Abstract
Cytochrome P450 oxidoreductase (CYPOR) is a microsomal electron-transferring enzyme containing both FAD and FMN as co-factors, which provides the reducing equivalents to various redox partners, such as cytochromes P450 (CYPs), heme oxygenase (HO), cytochrome b5 and squalene monooxygenase. Human patients with severe forms of CYPOR mutation show bone defects such as cranio- and humeroradial synostoses and long bone fractures, known as Antley-Bixler-like Syndrome (ABS). To elucidate the role of CYPOR in bone, we knocked-down CYPOR in multiple osteoblast cell lines using RNAi technology. In this study, knock-down of CYPOR decreased the expression of Connexin43 (Cx43), known to play a critical role in bone formation, modeling, and remodeling. Knock-down of CYPOR also decreased Gap Junction Intercellular Communication (GJIC) and hemichannel activity. Promoter luciferase assays revealed that the decrease in expression of Cx43 in CYPOR knock-down cells was due to transcriptional repression. Primary osteoblasts isolated from bone specific Por knock-down mice calvaria confirmed the findings in the cell lines. Taken together, our study provides novel insights into the regulation of gap junction function by CYPOR and suggests that Cx43 may play an important role(s) in CYPOR-mediated bone defects seen in patients.
Keywords: NADPH-cytochrome P450 reductase (CYPOR), Connexins, Gap Junctions, Hemichannels, Osteoblasts
Introduction
Cytochrome P450 oxidoreductase (CYPOR), an electron-transferring protein, donates reducing equivalents to different enzymes, including the microsomal cytochromes P450 (CYPs) [1,2,3], heme oxygenases-1 and -2 [4] [5], squalene monooxygenase [6] and fatty acid elongase [7]. There are over four dozen CYPs expressed in the endoplasmic reticulum, which are variously involved in cholesterol, steroid, drug and xenobiotic metabolism. Heme oxygenase (HO) maintains heme homeostasis by degrading heme into biliverdin, iron and carbon monoxide. Only heme oxygenase-1 (HO-1) is inducible, however, and it has been shown to respond to oxidative stress and inflammatory stimuli. Squalene monooxygenase is involved in the cholesterol biosynthesis pathway, and its dependence upon CYPOR has been demonstrated [8] The various electron-accepting partners of CYPOR suggest that it is an important player in a multitude of physiological and toxicological processes.
A critical role for CYPOR in embryogenesis and development is evidenced by the expression of the protein at the two-cell stage during embryonic development, and is also supported by the embryonic lethality of CYPOR knock-out mice. Examination of CYPOR knock-out embryos showed impairment in overall development of the neural tube, heart, eye, and limb buds. Tissue-specific CYPOR knock-out mice were developed using the Cre/loxP system to circumvent the lethality caused by global knock-outs. Although these mice grew normally and were fertile, they exhibited defective steroid and fatty acid metabolism and altered liver toxicity towards different drugs, which can be attributed to defects in CYPOR/CYP-mediated reactions [9,10]. Genetic screening of different populations with bone abnormalities and/or defective steroid metabolism has found 26 missense mutations in the CYPOR gene. Severe forms of CYPOR polymorphisms in humans lead to bone deformities, such as craniosynostoses, midface hypoplasia, long bone fractures, and femoral bowing, similar to the genetic disorder Antley-Bixler syndrome (ABS), along with abnormal genitalia and impaired steroidogenesis[11,12,13,14].
Various proteins and signaling molecules play critical roles in bone development, modeling, and remodeling, including connexin 43 (Cx43), a major gap junction-forming protein, that is abundantly expressed in osteoblasts and osteocytes. Gap junctions form the only known intercellular channels that allow direct communication between neighboring cells. Six connexin protein subunits form a hemichannel or connexon, and hemichannels from opposing surfaces of two cells form a gap junction channel. Gap junctions facilitate networking among the different cells of the bone, especially in osteocytes and osteoblasts, by allowing the passage of small molecules less than 1000 Daltons between cells [15]. Mutations in Cx43 affecting GJIC have been shown to cause Occulo-Dento-Digital Dysplasia (ODDD), which is an autosomal dominant human disease showing defects in eye and bone development, including skull and digits [16]. In vitro studies also showed that Cx43 mutations affecting GJIC and hemichannel function caused osteoblast dysfunction [17]. Connexin 43 null mice exhibit profound mineralization defects of skeletal elements derived from both endochondral and intramembranous ossification [15]. The expression of Cx43 and GJIC increases during osteoblast differentiation, and several studies suggest that Cx43 expression and GJIC modulate many osteoblast-specific genes such as ALP, collagen 1a1, bone sialoprotein and osteocalcin by positively regulating gene transcription [18,19,20]. Mice with osteoblast-specific deletion of Cx43 show lower bone mass and remain osteopenic with age [21].
In order to understand the role of CYPOR in bone development and its relationship with Cx43, we knocked down CYPOR by RNAi technology in osteoblast cell lines, MG63 and 2T3. We show for the first time that CYPOR can regulate Cx43 expression at the transcription level and that decrease in CYPOR reduces gap junctional intercellular communication, as well as hemi channel activity.
