Abstract
During surveys of dying vegetation in natural ecosystems and associated waterways in Australia many new taxa have been identified from Phytophthora ITS Clade 6. For representative isolates, the region spanning the internal transcribed spacer region of the ribosomal DNA, the nuclear gene encoding heat shock protein 90 and the mitochondrial cox1 gene were PCR amplified and sequenced. Based on phylogenetic analysis and morphological and physiological comparison, four species and one informally designated taxon have been described; Phytophthora gibbosa, P. gregata, P. litoralis, P. thermophila and P. taxon paludosa. Phytophthora gibbosa, P. gregata and P. taxon paludosa form a new cluster and share a common ancestor; they are homothallic and generally associated with dying vegetation in swampy or water-logged areas. Phytophthora thermophila and P. litoralis are sister species to each other and more distantly to P. gonapodyides. Both new species are common in waterways and cause scattered mortality within native vegetation. They are self-sterile and appear well adapted for survival in an aquatic environment and inundated soils, filling the niche occupied by P. gonapodyides and P. taxon salixsoil in the northern hemisphere. Currently the origin of these new taxa, their pathogenicity and their role in natural ecosystems are unknown. Following the precautionary principle, they should be regarded as a potential threat to native ecosystems and managed to minimise their further spread.
Keywords: aquatic habitat, breeding systems, evolution, phylogeny, radiation, sterility, survival
INTRODUCTION
During a recent re-evaluation of the Phytophthora collection maintained by the Vegetation Health Service of the Department of Environment and Conservation in Western Australia (WA), many new undescribed taxa and unique isolates were identified (Burgess et al. 2009) within what is known as ITS Clade 6 (Cooke et al. 2000). Prior to the advent of molecular systematics in Phytophthora, Clade 6 was represented by just three species: P. gonapodyides, P. humicola and P. megasperma (Erwin & Ribeiro 1996). However, an extensive review on the evolution, ecology, reproduction and impact of Clade 6 Phytophthoras (Brasier et al. 2003a) introduced eight new informally designated taxa, of which two have subsequently been described as Phytophthora inundata (Brasier et al. 2003b) and P. rosacearum (Hansen et al. 2009b). Additionally, P. taxon asparagi (Saude et al. 2008), a still unnamed pathogen of Asparagus officinalis, and P. pinifolia, a serious foliar pathogen of Pinus radiata in Chile (Durán et al. 2008), have also been described. Recently, further new taxa have been elucidated, but as yet not formally described (i.e. P. taxon hungarica and P. taxon sulawesiensis).
Clade 6 Phytophthoras show a strong association with both forests and riparian ecosystems and, with the exceptions of P. taxon asparagi, P. gonapodyides, P. megasperma and P. rosacearum, have limited association with agriculture and horticulture. The function of most of these taxa within the ecosystems is very unclear. Brasier et al. (2003a, b) hypothesized a saprotrophic lifestyle for taxa in this clade and their presence, and even dominance, in environmental water surveys is evidence for this (Hansen et al. 2009a, Hwang et al. 2009, Remigi et al. 2009, Hulvey et al. 2010, Reeser et al. 2011). However, some members of ITS Clade 6, such as P. pinifolia, P. inundata, P. taxon PgChlamydo and P. gonapodyides, can be opportunistic and sometimes aggressive tree pathogens (Brown & Brasier 2007, Durán et al. 2008, Jung & Nechwatal 2008, Jung 2009).
Based on ITS sequence data, Clade 6 can be divided into three sub-clades. Sub-clade III to date only contains P. taxon asparagi, while sub-clade I contains P. inundata, P. humicola, P. rosacearum and some undescribed taxa, separated by relatively long branch lengths. Sub-clade II contains P. megasperma, P. gonapodyides and the majority of the undescribed taxa, and is characterised by short branch lengths with high support for terminal clades, but weak support for deeper branches suggesting recent radiation from an ancestral type. Within sub-clade II there are several undescribed taxa, including P. sp. 3, P. sp. 7 and P. sp. 11, so far found only in Australian natural ecosystems where they are associated with plant mortalities (Burgess et al. 2009).
