Abstract
Accumulating evidence suggests that the endo-lysosomal system provides a substantial store of Ca2+ that is tapped by the Ca2+-mobilizing messenger, NAADP. In this article, we review evidence that NAADP-mediated Ca2+ release from this acidic Ca2+ store proceeds through activation of the newly described two-pore channels (TPCs). We discuss recent advances in defining the sub-cellular targeting, topology and biophysics of TPCs. We also discuss physiological roles and the evolution of this ubiquitous ion channel family.
Keywords: NAADP, Two-pore channels, TPC1, TPC2, TPCN1, TPCN2, Calcium, Endosomes, Lysosomes, Acidic calcium stores
1. Introduction
Changes in the concentration of cytosolic Ca2+ regulate a plethora of cellular events [1]. The importance of this pathway is exemplified by the many diseases that result from mis-regulated Ca2+ signals [2]. Given its pleiotropic actions, it is not surprising that changes in cytosolic Ca2+ concentration are tightly regulated. This is achieved through a rich portfolio of Ca2+ channels, pumps, transporters and buffers which underpin the spatio-temporally complex changes in Ca2+ concentration that typically result upon cell stimulation [3]. Fine tuning of these signals is thought to maintain the specificity of Ca2+-linked stimuli in regulating down-stream targets [1,3].
Many hormones and neurotransmitters evoke changes in cytosolic Ca2+ concentration through the production of intracellular messengers which, in turn, activate Ca2+-permeable channels located on intracellular stores [1,3]. By far the best studied of these pathways is that involving inositol trisphosphate (IP3). IP3 is produced by receptor-mediated activation of phospholipase C and mobilizes Ca2+ from endoplasmic reticulum (ER) Ca2+ stores through a well defined family of IP3-sensitive Ca2+ channels [4–6]. Cyclic ADP-ribose (cADPR) is another Ca2+-mobilizing messenger. It is produced by ADP-ribosyl cyclases and activates ryanodine receptors [7,8]. IP3 and ryanodine receptors are structurally and functionally related and both reside on ER Ca2+ stores [4–6,8]. A key feature of these channels is their biphasic regulation by cytosolic Ca2+ whereby low concentrations stimulate channel activity while higher concentrations inhibit [4–6,8]. This self regulatory mechanism is critical for the generation of spatiotemporally complex Ca2+ signals [1].
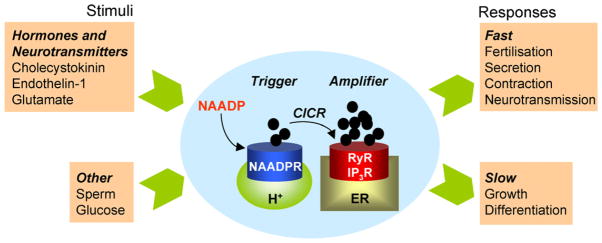
The most recently discovered Ca2+-mobilizing messenger is nicotinic acid adenine dinucleotide phosphate (NAADP) [9,10]. The Ca2+-mobilizing properties of NAADP were first recognized by Lee and colleagues using egg homogenates from sea urchin [11]. Much less is known about this pathway compared to IP3 and cADPR pathways. NAADP is produced upon cell stimulation with Ca2+-mobilizing agonists [12–14] although its mechanism of synthesis is uncertain. Like cADPR, NAADP may be synthesized by ADP-ribosyl cyclases [15,16]. Perhaps the most surprising feature of NAADP is its ability to target Ca2+-permeable channels on acidic Ca2+ stores that are clearly distinct from the ER [17]. Nevertheless, in intact cells, activation of these channels results in further release of Ca2+ from the ER [18]. NAADP is thus considered a “trigger” whereby it provides local release of Ca2+ that sensitizes neighbouring IP3 and ryanodine receptors to their respective messengers resulting in a larger Ca2+ release event (Fig. 1) [19].
Fig. 1.
NAADP-mediated Ca2+ signalling. Schematic depicting selected NAADP-linked stimuli (left) and NAADP-regulated Ca2+-dependent cellular responses (right). The intervening diagram summarizes the “trigger” hypothesis for NAADP action, whereby NAADP produced in response to cellular stimulation activates NAADP-sensitive Ca2+ channels located on acidic Ca2+ stores. The resulting local Ca2+ signal is then amplified by neighbouring IP3 and ryanodine receptors on the ER by Ca2+-induced Ca2+ release (CICR) to mediate a larger global Ca2+ signal.
The NAADP pathway is recruited in an agonist-selective manner notably by the same agonists that were thought previously to couple exclusively to IP3 production [20]. These include endothelin-1 [13], cholecystokinin [14], and glutamate [21]. Such differential recruitment of intracellular Ca2+ release channels by different extracellular cues provides a plausible basis for generating heterogeneity in the Ca2+ signal. The resulting Ca2+ signals have been implicated in a variety of cellular events including fertilisation [12,22], neuronal growth [23] and blood pressure control [24]. In this review, we discuss the evidence that NAADP mediates Ca2+ release from the endo-lysosomal system through activation of a novel family of Ca2+-permeable channels—the two-pore channels (TPCs).
2. NAADP mobilizes Ca2+ from endo-lysosomal Ca2+ stores
From early studies in sea urchin eggs, it was clear that the Ca2+ stores targeted by NAADP were distinct from the ER [25]. Thus, fractionation of egg homogenates on density gradients resulted in a broad distribution of vesicles that were capable of responding to NAADP [25]. In contrast, IP3- and cADPR-sensitive vesicles strictly co-migrated with markers for the ER [25]. In addition, thapsigargin, which depletes ER Ca2+ stores, effectively abolished Ca2+ release in response to IP3 and cADPR, but did not prevent Ca2+ release in response to NAADP [26]. Elegant experiments in intact eggs con- firmed that different stores were sensitive to NAADP and IP3/cADPR [27]. Gentle centrifugation resulted in stratification of organelles and global photo-release of the different messengers from inactive caged precursors showed that Ca2+ increases in response to NAADP originated from the opposite pole of the cell to those evoked by IP3 and cADPR [27]. Thus, NAADP releases Ca2+ from non-ER Ca2+ stores in sea urchin eggs.
Lysosomes are acidic organelles (pH ~ 4.8) that house a variety of hydrolytic enzymes. They receive input from the endosomal, autophagic and phagocytic routes. Lysosomes are traditionally viewed as terminal degradative compartments but much evidence indicates that they also form a mobilizable store of Ca2+ [28]. The use of glycyl-L-phenylalanine 2-naphthylamide (GPN) has provided important evidence that lysosomes represent a significant Ca2+ store. This compound is a cell-permeable di-peptide substrate for the acid hydrolase cathepsin C [29]. It therefore causes osmotic permeablisation of organelles housing the enzyme as it is degraded. Direct measurements of cytosolic Ca2+ reveal substantial Ca2+ signals in response to acute addition of GPN. This was first reported by Haller et al. in MDCK cells [30] and subsequently confirmed in a variety of other cells including neurons and glia [21]. The harsh luminal environment of the lysosome with its low pH and acid hydrolases, presents technical challenges for direct measurements of luminal Ca2+. Nevertheless elegant work by Christensen et al. who used endocytosed dextran-based Ca2+ indicators and performed careful calibration of the indicators with respect to pH, determined a luminal Ca2+ concentration of ~500 μM [31]. This value is well within the range reported for the luminal Ca2+ concentration of the ER [32]. A more recent study using fibroblasts obtained similar values for the lysosomal luminal Ca2+ concentration [33]. Endosomes are also likely to contain significant concentrations of Ca2+ since they are formed during invagination of the plasma membrane and thus will incorporate extracelluar Ca2+ which is present at ~1 mM. Direct measurements of endosomal Ca2+ are also limited. One study suggested that endosomal Ca2+ is rapidly lost as the endosomes acidify [34]. However, another study in pancreatic acinar cells suggested that the Ca2+ content of endosomal compartments is ~40 μM [35]. Thus, lysosomes and probably endosomes too are significant stores of Ca2+.