2. Materials and Methods
2.1 Cell Culture
MG63 cells, osteoblast-like cells derived from human osteosarcoma, were cultured in Dulbecco’s modified Eagle’s medium (DMEM), 2T3 cells, immortalized murine osteoblasts and mouse calvarial osteoblasts were cultured in MEM, with 10% fetal bovine serum (FBS) at 37°C under a humidified atmosphere of 5% CO2 in the presence of penicillin (1000u/ml) and streptomycin (100 µg/ml) and supplemented with glutamine. Cultures were maintained in T-75 flasks and media were replaced with fresh media every three days. Experiments were usually carried out in 35-mm dishes.
2.2 shRNA Stable Knock-down
Mission shRNA bacterial glycerol stock, mouse and human TRCN42140, TRCN46527 (Sigma Aldrich, St. Louis, MO) was used for downstream purification of lentiviral vector pLKO.1 (Puromycin-resistant) encoding the shRNA for CYPOR. pLKO.1 along with pMD2G and psPAX, envelope and packaging vectors respectively, were transfected into 293T cells using lipofectamine (Invitrogen, Carlsbad, CA) and viral supernatant was generated. MG63 cells and 293T cells were then infected with the viral supernatant and stable cells were selected with puromycin.
2.3 Immunoblot Analysis
Total cell lysates were collected and separated by SDS PAGE in 4–15% Ready Gels (Bio-Rad Laboratories, CA). The gels were electroblotted onto a PVDF membrane and proteins were detected by using appropriate primary antibodies and peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected using enhanced chemiluminescence (ECL) reagent (Perkin Elmer, Waltham, MA).
2.4 Immunocytochemistry
Control and CYPOR knock-down cells were grown and fixed on coverslips. Cells were exposed to Cx43 antibody (Zymed, Invitrogen, Carlsbad, CA) followed by goat anti-rabbit secondary antibody conjugated to Alexafluor 488 (Molecular probes, Eugene, OR). Coverslips were rinsed and mounted with prolong antifade kit (Molecular Probes, Eugene, OR) and viewed at 63X oil immersion using a Ziess Axiscop 2 HBO 100. Cells incubated with secondary antibody alone were used as the control for primary antibody specificity.
2.5 Dye Transfer Assay for GJIC (Microinjection)
A confluent 35-mm dish was placed in a temperature-and CO2-controlled chamber attached to the Nikon scope (Nikon TE-2000-E). Alexafluor 488 dye (0.25 mM) was microinjected into the cells using an Eppendorf Injectman NI2. Images of dye transfer from injected cell to surrounding cells are taken every 30 seconds after injection using a 1-second exposure on a Nikon TE2000 microscope. The data was analyzed using MetaMorph software version 7.1.4.0.
2.6 Dye Uptake Assay for Hemichannel Activity
2T3 cells plated at 50% confluency in 60 mm plates were rinsed with recording medium (MEM with 10 mM HEPES) and 200 µl of recording medium containing 50 µM ethidium bromide was dropped onto the cells with a pipette for mechanical stimulation. After 5 minutes, cells were rinsed with PBS and fixed with 4% paraformaldehyde. At least 4 different fields with fluorescent cells were imaged with a 10× objective in an inverted microscope (Carl Zeiss) with a rhodamine filter. The average pixel intensity of 15 random cells from each image was analyzed using ImageJ software (NIH).
2.7 Luciferase Assay
Wild Type and CYPOR knock-out 2T3 cells plated in 12-well plates were co-transfected in triplicates with vectors containing the Cx43 promoter (generous gift from Dr. Jean X. Jiang, UTHSCSA, San Antonio, TX) and β-galactosidase or pGL3 basic and β-galactosidase, respectively. Cells were lysed after 48 hours with the lysis buffer (Promega) and the luciferase assay was performed on the supernatant with the Luciferase Assay reagent (Invitrogen). The β-galactosidase activity was measured spectrophotometrically as an internal control using ortho-nitrophenyl-β-D-galactopyranoside (ONPG) as substrate. Relative luciferase activity was calculated after normalizing against β-Gal by dividing the luciferase activity with β-galactosidase activity.
2.8 Isolation of Primary Osteoblasts from Mouse Calvaria
Osteoblasts from mouse calvaria were isolated as in [22] with modifications. In short, calvaria from 3- to 5-day-old mouse pups were surgically removed and digested in 0.5% trypsin and 0.2% collagenase II by shaking at 37°C in a humidified 5% CO2 incubator for 15 min. each time. Cells were collected after discarding the first two digests and cultured in α MEM with 10% FBS with penicillin and streptomycin. The animals were maintained in the animal care facility in accordance with the protocols approved by the animal care committee at the University of Texas Health Science Center at San Antonio.
