Abstract
Background
Chronic alcohol consumption perturbs cellular function in a variety of organ systems. Previous studies have suggested that moderate alcohol consumption reduces vascular disease, whereas heavier alcohol consumption may worsen it. The mechanisms for these vascular effects of chronic alcohol ingestion continue to be defined and constitute the focus of this study.
Methods
Male Sprague Dawley rats were fed an isocaloric, Lieber-Decarli liquid diet containing either ethanol (36% calories) or Maltose–Dextrin (substituted for ethanol) for 6 weeks. Telemetric blood pressure measurements were taken before and after ethanol feeding. After the rats were killed, the aortas were analyzed for endothelial nitric oxide (NO) synthase expression and NO production.
Results
Chronic ethanol ingestion decreased mean arterial pressure and increased aortic NO production as demonstrated by direct ex vivo measurements using iron diethyldithio-carbamic acid as well as analysis of nitrosyl-hemoglobin (NO-Hb) levels. Consistent with these assays of vascular NO production, endothelium-dependent relaxation responses to acetycholine (Ach) were enhanced in ethanol-fed animals. Aortic endothelial nitric oxide synthase expression was also increased by chronic ethanol ingestion.
Conclusions
These findings demonstrate that a regimen of chronic alcohol ingestion in the rat produced generally salutary effects in the systemic vasculature following a 6-week treatment regimen. These findings extend previous in vitro studies to demonstrate that alcohol has potent effects on vascular endothelial nitric oxide synthase expression, NO production, and vascular function. Consistent with previous reports, these findings confirm that alcohol-induced alterations in the production of reactive nitrogen species play an important role in the pathogenesis of alcohol-mediated tissue effects.
Keywords: Nitric Oxide, Ethanol, Endothelial Nitric Oxide Synthase
Epidemiological Evidence Suggests that alcohol ingestion modulates the development of cardiovascular disease. Moderate alcohol consumption may prevent atherosclerotic vascular disease (Camargo et al., 1997; Hill, 2005; Thun et al., 1997), whereas excessive intake may enhance cardiovascular disease (Klatsky, 1996). Current evidence suggests that consumption of approximately 1 alcoholic beverage per day for women (~10 g ethanol) or 2 alcoholic beverages per day for men (~25 g ethanol) provides the maximum beneficial effect from coronary heart disease (CHD). With greater daily consumption, the effects of alcohol appear to shift from beneficial to detrimental effects suggesting that the relationship between alcohol consumption and CHD follows a “J-shaped” relationship (Corrao et al., 2000). Similarly, compared with elderly adults who abstain from alcohol, consumption of 1 to 6 drinks per week was inversely associated with carotid artery intima-media thickness, a surrogate marker of vascular disease, while consumption of 14 drinks or more per week was positively associated with carotid artery intima-media thickness (Mukamal et al., 2003).
Putative mechanisms for the vascular protective effects of moderate alcohol consumption include increases in high-density lipoprotein (Hulley and Gordon, 1981; Thornton et al., 1983), inhibition of platelet activation (Landolfi and Steiner, 1984; Renaud et al., 1992), improvements in insulin sensitivity (Kiechl et al., 1994; Stampfer et al., 1988), increased antioxidant activity (German and Walzem, 2000), and enhanced endothelial nitric oxide (NO) synthase expression and NO production (Abou-Agag et al., 2005). Similarly, recent studies have suggested that alcohol-induced alterations in vascular reactive oxygen species may also contribute to vascular dysfunction. For example, male Fisher rats treated with alcohol daily for 12-weeks via orogastric tube developed hypertension, impaired vascular relaxation, reduced vascular endothelial nitric oxide synthase (eNOS) expression, and evidence of increased vascular oxidative stress (Husain et al., 2005). Collectively these studies suggest, similar to epidemiological data in human subjects, that alcohol feeding regimens in animals may result in either beneficial or detrimental effects on vascular function depending on the amount and duration of alcohol consumption.
The current study employed a well established rat model of chronic ethanol ingestion to further explore the effects of a 6-week ethanol feeding regimen on blood pressure, as well as vascular reactivity and NO production in vivo. Our findings extend previous reports that alcohol stimulates eNOS expression and NO production in vascular endothelial cells in vitro and provides additional support for the dichotomous effects of alcohol on systemic vascular function.
MATERIALS AND METHODS
Ethanol Feeding
Adult male Sprague Dawley rats (150 to 200 g) were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, NJ) containing either ethanol (36% total calories), or an isocaloric substitution with Maltose–Dextrin (control diet) ad libitum for 6 weeks in a model that we have published previously (Polikandriotis et al., 2006). As previously reported, both the control and ethanol-fed rats gained weight steadily with approximate doubling of their body weight over the 6-week feeding period. The ethanol-fed rats had approximately 20% less weight gain, a consistent finding in chronic ethanol feeding studies, related to changes in intermediate metabolism. All studies were approved by the Institutional Animal Care and Use Committee of the Atlanta VA Medical Center.