The first evidence that NAADP mobilizes Ca2+ from the endo-lysosomal system was provided by Churchill and colleagues [17]. Using sea urchin eggs, they demonstrated that GPN-selectively blocked Ca2+ signals evoked by photorelease of NAADP, but not those evoked by IP3 or cADPR [17]. Subsequent studies showed that GPN also blocked NAADP-evoked Ca2+ signals in a variety of mammalian cells [20]. Additionally, Cancela and colleagues have provided evidence that endosomes might express functional NAADP-sensitive Ca2+-permeable channels [36]. Bafilomycin A1 has also been widely demonstrated to block NAADP-evoked Ca2+ signals [17]. Bafilomycin A1 is an inhibitor of V-type ATPases that are responsible for the acidification of organelles [37]. Although the mechanism of Ca2+ uptake into the endo-lysosomal system is obscure, it likely requires a proton gradient [31] because bafilomycin A1 causes loss of Ca2+. V-type ATPases are expressed on a variety of organelles including lysosomes and endosomes. Bafilomycin A1 does not therefore distinguish between them or indeed other acidic organelles such as secretory vesicles. Nevertheless, its inhibitory effect on NAADP-mediated Ca2+ signalling further supports the notion that NAADP mobilizes Ca2+ from acidic organelles.
3. NAADP activates two-pore channels
Considerable progress in defining the NAADP-sensitive Ca2+ store was not, until recently, matched by progress in defining the molecular target of NAADP. In the last year or so, however, three independent groups have converged on the TPCs as likely NAADP targets.
TPCs were cloned in 2000 by Ishibashi et al. from rat [38] and by Furuichi et al. from Arabidopsis [39]. Both proteins display significant sequence similarity (albeit modest) to voltage-gated Ca2+ and Na+ channels [38,39]. The localization of plant TPCs on the vacuole [40], an acidic Ca2+ store [28], prompted us and others to test the hypothesis that TPCs may be the elusive target of NAADP in animal cells.
Our studies have focussed on sea urchin and human TPCs [41–43]. Three genes are present in the sea urchin genome (SpTPC1–3), whereas only two (HsTPC1 and HsTPC2) are present in humans (see below). Overexpression of all isoforms in SKBR3 cells was found to markedly enhance NAADP-evoked Ca2+ signals consistent with a role as NAADP-sensitive Ca2+ channels [41–43]. In addition, TPC-evoked Ca2+ signals in response to NAADP were abolished by pre-treating cells with bafilomycin A1 thereby suggesting that the signals derived from acidic organelles [41–43]. Additionally, the Ca2+ signals evoked by NAADP were partially sensitive to ryanodine, consistent with the amplification of the initial Ca2+ signal by ryanodine receptors [41,43]. Notably the pharmacology of TPC-evoked Ca2+ signals, with respect to bafilomycin A1 and ryanodine, mirrored that of endogenous NAADP-evoked Ca2+ signals in these cells [44]. Accordingly, knockdown of endogenous TPC1 in SKBR3 cells using a siRNA-based approach substantially reduced Ca2+ signals evoked by NAADP [41]. Thus, in this cell type, TPC1 appears to mediate the effects of NAADP consistent with quantitative PCR analysis demonstrating levels of TPC1 transcripts are higher than those of TPC2 [41]. These studies strongly implicated TPCs in NAADP action [45].
Independent studies also support the notion that animal TPCs are NAADP-sensitive Ca2+ channels. Over-expression of sea urchin, human and mouse TPCs in HEK cells was generally associated with enhanced NAADP-evoked Ca2+ signals [46–49]. Calcraft et al. who focussed on human TPC2, found that overexpression of this isoform also enhanced NAADP binding (~3-fold) consistent with TPCs as direct targets for NAADP [46]. In accord, immunoprecipitates of endogenous SpTPC1 and SpTPC2 from sea urchin eggs bound NAADP in an essentially irreversible manner in the presence, but not absence, of K+ [48]. The regulation of NAADP dissociation by K+ is a peculiar feature of endogenous NAADP receptors in this cell type [50]. The purity of the preparation, however, was not reported. Thus, a potential role for tightly associated accessory binding proteins cannot be excluded. Importantly, Calcraft et al. also showed that NAADP-evoked Ca2+-dependent ion currents in pancreatic beta cells were lacking in TPC2 KO mice [46] suggesting that TPC2 mediates NAADP-evoked Ca2+ release in this cell type.
In HEK cells expressing human TPC2, the NAADP responses were markedly biphasic comprising an initial relatively small and slow release of Ca2+ followed by a larger more abrupt Ca2+ signal [46]. Bafilomycin A1 abolished the Ca2+ signals, whereas thapsigargin blocked only the second phase [46]. TPC1-mediated Ca2+ signals appeared to support only small localized changes [46]. The authors rationalized these findings in the context of the trigger hypothesis, whereby the first and second phases represent release of Ca2+ from acidic organelles and amplification by the ER, respectively [46]. This is an attractive proposal but the TPC-mediated Ca2+ signals appear remarkably slow taking several minutes to peak compared to endogenous NAADP-evoked Ca2+ signals, which peak in seconds [51]. The kinetics also differed to those reported for human TPCs expressed in SKBR3 cells, where rapid and robust (global) responses were observed upon over-expression of either TPC1 or TPC2 [41–43]. This difference might reflect the different cell lines used for heterologous expression. It is notable that SKBR3 cells express functional ryanodine receptors [44], whereas HEK cells do not, and that in several cell types NAADP preferentially recruits ryanodine receptors [13,24,52]. Consequently, the absence of functional ryanodine receptors in HEK cells may have “loosened” coupling between activation of TPCs. Indeed, over-expression of mouse TPC2 in HEK cells appeared to be completely uncoupled from Ca2+ release from the ER (given its insensitivity to thapsigargin) and TPC1-evoked signals were not resolvable [47]. However, a recent re-examination using the same HEK cells expressing HsTPC1 and HsTPC2 indicates that NAADP-evoked signals are rapid, robust and mono-phasic [49] and thus more comparable to those in SKBR3 cells [41–43] than initially reported [46]. A somewhat perplexing finding that remains to be explained is the apparent lack of functional activity of SpTPC3 expressed in HEK cells [48], which contrasts with our findings in SKBR3 cells [42]. Taken together, ample evidence from independent laboratories implicate TPCs in NAADP action, although the nature of the NAADP-evoked Ca2+ signals differ between studies possibly as a result of the different cell types used for analysis.
4. Sub-cellular targeting of TPCs
Befitting their role as NAADP-sensitive Ca2+ channels responsible for Ca2+ release from acidic Ca2+ stores, animal TPCs localize to the endo-lysosomal system. In our studies using heterologously expressed TPCs, we found a punctate intracellular distribution for human TPCs [41]. The acidic nature of the labelled vesicles was confirmed by the overlap in distribution of the TPCs with lysotracker red, a fluorescent weak base [45]. Co-expression with markers for endosomes (rhoB) and lysosomes (LAMP1) indicated that TPC1 is expressed on both organelles [41]. In contrast, TPC2 appeared to be almost exclusively localized on lysosomes [41,43]. Essentially similar results were obtained by Calcraft et al. [46]. Quantitative analysis of TPC1 distribution showed a similar overlap with markers for endosomes and lysosomes (see Supplementary Table 1 in [46]) whereas TPC2 overlapped preferentially with the lysosomal marker. The lysosomal distribution of TPC2 was confirmed for the endogenous protein expressed in HEK cells using an anti-TPC2 antibody [46].
Many integral membrane proteins destined for the endo-lysosomal system are targeted by dileucine motifs [53]. Two classes of dileucine motifs have been described conforming to either the DXXLL or D/E-XXX-LL consensus sequence [53]. We noted the presence of conserved dileucine motifs in vertebrate TPCs [43]. One of these (corresponding to the latter motif) was located in the N-terminus and was conserved in both TPC1 and TPC2 [43]. Deletion of the N-terminus of human TPC2 or mutation of the leucine residues to alanine resulted in a predominant plasma membrane distribution [43]. These data suggest that the dileucine motif is normally responsible for targeting of TPC2 to lysosomes and that interfering with it results in default trafficking of TPC2 to the cell surface. Similar experiments using truncated TPC1, however, failed to result in plasma membrane targeting [43]. Mutation of a second dileucine motif found in the C-terminus of TPC1 only was also without effect as was combining both mutations [43]. These data suggest that TPC1 is targeted by very different means to TPC2, possibly through tyrosine-based motifs.