In this study DNA sequence data from the rDNA internal transcribed spacer regions (ITS) and part of the nuclear heat shock protein 90 (HSP90) and the mitochondrial cox1 genes were used in combination with morphological and physiological characteristics to describe four new species and a new taxon within sub-clade II: P. gibbosa, P. gregata, P. litoralis, P. thermophila and P. taxon paludosa.
MATERIAL AND METHODS
Sampling and Phytophthora isolation
Soil and root samples were collected from beneath dying, Phytophthora-sensitive ‘indicator species’ in native ecosystems. Samples were baited with Eucalyptus sieberi cotyledons (Marks & Kassaby 1974) that were plated after 5 d and 10 d onto NARPH (Hüberli et al. 2000), or P10VPH (Tsao & Guy 1977) selective media, from which pure cultures of Phytophthora were then isolated. In some cases plant roots were surface-sterilised in 70 % ethanol for 30 s followed by four rinses in distilled water, and plated directly onto selective media. Some isolates were derived from stream or water baiting. Leaves of several plant species, including Citrus limon, Quercus robur and Pittosporum undulatum, were suspended in mesh bags in streams, rivers or other water bodies for 3–5 d. Small sections of necrotic lesions on leaves were then plated onto selective media. Cultures derived from earlier research and surveys have been incorporated (Table 1).
Table 1.
Identity, host, location, isolation information and GenBank accession numbers for Clade 6 Phytophthora isolates used in this study.
| Reference collection no. 1 , 2 | Other collection no. | Identity | Substrate | Host | Location | Isolated by | Date | GenBank Accession No.
3
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| ITS | cox1 | HSP90 | ||||||||
| VHS21998§* | ||||||||||
| CBS127951 | P. gibbosa | Soil | Acacia pycnantha | Australia, WA, Scott River | VHS | 2009 | HQ012933 | HQ012846 | HQ012892 | |
| VHS21999* | P. gibbosa | Soil | Xanthorrhoea gracilis | Australia, WA, Scott River | VHS | 2009 | HQ012934 | HQ012847 | HQ012893 | |
| VHS22007* | P. gibbosa | Soil | A. pycnantha | Australia, WA, Scott River | VHS | 2009 | HQ012935 | HQ012848 | HQ012894 | |
| VHS22008* | P. gibbosa | Soil | Grevillea sp. | Australia, WA, Scott River | VHS | 2009 | HQ012936 | HQ012849 | HQ012895 | |
| DCE68* | P. gregata | Soil | Road-side (highway) | Australia, WA, Byford | 1965 | EU301171 | HQ012851 | HQ012897 | ||
| HSA2356* | P. gregata | Roots | B. prionotes | Australia, WA, Cataby | R Hart | 1996 | HQ012938 | HQ012852 | HQ012898 | |
| MJS235* | P. gregata | Soil | Pinus radiata | Australia, WA, Nannup | MJC Stukely | 1982 | EU301172 | HQ012853 | HQ012899 | |
| MJS238* | P. gregata | Root collar | P. radiata | Australia, WA, Nannup | MJC Stukely | 1981 | EU301173 | HQ012854 | HQ012900 | |
| MUCC759 | P. gregata | Soil | Eucalyptus sp. | Australia, VIC, Toolangi North State Forest | WA Dunstan | 2008 | HQ012939 | |||
| MUCC760* | P. gregata | Soil | Pasture | Australia, VIC, Devlins Bridge | WA Dunstan | 2008 | HQ012940 | HQ012855 | HQ012901 | |
