Abstract
Background
Carcinoid tumors are associated with the carcinoid syndrome, a set of symptoms resulting from the peptide and amine products, including serotonin, secreted from the cancer cells. The purpose of our study was to investigate the relationship between the PI3K/Akt inhibitor PTEN (phosphatase and tensin homolog deleted on chromosome ten) and serotonin synthesis and secretion in the carcinoid cancer cell line BON.
Methods
PTEN was inhibited by pharmacological and molecular approaches, and the resultant secretion of serotonin and expression of tryptophan hydroxylase 1 (TPH1), the rate limiting enzyme in serotonin synthesis, was assessed.
Results
Inhibition of PTEN in vitro, with concomitant increased Akt signaling, resulted in decreased secretion of serotonin, as well as decreased serotonin synthesis, as confirmed by reduced expression of TPH1. Inhibition of PTEN in BON cells in an animal model resulted in decreased serum serotonin.
Conclusion
By inhibiting signaling through Akt, PTEN indirectly promotes serotonin synthesis and secretion.
Keywords: carcinoid, PTEN, serotonin
INTRODUCTION
Carcinoid tumors are slow growing neuroendocrine neoplasms that have been increasing in incidence since the 1970s (1). These tumors arise from enterochromaffin cells lining the gut and secrete amine and peptide products, including chromogranin A (CgA) and serotonin (2). Derived from the amino acid tryptophan, serotonin is a neurotransmitter that enhances bowel motility and, in excess, can lead to severe diarrhea (3). Serotonin is also hypothesized to promote vasodilation, resulting in flushing, and to induce cardiac fibrosis (3). Unfortunately, due to their indolent nature, many carcinoids are not detected until they have reached an advanced stage (4) and generally do not respond well to standard chemotherapeutic agents (5).
The development of novel treatments for carcinoid has focused on targeting growth factor receptors and oncogenic cellular signaling pathways (5). Phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) signaling is over-activated in many types of cancers, including carcinoid tumors (6,7), through either constitutive activation of PI3K, which indirectly promotes Akt phosphorylation and activation, or downregulation or mutation of the endogenous inhibitor of PI3K/Akt signaling PTEN (phosphatase and tensin homolog deleted on chromosome ten). PTEN converts the membrane phospholipid phosphatidylinositol-3,4,5-triphosphate to phosphatidylinositol-4,5-bisphosphate, thereby removing the binding site for Akt, where it is phosphorylated and activated (8,9). While the role of Akt signaling in tumor progression and metastasis is well established, the role of the PI3K/Akt pathway in carcinoid secretion or the carcinoid syndrome has not been investigated.
Our laboratory established and characterized the BON cell line, which was derived from a lymph node metastasis of a pancreatic carcinoid tumor (10,11). BON cells synthesize and secrete neurotensin, pancreastatin, CgA, and serotonin (10,12-14). Various growth and cell signaling inhibitors are noted to inhibit BON cell growth in vitro and in vivo (11). Recently, we have developed an animal model in which splenic injection of BON cells into athymic nude mice leads to the development of primary and metastatic tumors and produces symptoms consistent with carcinoid syndrome (15). Additionally, we previously demonstrated that BON cells with stable reduction of PTEN have a higher metastatic potential using an in vivo model (16).
The purpose of our current study was to assess the relationship between PI3K/Akt/PTEN signaling and serotonin secretion and synthesis in the BON carcinoid cell line. While enhanced Akt signaling, through PTEN inhibition, increases the metastatic potential of BON cells, decreased PTEN expression reduces serotonin synthesis and secretion. Our findings demonstrate a positive relationship between PTEN expression and serotonin secretion in carcinoid tumors and will be valuable in the development of novel treatment strategies that balance the inhibition of carcinoid cell growth and secretion.
MATERIALS AND METHODS
Cell culture and establishment of a stable PTEN knockdown cell line
The BON cell line was last authenticated in October 2009 at the Johns Hopkins Genetic Resources Core Facility with short tandem repeat analysis using an Identifiler identification kit (Applied Biosystems, Carlsbad, CA). BON cells are maintained in DMEM/F12K (50/50) media (Mediatech, Inc., Herndon, VA) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) in 5% CO2 at 37°C. To reduce PTEN expression, BON cells were transfected with GFP-tagged shRNA to PTEN (Open Biosystems, Huntsville, AL) and selected in medium containing puromycin (2 μg/ml).