Although much experimental evidence is consistent with the trigger hypothesis for explaining NAADP-mediated Ca2+ signals, direct measurements of the trigger in isolation are limited. Indeed, lack of putative trigger events in T-lymphocytes following ryanodine receptor blockade has been interpreted as evidence of a direct effect of NAADP on ryanodine receptors [54]. As noted above, even after overexpression of TPCs, a substantial component of the resulting NAADP-evoked Ca2+ signal is sensitive to interfering with ER Ca2+ stores [41,43,46–48] (but see [47]). Targeting of TPC2 to the plasma membrane by manipulation of its di-leucine motif provided a unique opportunity to characterize this trigger event in isolation. We reported that NAADP evoked Ca2+ signals in cells expressing plasma membrane-targeted TPC2 [43]. Importantly, in contrast to cells expressing full-length TPC2, the Ca2+ signals were largely insensitive to bafilomycin A1 and ryanodine, but they were abolished by removal of extracellular Ca2+ [43]. These data provide evidence that plasma membrane-targeted TPC2 mediates Ca2+ influx, and that the resulting Ca2+ signals are not subject to appreciable amplification. Thus, by manipulating the subcellular distribution of TPC2, we were able to uncouple trigger from amplification pathways [43]. Such data argue against a direct effect of NAADP on ER Ca2+ channels. Intriguingly, the Ca2+ signals in cells expressing plasma membrane targeted TPC2 were smaller and much slower to peak than those expressing wild type TPC2. This is perhaps a consequence of the lack of amplification. Indeed, such modest sluggish signals resemble endogenous NAADP-evoked Ca2+ signals in sea urchin eggs treated with heparin and 8-amino cADPR to block Ca2+ release from the ER [55]. Alternatively, the differences might result from the different lipid composition of the host membranes or the environments to which the luminal surface of TPC2 is exposed.
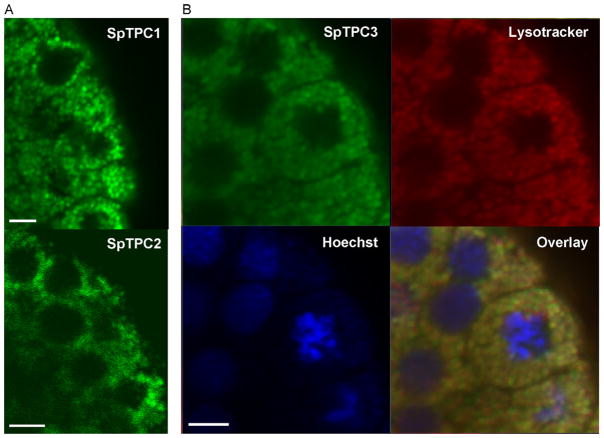
Sea urchin TPCs also localize to acidic organelles when heterologously expressed in mammalian cells [42,48]. Interestingly, heterologous expression studies in oocytes from the closely related starfish, indicate that all three isoforms localize to the cortex—a finding confirmed for endogenous TPC3 in sea urchin eggs [48]. Such a distribution is consistent with the initiation of endogenous NAADP-evoked Ca2+ signals in the cortex of both sea urchin eggs [12] and starfish oocytes [56]. However, these cortical Ca2+ signals are thought to arise from NAADP-evoked Ca2+ influx [12,57]. Moreover, the cortical distribution of SpTPCs is difficult to reconcile with the localisation of endogenous NAADP-sensitive Ca2+ channels to reserve granules [17] which are distributed throughout the egg. As shown in Fig. 2, SpTPCs over expressed in sea urchin embryos show a punctate perinuclear distribution typical of the endo-lysosomal system. Clearly further work is required to determine the localisation of SpTPCs which may be dynamically regulated throughout development. Nevertheless, current evidence obtained using a variety of preparations place TPCs within the endo-lysosomal system—a location which seems necessary for subsequent recruitment of ER Ca2+ channels upon NAADP stimulation.
Fig. 2.
Sub-cellular distribution of sea urchin TPCs. Confocal images of Stronglylocentrotus purpuratus embryos (19 h post fertilisation) that had been injected with mRNA encoding for GFP-tagged SpTPC1 (A, top), SpTPC2 (A, bottom) and SpTPC3 (B). In B, the embryos were counterstained with lysotracker red (red) and Hoechst (blue) to label acidic organelles and nuclei, respectively. An overlay of the images is shown in the bottom right panel. All scale bars = 5 μm.
5. Structure of TPCs
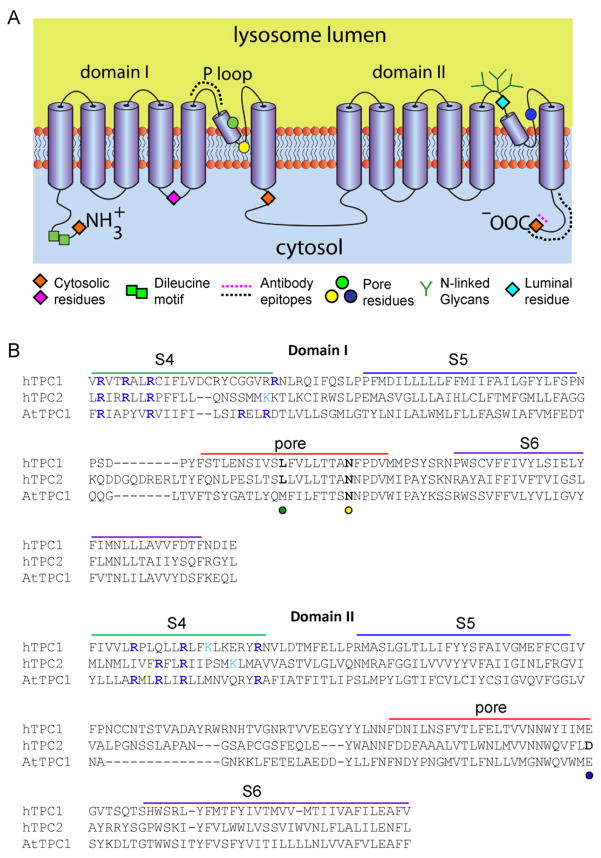
Based on their sequence similarity to voltage-gated Ca2+/Na+ channels, TPCs are predicted to comprise two homologous domains each consisting of 6 trans-membrane regions with a putative pore-forming domain located between the 5th and 6th membrane-spanning regions [38,39]. They thus have a unique predicted structure corresponding to approximately half of a voltage-sensitive Ca2+/Na+ channel (Fig. 3A). Consistent with this predicted topology, fluorophores placed at either terminus or after trans-membrane regions 4 and 6 of domain I are all accessible to trypsin upon selective permeabilisation of the plasma membrane [58]. Mutation of a highly conserved leucine residue in domain I ablates NAADP-mediated Ca2+ release by both HsTPC1 [41] and HsTPC2 [43]. These data are consistent with the position of this residue within the first predicted pore. Indeed, the predicted luminal region immediately upstream of the pore has been confirmed for HsTPC2 based on accessibility to an anti-TPC2 antibody [58]. The corresponding region preceding pore 2 harbours a cluster of N-linked glycosylation sites. Mutation of these sites in HsTPC1 [58] and mouse TPC2 [47] prevents N-glycosylation, thereby confirming their luminal location. Indeed, a fluorophore placed at this position in TPC1 shows only limited accessibility to trypsin [58]. Interestingly, several ion channels are regulated by N-glycosylation through sites close to their pores [59–61]. TPCs also appear to be regulated in this fashion. Thus, mutation of the N-glycosylation sites in TPC1 preceding the second pore enhances NAADP-evoked Ca2+ release [58]. Glycosylation thus serves to inhibit TPC function. The functional architecture of TPCs is thus emerging and consistent with the structural assignment of TPCs to the family of voltage-gated ion channels.