Blood Pressure Data Acquisition
Anesthesia was induced with 5% inhalational isoflurane and maintained at 1 to 1.5% isoflurane for the remainder of the procedure. The lower abdomen was shaved and disinfected. A 1.5 to 2.5-inch ventral midline incision was made from the bottom of the sternum and continuing caudally to reveal a layer of muscle. Another incision, approximately 1.5 inches in length was made to penetrate the abdominal wall. The intestines were then gently pushed to the side and kept in place by a rib separator. Using blunt dissection, the abdominal aorta was gradually exposed, and blood flow was temporarily occluded using 4–0 silk suture. A 25-gauge bent needle was used to cannulate the artery with a sterile TA11PA-C40 transmitter (Data Sciences International, St. Paul, MN). The catheter was then advanced approximately 1.5 inches and held in place by Vetbond adhesive. The transmitter was placed inside the abdominal cavity. The muscle wall was closed using separated knots of 4–0 silk suture, with 3 of the loops attaching to the transmitter. The outer layer of skin was closed using continuous suture. Postoperatively, the rats received 1 cc of warmed lactated Ringer’s solution along with 0.5 mg/kg buprenorphine for 3 days. The rats were allowed to recover from surgery for 1 week before measurements of blood pressure were initiated. Blood pressure measurements were then telemetrically collected for 10 seconds each minute during a 24-hour period at baseline prior to the initiation of control or ethanol diets, and then weekly during the 6-week feeding regimen. Blood pressure measurements at each time-point for each animal were then averaged for comparative purposes.
Measurement of Aortic NO Production Using Fe(DETC)2
Animals were fed control or ethanol-containing Lieber-DeCarli diets for 6 weeks and then killed. The aortas were rapidly dissected and placed in cold Krebs–Hepes buffer (pH 7.4), and any extraneous peri-adventitial fat was removed. Each aorta (~15 mm in length) was cut into 2-mm sections and placed into a single well of a 12-well plate. The NO spin trap, iron diethyldithio-carbamic acid (Fe(DETC)2) was added to each well, and the arteries were incubated at 37°C for 60 minutes (Dikalov and Fink, 2005). After incubation with Fe(DETC)2, aortic segments were collected and snap frozen in liquid nitrogen in a 1-cc syringe until analysis with electron spin resonance (ESR). ESR spectroscopy was performed with a Magnatech Miniscope (Magnatech, Berlin, Germany) with the following settings: field sweep, 300 G; microwave frequency, 9.78 GHz; microwave power, 10 mW; modulation amplitude, 3 G; conversion time, 2624 ms; time constant, 5248 ms; receiver gain, 1 × 105 (Dikalov and Fink, 2005). The signal from Fe(DETC)2 was derived from measuring the amplitude of the peaks from the triplet ESR signal characteristic of the NO–Fe(DETC)2 complex. Equivalent amounts of aorta (~15 mm) were used for each measurement.
Endothelium-Dependent Relaxation Responses in Aortic Rings
Isometric forces in aortic rings were measured as described previously (Sutliff et al., 2002). Aortic rings were threaded with 2 triangular stainless steel wires and then mounted on the hooks that were attached to a differential capacitor force transducer (Harvard Apparatus, Hollister, MA). Resting tension on each aortic ring was set to 40 mN, and this passive tension was maintained throughout the experiment. Each ring was submersed in a 50-ml bath kept at 37°C with circulating water. Each bath was filled with Krebs–HEPES buffer and aerated with a steady stream of 95% O2 and 5% CO2. Relaxation responses to acetylcholine (Ach, 1 × 10−9 to 1 × 10−5 M) and sodium nitroprusside (SNP, 1 × 10−10 to 3 × 10−7 M) were determined in aortas precontracted with L-phenylephrine (3 μM) to 80% maximal contraction. Data were obtained using MP100W hardware (Biopac, Goleta, CA) and analyzed using AcqKnowledge software (Biopac, Goleta, CA).
Nitrosyl-Hemoglobin (NO-Hb) Analysis
On the day of killing, blood was taken (~1 ml) via cardiac puncture using a heparinized 1-cc syringe. The blood was centrifuged at 2,000 × g for 10 minutes at 4°C. After centrifugation, the serum was aspirated and an equal volume of phosphate buffered saline aerated with nitrogen for ≥20 min was mixed with the remaining red blood cells (RBCs) and snap frozen in liquid nitrogen. ESR measurements were carried out using an EMX ESR spectrometer (Bruker, Karlruhe, Germany) with a super-high Q microwave cavity. The ESR settings for detection of NO-Hb were as follows: field sweep, 300 G; microwave frequency, 9.78 GHz; microwave power, 10 mW; modulation amplitude, 3 G; conversion time, 2624 ms; time constant, 5248 ms; receiver gain, 1 × 105 (Landmesser et al., 2003).