3. Results
3.1. Effect of CYPOR Knock-down on Connexin 43 (Cx43) Protein Levels
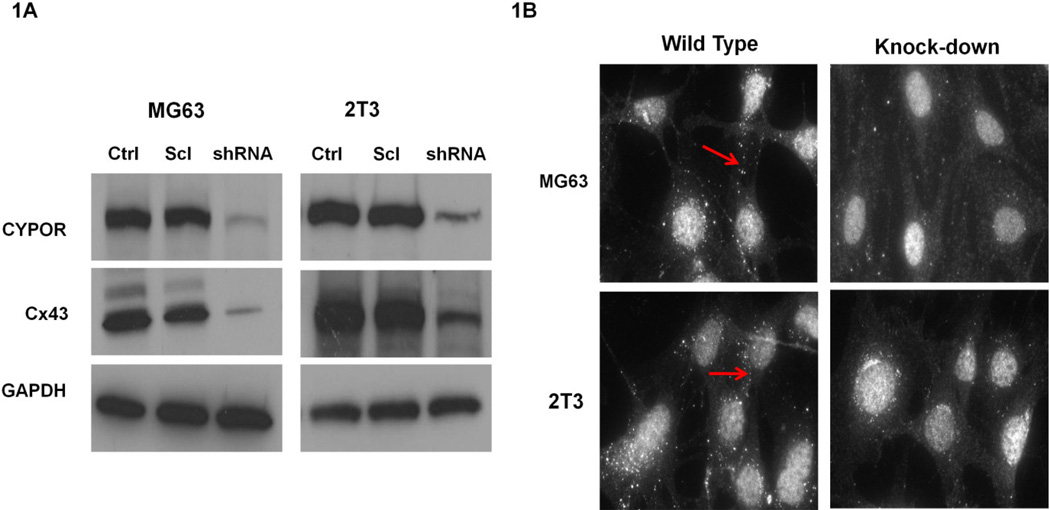
To investigate the effect of CYPOR on Cx43 expression, stable CYPOR knock-down MG63 (human osteosarcoma cell line) and 2T3 cells (immortalized murine osteoblasts) were generated by lentiviral transduction. Immunoblots (Figure 1A) showed decreased Connexin43 (Cx43) protein expression on CYPOR knock-down with shRNA in both MG63 and 2T3 cell lines. Control (cells transfected with just the cloning vector) and Scrambled (cells transfected with the scrambled shRNA-containing vector) did not show any decrease in the protein levels of CYPOR, or Connexin 43 (Cx43). Immunocytochemistry (Figure 1B) showed decreased gap junction plaque formation on the membranes of (arrows) MG63 and 2T3 cells when CYPOR was knocked-down compared to the control cells. Together these data suggest that knock-down of CYPOR causes a decrease in the Connexin 43 (Cx43) protein levels in osteoblasts from two different sources.
Figure 1.
1A. Immunoblots showing the knock-down of CYPOR and a concurrent reduction in the Connexin 43 (Cx43) levels in stably generated CYPOR knock-down 2T3 and MG63 cell lines. (shRNA- cells treated with POR shRNA vector, Ctrl – cells with cloning vector, Scl – Scrambled shRNA vector). 1B. Immunofluorescence images of gap junction plaques on the cell membranes of MG63 cells and 2T3 cells. The CYPOR knock-down MG63 and 2T3 cells show decreased membrane-localized Cx43 gap junction plaques and intracellular Cx43 compared to the wild type cells (arrows)
3.2. Effect of CYPOR Knock-down on Gap Junction Intercellular Communication (GJIC) and Hemichannel Activity
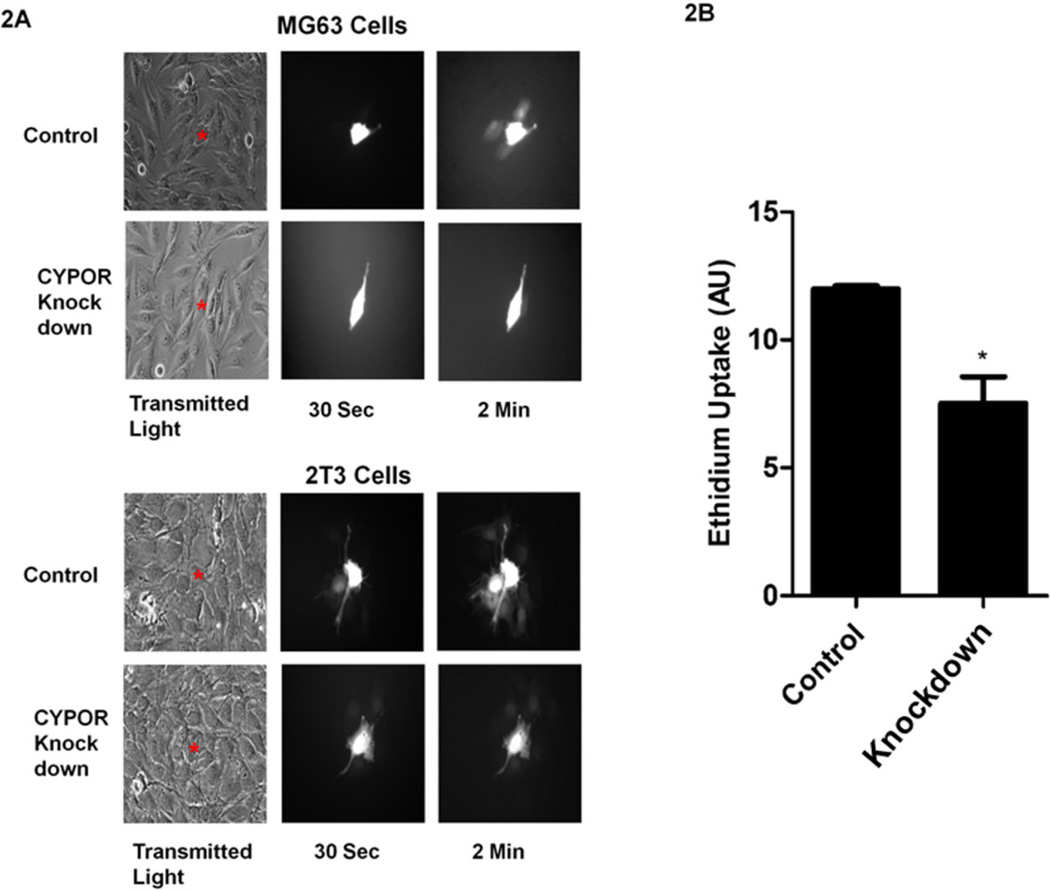
Gap Junction Intercellular Communication (GJIC) has been hypothesized to play a critical role in the coordination of bone development by the ability of gap junctions to permit diffusion of ions, metabolites and small signaling molecules. To measure functional activity of Cx43, GJIC was measured in stable CYPOR knock-down MG63 and 2T3 cells to investigate how CYPOR regulates intercellular