Fig. 3.
Structural and biophysical properties of TPCs. (A) Proposed topology of TPCs. TPCs are predicted to contain two repeated domains each comprising 6 trans-membrane regions and a putative pore-loop between the fifth and sixth transmembrane regions. Both termini and the loop connecting the domains are predicted to be cytosolic. Positions at which fluorophores were introduced and their accessibility to trypsin assessed in fluorescence protease protection assays [58] are shown by diamonds. The results confirmed the positions of the indicated regions to either the cytosol (orange for both HsTPC1 and HsTPC2; pink for HsTPC1) or lumen (cyan; HsTPC1). An N-terminal dileucine endo-lysosomal targeting motif is shown by green squares. The dotted lines represent the epitopes in TPC1 (pink) and TPC2 (black) to which antibodies used for topology mapping were raised [58]. Pore residues are highlighted by circles (see B for further detail). Green branches indicate N-linked glycosylation sites within the pore loop of domain II [47,58]. (B) Multiple sequence alignment (using ClustalW2) of putative pore regions of human TPC1 (HsTPC1, NP 001137291.1) and TPC2 (HsTPC2, NP 620714.2), and of Arabidopsis TPC1 (AtTPC1, NP 567258.1) from domains I and II. Possible positions of the S4–S6 membrane-spanning regions are from [38]. For voltage-gated cation channels, positively charged residues (usually arginine) aligned on one side of the S4 helix provide the voltage-sensor. Residues that might provide such an arrangement in TPC channels are highlighted in blue (arginine) and cyan (lysine) in each of the S4 regions. There are fewer such residues in HsTPC2 than in HsTPC1 and AtTPC1. Mutation of a conserved Leu (L273 for HsTPC1 and L265 for HsTPC2, green circle) [41,43] or Asn (N257 for mouse TPC2, equivalent to N273 of HsTPC2, yellow circle) [69] within the putative P-loop of domain I affect either Ca2+ release, conductance or ion selectivity, consistent with their proposed positions within the pore. A conserved acidic residue (D660 for HsTPC2 and E643 for mTPC2, blue circle) within the proposed P loop of domain II also appears to be critical for channel function [69], suggesting that the second putative P loop also contributes to formation of the pore.
6. Electrophysiological properties of TPCs
The electrophysiological characterization of TPCs is at present more advanced in higher plants than animals. Plant TPCs form the ubiquitous SV (slowly activating vacuolar) channels within the vacuolar membrane. SV channels are the most abundant ion channels in the vacuolar membrane and so amenable to patch-clamp recording from isolated vacuoles [40,62,63]. They are almost entirely cation-selective with large single-channel conductances (γ K ~ 155pS) and they are Ca2+-permeable, but select weakly between cations (PCa/PK ~ 3) [62–65]. Plant TPC1 has sufficient sequence similarity with animal TPCs [39], particularly within the likely pore regions (Fig. 3B), to suggest that they may share some common properties. However there is presently no evidence to suggest that plant TPCs are gated by NAADP, although NAADP has been reported to release Ca2+ from the ER of plants [66].
Whereas the large central vacuole of plants lends itself readily to patch-clamp recording, the small acidic organelles, within which animal TPC proteins are expressed, are smaller (diameter <0.5 μm) than the tip of a typical patch pipette (~1 μm) and so present more formidable practical problems. Three different approaches, each with their strengths and weaknesses, have been applied to electrophysiological analysis of animal TPCs. We mutated residues within the N-terminus of human TPC2 that mediate its targeting to lysosomes, and thereby achieved functional expression of TPC2 within the plasma membrane. Here it becomes accessible to conventional patch-clamp recording methods, with all the opportunities they provide for exquisitely high-resolution recording and rapid changes of media [43]. This approach requires mutation of targeting residues with the attendant possibility that the same residues might also influence gating; and re-targeting may separate TPC2 from regulatory proteins within the endo-lysosomal lumen, but the approach is otherwise minimally disruptive. The protein remains within a cellular membrane and it allows the behavior of TPC2 in exactly the same setting to be compared in intact cells (using conventional Ca2+ indicators) and at the single-channel level.
Others have reconstituted partially purified human TPC2 into artificial lipid bilayers, from which single-channel activity can be recorded [67]. This approach, at least when applied to completely purified protein, has the merit of allowing the behavior of a fully defined protein complex to be examined. The many disadvantages include the risk of damage during purification, the loss of essential accessory proteins, the need to reconstitute the protein into an entirely artificial bilayer and the limited opportunity for rapid media changes that bilayers afford. A similar approach, but using a crude lysosomal fraction fused with a bilayer, was used to examine the effects of NAADP on endogenous channels from liver [68]. The third approach was to record murine TPC2 activity from artificially enlarged lysosomes [69]. With this method, cells are first treated for several hours with vacuolin to cause lysosome fusion [70], the enlarged lysosomes are then isolated in media containing mM Ca2+ from cell lysates, and fused with a planar patch-clamp chip to allow recording [69]. This method, which has so far been applied only to analysis of whole-lysosome currents, has the merit of retaining TPC2 within a native lysosomal membrane (albeit modified by vacuolin), but it requires rather harsh, and potentially damaging, isolation methods, luminal proteins are likely to be lost when the patches are formed, and, as with bilayers, there is limited opportunity to change media rapidly. Although it is still very early days for the electrophysiological analysis of TPC2 channels from animals – the only reports were published in 2010 – it is worth comparing the results obtained with the different approaches.
Perhaps the most important conclusion from these studies is that mutations within either the first or second putative pore loop (the S5–6 loops of each domain) (Fig. 3) affect single-channel conductance [43] or ion selectivity [69], thereby establishing that TPC proteins are indeed pore-forming subunits of an NAADP-gated channel. The following features were observed in each of the three studies of TPC2 [43,67,69]. Channel activity was stimulated by sub-μM concentrations of NAADP and in two studies blocked by the antagonist, Ned-19 [43,67]. In bilayers, activation by NAADP was inexplicably irreversible [67], whereas it rapidly reversed upon either removal of NAADP or addition of Ned-19 to the plasma membrane patches [43]. The disparity may simply arise from inadequate washout of NAADP from the bilayer chamber [67]. In all three studies, the currents had near linear current–voltage (i–V) relationships, although we reported a slight inward rectification [43]. This contrasts with the striking outwardly rectifying behavior of plant TPC1 [64]. The difference, by analogy to the structures of conventional voltage-gated ion channels, where positively charged residues (usually arginine) within the fourth transmembrane region (S4) comprise the voltage-sensor, may be due to the lesser numbers of such residues in the S4 segments of animal TPC2 versus plant TPC proteins (Fig. 3B). The similar numbers of positively-charged residues in animal and plant TPC1, however, suggests that animal TPC1 may be voltage-sensitive (Fig. 3B). The single-channel studies concur in suggesting that TPC2 forms cation-selective channels with large monovalent cation conductances (γK ~ 300pS and γCs ~ 130pS), lesser Ca2+ conductances (γCa 15–40pS) and weak selectivity between cations (PCa/PK ~ 2.6) [43,67]. These ion-handing properties are broadly similar to those of plant TPC1 [64], but they differ markedly from the third study of animal TPC2, which reported a permeability ratio (PCa/PK > 1000) comparable to that of the most Ca2+-selective plasma membrane Ca2+ channels [69]. It is noteworthy that here, ion selectivity was measured at a luminal pH that mimics that of lysosomes (pH 4.6), perhaps suggesting that luminal H+ influences ion selectivity, but that interpretation is difficult to reconcile with luminal pH having no obvious effect on the amplitude of the NAADP-evoked K+ currents in bilayer recordings [67]. In summary, and while we await further analyses, it seems reasonable to suppose that TPC2 forms a large-conductance, non-selective, Ca2+-permeable, cation channel that is minimally regulated by membrane potential. The finer details of the single-channel behavior of animal TPCs are largely unexplored. There is evidence for sub-conductance states [67] and substantial open-channel noise is suggestive of either rapid flickering between conductances or block by permeating cations [43,67]. Openings that occur in bursts, effects of permeating cations on gating behavior, and the suggestion that openings may be coupled indicate that the gating mechanism for TPC channels is more complex than a simple switch between a single closed and open state. This complexity also finds a parallel in the gating behavior of plant TPC1 [63].