Real-Time PCR
Whole aortas were collected, homogenized, and RNA isolation was performed using an RNeasy Fibrous Tissue kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Total RNA (5 μg) was reverse transcribed using random nanomer primers. Real-time PCR was then performed using 18S for normalization and eNOS specific primers.
Western Analysis of Aortic Tissue
Immediately following killing, selected aortas were ground using a Pro 200 tissue homogenizer (Pro Scientific, Oxford, CT) in lysis buffer (20 mM Tris pH 7.4, 2.5 mM EDTA, 100 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 1% Triton X-100, 0.1% SDS, 1% Na deoxycholate, 1 tablet/10 ml EDTA-free Complete protease inhibitor cocktail (Roche, Indianapolis, IN), 1 mM β-glycerolphosphate, 2.5 mM Na pyrophosphate) (Sessa et al., 1995) followed by sonication (10 × 2 seconds burst at low power). The lysate was spun at 28,000 × g for 15 minutes, and the supernatants were then transferred to new tubes, and protein concentrations were determined using a bicinchoninic acid assay (Pierce, Rockford, IL). Equal amounts of sample protein (50 μg/lane) were loaded into wells of a 4 to 12% bis–tris PAGE mini gel. Proteins were separated by electrophoresis and blotted to polyvinylidene difluoride membranes. Membranes were incubated overnight at 4°C in a 1:1000 dilution of primary antibodies directed against eNOS (BD-Transduction, Lexington, KY) or actin (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were visualized using a peroxidase-coupled anti-mouse IgG in the presence of LumiGlo reagent while exposing in a Biorad Chemidoc XRS/HQ (Hercules, CA). Densitometric analysis was accomplished using Biorad Quantity One (Version 4.5.0) software. The densitometric intensity of eNOS in each sample was normalized to the density of actin in that sample.
Statistical Analysis
The data in Fig. 1 were analyzed using analysis of variance (ANOVA). Post hoc analysis to detect differences between specific groups was accomplished with the Student Neuman Keuls test. In all other experiments, data were analyzed with Student’s t-test to determine the significance of treatment effects. The level of statistical significance was taken as p < 0.05.
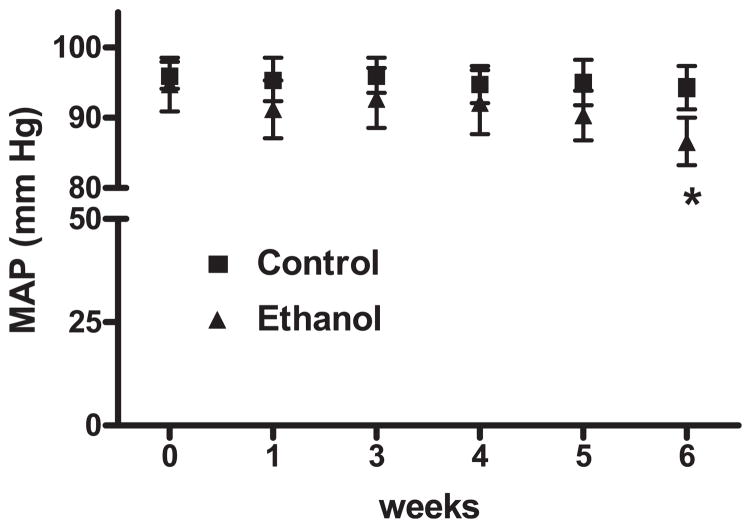
Fig. 1.
Chronic ethanol ingestion decreases blood pressure. Telemetric blood pressure devices were inserted into rats and baseline blood pressure readings were recorded (0 weeks). Each animal was then fed ethanol or control diets for 6 weeks, and blood pressures were recorded weekly. Each point represents the average mean arterial pressure (MAP) ± SEM. *p < 0.05 versus control. n = 4 to 7.
RESULTS
The effects of alcohol consumption on mean arterial pressure (MAP) are shown in Fig. 1. Baseline arterial pressure was not different between control and ethanol-fed rats. MAP in the ethanol-fed rats decreased slightly within 1 week of addition of ethanol and reached a significant decrease from baseline in the sixth week of treatment.