communication through Cx43 channels. Transfer of the dye, Alexa-488 (MW 640 Daltons), from the injected cell (donor cell) to the surrounding cells (recipient cells) was monitored in both wild type and CYPOR knock-down MG63 and 2T3 cells by taking images over time (Figure 2A). Wild Type MG63 and 2T3 cells showed a rapid transfer of Alexa-488 from the donor cells to the recipient cells compared to the knock-down cells, suggesting that GJIC is reduced in cells in which CYPOR is knocked-down by shRNA. Unpaired hemichannels can also communicate with the extracellular milieu providing an alternative mechanism for connexin function. Hemichannels have been shown to regulate the release of NAD+, ATP and prostaglandinE2 (PGE2) [23,24]. To test for hemichannel function, ethidium bromide dye uptake assay performed on wild-type and CYPOR knock-down 2T3 cells (Figure 2B). The CYPOR knock-down cells showed a 30% reduction in hemichannel activity compared to the wild-type 2T3 cells. Taken together, these data show that the decreased expression of Cx43 protein upon CYPOR knock-down affects both the gap junction as well as the hemichannel function.
Figure 2.
2A. Dye transfer analysis by microinjection of Alexa 488 to test for GJIC in wild-type and CYPOR knock-down MG63 (upper panel) and wild-type and CYPOR knock-down 2T3 cells (lower panel). Increased dye transfer is seen in the wild-type cells compared to the CYPOR knock-down cells confirming the loss of gap junctions and GJIC in CYPOR knock-out cells.(* injected cell, representative images, n=12). 2B. Dye-uptake assay to test for hemichannel function in wild type and CYPOR knock-down 2T3 cells. CYPOR knock-down cells show decreased ethidium bromide uptake and hence decreased hemichannel function compared to the wild-type cells. (n=3)
3.3. Luciferase Assay to Determine Effect of CYPOR knock-down on Connexin43 (Cx43) at the Transcriptional Level
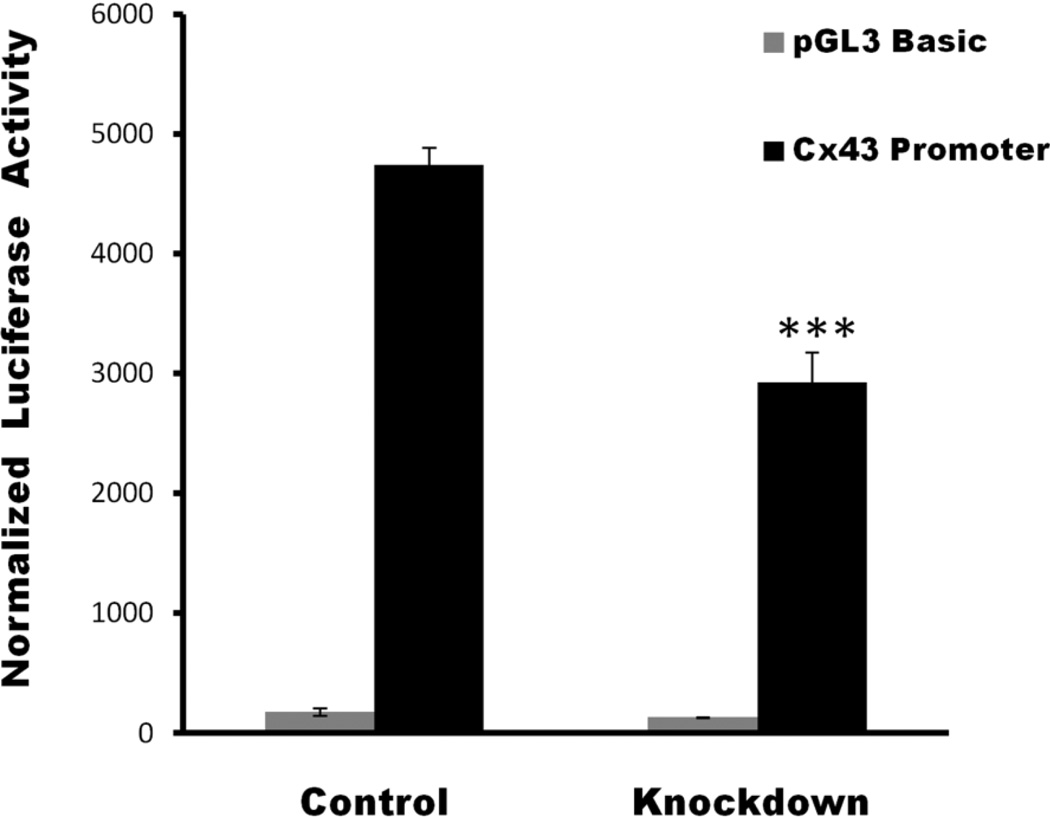
