
- 27.Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Am. J. Physiol. 2005;289:R851. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.