Western blot analysis
Total protein was resolved on NuPAGE® 4-12% Bis-Tris gels (Invitrogen) and transferred to Sequi-Blot™ PVDF membranes (Bio-Rad, Hercules, CA). Membranes were incubated with specific primary antibodies (Cell Signaling Technology, Inc., Danvers, MA). Antibody against tryptophan hydroxylase 1 (TPH1) was obtained from Abcam Inc. (Cambridge, MA). Following incubation with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), proteins were visualized using an enhanced chemiluminescence (ECL™) detection system (GE Healthcare, Buckinghamshire, United Kingdom).
Serotonin and 5-HIAA ELISA
To quantitate the amount of serotonin produced by BON cells a serotonin ELISA (Immuno-Biological Laboratories, Inc., Minneapolis, MN) was performed with BON cell conditioned media according to the manufacturer's directions. BON cells were plated at a density of 5 × 104 cells/cm2. After 24 h media was removed, and cells were washed and serum starved in serum-free McCoy's 5A medium for 24 h. McCoy's 5A media contains ascorbic acid, which protects serotonin from oxidative degradation. After the media was collected, the cells in each flask were counted, and serotonin concentration in the media was normalized to the number of cells. From these values, the amount of serotonin in the media relative to the control groups was calculated. For assessment of serotonin in the serum, blood was collected from mice following sacrifice. The blood samples were centrifuged, and the top layer (serum) was collected for analysis via serotonin ELISA. Urine was collected from the mice immediately prior to sacrifice, and a 5-hydroxyindoleacetic acid (5-HIAA) ELISA kit from DRG Diagnostics (Mountainside, NJ) was used to evaluate 5-HIAA in the urine. The ELISA with the BON cell supernatant was performed in quadruplicate, while the ELISAs with the animal samples were performed in duplicate.
Immunohistochemistry
Serial tissue sections were incubated overnight in TPH1 or CgA primary antibodies diluted in antibody diluent, as described previously (17). Briefly, the sections were stained, and the intensity of TPH1 staining relative to CgA expression was assessed in a blinded fashion by an experienced pathologist. Protein staining was performed using an Envision+® System-HRP (DAB) kit (Dako, Carpinteria, CA); samples were counterstained with hematoxylin.
Animal studies
Male athymic nude mice (4-6 weeks) were purchased from Harlan-Sprague-Dawley. Mice were anesthetized with isoflurane, and BON cells (1 × 107 per 100 μl) were injected into the pancreas or spleen with a 27-gauge needle. Mice were sacrificed 10 weeks following injection. All studies were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Statistical analysis
Descriptive statistics including means and standard deviations were calculated and displayed in bar graphs to summarize ELISA measurements across cell culture and animal treatment and control groups. Two-sample t-test or analysis of variance was performed for comparison of two or multiple groups, respectively. Assumptions on data normality and equality of variance were verified to determine validity of the statistical tests. The null hypothesis was rejected when p < 0.05. Data analysis was conducted using statistical software, SAS®, Release 9.2.
RESULTS
The expression of PTEN is directly related to serotonin secretion and synthesis
We previously established an animal model of carcinoid syndrome, where we observed a significant increase in serum serotonin concentration in athymic nude mice following splenic injection of BON cells (15). To further study the mechanism of carcinoid-induced serotonin secretion, we assessed the secretion of serotonin in BON cells with stable reduction of PTEN. The results of a serotonin ELISA demonstrated that BON shPTEN cells secrete approximately half the amount of serotonin compared to BON shControl cells (Fig. 1A; p = 0.002). The amount of serotonin secretion from BON shPTEN metastasis cells from two different passages was approximately 100 times less than that of BON shControl cells (Fig. 1A; p < 0.0001).
Fig. 1. Reduction in PTEN expression and activity decreases serotonin synthesis and secretion.