Decreased expression of Cx43 upon CYPOR knock-down could be at the level of transcription or at the protein level. To understand if the knock-down of CYPOR affects Cx43 production at the transcriptional level, luciferase activity was assayed in wild type and CYPOR knock-out 2T3 cells after transfecting the cells with either the vector containing Cx43 promoter or a pGL3 basic vector. Relative luciferase activity (Figure 3) calculated after normalizing against β-Galactosidase showed a significant decrease (1.5-fold) in the promoter activity of the 2T3 cells in which CYPOR was knocked down compared to the wild type cells. This shows that the decrease in Cx43 upon CYPOR knock-down is at least partially due to regulation at the transcriptional level.
Figure 3.
The relative luciferase activity in CYPOR knock-down 2T3 cells (KD) compared to wild type 2T3 cells. CYPOR knock-down 2T3 cells show a 1.5 fold decrease in luciferase activity compared to wild-type 2T3 cells, indicating a decrease in the transcription of Cx43 mRNA in CYPOR knock-down cells compared to the wild type cells. (n=3)
3.4. Expression of Cx43 in CYPOR Knock-Out Mouse Primary Calvarial Osteoblasts
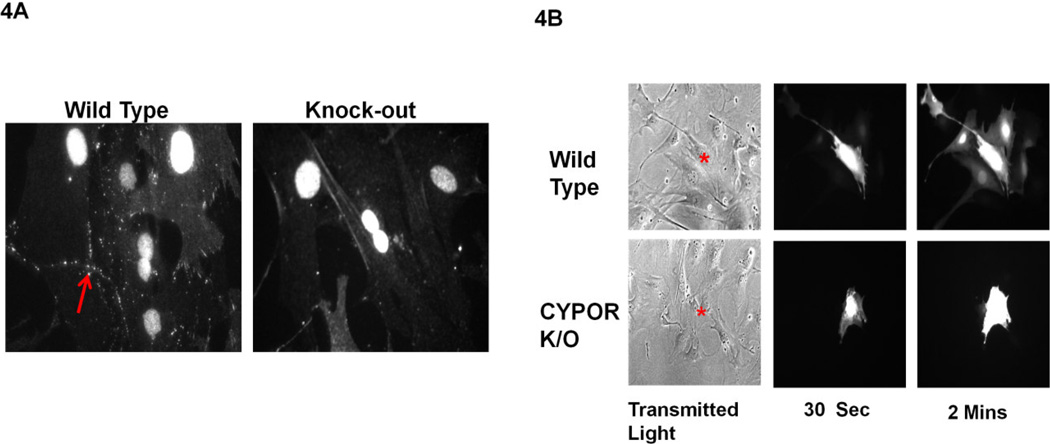
To confirm that the decreased Cx43 level in CYPOR knock-down cells was not merely a characteristic of transformed cell lines, Cx43 levels as well as GJIC were measured in primary osteoblast cells isolated from mouse calvaria of bone-specific knock-out of CYPOR mice generated by crossing Dermo Cre and POR lox/lox mice (unpublished data). The expression of Cx43 was verified by immunofluorescence using antibodies against Cx43. Reduced gap junction plaque formation was observed in the osteoblasts isolated from bone specific knock-out mouse compared to its littermate wild-type control cells (Figure 4A). GJIC was also measured in these cells by dye transfer to determine the effect of CYPOR knock-down on intercellular communication; this assay confirmed that dye transfer from donor cell to surrounding acceptor cells was negligible as compared to the wild type cells (Figure 4B). Decreased gap junction plaque formation and dye transfer from bone-specific knock-out of CYPOR mice compared to wild-type cells shows that the effect of CYPOR on Cx43 expression is also observed in primary cells.