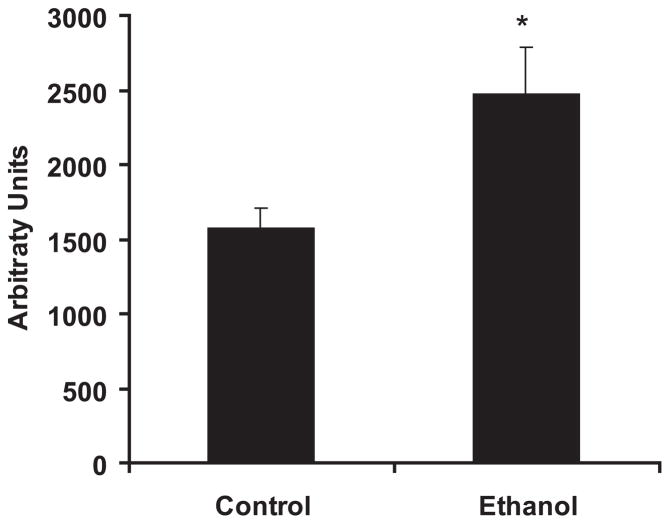
Although previous investigations have examined the effect of chronic alcohol ingestion on vascular function, to our knowledge, this is the first report to directly measure the production of NO by vascular tissue following chronic in vivo ethanol ingestion. Aortic segments harvested from rats fed control or ethanol diets for 6-weeks were subjected to ESR spectroscopic analysis using the spin trap, Fe(DETC)2, which permits detection of NO (Dikalov and Fink, 2005). The 6-week ethanol treatment regimen employed in the current study increased basal NO production compared with aortic segments from control animals (Fig. 2). Signal enhancement detected following the ex vivo treatment of aortic segments from control animals with calcium ionophore (5 μM) as well as the abrogation of the signal by ex vivo treatment with Nω-nitro-L-arginine-methyl ester (1 mM) supported the specificity of this assay for detecting NO (data not shown).
Fig. 2.
Chronic ethanol ingestion increases aortic nitric oxide production. Rats were fed control or ethanol diets for 6 weeks. Nitric oxide production by aortic segments was measured using electron spin resonance (ESR) spectroscopy and the spin trap iron diethyldithio-carbamic acid (Fe(DETC)2). Each bar represents mean nitric oxide (NO) production in arbitrary units ± SEM. *p < 0.05 versus control. n = 7 to 9.
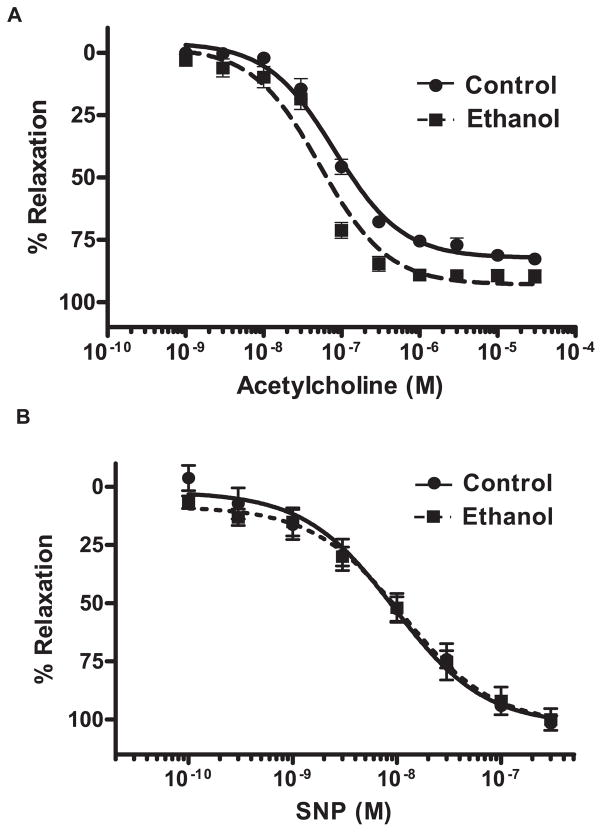
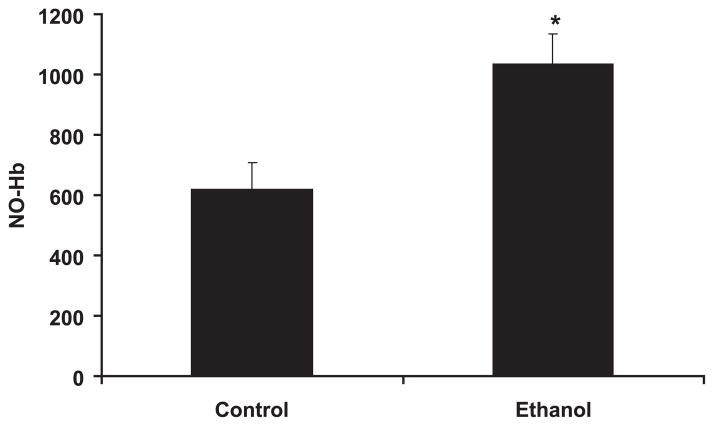
The functional correlate of these ethanol-induced increases in aortic NO production was examined by investigating endothelium-dependent and -independent vasorelaxation responses ex vivo. As illustrated in Fig. 3, aortic rings from ethanol-fed animals tended to demonstrate greater endothelium-dependent relaxation in response to graded concentrations of acetylcholine than did aortic rings from control animals, although this effect did not achieve statistical significance. In contrast, endothelium-independent vasorelaxation responses in aortic rings from control and ethanol-fed rats to the NO generating, SNP, were nearly identical (Fig. 3). To further assess the impact of chronic ethanol ingestion on vascular NO production, NO-Hb was measured as a global indicator of bioavailable NO (Dikalov and Fink, 2005). NO binds to hemoglobin in RBCs forming a complex that is detectable using ESR spectroscopy. As illustrated in Fig. 4, compared with the control diet, chronic ethanol feeding increased NO-Hb levels nearly 2-fold.