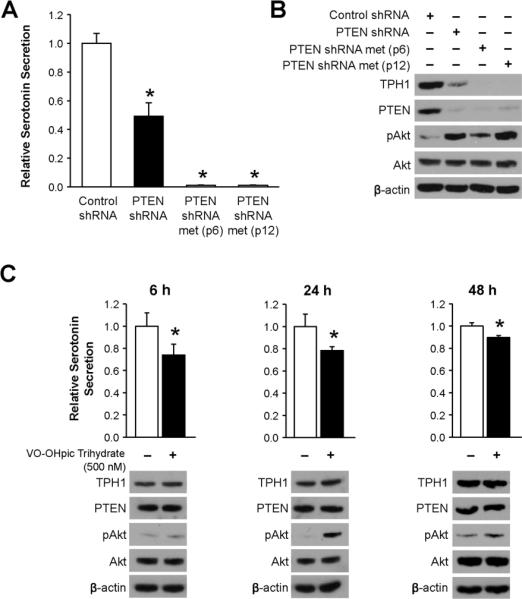
A.) The relative secretion of serotonin is approximately half in BON cells with stable reduction of PTEN compared to control BON cells (*, p = 0.002 vs. treatment with Control shRNA). BON cells with reduced PTEN expression were injected into the pancreas of athymic nude mice. Cells from the resulting liver metastases (designated BON shPTEN metastasis) were harvested, cultured, and assessed for serotonin secretion. (The passage number [p] listed for the BON shPTEN metastasis cells is the number of passages after being harvested from a liver metastasis.) BON shPTEN metastasis cells secreted approximately 100 times less serotonin compared to control BON cells (*, p < 0.0001 vs. treatment with Control shRNA). B.) PTEN reduction in BON cells also resulted in decreased synthesis of serotonin, as indicated by a concomitant decrease in TPH1 protein expression. C.) Inhibition of PTEN activity with the small molecule VO-OHpic trihydrate decreased serotonin secretion, as assessed in BON cell conditioned media, at 6, 24, and 48 h following treatment (*, p = 0.016 and 0.026 at 6 h and 24 h, respectively, and p < 0.0001 at 48 h vs. treatment with vehicle control). However, VO-OHpic trihydrate treatment had no effect on TPH1 expression, as indicated in the corresponding western blots.
Serotonin is derived from the amino acid tryptophan, and the rate-limiting enzyme in serotonin synthesis is tryptophan hydroxlase (TPH), which converts tryptophan to 5-hydroxy-L-tryptophan (5-HTP). Two isoforms of TPH exist: TPH1 is normally only expressed in the pineal gland, spleen, thymus, and enterochromaffin cells of the gut, while TPH2 is generally confined to the brainstem (18). We assessed the expression of TPH1 in BON shControl and BON shPTEN cells and observed that PTEN reduction, associated with a concomitant induction of phospho-Akt, results in decreased TPH1 production (Fig. 1B). These results suggest that the synthesis of serotonin from BON cells is affected by the balance of PTEN expression and Akt activity and that BON cells lose their endocrine properties as they become more metastatic.
Pharmacologic inhibition of PTEN reduces serotonin secretion
Our above experiments suggest that PTEN inhibition by shRNA-mediated knockdown decreases serotonin secretion by reducing the expression of TPH1 and, thus, the synthesis of serotonin. We next evaluated whether pharmacologic inhibition of PTEN activity affects serotonin secretion in BON cells. The phosphatase activity of PTEN can be inhibited with the 3-hydroxypicolinate vanadium (IV) complex VO-OHpic trihydrate (19) and is verified by an increase in phospho-Akt. A single treatment with VO-OHpic trihydrate (Sigma, St. Louis, MO) decreased BON cell serotonin secretion to approximately 74%, 78%, and 90% of vehicle-treated cells for treatment times of 6, 24, and 48 h, respectively (Fig. 1C; p = 0.016 and 0.026 at 6 h and 24 h, respectively, and p < 0.0001 at 48 h). While pharmacologic inhibition of PTEN, with concurrent enhancement of Akt signaling, significantly reduced serotonin secretion, it did not alter serotonin secretion to the same extent as with inhibition of PTEN using shRNA (Fig. 1A). Additionally, the expression of TPH1 was not altered following treatment with VO-OHpic trihydrate (Fig. 1C; bottom). Taken together these results suggest that transient PTEN inhibition, with concomitant Akt induction, reduces serotonin secretion, while stable reduction in PTEN expression decreases serotonin secretion and synthesis.