Figure 4.
4A. Immunofluorescent staining for Cx43 in wild-type and CYPOR knock-out mouse primary calvarial osteoblasts. The CYPOR knock-out primary osteoblasts show decreased membrane-localized Cx43 gap junction plaques compared to the wild type cells (arrows). 4B. Dye transfer assay to determine GJIC in wild-type and CYPOR knock-out mouse primary calvarial osteoblasts. Decreased dye transfer in CYPOR knock-out primary calvarial osteoblasts compared to wild can be observed over 2 minutes. (* injected cell, representative image, n=8)
4. Discussion
In this study we show, for the first time, regulation of Cx43, GJIC and hemichannel function by NADPH-cytochrome P450 reductase in osteoblast cells. Knock-down of CYPOR in osteoblasts clearly showed a decrease in the Cx43 protein level in both transformed and primary cells. The observed decrease in the levels of Cx43 in immunoblots was confirmed by immunostaining of the cells. This decrease in Cx43 level was reflected in the functional activities measured by both GJIC and hemichannel dye uptake. Further examination, using the luciferase promoter assay, showed down regulation of Cx43 upon CYPOR knock-down at the transcriptional level.
Cx43 has been shown to be regulated by various factors, such as nitric oxide [25], and growth factors, such as EGF [26], FGF [27], TGF-β [28], and PDGF [29]. Bone anabolic factors such as bone morphogenetic protein (BMP2), PGE2 and parathyroid hormone upregulate Cx43 protein and GJIC [15]. As CYPOR is the obligate electron donor for many enzymes, including all microsomal CYPs and heme oxygenase, as well as enzymes involved in lipid metabolism, it is predictable that deletion of CYPOR will have a multitude of effects on various physiological processes. Retinoic acid, which is hydroxylated by CYP26, a CYPOR-requiring enzyme, is known to upregulate Cx43 at both protein and mRNA levels [30]. Studies by You et al. [31] in rat Leydig cells have shown that addition of human chorionic gonadotropin increased cytochrome P450 side-chain cleavage and steroidogenic acute regulatory protein mRNA and testosterone formation, leading to a decrease in Cx43 mRNA levels in a time- and dose-dependent manner. Liarozole, a derivative of the anti-fungal drug ketoconazole, increases Cx43 expression and GJIC presumably by inhibiting a P450 enzyme responsible for 4-hydroxylation of retinoic acid; a major pathway for catabolism [32]. The upregulation of Cx43 expression by retinoic acid per se suggests that deletion of CYPOR should increase the Cx43 level since retinoic acid catabolism would be inhibited. The result presented here is in stark contrast to this retinoic acid-related mechanism of Cx43 regulation. Our results indicate that these cells produce CYPOR/CYP metabolites that positively regulate expression of Cx43. Increased oxidative stress response gene expression in liver from liver-specific CYPOR deletion mouse models suggests that CYPOR might play a role in maintaining the redox status of the cell [33,34]. It is also known that oxidative stress damages connexins and alters the GJIC [35]. Deletion of CYPOR may lead to oxidative stress, which could play a role in the regulation of expression and function of Cx43.
Human patients with severe forms of CYPOR mutation show bone defects similar to those of Antley-Bixler syndrome [36,37]. Antley-Bixler syndrome is associated with a gain of function mutation in FGFR2 [38]. Though CYPOR variant- and FGFR2 mutation-related cases segregate, both the syndromes present similar bone deformities. The convergence of FGF- and CYPOR-mediated cellular phenomena is not understood and is a topic of active research. If CYPOR plays some role in FGF-mediated signaling, and FGF mediates Cx43 expression [27], it might also answer, in part, the regulation of Cx43 expression. Cellular and animal models have been developed to understand the role of CYPOR in bone development. Bone- specific CYPOR knock-out animals generated by crossing Prx1 Cre and Por floxed mice were shown to have abnormal limb bud development, fused joints and splayed, stunted and fused digits [39]. Retinoic acid, a known teratogen, is upregulated in these mice. However, a Vitamin A-deficient (VAD) diet was not able to rescue the phenotype. Upregulation of various genes involved in lipid metabolism, including the cholesterol biosynthetic pathway, was shown in limb buds in these bone-specific Por knock-out mice. Cholesterol modification of the amino terminus of Hedgehog is an essential step in the Hedgehog signaling pathway [40], which is known to play important role during bone development[41]. A cholesterol-supplemented diet fed to pregnant mice reverted the webbed-foot phenotype. Other bone defects in these mice were not fully rescued by the cholesterol diet, which could be due to ineffective uptake during embryogenesis, or due to alterations in other non-Hedgehog signaling pathways. Deletion of Por from rat primary chondrocytes leads to decreased cholesterol levels, decreased differentiation and increased apoptosis [42]. Addition of cholesterol to the culture media was able to revert the phenotype almost to normal.