Fig. 3.
Chronic ethanol ingestion tends to increase endothelium-dependent relaxation in aortic rings. Rats were fed control or ethanol for 6 weeks. After killing, aortic rings were prepared and mounted on force transducers in a muscle bath. After contracting each ring with L-phenylepherine to 80% maximal contraction, relaxation was measured in response to graded concentrations of (A) acetylcholine (1 × 10−9 to 3 × 10−5 M) or (B) sodium nitroprusside (SNP, 1 × 10−10 to 3 − 10−7 M). Each symbol represents mean percent relaxation from 80% maximal contraction ± SEM from 5 individual animals.
Fig. 4.
Chronic ethanol ingestion increased nitrosyl-hemoglobin (NO-Hb). Rats were fed control or ethanol diets for 6 weeks. Blood was taken on the day of killing, and red blood cells were collected for ESR analysis of NO-Hb. Each bar represents the mean NO-Hb in arbitrary units ± SEM. *p < 0.05 versus control. n = 5 to 7.
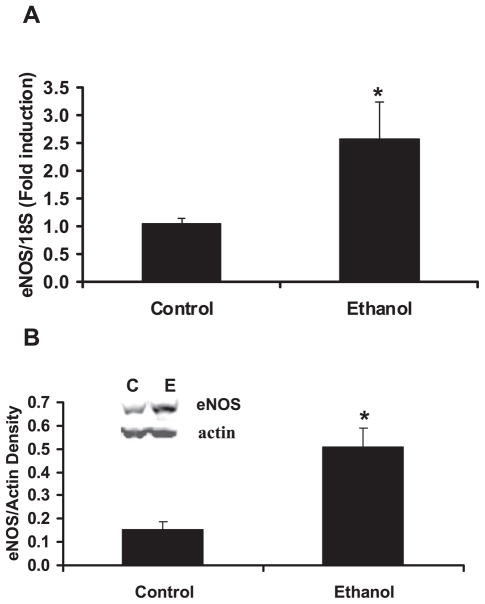
To examine the mechanisms of ethanol-mediated decreases in MAP, and increases in vascular NO production (Figs 2–4), the expression of eNOS in aortas from control- and ethanol-fed rats was investigated. As illustrated in Fig. 5, compared with the control diet, chronic ethanol ingestion increased both eNOS mRNA and protein levels by approximately 2.5-fold.
Fig. 5.
Chronic ethanol ingestion increased aortic endothelial nitric oxide synthase (eNOS) expression. Rats were fed control or ethanol diets for 6 weeks. On killing, the aortas were dissected and homogenized in lysis buffer, or the RNA was isolated for real time PCR. In (A), real time PCR was performed using 18S normalization and eNOS specific primers. Each bar represents the mean copy number for eNOS relative to 18S as % control ± SEM. n = 6. In (B), aortic eNOS protein expression was examined by Western blotting the aortic homogenates (50 μg/lane) for eNOS and actin followed by laser densitometry. Each bar represents the mean density of eNOS relative to actin in each sample ± SEM. n = 5. A representative blot is shown (inset) for eNOS and actin from animals fed control (C) or ethanol (E) diets. *p < 0.05 versus control. n = 5 to 7.
DISCUSSION
The current study employed direct and unequivocal parameters of in vivo vascular NO production to demonstrate that, in male Sprague-Dawley rats, ingestion of the Lieber-DeCarli diet containing 36% of calories as ethanol for 6-weeks decreased blood pressure and increased vascular NO production and NO bioavailability. This ethanol feeding regimen also increased both eNOS mRNA and protein levels in vascular tissue. Evidence that enhanced vascular eNOS expression reduced blood pressure (van Haperen et al., 2002) suggests that the ethanol-induced reductions in blood pressure observed in the current study were mediated, at least in part, by increased vascular eNOS expression and NO production.
Our findings are consistent with previous studies demonstrating that ethanol enhanced NO production by vascular cells in vitro. For example, stimulation with ethanol in vitro for 3 to 6 hours increased NO production in bovine aortic endothelial cells (Venkov et al., 1999) and human umbilical vein endothelial cells (Acevedo et al., 2001). Recently, we demonstrated that more prolonged exposure to clinically relevant concentrations of ethanol for 72 hours increased NO production by pulmonary endothelial cells in vitro by increasing eNOS protein levels through phosphatidylinositol-3 kinase-dependent up regulation of eNOS gene transcription and by increasing interactions between eNOS and heat shock protein 90 (Polikandriotis et al., 2005). Ethanol also produced time-dependent increases in eNOS responsiveness to agonists in cultured endothelial cells (Davda et al., 1993). The role that these in vitro biochemical pathways and mechanisms play in the vascular effects of chronic alcohol ingestion in vivo remains to be defined and constitutes an area of active investigation in our laboratories.