| VHS9854* | P. gregata | Soil | X. preissii | Australia, WA, Lancelin | VHS | 2001 | EU301174 | HQ012856 | HQ012902 | |
| VHS21961 | P. gregata | Soil | Hakea sp. | Australia, WA, Busselton | VHS | 2009 | HQ012941 | HQ012857 | HQ012903 | |
| VHS21962§* | ||||||||||
| CBS127952 | P. gregata | Soil | Patersonia sp. | Australia, WA, Busselton | VHS | 2009 | HQ012942 | HQ012858 | HQ012904 | |
| VHS21992* | P. gregata | Soil | Native forest | Australia, WA, Scott River | VHS | 2009 | HQ012943 | HQ012859 | HQ012905 | |
| IMI389723 | P1047 | P. gonapodyides | Soil | Quercus robur | Germany, Rhineland, Bienwald | T Jung | 1996 | AF541889 | ||
| IMI389727 | P897 | P. gonapodyides | Soil | Native forest | Australia, TAS, Pine Lake | K Shanahan | 1996 | AF541888 | ||
| MUCC761* | P. gonapodyides | Water | E. obliqua forest | Australia, VIC, Toolangi North | WA Dunstan | 2008 | HQ012937 | HQ012850 | HQ012896 | |
| NY393 | P. gonapodyides | Malus sylvestris | USA, New York | AY129175 | ||||||
| IMI302303 | ||||||||||
| CBS200.81 | P. humicola | Soil | Citrus | Taiwan | PJ Ann & WH Ko | 1981 | AF266792 | EU080172 | ||
| HUMI-P6701 | P. humicola | AJ Uddin | AB367496 | |||||||
| VHS16836 | P. inundata | Soil | X. preissii | Australia, WA, Boyup Brook | VHS | 2007 | HQ012944 | HQ012860 | ||
| VHS19081 | P. inundata | Soil | B. attenuata | Australia, WA, Bold Park | VHS | 2008 | HQ012945 | HQ012861 | ||
| IMI389750 | P210 | P. inundata | Roots | Aesculus hippocastanum | UK, Buckinghamshire, Claydon | CM Brasier | 1970 | AF541912 | EU079945 | |
| MUCC762* | P. litoralis | Water | Stream baiting | Australia, WA, Borden | D Hüberli | 2008 | HQ012946 | HQ012862 | HQ012907 | |
| MUCC763* | P. litoralis | Water | Stream baiting | Australia, WA, Borden | D Hüberli | 2008 | HQ012947 | HQ012863 | HQ012908 | |
| VHS17085* | P. litoralis | Soil | Banksia sp. | Australia, WA, Hopetoun | VHS | 2007 | EU593262 | HQ012864 | HQ012909 | |
| VHS19173 | P. litoralis | Soil | X. preissii | Australia, WA, Wilga | VHS | 2008 | EU869119 | HQ012865 | HQ012910 | |
| VHS20763§* | ||||||||||
| CBS127953 | P. litoralis | Soil | Banksia sp. | Australia, WA, Ravensthorpe | VHS | 2008 | HQ012948 | HQ012866 | HQ012911 | |
| DDS3432* | P. megasperma | Soil | Banksia sp. | Australia, WA, North Dinninup | VHS | 1992 | HQ012949 | HQ012867 | HQ012906 | |
| VHS17183 | P. megasperma | Soil | X. platyphylla | Australia, WA, Esperance | VHS | 2007 | EU301166 | HQ012868 | HQ012912 | |
| IMI389741 | P1278 | P. megasperma | M. sylvestris | Australia, WA, | HL Harvey | 1968 | AF266794 | AY129211 | ||
| P476 | P. megasperma | Apricot | USA, California | SM Mircetich | AF514897 | |||||
| P3136 | P. megasperma | Brassica napus | Australia | 1985 | EU080062 | |||||
| CMW26667 | P. pinifolia | Needles | P. radiata | Chile, Arauco, Llico plantation | A Durán | 2007 | EU725805 | |||
| CMW26668 | P. pinifolia | Needles | P. radiata | Chile, Arauco, Llico plantation | A Durán | 2007 | EU725806 | |||
| IMI389749 | P462 | P. rosacearum | Malus sp. | USA, California, Sonoma County | SM Mircetich | 1979 | AF541911 | |||