Finally, electrophysiological analyses provide access to the luminal surface of TPC2 and thereby allow effects of luminal regulators to be explored in ways that would be difficult in intact cells. As with plant TPC1 [64], both luminal Ca2+ and H+ may regulate TPC2 [67,69]. Pitt et al. have suggested that high levels of luminal Ca2+ (100 μM to 1 mM) are required for NAADP to gate TPC2, while luminal H+ modestly reduced activity [67]. By contrast, channels in the planar patch-clamp were detected at acidic luminal pH, but not at pH 7.2 [69]. This requirement for luminal H+ contrasts with the inhibitory effect of H+ on plant TPC1 [64], and nor can it be reconciled with the other studies in which NAADP stimulated TPC2 activity at neutral luminal pH [43,67]. One possibility is that the very high concentrations of luminal Ca2+ (60 mM) used in [69] may, by analogy with plant TPC1 [64], have inhibited channel activity, and H+ might then partially alleviate that inhibition. Further work is clearly needed to resolve, ideally in a setting that retains luminal proteins, the possible roles of luminal Ca2+ and H+ in modulating the gating of TPC2 by NAADP.
7. Functional roles of TPCs
NAADP has been shown to regulate a variety of cellular functions that include muscle contraction and differentiation. Here we discuss the role of TPCs in these established NAADP-dependent processes, and also their role in trafficking and pigmentation in which NAADP signalling had not previously been implicated.
Much evidence indicates that NAADP regulates muscle contractility. In pulmonary artery smooth muscle cells, NAADP generates local Ca2+ signals from bafilomycin A1-sensitive Ca2+ stores. These are subsequently amplified by ryanodine, but not IP3 receptors [13]. The resulting global Ca2+ signals are sufficient to cause contraction [13]. Endothelin-1 elevates cellular levels of NAADP and its effect on cytosolic Ca2+ concentration was abolished by bafilomycin A1 [13]. A recent study has confirmed the role of TPCs in contraction of smooth muscle. Thus, in TPC2-deficient mice, NAADP-evoked contractions of detrusor and taenia caecum muscle were absent [71]. Carbachol-evoked contractions in detrusor muscle from wild-type mice were partially sensitive to NAADP-blockade. However, agonist-evoked contractions were not reduced in muscle from TPC2 knock-out mice although the contractions were insensitive to pharmacological blockade of NAADP [71]. These data suggest compensation may have occurred in the TPC2 knock-out mice to maintain agonist-evoked contractions. Notably, the contribution of ryanodine receptors to carbachol-evoked Ca2+ signals was increased in the transgenic animals [71]. In this context, it is interesting to note a recent comparison of Ca2+ signals in pancreatic acinar cells from wild type and CD38 knock-out mice [16]. Previous studies had provided evidence that cholecystokinin is an NAADP-linked agonist based on the blockade of cholecystokinin-evoked Ca2+ signals by NAADP desensitization [18] and lysosomotropic agents [72], and the ability of cholecystokinin to elevate NAADP levels [14]. In CD38-kockout mice, cholecystokinin-evoked NAADP elevations were abolished [16] suggesting that CD38 is responsible for NAADP production in these cells. However, the cholecystokinin-evoked Ca2+ signals appeared not to be affected although as in the case of agonist-evoked contractions in TPC2 knock-out mice [71], they became insensitive to depletion of acidic organelles [16]. These observations once again suggest that compensation may have occurred upon depletion of CD38 to maintain cholecystokinin-evoked Ca2+ signalling. In this case, this may be due to enhanced Ca2+ influx since removal of extracellular Ca2+ did reveal reduced cholecystokinin-evoked Ca2+ signals in the CD38-deficient mice [16]. Such compensation perhaps highlights the importance of the Ca2+-dependent function under NAADP control. It might also reflect coordinated regulation of the expression of the various Ca2+ channels and synthetic enzymes in normal cells [73].
Differentiation is another process in which NAADP has been implicated. Using the PC12 cell line, an extensively used model system to study neuronal differentiation, cellular delivery of NAADP using NAADP-filled liposomes was sufficient to induce differentiation [74]. In parallel experiments, IP3 delivery was ineffective [74]. Direct measurements of cytosolic Ca2+ concentration showed that NAADP-induced Ca2+ signals were reliant on both acidic Ca2+ stores (based on their inhibition by bafilomycin A1 and GPN) and the ER (based on their partial inhibition by thapsigargin) [74]. Kinetic com-parison of the signals in response to NAADP and IP3 showed that the former were more sustained [74]. Consequently, these unique Ca2+ signatures likely underlie the very different effect of the messengers on Ca2+-dependent output. Such data provide evidence to support the idea that differential recruitment of Ca2+-mobilizing messengers may provide a mechanism for fine tuning Ca2+ signals, which are in turn differentially decoded. A recent study using a cell-permeable analogue of NAADP, NAADP-AM [75], supports a role for NAADP in mediating differentiation [76]. Thus, as in PC12 cells, elevation of cellular NAADP levels in skeletal muscle cells promoted differentiation and siRNA-mediated inhibition of either TPC1 or TPC2 inhibited it [76]. This study provides further evidence for a functional role for TPCs in mediating NAADP action.
The endo-lysosomal system is a highly dynamic organelle network requiring fusion events between organelles [77]. A role for Ca2+ in these “constitutive” events has been appreciated for some time [78] and certainly its role in regulated secretion is established. Ca2+ is thought to be released from acidic organelles close to the fusion machinery thereby accounting for the block of these events by the fast Ca2+ chelator, BAPTA, but not by the slower chelator, EGTA [79,80]. The identity of the target channel responsible for this local release of Ca2+ however has not been established but a clear candidate is TRP mucolipin-1 (TRPML1). TRPML1 derives its name from the finding that mutation of its gene results in the lysosomal storage disorder, mucolipidosis IV (MLIV) [81]. That TRPML1 is likely a non-selective cation channel (and thus permeable to Ca2+), and that MLIV is characterised by trafficking defects [82] support the contention that Ca2+-dependent fusion events required for normal endo-lysosomal trafficking are mediated by TRPML1, possibly in response to PI(3,5)P2 [83].
A recent study also suggested a role for TPCs in trafficking within the endo-lysosomal system [48]. Cholera toxin B normally enters cells by endocytosis and is trafficked to the Golgi complex by retrograde transport. Over-expression of sea urchin TPC1 and TPC2 was suggested to disrupt this pathway resulting in accumulation of the toxin in endosomes (although its exact localisation using markers was not reported) [48]. These data implicate TPCs in transport of cargo from endosomes to the trans-Golgi network, perhaps because local Ca2+ signals are required for fusion of endosomes or endosome-derived vesicles within the trans-Golgi network. Disrupting trafficking from endosomes to the Golgi may affect recycling of the mannose-6-phosphate receptor. This protein mediates delivery of lysosomal hydrolases from the Golgi to endosomes prior to their delivery to lysosomes. Consequently disruption of this pathway may alter lysosome biogenesis. Indeed, overexpression of TPCs was also associated with increased staining of cells with Lysotracker and an enlargement of individual lysosomes [48]. This phenotype is reminiscent of that we reported previously in cells where lysosomal dysfunction was imposed by pharmacological inhibition of select lysosomal hydrolases [84]. TPC dysfunction may thus precipitate lysosomal dysfunction. Conversely, lysosomal dysfunction may disrupt TPC function. For example in fibroblasts from patients suffering from the lysosomal storage disease, Nieman-Pick type C, lysosomal Ca2+ concentration and associated global NAADP-evoked Ca2+ signals are reduced [33]. Lysosomal dysfunction imposed by pharmacological means in neurons is also associated with deviant NAADP-evoked Ca2+ signals, although in this case they are enhanced [84]. NAADP-evoked Ca2+ signals mediated by TPCs may therefore be intimately linked to both lysosomal function and dysfunction.