PTEN reduction decreases serotonin secretion in vivo
Previously, we noted that PTEN reduction in BON cells resulted in increased liver metastases in an animal model (16). To evaluate the effect of PTEN reduction on serotonin secretion in vivo, we injected BON shControl and BON shPTEN cells into the pancreas of athymic nude mice, as described previously (16). Immediately prior to sacrifice we collected urine for assessment of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA). Following sacrifice trunk blood was collected, and serum was assessed for serotonin. To account for differences in the number of physiologic and pathologic serotonin secreting cells in each mouse, serotonin concentration was normalized to total body weight. The average serum serotonin concentration relative to total body weight in mice injected with BON shPTEN cells was significantly less than in mice injected with BON shControl cells (Fig. 2A, p = 0.012). Consistent with this observation, the concentration of 5-HIAA in the urine relative to total body was significantly lower in mice that received injections of BON shPTEN cells compared to those injected with BON shControl cells (Fig. 2B, p = 0.0059). Primary and metastatic tumors were excised from the mice and paraffin embedded. Immunohistochemical analysis of tissue sections demonstrates representative expression of TPH1 in primary tumors derived from BON shControl cells compared to tumors derived from BON shPTEN cells (Fig. 2C). Relative to CgA expression, the expression of TPH1 is similar in primary tumors derived from BON shControl cells and BON shPTEN cells. However, metastatic tumors derived from BON shPTEN cells demonstrated decreased expression of TPH1, relative to CgA, compared to primary tumors, consistent with the results in Figure 1B. To further evaluate differences in TPH1 expression in primary and metastatic tumors, parental BON cells were injected into the spleen of athymic nude mice. Liver metastases derived from parental BON cells demonstrated decreased TPH1 expression compared to the corresponding primary tumors (Fig. 2D). The enhanced aggressiveness of BON cells with concomitant decreases in serotonin secretion and 5-HIAA excretion suggest that as carcinoid cells become more metastatic they lose their secretory properties.
Fig. 2. Reduction in PTEN expression decreases serotonin secretion in an in vivo model.

A.) Control shRNA and PTEN shRNA BON cells were injected into the pancreas of athymic nude mice (n = 10). Following sacrifice, serum was obtained from each mouse, and serotonin concentration was assessed. Serotonin concentration in the serum relative to total body weight was significantly less in the mice with tumors derived from BON shPTEN cells (*, p = 0.012). B.) The urine concentration of the serotonin metabolite 5-HIAA relative to total body weight was decreased in mice with BON shPTEN-derived tumors (*, p = 0.0059). C.) Immunohistochemical analysis of representative tumor sections demonstrated similar staining of TPH1 in primary tumors derived from BON shPTEN and BON shControl cells and decreased expression of TPH1 in liver metastases compared to primary tumors (100x). D.) Parental BON cells were injected into the spleen of athymic nude mice, and TPH1 expression was less in the metastatic tumors compared to the corresponding primary tumors (200x).
Inhibition of Akt2, but not Akt1, increases serotonin secretion
Since PTEN inhibits Akt activation, we next evaluated the role of Akt on serotonin secretion from BON cells. Three isoforms of Akt exist, and we have previously determined that Akt2 promotes colon cancer metastasis (20). We assessed the effect of Akt1 and Akt2 on BON cell serotonin secretion by inhibiting Akt with the isoform-selective inhibitors, Akt Inhibitor VIII and Akt Inhibitor XII (Calbiochem ®, San Diego, CA). Akt Inhibitor VIII has a higher selectivity for Akt1, while Akt Inhibitor XII is more selective for Akt2. At 6 h and 24 h following a single treatment with 500 nM Akt inhibitor VIII, there was no difference in serotonin secretion compared to vehicle treatment (Fig. 3A, p = 0.237 and 0.812, respectively). However, treatment with 1 μM Akt Inhibitor XII significantly increased serotonin secretion after 6 h and 24 h (Fig. 3B, p = 0.003 at 6 h and p < 0.0001 at 24 h) compared to vehicle treatment. Together, our results suggest that PTEN inhibits serotonin synthesis and secretion through Akt2.
Fig. 3. Akt2, but not Akt1, affects serotonin secretion.