| MUCC764* | P. thermophila | Water | Stream baiting | Australia, WA, Brunswick | GEStJ Hardy | 2008 | HQ012950 | HQ012869 | HQ012913 | |
| VHS3655 | P. thermophila | Soil | Native forest | Australia, WA, Quinninup | VHS | 1998 | HQ012951 | HQ012870 | HQ012914 | |
| VHS7474* | P. thermophila | Soil | Native forest | Australia, WA, Manjimup | VHS | 2000 | HQ012952 | HQ012871 | HQ012915 | |
| VHS13530§* | ||||||||||
| CBS127954 | P. thermophila | Soil | E. marginata | Australia, WA, Dwellingup | VHS | 2004 | EU301155 | HQ012872 | HQ012916 | |
| VHS13567 | P. thermophila | Roots | E. marginata | Australia, WA, Dwellingup | VHS | 2004 | EU301156 | HQ012873 | HQ012917 | |
| VHS13761 | P. thermophila | Soil | E. marginata | Australia, WA, Dwellingup | VHS | 2004 | EU301157 | HQ012874 | HQ012918 | |
| VHS16164* | P. thermophila | Soil | B. grandis | Australia, WA, Pemberton | VHS | 2006 | EU301158 | HQ012875 | HQ012919 | |
| VHS17175 | P. taxon asparagi | Soil | B. media | Australia, WA, Esperance | VHS | 2007 | EU301167 | HQ012844 | HQ012890 | |
| VHS17644 | P. taxon asparagi | Soil | Lomandra sonderi | Australia, WA, Murdoch | VHS | 2007 | EU301168 | HQ012845 | HQ012891 | |
| P10690 | P. taxon asparagi | Asparagus officinalis | New Zealand, Whakatane | 1986 | FJ801481 | EU080568 | ||||
| IMI389747 | P1054 | P. taxon forestsoil | Soil | Native forest | France, Alsace, Illwald forest | EM Hansen | 1998 | AF541908 | ||
| UASWS0315 | P. taxon forestsoil | Soil | Alnus glutinosa | Poland, Kolo | L Belbahri | 2006 | EF522138 | |||
| MUCC768 | P. taxon humicola-like | Water | Stream baiting | Australia, WA, Esperance | D Hüberli | 2008 | HQ012959 | HQ012883 | HQ012927 | |
| MUCC769 | P. taxon humicola-like | Water | Stream baiting | Australia, WA, Esperance | D Hüberli | 2008 | HQ012960 | HQ012884 | HQ012928 | |
| UASWS0318 | P. taxon hungarica | Soil | A. glutinosa | Poland, Kolo | L Belbahri | 2006 | EF522141 | |||
| UASWS0321 | P. taxon hungarica | Soil | A. glutinosa | Poland, Adamowizna | L Belbahri | 2006 | EF522144 | |||
| MUCC770 | P. taxon kwongan | Soil | Hibbertia sp. | Australia, WA, Cooljarloo | WA Dunstan | 2008 | HQ012957 | HQ012881 | HQ012925 | |
| IMI329669 | TCH009 | P. taxon kwongan | Roots | B. prionotes | Australia, WA, Cervantes | TC Hill | 1986 | EU593265 | HQ012889 | HQ012932 |
| HAS2313 | P. taxon kwongan | Water | Baiting | Australia, WA, Cooljarloo | R Hart | 1996 | HQ012961 | HQ012885 | HQ012929 | |
| IMI389733 | P1055 | P. taxon oaksoil | Soil | Q. robur | France, Alsace, Illwald Forest | EM Hansen | 1998 | AF541906 | ||
| MUCC765* | P. taxon paludosa | Water | Pond baiting | Australia, VIC, Sugarloaf Reservoir Reserve | WA Dunstan | 2008 | HQ012953 | HQ012876 | HQ012920 | |
| IMI389730 | P236 | P. taxon PgChlamydo | Roots | Prunus sp. | UK, Cheltenham | CM Brasier | 1982 | AF541900 | ||
| P10456 | P. taxon PgChlamydo | USA, California | D Ferrin | 2002 | EU079573 | |||||
| DDS3753 | P. taxon PgChlamydo | Soil | Native forest | Australia, WA, Manjimup | VHS | 1995 | EU301160 | HQ012878 | HQ012922 | |
| VHS6595 | P. taxon PgChlamydo | Soil | Native forest | Australia, WA, Manjimup | VHS | 1999 | EU301159 | HQ012879 | HQ012923 | |