Numerous studies in animal models have provided additional support for the ability of ethanol to modulate vascular NO metabolism. For example, ingestion of Lieber-DeCarli diets containing ethanol comprising 9 or 18% of total dietary calories for 8-weeks altered endothelium-dependent vasorelaxation responses in aortic rings from male Sprague Dawley rats (Abou-Agag et al., 2005). Interestingly, in that study, ingestion of 9% ethanol in the liquid diet for 8-weeks enhanced endothelium-dependent vasorelaxation whereas 18% ethanol for 8-weeks impaired relaxation. Similar to our results, neither concentration of ethanol altered endothelium-independent relaxation responses to SNP. An alternative ethanol feeding regimen (7.5% ethanol in drinking water for 8 weeks) also enhanced endothelium-dependent vasorelaxation responses and increased eNOS protein in the vasculature and NO metabolites in the blood. These findings indicate that the concentration and duration of ethanol ingestion have an important impact on vascular outcomes in animal models.
Similarly, when male Fisher rats were treated with graded concentrations of alcohol for 12-weeks, higher concentrations of alcohol were associated with hypertension, reduced plasma levels of NO metabolites, and enhanced plasma indices of oxidative stress, whereas only the lowest level of alcohol ingestion studied was associated with increased plasma levels of NO metabolites (Husain et al., 2005). This group recently clarified that daily oral treatment with ethanol for 12-weeks caused significant decreases in aortic NO production, reduced endothelium-dependent vasorelaxation, increased indices of vascular oxidative stress, and increased blood pressure that were due in part to alcohol-induced reductions in eNOS and vascular endothelial growth factor expression (Husain, 2007; Husain et al., 2007). It is important to emphasize that differences between models of chronic ethanol ingestion may also contribute to differences in vascular outcomes between studies. For example, in the model of ethanol ingestion employed in the current study, animals consume alcohol in the diet on an ad libitum basis. In contrast, the orogastric approach employed in the studies by Husain et al. (2007) likely produces very different alcohol pharmacokinetics that may have important influences on ethanol-induced gene expression and signaling in the vasculature. In addition, inherent differences between strains of rats employed in these studies could also contribute to differences in vascular responses to alcohol. Despite these technical considerations, the current study along with published reports suggest that ingestion of lower concentrations of ethanol for shorter durations promotes vascular NO bioavailability, whereas ingestion of higher concentrations of alcohol for longer durations promotes vascular oxidative stress and endothelial dysfunction.
The mechanism by which chronic ethanol ingestion increased vascular NO production is not completely defined in the current studies, although ethanol-induced increases in both eNOS mRNA and protein expression suggest that increased vascular eNOS expression and activity contribute to the observed increases in aortic NO production and reductions in blood pressure. Activation of the PI3 kinase/Akt signaling pathways has been implicated in ethanol-induced increases in vascular cell eNOS expression in vitro (Liu et al., 2002; Polikandriotis et al., 2005). Furthermore, using the current model of chronic ethanol ingestion in the rat, we previously reported that ethanol also increased NADPH oxidase expression and oxidative stress in the lung by increasing the activity of components of the renin–angiotensin system (Polikandriotis et al., 2006). More recently, we have reported that this ethanol-feeding regimen also increased eNOS expression and activity in the lung (Polikandriotis et al., 2007). Coupled with previous evidence that angiotensin II increases eNOS expression and endothelial NO production by activating the angiotensin II type 2 receptor (Olson et al., 2004), we postulate that ethanol-induced increases in the activity of the renin–angiotensin system contribute to enhanced vascular eNOS expression and NO production. Furthermore, evidence that angiotensin II-induced increases in endothelial hydrogen peroxide (H2O2) production stimulated eNOS expression in vitro (Drummond et al., 2000), suggests an additional mechanism by which chronic ethanol ingestion may lead to enhanced vascular eNOS expression and aortic NO production.