A.) Treatment of BON cells with the Akt1 selective inhibitor Akt Inhibitor VIII does not affect the secretion of serotonin at 6 h or 24 h following treatment. B.) Selective inhibition of Akt2 with Akt Inhibitor XII increases the secretion of serotonin 1.4 and 1.5 times at 6 h and 24 h, respectively, compared to treatment with vehicle control (*, p = 0.003 at 6 h and p < 0.0001 at 24 h).
DISCUSSION
Serotonin is hypothesized to play a significant role in the development of carcinoid syndrome, and many treatments for the symptoms inhibit the release of serotonin and other hormones from carcinoid tumors. Our results demonstrate an inverse relationship between PI3K/Akt signaling and serotonin synthesis and secretion from the BON carcinoid cell line. Our in vivo studies further suggest that the secretion of serotonin is decreased in more aggressive carcinoid tumors.
PTEN activity directly correlates with serotonin secretion and synthesis. One of the central hypotheses of cancer biology is that cellular dedifferentiation is concomitant with tumor progression (21), and deletion of Pten has been demonstrated to induce the dedifferentiation of cells into a “cancer stem cell” phenotype (22). By inhibiting the tumorigenic activity of PI3K/Akt signaling, PTEN may also contribute to the maintenance of the differentiated, secretory state of BON cells. One of the markers of the differentiated carcinoid phenotype TPH1, the rate-limiting enzyme in the synthesis of serotonin, is expressed in the enterochromaffin cells of the gastrointestinal tract (18), from which carcinoid tumors arise. Overactivation of the Notch signaling pathway, known to inhibit endocrine differentiation, represses TPH1 expression in BON cells (23). Furthermore, TPH expression is restricted to cells whose distinct function is serotonin synthesis, and in serotonergic neurons, its expression is regulated by several transcription factors (24,25). Gata3, a transcription factor that regulates TPH expression, is downregulated, while PI3K/Akt signaling is enhanced, in metastatic breast cancers (26). Interestingly, we found that inhibition of Akt1 had no effect on serotonin secretion, while inhibition of Akt2 increased serotonin secretion from BON cells. While the role of Akt2 in cancer progression and metastasis is well-established, its role in secretion is less well understood, and further investigation is warranted. Similar to our results of Akt2-mediated inhibition of BON cell serotonin secretion, Akt2 was recently shown to be a suppressor of gastric acid secretion (27,28).
We demonstrated that molecular inhibition of PTEN concomitantly reduced TPH1 expression, while pharmacological inhibition of PTEN decreased serotonin secretion but not its synthesis. A possible explanation is that pharmacological inhibition of PTEN only transiently increases PI3K/Akt signaling, due to degradation of the inhibitor, thereby affecting secretion but not gene transcription. Consistent with our in vitro studies, mice with tumors derived from BON shPTEN cells secreted less serotonin and excreted less 5-HIAA than mice with tumors derived from BON shControl cells. Interestingly, the expression of TPH1 in primary tumors derived from BON shControl and BON shPTEN cells was similar. However, we observed that metastatic tumors had decreased expression of TPH1 compared to primary tumors, suggesting that as carcinoid cells adopt a more metastatic phenotype there is a concurrent loss in the secretory phenotype. This can be explained by the complexity of the regulation of serotonin synthesis and secretion in cancer cells. It has been demonstrated in breast cancers that serotonin has tumor suppressive and tumor promoting actions and that the expression of TPH1 has a nonlinear association with tumor stage (29). As breast tumors grow, they lose expression of TPH1, followed by a regain in TPH1 expression as the tumors become invasive (29). The hypothesis for this phenomenon is that early on in breast tumor development, TPH1 expression is reduced so that tumors can evade the tumor-suppressive functions of serotonin, such as growth inhibition and apoptosis, and maintain a proliferative state. Since serotonin has also been demonstrated to promote epithelial-to-mesenchymal transition (EMT) (30,31), the increase in TPH1 expression as tumors expand is proposed to be a marker of increased metastatic capability (29). We noted previously that carcinoid tumors with reduced PTEN expression have an increased metastatic potential (16). Thus, in the more aggressive BON shPTEN primary tumors, it is possible that during the first weeks of tumor growth, the expression of TPH1, and, hence, secretion of serotonin, was much lower than at the time of sacrifice and more comparable to our in vitro results, shown in Fig. 1. At the time of sacrifice, cells from the BON shPTEN tumors are becoming more invasive and may have increased the expression of TPH1. After tumor cells migrate to a distant site, they undergo mesenchymal-to-epithelial transition (MET) to form a micrometastasis resembling the primary tumor (32). Thus, the decreased expression of TPH1 in the carcinoid liver metastases may be associated with the resurgence of a proliferative, non-invasive phenotype.