Mutant Cx43 mice with decreased GJIC and hemichannel activity show bone defects in the skull and digits. Such defects have been observed in human patients with severe CYPOR defects and in mouse models with bone-specific CYPOR knock-out. Here, we show that CYPOR plays a role in transcriptional regulation of Cx43 in osteoblasts and, as a consequence could be an important player in CYPOR-mediated bone development.
Highlights.
Humans with severe forms of Cytochrome P450 oxidoreductase (CYPOR) mutations show bone defects as observed in Antley-Bixler Syndrome
First report showing knockdown of CYPOR in osteoblasts decreased Connexin43 (Cx43) protein levels. Cx43 is known to play an important role in bone modeling
Knockdown of CYPOR decreased Gap Junctional Intercellular Communication and Hemichannel activity
Knockdown of CYPOR decreased Cx43 in mouse primary calvarial osteoblasts
Decreased Cx43 expression was observed at the transcriptional level
Acknowledgements
This work was supported in part by NIH Grant GM081568 (to BSSM. who is The Robert A. Welch Distinguished Professor in Chemistry, AQ-0012)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuriyama Y, Omura T, Siekevitz P, et al. Effects of phenobarbital on the synthesis and degradation of the protein components of rat liver microsomal membranes. J Biol Chem. 1969;244:2017–2026. [PubMed] [Google Scholar]
- 2.Lu AY, Junk KW, Coon MJ. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969;244:3714–3721. [PubMed] [Google Scholar]
- 3.Masters BS, Baron J, Taylor WE, et al. Immunochemical studies on electron transport chains involving cytochrome P-450. I. Effects of antibodies to pig liver microsomal reduced triphosphopyridine nucleotide-cytochrome c reductase and the non-heme iron protein from bovine adrenocortical mitochondria. J Biol Chem. 1971;246:4143–4150. [PubMed] [Google Scholar]
- 4.Schacter BA, Nelson EB, Marver HS, et al. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- 5.Maines MD, Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974;71:4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Porter TD. Hepatic cytochrome P450 reductase-null mice reveal a second microsomal reductase for squalene monooxygenase. Arch Biochem Biophys. 2007;461:76–84. doi: 10.1016/j.abb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Ilan Z, Ilan R, Cinti DL. Evidence for a new physiological role of hepatic NADPH:ferricytochrome (P-450) oxidoreductase. Direct electron input to the microsomal fatty acid chain elongation system. J Biol Chem. 1981;256:10066–10072. [PubMed] [Google Scholar]
- 8.Ono T, Takahashi K, Odani S, et al. Purification of squalene epoxidase from rat liver microsomes. Biochem Biophys Res Commun. 1980;96:522–528. doi: 10.1016/0006-291x(80)91245-0. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Weng Y, Zhang QY, et al. Liver-specific deletion of the NADPH-cytochrome P450 reductase gene: impact on plasma cholesterol homeostasis and the function and regulation of microsomal cytochrome P450 and heme oxygenase. J Biol Chem. 2003;278:25895–25901. doi: 10.1074/jbc.M303125200. [DOI] [PubMed] [Google Scholar]
- 10.Henderson CJ, Otto DM, Carrie D, et al. Inactivation of the hepatic cytochrome P450 system by conditional deletion of hepatic cytochrome P450 reductase. J Biol Chem. 2003;278:13480–13486. doi: 10.1074/jbc.M212087200. [DOI] [PubMed] [Google Scholar]
- 11.Adachi M, Asakura Y, Matsuo M, et al. POR R457H is a global founder mutation causing Antley-Bixler syndrome with autosomal recessive trait. Am J Med Genet A. 2006;140:633–635. doi: 10.1002/ajmg.a.31112. [DOI] [PubMed] [Google Scholar]
- 12.Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135. doi: 10.1016/S0140-6736(04)16503-3. [DOI] [PubMed] [Google Scholar]
- 13.Fukami M, Horikawa R, Nagai T, et al. Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab. 2005;90:414–426. doi: 10.1210/jc.2004-0810. [DOI] [PubMed] [Google Scholar]
- 14.Kelley RI, Kratz LE, Glaser RL, et al. Abnormal sterol metabolism in a patient with Antley-Bixler syndrome and ambiguous genitalia. Am J Med Genet. 2002;110:95–102. doi: 10.1002/ajmg.10510. [DOI] [PubMed] [Google Scholar]
- 15.Stains JP, Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta. 2005;1719:69–81. doi: 10.1016/j.bbamem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLachlan E, Plante I, Shao Q, et al. ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. J Bone Miner Res. 2008;23:928–938. doi: 10.1359/jbmr.080217. [DOI] [PubMed] [Google Scholar]