The dichotomous effects of ethanol on vascular NO production may not only relate to alterations in the expression of eNOS, but also to the regulation of this critical enzyme’s activity. Recent evidence indicates that ethanol can oxidize the eNOS cofactor, tetrahydrobiopterin (Sun et al., 2001, 2006). Oxidation of tetrahydrobiopterin leads to uncoupling of eNOS and production of superoxide rather than NO (Vasquez-Vivar et al., 1998). This concept was supported by evidence that ethanol ingestion for 2 to 3 months in the rat impaired endothelium-dependent vasodilation in cerebral arterioles without altering vascular eNOS expression. Administration of tetrahydrobiopterin to alcohol-fed rats restored endothelium-dependent vasodilation (Sun et al., 2001). More recently, these investigators provided evidence that alcohol stimulated vascular NADPH oxidase-derived reactive oxygen species that participated in tetrahydrobiopterin oxidation (Sun et al., 2006). These results suggest that at higher concentrations, chronic ethanol ingestion may decrease the production of NO by eNOS through NADPH oxidase-induced oxidation of tetrahydrobiopterin leading to reduced NO bioavailability, endothelial dysfunction, and enhanced predilection for vascular disease. Previous evidence, that the alcohol regimen employed in the current model stimulates the simultaneous production of superoxide (Polikandriotis et al., 2006) and nitric oxide (Polikandriotis et al., 2007) in the lung and increases the levels of nitrated and oxidized proteins (Polikandriotis et al., 2007), further suggests that chronic ethanol ingestion can stimulate peroxynitrite formation in vivo. Peroxynitrite leads to vascular dysfunction through a variety of complex mechanisms (Pacher et al., 2007). On the basis of these observations and our current results, we speculate that while the duration and concentration of ethanol ingestion employed in the current report were sufficient to induce both oxidative and nitrosative stress in the lung, the vascular compartment at this time point was relatively protected from these derangements as demonstrated by lack of endothelial dysfunction, enhanced NO bioavailability, and mild reductions in blood pressure.
In summary, the current findings have carefully characterized the impact of a regimen of chronic ethanol ingestion on vascular eNOS expression and NO bioavailability. These findings will be critical for future studies to determine whether prolonged exposure to this ethanol feeding regimen produces progressive vascular oxidative stress sufficient to cause eNOS uncoupling, reduced eNOS expression, or impaired NO bio-availability resulting in endothelial dysfunction and reversal of the observed blood pressure lowering effects. The current report and related publications are consistent with the proposed “J-shaped” relationship between alcohol consumption and cardiovascular disease reported in human epidemiological studies. Evidence that direct intra-arterial ethanol infusion potentiated both endothelium-dependent and endothelium-independent vasodilation in healthy human subjects (Tawakol et al., 2004) emphasizes that ethanol may not only regulate NO metabolism but also the other critical pathways involved in vascular homeostasis. Thus, further studies examining chronic ethanol ingestion and its effects on vascular function will be required to fully define how chronic alcohol ingestion modulates vascular disease.
Acknowledgments
Supported by grants from the Veterans Affairs Research Service (CMH, DMG) and the National Institutes of Health (DK 61274).
The authors gratefully acknowledge the expert technical assistance of Ms. Heidi Rupnow and Mr. Joshua Boutwell.
References
- Abou-Agag LH, Khoo NK, Binsack R, White CR, Darley-Usmar V, Grenett HE, Booyse FM, Digerness SB, Zhou F, Parks DA. Evidence of cardiovascular protection by moderate alcohol: role of nitric oxide. Free Radic Biol Med. 2005;39:540–548. doi: 10.1016/j.freeradbiomed.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Acevedo CG, Carrasco G, Burotto M, Rojas S, Bravo I. Ethanol inhibits L-arginine uptake and enhances NO formation in human placenta. Life Sci. 2001;68:2893–2903. doi: 10.1016/s0024-3205(01)01070-0. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Stampfer MJ, Glynn RJ, Gaziano JM, Manson JE, Goldhaber SZ, Hennekens CH. Prospective study of moderate alcohol consumption and risk of peripheral arterial disease in US male physicians. Circulation. 1997;95:577–580. doi: 10.1161/01.cir.95.3.577. [DOI] [PubMed] [Google Scholar]
- Corrao G, Rubbiati L, Bagnardi V, Zambon A, Poikolainen K. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95:1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- Davda RK, Chandler LJ, Crews FT, Guzman NJ. Ethanol enhances the endothelial nitric oxide synthase response to agonists. Hypertension. 1993;21 (6 Pt 2):939–943. doi: 10.1161/01.hyp.21.6.939. [DOI] [PubMed] [Google Scholar]
- Dikalov S, Fink B. ESR techniques for the detection of nitric oxide in vivo and in tissues. Methods Enzymol. 2005;396:597–610. doi: 10.1016/S0076-6879(05)96052-7. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- German JB, Walzem RL. The health benefits of wine. Annu Rev Nutr. 2000;20:561–593. doi: 10.1146/annurev.nutr.20.1.561. [DOI] [PubMed] [Google Scholar]