While carcinoid tumors generally maintain a differentiated phenotype, the carcinoid syndrome does not present until liver metastases have developed and the hormone products secreted by the cancer cells are able to bypass degradation in the liver. Paradoxically, we observed that metastatic tumors in the liver had decreased expression of TPH1. A possibility for this paradox is that the secretory products responsible for carcinoid syndrome are actually secreted from the primary tumor. Carcinoid tumors are highly vascular (33,34) and upon angiogenesis may receive blood from the systemic, in addition to the portal circulation, enabling the secreted products to circulate throughout the body without being degraded in the liver.
In summary, we have identified a unique relationship between PI3K/Akt/PTEN signaling and the secretion and synthesis of serotonin in the BON carcinoid cell line. Furthermore, the expression of TPH1 and secretion of serotonin from carcinoid cells is directly related to the activity of PTEN. With the evolving complexity of PI3K/Akt/PTEN signaling, we have identified a novel function for this cell signaling pathway, which may be utilized in the development of new treatments for carcinoid disease and the carcinoid syndrome. Our findings suggest the need for a multi-targeted approach in treating carcinoid tumors, as targeting the PI3K/Akt pathway may inhibit the growth of the tumor but also results in enhanced secretion of serotonin and other peptide products which may have deleterious clinical effects.
ACKNOWLEDGEMENTS
The authors would like to thank Donna Gilbreath and Nathan Vanderford for manuscript preparation.
Grant Support: This work was supported by National Institutes of Health grants R37 AG10885, R01 DK48489, T32DK07639, and R01 CA104748.
Footnotes
Disclosure Summary: The authors have nothing to disclose
REFERENCES
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255–69. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP, Moss SF, Nilsson O, Rindi G, Salazar R, Ruszniewski P, Sundin A. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Kidd M, Drozdov I, Siddique ZL, Gustafsson BI. Pharmacotherapy of neuroendocrine cancers. Expert Opin Pharmacother. 2008;9:2617–26. doi: 10.1517/14656566.9.15.2617. [DOI] [PubMed] [Google Scholar]
- 6.Pitt SC, Davis R, Kunnimalaiyaan M, Chen H. AKT and PTEN expression in human gastrointestinal carcinoid tumors. Am J Transl Res. 2009;1:291–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Voortman J, Lee JH, Killian JK, Suuriniemi M, Wang Y, Lucchi M, Smith WI, Jr., Meltzer P, Giaccone G. Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumors. Proc Natl Acad Sci U S A. 2010;107:13040–5. doi: 10.1073/pnas.1008132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–52. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 9.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evers BM, Ishizuka J, Townsend CM, Jr., Thompson JC. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann N Y Acad Sci. 1994;733:393–406. doi: 10.1111/j.1749-6632.1994.tb17289.x. [DOI] [PubMed] [Google Scholar]
- 11.Evers BM, Townsend CM, Jr., Upp JR, Allen E, Hurlbut SC, Kim SW, Rajaraman S, Singh P, Reubi JC, Thompson JC. Establishment and characterization of a human carcinoid in nude mice and effect of various agents on tumor growth. Gastroenterology. 1991;101:303–11. doi: 10.1016/0016-5085(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 12.Evers BM, Ishizuka J, Townsend CM, Jr., Rajaraman S, Thompson JC. Expression of neurotensin messenger RNA in a human carcinoid tumor. Ann Surg. 1991;214:448–54. doi: 10.1097/00000658-199110000-00009. discussion 454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germano PM, Lieu SN, Xue J, Cooke HJ, Christofi FL, Lu Y, Pisegna JR. PACAP induces signaling and stimulation of 5-hydroxytryptamine release and growth in neuroendocrine tumor cells. J Mol Neurosci. 2009;39:391–401. doi: 10.1007/s12031-009-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeng YJ, Townsend CM, Jr., Nagasawa S, Chuo S, Kern K, Yanaihara N, Ferrar RS, Hill FL, Thompson JC, Greeley GH., Jr. Regulation of pancreastatin release from a human pancreatic carcinoid cell line in vitro. Endocrinology. 1991;128:220–5. doi: 10.1210/endo-128-1-220. [DOI] [PubMed] [Google Scholar]