| MUCC766 | P. taxon PgChlamydo | Water | Stream baiting | Australia, VIC, Yea Wetlands | WA Dunstan | 2008 | HQ012955 | |||
| P11555 | P. taxon personii | Nicotiana tobacum | USA | 2006 | EU080316 | |||||
| VHS14801 | P. taxon personii | Soil | Grevillea mccutcheonii | Australia, WA, Busselton | VHS | 2005 | EU301169 | HQ012877 | HQ012921 | |
| MUCC767 | P. taxon personii | Water | Stream baiting | Australia, VIC, Ti-Tree Creek, Melba Highway | WA Dunstan | 2008 | HQ012954 | |||
| IMI389745 | P1049 | P. taxon raspberry | Roots | Rubus idaeus | Australia, VIC, | G McGregor | 1996 | AF541904 | ||
| IMI389744 | P896 | P. taxon raspberry | Soil | Dying vegetation | Australia, TAS, Pine Lake | K Shanahan | 1997 | AF541903 | ||
| CH97TUL2 | P. taxon raspberry | Tulipa gesneriana | Japan, Chiba, Shirako | AJ Uddin | AB367377 | |||||
| IMI389746 | P1050 | P. taxon raspberry | Roots | R. idaeus | Sweden, Scania | CHB Olsson | 1994 | AF541905 | ||
| RAS1 | P. taxon raspberry | Soil | Betula pendula | Germany, Bavaria, Neuburg | T Jung | 2006 | HQ012964 | HQ012888 | ||
| 92-209C | P. taxon raspberry | Ilex aquifolia | Switzerland | EU106591 | ||||||
| UASWS0213 | P. taxon raspberry | Soil | A. glutinosa | Poland | L Belbahri | DQ396425 | ||||
| 2FFL-2008 | P. taxon raspberry | Hungary | I Szabo | 2008 | EU594600 | |||||
| P1044 | P. taxon riversoil | Soil | Riparian vegetation | UK, Worcestershire, Riverbank | J Delcan | 1997 | AF541907 | |||
| DDS2909 | P. taxon rosacearum-like | Soil | P. radiata | Australia, WA, Albany | MJC Stukely | 1989 | HQ012958 | HQ012882 | HQ012926 | |
| HAS2529 | P. taxon rosacearum-like | Water | Baiting | Australia, WA, Cooljarloo | R Hart | 1998 | HQ012962 | HQ012886 | HQ012930 | |
| HSA2530 | P. taxon rosacearum-like | Water | Baiting | Australia, WA, Cooljarloo | R Hart | 1998 | HQ012963 | HQ012887 | HQ012931 | |
| IMI389726 | P878 | P. taxon salixsoil | Root | Alnus sp. | Denmark, Funen, Odense | K Thinggaard | 1995 | AF541909 | ||
| HSA1959 | P. taxon salixsoil | Water | Road drainage sump baiting | Australia, WA, Welshpool | R Hart | 1994 | HQ012956 | HQ012880 | HQ012924 | |
| IMI389725 | P245 | P. taxon salixsoil | Roots | Salix sp. | UK, Kent, Bexley Heath | CM Brasier | 1972 | AF266793 | EU080534 | |
| P6306 | P. taxon sulawesiensis | Root | Syzygium aromaticum | Indonesia, Sulawesi | MD Coffey | 1989 | FJ801912 | |||
| IMI389735 | P532 | P. taxon walnut | Juglans hindsii | USA, California, Merced County, | SM Mircetich | 1988 | AF541910 | |||
1 Abbreviations of isolates and culture collections: CBS = Centraalbureau voor Schimmelcultures Utrecht, Netherlands; IMI = CABI Bioscience (International Mycological Institute), UK; VHS = Vegetation Health Service Collection, Department of Environment and Conservation, Perth, Australia; DDS = earlier prefix of VHS Collection; TCH = TC Hill, in VHS Collection; MJS = MJC Stukely, in VHS Collection; HSA = Hart, Simpson and Associates, in VHS Collection; DCE = EM Davison, in VHS Collection; MUCC = Murdoch University Culture Collection.