Prior to identification of TPCs as NAADP targets, single nucleotide polymorphisms in the human TPC2 gene were associated with hair colour in northern Europeans [85]. Two non-synonymous variants were identified that associated with blond versus brown hair. Pigmentation is a complex process that involves the synthesis of melanins in lysosome-related organelles known as melanosomes [86]. Melansosomes are then transferred by an ill-defined mechanism from the melanocyte to neighbouring keratinocytes. The mechanism whereby TPC2 and its variants regulate pigmentation is not known, but it is tempting to speculate that TPC2 may be localized to the melanosome. Indeed, melanosomes may serve as Ca2+ stores [87] and there is evidence for the presence of functional NAADP-sensitive Ca2+ channels on other lysosome-related organelles [88]. Consequently, TPC2-mediated Ca2+ signals may regulate either the function of the melanosome or perhaps trafficking of melanosomes to keratinocytes. Alternatively, TPC2 may regulate melanogenesis via changes in melanosomal pH since melanin synthesis is regulated by pH [89] and mobilisation of acidic Ca2+ stores by NAADP is associated with luminal alkanization [90]. Intriguingly, mutations/variants of several other proteins such as TRPML3, SLC24A5 and TRPM1 have also been associated with pigmentation phenotypes [28,91]. Consequently, ion channel fluxes within the endo-lysosomal system may be a general mechanism involved in pigmentation [28,91].
8. Evolution of TPCs
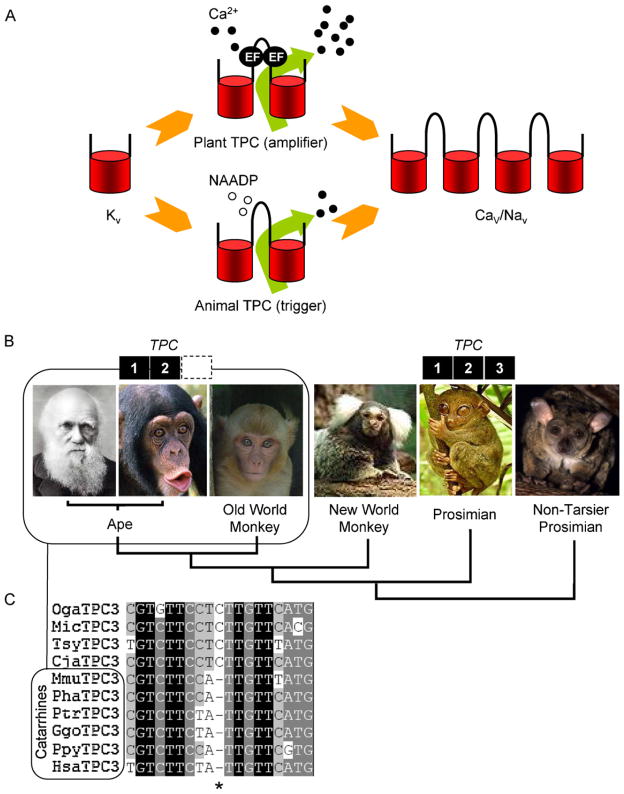
As mentioned above, structurally speaking, the TPCs correspond to approximately one-half of a voltage sensitive Ca2+/Na+ channel. Since the latter are thought to have evolved from K+ channels [92], TPCs may represent an evolutionary intermediate between the two. Thus the single repeat of the K+ channels may have been duplicated to generate TPCs, which may then have undergone another round of duplication to generate 4-repeat Ca2+/Na+ channels (Fig. 4A).
Fig. 4.
Evolution of TPCs. (A) Schematic outlining a possible trajectory for the evolution of voltage-gated ion channels and TPCs. Voltage-sensitive K+ channels (Kv; one-repeat) may have duplicated to form TPCs (two-repeat) and the TPCs duplicated to form voltage-sensitive Ca2+ (Cav) and Na+ (Nav) channels (four-repeat). Plant TPCs by virtue of their EF hands may serve to amplify Ca2+ signals (middle top) whereas animal TPCs (which lack EF hands) may have evolved to trigger Ca2+ signals in response to NAADP (middle bottom). (B) TPC gene complement of selected primates (from left to right; Homo sapiens, Pan troglodytes, Maccaca mulatta, Callithrix jacchus, Tarsius syrichta, Otolemur garnettii) highlighting lack of a functional TPC3 gene in Catarrhines (box). (C) Multiple sequence alignment of primate genomic sequences. A conserved cytosine residue within the TPC3 gene (*) is deleted in Cattarhines [98]. Abbreviations used are: Oga, Otolemur garnettii; Mic, Microcebus murinus; Tsy, Tarsius syrichta; Cja, Callithrix jacchus; Mmu, Maccaca mulatta; Pha, Papio hamadryas; Ptr, Pan troglodytes; Ggo, Gorilla gorilla; Ppy, Pongo pygmaeus; and Hsa, Homo sapiens.
A distinguishing feature between plant and animal TPCs is the presence in the linker region connecting the two domains in plants of two predicted EF hands [40], which in other proteins bind Ca2+. Indeed, it is established that the SV currents in plants which are mediated by TPCs are Ca2+-regulated [40]. The lack of EF hands in animal TPCs is consistent with the insensitivity of NAADP-evoked Ca2+ release to cytosolic Ca2+ [93]. Thus, whereas plant TPCs may amplify Ca2+ signals, animal TPCs likely trigger them (Fig. 4A).
In plants, a single TPC has been described in Arabidopsis [39], rice [94] and wheat [95] and two closely related genes have been described in tobacco [96]. In animals, there is evidence for both gene loss, and gene multiplication and divergence [41]. Most animal species within the protostome super phylum, for example, appear not to possess TPC genes [41,45,46]. These include key model organisms such as worms and flies. In contrast, most deuterostomes possess three TPC genes [42]. These include sea urchins, which are basal deuterostomes that have been extensively used for NAADP research [97]. The sequence similarity between TPC isoforms in animals is relatively low (30–40% for sea urchins) compared to other intracellular channels such as IP3 receptors indicating substantial divergence.
The complete (three-member) ancestral family is retained in chordates such as frogs, fish, birds and most mammals, but strikingly, the TPC3 gene is absent in rodents and humans [42]. In cows, dogs and horses which possess the full TPC complement, the TPC3 gene is located between NPHP1 and BUB1 [42]. Inspection of the corresponding human genomic sequences reveals TPC3-related sequences corresponding to the N-terminal third of TPC3 [42]. However, there is no clear start site and there are multiple nucleotide insertions and deletions that would result in the production of a truncated protein if transcribed and translated [42]. Partial TPC3-related sequences were identified in several other apes including our closest relative, the chimpanzee and also in the Rhesus monkey (an Old World Monkey) [42]. This analysis provides evidence that the TPC3 gene is undergoing loss in the primate lineage.
Further inspection of more distantly-related primate genome sequences identified sequences likely corresponding to full-length TPC3 in a New World Monkey [98]. Near complete sequences were also found in both tarsier and non-tarsier Prosimians [98]. Importantly, no deleterious mutations were present. The TPC3 gene thus appears to be retained in these organisms (Fig. 4B) and suggests that degeneration of the gene in the primate lineage began in the common ancestor of Cattarhines (apes and Old World monkeys) ~25 to 40 million years ago [98]. The TPC3 gene is therefore of major interest in an evolutionary context because it is a rare example of an ion channel undergoing degeneration that began relatively recently. In summary, the TPC gene family has undergone remarkable diversi-fication during evolution.