- 18.Lecanda F, Towler DA, Ziambaras K, et al. Gap junctional communication modulates gene expression in osteoblastic cells. Mol Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Zhou Z, Yellowley CE, et al. Inhibiting gap junctional intercellular communication alters expression of differentiation markers in osteoblastic cells. Bone. 1999;25:661–666. doi: 10.1016/s8756-3282(99)00227-6. [DOI] [PubMed] [Google Scholar]
- 20.Schiller PC, D'Ippolito G, Balkan W, et al. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–369. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- 21.Chung DJ, Castro CH, Watkins M, et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 22.Yeh LC, Adamo ML, Kitten AM, et al. Osteogenic protein-1-mediated insulin-like growth factor gene expression in primary cultures of rat osteoblastic cells. Endocrinology. 1996;137:1921–1931. doi: 10.1210/endo.137.5.8612532. [DOI] [PubMed] [Google Scholar]
- 23.Cherian PP, Siller-Jackson AJ, Gu S, et al. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 25.Yao J, Hiramatsu N, Zhu Y, et al. Nitric oxide-mediated regulation of connexin43 expression and gap junctional intercellular communication in mesangial cells. J Am Soc Nephrol. 2005;16:58–67. doi: 10.1681/ASN.2004060453. [DOI] [PubMed] [Google Scholar]
- 26.Lau AF, Kanemitsu MY, Kurata WE, et al. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992;3:865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiokawa-Sawada M, Mano H, Hanada K, et al. Down-regulation of gap junctional intercellular communication between osteoblastic MC3T3-E1 cells by basic fibroblast growth factor and a phorbol ester (12-O-tetradecanoylphorbol-13-acetate) J Bone Miner Res. 1997;12:1165–1173. doi: 10.1359/jbmr.1997.12.8.1165. [DOI] [PubMed] [Google Scholar]
- 28.van Zoelen EJ, Tertoolen LG. Transforming growth factor-beta enhances the extent of intercellular communication between normal rat kidney cells. J Biol Chem. 1991;266:12075–12081. [PubMed] [Google Scholar]
- 29.Burt JM, Steele TD. Selective effect of PDGF on connexin43 versus connexin40 comprised gap junction channels. Cell Commun Adhes. 2003;10:287–291. doi: 10.1080/cac.10.4-6.287.291. [DOI] [PubMed] [Google Scholar]
- 30.Rogers M, Berestecky JM, Hossain MZ, et al. Retinoid-enhanced gap junctional communication is achieved by increased levels of connexin 43 mRNA and protein. Mol Carcinog. 1990;3:335–343. doi: 10.1002/mc.2940030605. [DOI] [PubMed] [Google Scholar]
- 31.You S, Li W, Lin T. Expression and regulation of connexin43 in rat Leydig cells. J Endocrinol. 2000;166:447–453. doi: 10.1677/joe.0.1660447. [DOI] [PubMed] [Google Scholar]
- 32.Acevedo P, Bertram JS. Liarozole potentiates the cancer chemopreventive activity of and the up-regulation of gap junctional communication and connexin43 expression by retinoic acid and beta-carotene in 10T1/2 cells. Carcinogenesis. 1995;16:2215–2222. doi: 10.1093/carcin/16.9.2215. [DOI] [PubMed] [Google Scholar]
- 33.Wang XJ, Chamberlain M, Vassieva O, et al. Relationship between hepatic phenotype and changes in gene expression in cytochrome P450 reductase (POR) null mice. Biochem J. 2005;388:857–867. doi: 10.1042/BJ20042087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng Y, DiRusso CC, Reilly AA, et al. Hepatic gene expression changes in mouse models with liver-specific deletion or global suppression of the NADPH-cytochrome P450 reductase gene. Mechanistic implications for the regulation of microsomal cytochrome P450 and the fatty liver phenotype. J Biol Chem. 2005;280:31686–31698. doi: 10.1074/jbc.M504447200. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxid Redox Signal. 2009;11:339–353. doi: 10.1089/ars.2008.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fluck CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230. doi: 10.1038/ng1300. [DOI] [PubMed] [Google Scholar]
- 37.Huang N, Pandey AV, Agrawal V, et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet. 2005;76:729–749. doi: 10.1086/429417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chun K, Siegel-Bartelt J, Chitayat D, et al. FGFR2 mutation associated with clinical manifestations consistent with Antley-Bixler syndrome. Am J Med Genet. 1998;77:219–224. doi: 10.1002/(sici)1096-8628(19980518)77:3<219::aid-ajmg6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt K, Hughes C, Chudek JA, et al. Cholesterol metabolism: the main pathway acting downstream of cytochrome P450 oxidoreductase in skeletal development of the limb. Mol Cell Biol. 2009;29:2716–2729. doi: 10.1128/MCB.01638-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 41.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguilar A, Wu S, De Luca F. P450 oxidoreductase expressed in rat chondrocytes modulates chondrogenesis via cholesterol- and Indian Hedgehog-dependent mechanisms. Endocrinology. 2009;150:2732–2739. doi: 10.1210/en.2009-0043. [DOI] [PubMed] [Google Scholar]