2 Numbers in bold are isolates used in the morphological studies; numbers with asterisk are isolates used in the growth rate studies; § denotes type isolates.
3 GenBank numbers in italics are from previous studies.
DNA isolation, amplification and sequencing
The Phytophthora isolates were grown on half-strength potatodextrose agar (PDA, Becton, Dickinson and Company, Sparks, MD 21152 USA; 19.5 g PDA, 7.5 g of agar and 1 L of distilled water) at 20 °C for 2 wk and the mycelium was harvested by scraping from the agar surface with a sterile blade and placed in a 1.5 mL sterile Eppendorf® tube. Harvested mycelium was frozen in liquid nitrogen, ground to a fine powder and genomic DNA was extracted according to Andjic et al. (2007).
The region spanning the internal transcribed spacer (ITS1–5.8S–ITS2) region of the ribosomal DNA was PCR amplified and sequenced using the primers ITS6 (Cooke et al. 2000) and ITS4 (White et al. 1990). For representative isolates from each species, heat shock protein 90 (HSP90) gene was amplified with HSP90_F1 and HSP90_R2 (Blair et al. 2008). Templates were sequenced in both directions with primers HSP90_F1int, HSP90_F3, HSP90_F2, HSP90_R1 and HSP90_R2 (Blair et al. 2008). After an initial comparison of sequences the first half of the HSP90 gene was found to be more variable for ITS Clade 6 Phytophthoras, and remaining isolates were sequenced with only HSP90_F1int and HSP90_R1. The mitochondrial gene cox1 was amplified with primers FM84 and FM83 (Martin & Tooley 2003). Templates were sequenced in both directions with primers used in amplification, as well as primers FM 85 and FM 50 (Martin & Tooley 2003).
The PCR reaction mixture to amplify the three gene regions, the clean-up of products and sequencing were as described by Andjic et al. (2007). The PCR conditions for ITS, HSP90 and cox1 amplification were as described by Andjic et al. (2007), Blair et al. (2008) and Martin & Tooley (2003), respectively. All sequences derived in this study were deposited in GenBank and accession numbers are given in Table 1.
Phylogenetic analysis
The sequence data of Phytophthora isolates used in this study were compared with other closely related species (ITS Clade 6) including undescribed taxa available from GenBank (http://www.ncbi.nlm.nih.gov/). Sequence data for the ITS region were initially aligned and subsequent manual adjustments made using Geneious Pro v4.8.1 (Drummond et al. 2010).
Using the same aligned datasets, parsimony analysis and partition homogeneity tests were performed in PAUP (Phylogenetic Analysis Using Parsimony) v4.0b10 (Swofford 2003) and Bayesian analysis was conducted with MrBayes v3.1 (Ronquist & Huelsenbeck 2003) using the same constraints and models as described previously (Jung & Burgess 2009). All datasets and trees derived from parsimony and Baysian analyses are available from TreeBASE (10764; http://www.treebase.org/).