- van Haperen R, De Waard M, Van Deel E, Mees B, Kutryk M, Van Aken T, Hamming J, Grosveld F, Duncker DJ, De Crom R. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277:48803–48807. doi: 10.1074/jbc.M209477200. [DOI] [PubMed] [Google Scholar]
- Hill JA. In vino veritas: alcohol and heart disease. Am J Med Sci. 2005;329:124–135. doi: 10.1097/00000441-200503000-00003. [DOI] [PubMed] [Google Scholar]
- Hulley SB, Gordon S. Alcohol and high-density lipoprotein cholesterol: causal inference from diverse study designs. Circulation. 1981;64(3 Pt 2):III, 57–63. [PubMed] [Google Scholar]
- Husain K. Vascular endothelial oxidative stress in alcohol-induced hypertension. Cell Mol Biol (Noisy-le-grand) 2007;53:70–77. [PubMed] [Google Scholar]
- Husain K, Mejia J, Lalla J, Kazim S. Dose response of alcohol-induced changes in BP, nitric oxide and antioxidants in rat plasma. Pharmacol Res. 2005;51:337–343. doi: 10.1016/j.phrs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Husain K, Vazquez-Ortiz M, Lalla J. Down regulation of aortic nitric oxide and antioxidant systems in chronic alcohol-induced hypertension in rats. Hum Exp Toxicol. 2007;26:427–434. doi: 10.1177/0960327106072993. [DOI] [PubMed] [Google Scholar]
- Kiechl S, Willeit J, Egger G, Oberhollenzer M, Aichner F. Alcohol consumption and carotid atherosclerosis: evidence of dose-dependent atherogenic and antiatherogenic effects. Results from the Bruneck Study. Stroke. 1994;25:1593–1598. doi: 10.1161/01.str.25.8.1593. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol, coronary disease, and hypertension. Annu Rev Med. 1996;47:149–160. doi: 10.1146/annurev.med.47.1.149. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi R, Steiner M. Ethanol raises prostacyclin in vivo and in vitro. Blood. 1984;64:679–682. [PubMed] [Google Scholar]
- Liu J, Tian Z, Gao B, Kunos G. Dose-dependent activation of antiapoptotic and proapoptotic pathways by ethanol treatment in human vascular endothelial cells: differential involvement of adenosine. J Biol Chem. 2002;277:20927–20933. doi: 10.1074/jbc.M110712200. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kronmal RA, Mittleman MA, O’Leary DH, Polak JF, Cushman M, Siscovick DS. Alcohol consumption and carotid atherosclerosis in older adults: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2003;23:2252–2259. doi: 10.1161/01.ATV.0000101183.58453.39. [DOI] [PubMed] [Google Scholar]
- Olson S, Oeckler R, Li X, Du L, Traganos F, Zhao X, Burke-Wolin T. Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L559–L568. doi: 10.1152/ajplung.00312.2003. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Brown LA, Hart CM. Chronic ethanol ingestion increases nitric oxide production in the lung. Alcohol. 2007;41:309–316. doi: 10.1016/j.alcohol.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Hart CM. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase-and hsp90-dependent mechanisms. Alcohol Clin Exp Res. 2005;29:1932–1938. doi: 10.1097/01.alc.0000187597.62590.a4. [DOI] [PubMed] [Google Scholar]
- Renaud SC, Beswick AD, Fehily AM, Sharp DS, Elwood PC. Alcohol and platelet aggregation: the Caerphilly Prospective Heart Disease Study. Am J Clin Nutr. 1992;55:1012–1017. doi: 10.1093/ajcn/55.5.1012. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Garcia-Cardena G, Liu J, Keh A, Pollock JS, Bradley J, Thiru S, Braverman IM, Desai KM. The Golgi association of endothelial nitric oxide synthase is necessary for the efficient synthesis of nitric oxide. J Biol Chem. 1995;270:17641–17644. doi: 10.1074/jbc.270.30.17641. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- Sun H, Patel KP, Mayhan WG. Tetrahydrobiopterin, a cofactor for NOS, improves endothelial dysfunction during chronic alcohol consumption. Am J Physiol Heart Circ Physiol. 2001;281:H1863–H1869. doi: 10.1152/ajpheart.2001.281.5.H1863. [DOI] [PubMed] [Google Scholar]
- Sun H, Zheng H, Molacek E, Fang Q, Patel KP, Mayhan WG. Role of NAD(P)H oxidase in alcohol-induced impairment of endothelial nitric oxide synthase-dependent dilation of cerebral arterioles. Stroke. 2006;37:495–500. doi: 10.1161/01.STR.0000199033.06678.c3. [DOI] [PubMed] [Google Scholar]
- Sutliff RL, Dikalov S, Weiss D, Parker J, Raidel S, Racine AK, Russ R, Haase CP, Taylor WR, Lewis W. Nucleoside reverse transcriptase inhibitors impair endothelium-dependent relaxation by increasing superoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2363–H2370. doi: 10.1152/ajpheart.00151.2002. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Omland T, Creager MA. Direct effect of ethanol on human vascular function. Am J Physiol Heart Circ Physiol. 2004;286:H2468–H2473. doi: 10.1152/ajpheart.01207.2003. [DOI] [PubMed] [Google Scholar]
- Thornton J, Symes C, Heaton K. Moderate alcohol intake reduces bile cholesterol saturation and raises HDL cholesterol. Lancet. 1983;2:819–822. doi: 10.1016/s0140-6736(83)90738-9. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkov CD, Myers PR, Tanner MA, Su M, Vaughan DE. Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost. 1999;81:638–642. [PubMed] [Google Scholar]