9. Outlook
The molecular basis for Ca2+ release by NAADP has been subject to controversy [99,100], with competing claims for activation of Ca2+-permeable channels located on the ER, the plasma membrane, and as discussed in this review, acidic Ca2+ stores. Multiple lines of evidence from independent groups are now converging on the TPCs as the target for NAADP on acidic stores of Ca2+. Indeed, the localisation of TPCs to the endo-lysosomal system, within which there is much protein trafficking, might well reconcile at least some of the apparent discrepant findings in the literature regarding the functional localisation of NAADP targets (discussed in [45]). Clearly, over-expression of TPCs enhances cellular sensitivity to NAADP, but how NAADP binding is linked to activation (and inactivation) of Ca2+ release is not yet clear. The field is now ripe for structure–function analysis, and high-level expression and analysis of TPCs in a null background will certainly facilitate this. The basic topology of TPCs has now been defined, but not the quaternary structure. The basic biophysical properties of TPCs are also emerging, but much more analysis is required to define ion selectivity, gating mechanisms and regulation by luminal ions of the various isoforms. Do TPCs interact with other proteins? Defining the TPC interactome will provide important information regarding the regulation of TPCs of which little is known at present. NAADP, via TPCs, recruits the activity of other Ca2+ channels, but until now this “chatter” [101] has been imaged using relatively low resolution microscopy methods. Can higher resolution methods be applied in cells expressing defined levels of TPCs to probe the microarchitecture of the underlying elementary Ca2+ signals? Finally, armed with not only molecular tools to manipulate NAADP-mediated Ca2+ signals but also new chemical tools [102,103], we now have an ideal opportunity to probe the physiological role of TPCs. Might functions already ascribed to the endo-lysosomal system uncover new roles for TPCs? Are TPCs mis-regulated in disease states in which the endo-lysosomal system is perturbed, and might manipulating their levels/activity represent a novel therapeutic strategy? Answers to these questions are likely to be forthcoming in the near future in what is now a rapidly advancing field.
Acknowledgments
We thank Dev Churamani, Robert Hooper, Chi Li and Desmond Tobin for useful discussion, and the Bogue foundation (University College London) for facilitating staff exchange. Work in SP’s laboratory is funded by the BBSRC. Work in CWT’s laboratory is funded by the Wellcome Trust. TR is a Drapers’ Research Fellow at Pembroke College, Cambridge.
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Rizzuto R, Pozzan T. When calcium goes wrong: genetic alterations of a ubiquitous signaling route. Nat Genet. 2003;34:135–141. doi: 10.1038/ng0603-135. [DOI] [PubMed] [Google Scholar]
- 3.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- 5.Taylor CW, Genazzani AA, Morris SA. Expression of inositol trisphosphate receptors. Cell Calcium. 1999;26:237–251. doi: 10.1054/ceca.1999.0090. [DOI] [PubMed] [Google Scholar]
- 6.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 8.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 9.Lee HC. Calcium signaling: NAADP ascends as a new messenger. Curr Biol. 2003;13:R186–R188. doi: 10.1016/s0960-9822(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 10.Patel S. NAADP-induced Ca2+ release—a new signaling pathway. Biol Cell. 2004;96:19–28. doi: 10.1016/j.biolcel.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 12.Churchill GC, O‘Neil JS, Masgrau R, et al. Sperm deliver a new messenger: NAADP. Curr Biol. 2003;13:125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 13.Kinnear NP, Boittin FX, Thomas JM, Galione A, Evans AM. Lysosome-sarcoplasmic reticulum junctions: a trigger zone for calcium signalling by NAADP and endothelin-1. J Biol Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki M, Thomas JT, Churchill GC, et al. Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ Spiking in mouse pancreatic acinar cells. Curr Biol. 2005;15:874–878. doi: 10.1016/j.cub.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Cho BH, Kim UH. CD38-mediated Ca2+ signaling contributes to angiotensin II-induced activation of hepatic stellate cells: attenuation of hepatic fibrosis by CD38 ablation. J Biol Chem. 2010;285:576–582. doi: 10.1074/jbc.M109.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosker F, Cheviron N, Yamasaki M, et al. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem. 2010;285:38251–38259. doi: 10.1074/jbc.M110.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 18.Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 19.Guse AH, Lee HC. NAADP a universal Ca2+ trigger. Sci Signal. 2008;1:re10. doi: 10.1126/scisignal.144re10. [DOI] [PubMed] [Google Scholar]
- 20.Galione A, Morgan AJ, Arredouani A, et al. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem Soc Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- 21.Pandey V, Chuang CC, Lewis AM, et al. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem J. 2009;422:503–512. doi: 10.1042/BJ20090194. [DOI] [PubMed] [Google Scholar]
- 22.Lim D, Kyozuka K, Gragnaniello G, Carafoli E, Santella L. NAADP+ initiates the Ca2+ response during fertilization of starfish oocytes. FASEB J. 2001;15:2257–2267. doi: 10.1096/fj.01-0157com. [DOI] [PubMed] [Google Scholar]
- 23.Brailoiu E, Hoard JL, Filipeanu CM, et al. NAADP potentiates neurite outgrowth. J Biol Chem. 2005;280:5646–5650. doi: 10.1074/jbc.M408746200. [DOI] [PubMed] [Google Scholar]
- 24.Brailoiu GC, Gurzu B, Gao X, et al. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J Biol Chem. 2010;285:37133–37137. doi: 10.1074/jbc.C110.169763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- 26.Genazzani AA, Galione A. Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem J. 1996;315:721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HC, Aarhus R. Functional visualisation of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. J Cell Sci. 2000;113:4413–4420. doi: 10.1242/jcs.113.24.4413. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadot M, Colmant C, Wattiaux-de CS, Wattiaux R. Intralysosomal hydrolysis of glycyl-L-phenylalanine 2-naphthylamide. Biochem J. 1984;219:965–970. doi: 10.1042/bj2190965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haller T, Dietl P, Deetjen P, Volkl H. The lysosomal compartment as intracellular calcium store in MDCK cells: a possible involvement in InsP3-mediated Ca2+ release. Cell Calcium. 1996;19:157–165. doi: 10.1016/s0143-4160(96)90084-6. [DOI] [PubMed] [Google Scholar]
- 31.Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 32.Miyawaki A, llopis J, Heim R, McCaffery JM, Adams JA, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Evans E, Morgan AJ, He X, et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 34.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood MW, Prior IA, Voronina SG, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Curr Biol. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 37.Huss M, Wieczorek H. Inhibitors of V-ATPases: old and new players. J Exp Biol. 2009;212:341–346. doi: 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 39.Furuichi T, Cunningham KW, Muto S. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 2001;42:900–905. doi: 10.1093/pcp/pce145. [DOI] [PubMed] [Google Scholar]
- 40.Peiter E, Maathuis FJ, Mills LN, et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 41.Brailoiu E, Churamani D, Cai X, et al. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brailoiu E, Hooper R, Cai X, et al. An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem. 2010;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brailoiu E, Rahman T, Churamani D, et al. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrlau MG, Brailoiu E, Patel S, Gogotsi Y, Dun NJ, Bau HM. Carbon nanopipettes characterize calcium release pathways in breast cancer cells. Nanotechnology. 2008;19:325102. doi: 10.1088/0957-4484/19/32/325102. [DOI] [PubMed] [Google Scholar]
- 45.Patel S, Marchant JS, Brailoiu E. Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium. 2010;47:480–490. doi: 10.1016/j.ceca.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zong X, Schieder M, Cuny H, et al. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruas M, Rietdorf K, Arredouani A, et al. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endo-lysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogunbayo OA, Zhu Y, Rossi D, Sorrentino V, Ma J, Zhu MX, Evans AM. Cyclic adenosine diphosphate ribose activates ryanodine receptors, whereas NAADP activates two-pore domain channels. J Biol Chem. 2011;286:9136–9140. doi: 10.1074/jbc.M110.202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickinson GD, Patel S. Modulation of NAADP receptors by K+ ions: evidence for multiple NAADP receptor conformations. Biochem J. 2003;375:805–812. doi: 10.1042/BJ20030672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genazzani AA, Mezna M, Summerhill RJ, Galione A, Michelangeli F. Kinetic properties of nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. J Biol Chem. 1997;272:7669–7675. doi: 10.1074/jbc.272.12.7669. [DOI] [PubMed] [Google Scholar]