- 15.Jackson LN, Chen LA, Larson SD, Silva SR, Rychahou PG, Boor PJ, Li J, Defreitas G, Stafford WL, Townsend CM, Jr., Evers BM. Development and characterization of a novel in vivo model of carcinoid syndrome. Clin Cancer Res. 2009;15:2747–55. doi: 10.1158/1078-0432.CCR-08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva SR, Bowen KA, Rychahou PG, Jackson LN, Weiss HL, Lee EY, Townsend CM, Jr., Evers BM. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer. 2011;128:1045–1056. doi: 10.1002/ijc.25441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen KA, Silva SR, Johnson JN, Doan HQ, Jackson LN, Gulhati P, Qiu S, Riall TS, Evers BM. An analysis of trends and growth factor receptor expression of GI carcinoid tumors. J Gastrointest Surg. 2009;13:1773–80. doi: 10.1007/s11605-009-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–80. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 19.Rosivatz E, Matthews JG, McDonald NQ, Mulet X, Ho KK, Lossi N, Schmid AC, Mirabelli M, Pomeranz KM, Erneux C, Lam EW, Vilar R, Woscholski R. A small molecule inhibitor for phosphatase and tensin homologue deleted on chromosome 10 (PTEN). ACS Chem Biol. 2006;1:780–90. doi: 10.1021/cb600352f. [DOI] [PubMed] [Google Scholar]
- 20.Rychahou PG, Kang J, Gulhati P, Doan HQ, Chen LA, Xiao SY, Chung DH, Evers BM. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:20315–20. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abelev GI, Lazarevich NL. Control of differentiation in progression of epithelial tumors. Adv Cancer Res. 2006;95:61–113. doi: 10.1016/S0065-230X(06)95003-9. [DOI] [PubMed] [Google Scholar]
- 22.Hill R, Wu H. PTEN, stem cells, and cancer stem cells. J Biol Chem. 2009;284:11755–9. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, Hsiao EC, Schuebel KE, Borges MW, Jin N, Collins BJ, Nelkin BD, Chen H, Ball DW. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–6. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 24.Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. doi: 10.1007/s12015-006-0002-2. [DOI] [PubMed] [Google Scholar]
- 25.Dolmazon V, Alenina N, Markossian S, Mancip J, Van de Vrede Y, Fontaine E, Dehay C, Kennedy H, Bader M, Savatier P, Bernat A. Forced expression of Lmx1b enhances differentiation of mouse ES cells into serotonergic neurons. Stem Cells Dev. doi: 10.1089/scd.2010.0224. Epub Oct. 12, 2010. PMID 20649486. [DOI] [PubMed] [Google Scholar]
- 26.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–24. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotte A, Pasham V, Bhandaru M, Eichenmuller M, Yang W, Qadri SM, Kempe DS, Puchchakayala G, Pearce D, Birnbaum MJ, Lang F. Regulation of gastric acid secretion by PKB/Akt2. Cell Physiol Biochem. 2010;25:695–704. doi: 10.1159/000315089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotte A, Pasham V, Eichenmuller M, Yang W, Qadri SM, Bhandaru M, Lang F. Regulation of basal gastric acid secretion by the glycogen synthase kinase GSK3. J Gastroenterol. 2010;45:1022–1032. doi: 10.1007/s00535-010-0260-2. [DOI] [PubMed] [Google Scholar]
- 29.Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:R81. doi: 10.1186/bcr2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem. 2008;283:30901–10. doi: 10.1074/jbc.M802476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stull MA, Pai V, Vomachka AJ, Marshall AM, Jacob GA, Horseman ND. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc Natl Acad Sci U S A. 2007;104:16708–13. doi: 10.1073/pnas.0708136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 33.Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–10. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 34.Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, Hess K, Ng C, Abbruzzese JL, Ajani JA. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–23. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]