- 52.Brailoiu GC, Brailoiu E, Parkesh R, et al. NAADP-mediated channel “chatter” in neurons of the rat medulla oblongata. Biochem J. 2009;419:91–97. doi: 10.1042/BJ20081138. [DOI] [PubMed] [Google Scholar]
- 53.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 54.Dammermann W, Guse AH. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J Biol Chem. 2005;280:21394–21399. doi: 10.1074/jbc.M413085200. [DOI] [PubMed] [Google Scholar]
- 55.Churchill GC, Galione A. Spatial control of Ca2+ signalling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J Biol Chem. 2000;275:38687–38692. doi: 10.1074/jbc.M005827200. [DOI] [PubMed] [Google Scholar]
- 56.Santella L, Kyozuka K, Genazzani AA, De Riso L, Carafoli E. Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. J Biol Chem. 2000;275:8301–8306. doi: 10.1074/jbc.275.12.8301. [DOI] [PubMed] [Google Scholar]
- 57.Moccia F, Lim D, Nusco GA, Ercolano E, Santella L. NAADP activates a Ca2+ current that is dependent on F-actin cytoskeleton. FASEB J. 2003;13:1907–1909. doi: 10.1096/fj.03-0178fje. [DOI] [PubMed] [Google Scholar]
- 58.Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett ES. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: functional sialic acids are localized to the S5-S6 loop of domain I. J Physiol. 2002;538:675–690. doi: 10.1113/jphysiol.2001.013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noma K, Kimura K, Minatohara K, et al. Triple N-glycosylation in the long S5-P loop regulates the activation and trafficking of the Kv12.2 potassium channel. J Biol Chem. 2009;284:33139–33150. doi: 10.1074/jbc.M109.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16:997–1001. doi: 10.1097/00001756-200506210-00023. [DOI] [PubMed] [Google Scholar]
- 62.Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scholz-Starke J, Naso A, Carpaneto A. A perspective on the slow vacuolar channel in vacuoles from higher plant cells. J Chem Inf Model. 2005;45:1502–1506. doi: 10.1021/ci050218a. [DOI] [PubMed] [Google Scholar]
- 64.Pottosin II, Schonknecht G. Vacuolar calcium channels. J Exp Bot. 2007;58:1559–1569. doi: 10.1093/jxb/erm035. [DOI] [PubMed] [Google Scholar]
- 65.Schulz-Lessdorf B, Hedrich R. Protons and calcium modulate SV-type channels in the vacuolar-lysosomal compartment—channel interaction with calmodulin inhibitors. Planta. 1995;197:655–671. [Google Scholar]
- 66.Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitt SJ, Funnell T, Sitsapesan M, et al. TPC2 is a novel NAADP-sensitive Ca2+-release channel, operating as a dual sensor of luminal pH and Ca2+ J Biol Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang F, Li PL. Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J Biol Chem. 2007;282:25259–25269. doi: 10.1074/jbc.M701614200. [DOI] [PubMed] [Google Scholar]
- 69.Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J Biol Chem. 2010;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong XP, Cheng X, Mills E, et al. The type IV mucolipidosis-associated protein TRPML1 is an endo-lysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tugba Durlu-Kandilci N, Ruas M, Chuang KT, Brading A, Parrington J, Galione A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J Biol Chem. 2010;285:24925–24932. doi: 10.1074/jbc.M110.129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamasaki M, Masgrau R, Morgan AJ, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 73.Ritchie MF, Zhou Y, Soboloff J. Transcriptional mechanisms regulating Ca2+ homeostasis. Cell Calcium. doi: 10.1016/j.ceca.2010.10.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brailoiu E, Churamani D, Pandey V, et al. Messenger-specific role for NAADP in neuronal differentiation. J Biol Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 75.Parkesh R, Lewis AM, Aley PK, et al. Cell-permeant NAADP: a novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell Calcium. 2007;43:531–538. doi: 10.1016/j.ceca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 76.Aley PK, Mikolajczyk AM, Munz B, Churchill GC, Galione A, Berger F. Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc Natl Acad Sci USA. 2010;107:19927–19932. doi: 10.1073/pnas.1007381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 78.Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007;35:1088–1091. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- 79.Holroyd C, Kistner U, Annaert W, Jahn R. Fusion of endosomes involved in synaptic vesicle recycling. Mol Biol Cell. 1999;10:3035–3044. doi: 10.1091/mbc.10.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pryor PR, Mullock BM, Bright NA, Gray SR, Luzio JP. The role of intraor-ganellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bargal R, Avidan N, Ben-Asher E, et al. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–123. doi: 10.1038/79095. [DOI] [PubMed] [Google Scholar]
- 82.Puertollano R, Kiselyov K. TRPMLs: in sickness and in health. Am J Physiol Renal Physiol. 2009;296:F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong XP, Shen D, Wang X, et al. PI(3,5)P2 controls membrane traffic by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dickinson GD, Churchill GC, Brailoiu E, Patel S. Deviant NAADP-mediated Ca2+-signalling upon lysosome proliferation. J Biol Chem. 2010;285:13321–13325. doi: 10.1074/jbc.C110.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sulem P, Gudbjartsson DF, Stacey SN, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 86.Tobin DJ. The cell biology of human hair follicle pigmentation. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 87.Salceda R, Sanchez-Chavez G. Calcium uptake, release and ryanodine binding in melanosomes from retinal pigment epithelium. Cell Calcium. 2000;27:223–229. doi: 10.1054/ceca.2000.0111. [DOI] [PubMed] [Google Scholar]
- 88.Rosado JA. Acidic Ca2+ stores in platelets. Cell Calcium. 2010 doi: 10.1016/j.ceca.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 89.Ito S, Wakamatsu K. Human hair melanins: what we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- 90.Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007;402:301–310. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel S, Docampo R. In with the TRP channels: intracellular functions for TRPM1 and TRPM2. Sci Signal. 2009;2:e69. doi: 10.1126/scisignal.295pe69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strong M, Chandy KG, Gutman GA. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol Biol Evol. 1993;10:221–242. doi: 10.1093/oxfordjournals.molbev.a039986. [DOI] [PubMed] [Google Scholar]
- 93.Chini EN, Dousa TP. Nicotinate-adenine dinucleotide phosphate-induced Ca2+ release does not behave as a Ca2+-induced Ca2+-release system. Biochem J. 1996;316:709–711. doi: 10.1042/bj3160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurusu T, Sakurai Y, Miyao A, Hirochika H, Kuchitsu K. Identification of a putative voltage-gated Ca2+-permeable channel (OsTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant Cell Physiol. 2004;45:693–702. doi: 10.1093/pcp/pch082. [DOI] [PubMed] [Google Scholar]
- 95.Wang YJ, Yu JN, Chen T, et al. Functional analysis of a putative Ca2+ channel gene TaTPC1 from wheat. J Exp Bot. 2005;56:3051–3060. doi: 10.1093/jxb/eri302. [DOI] [PubMed] [Google Scholar]
- 96.Kadota Y, Furuichi T, Ogasawara Y, et al. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem Biophys Res Commun. 2004;317:823–830. doi: 10.1016/j.bbrc.2004.03.114. [DOI] [PubMed] [Google Scholar]
- 97.Galione A, Patel S, Churchill GC. NAADP-induced calcium release in sea urchin eggs. Biol Cell. 2000;92:197–204. doi: 10.1016/s0248-4900(00)01070-4. [DOI] [PubMed] [Google Scholar]
- 98.Cai X, Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol Biol Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- 99.Galione A, Petersen OH. The NAADP receptor: new receptors or new regulation? Mol Interv. 2005;5:73–79. doi: 10.1124/mi.5.2.4. [DOI] [PubMed] [Google Scholar]
- 100.Guse AH. Second messenger signaling: multiple receptors for NAADP. Curr Biol. 2009;19:R521–R523. doi: 10.1016/j.cub.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 101.Patel S, Churchill GC, Galione A. Coordination of Ca2+ signalling by NAADP. Trends Biochem Sci. 2001;26:482–489. doi: 10.1016/s0968-0004(01)01896-5. [DOI] [PubMed] [Google Scholar]
- 102.Naylor E, Arredouani A, Vasudevan SR, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dammermann W, Zhang B, Nebel M, et al. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc Natl Acad Sci USA. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]