Morphology of asexual and sexual structures
Sporangia, hyphal swellings, chlamydospores and gametangia of four isolates of P. gibbosa, nine isolates of P. gregata, five isolates of both P. litoralis and P. thermophila, two isolates of P. megasperma and one isolate of P. taxon paludosa (Table 1) were measured on V8 agar (V8A) (16 g agar, 3 g CaCO3, 100 mL Campbell’s V8 juice, 900 mL distilled water), as described in detail by Jung et al. (1999). Sporangia were produced by flooding 15 × 15 mm agar squares taken from growing margins of 3–5 d old colonies, so that their surfaces were just covered with non-sterile soil extract (100 g of soil from a Eucalyptus marginata stand suspended in 1 L distilled water, incubated for 24 h at 20 °C and then filtered through cheesecloth followed by Whatman no. 1 paper) in 9 cm Petri dishes which were incubated at 18–22 °C in natural daylight. The soil extract was decanted and replaced again after 6 and 12 h. After 24–36 h dimensions and characteristic features of 50 mature sporangia and 25 exit pores, and zoospore cysts per isolate, chosen at random, were determined at ×400 magnification (BX51, Olympus). After 5–7 d, 25 hyphal swellings and 50 chlamydospores, if formed, were also measured.
Isolates grown in the dark on V8A plates at 20 °C for 14–21 d were examined for the presence of oogonia. Isolates which had either a high incidence of oogonial abortion or did not produce, or only inconsistently produced oogonia in single culture were paired on V8A with isolates of the same species, with A1 and A2 tester strains of P. cambivora (MP45, MP73), P. cinnamomi (MP75, DCE60) and P. cryptogea (MP21, MP22), and with Trichoderma reesei (Brasier 1972). Inoculum plugs (5 mm diam) of the isolate to be tested and the tester isolate were placed on opposite sides of a 9 cm Petri dish, 2 cm from the edge. The plates were incubated at 20 °C in darkness and scored for oogonial formation 30 d after the two colonies had met. For each isolate producing oogonia (either in single culture or when paired), dimensions and characteristic features of 50 mature oogonia, oospores and antheridia chosen at random were measured at ×400. The oospore wall index was calculated as the ratio between the volume of the oospore wall and the volume of the entire oospore (Dick 1990). Descriptions, illustrations and nomenclatural data were deposited in MycoBank (www.mycobank.org; Crous et al. 2004).
Colony morphology, growth rates and cardinal temperatures
Hyphal morphology and colony growth patterns were described from 7 d old cultures grown at 20 °C in the dark on V8A, malt extract agar (MEA), and half-strength PDA (all from BBL, Becton, Dickinson & Co, Sparks MD 21152 USA). Colony morphologies were described according to patterns observed previously (Erwin & Ribeiro 1996, Brasier et al. 2003a, Jung et al. 2003).
For temperature-growth relationships, representative isolates (Table 1) were sub-cultured onto V8A plates and incubated for 24 h at 20 °C to stimulate onset of growth (Hall 1993). Then three replicate plates per isolate were transferred to 15, 20, 25, 30, 32.5, 35 and 37 °C. Radial growth was recorded after 5–7 d later along two lines intersecting the centre of the inoculum at right angles and the mean growth rates (mm per day) were calculated. Plates showing no growth at 35 and 37 °C were returned to 20 °C to determine isolate viability.
RESULTS
Phylogenetic analysis
Within the ITS region, the majority of mutations are single base pair mutations and there are occasional small indels of 1–3 bp. There were no gaps in the cox1 and HSP90 alignments. Excluding outgroups, the aligned datasets for ITS (79 sequences), HSP90 (51 sequences) and cox1 (49 sequences) consisted of 846, 1 024 and 1 189 characters, respectively. Based on partition homogeneity tests in PAUP, the ITS and HSP datasets were congruent (P = 0.12), but they were not combined due to the lack of HSP90 sequence data for many of the designated taxa. The cox1 dataset was incongruent with both the nuclear gene regions (P < 0.01).
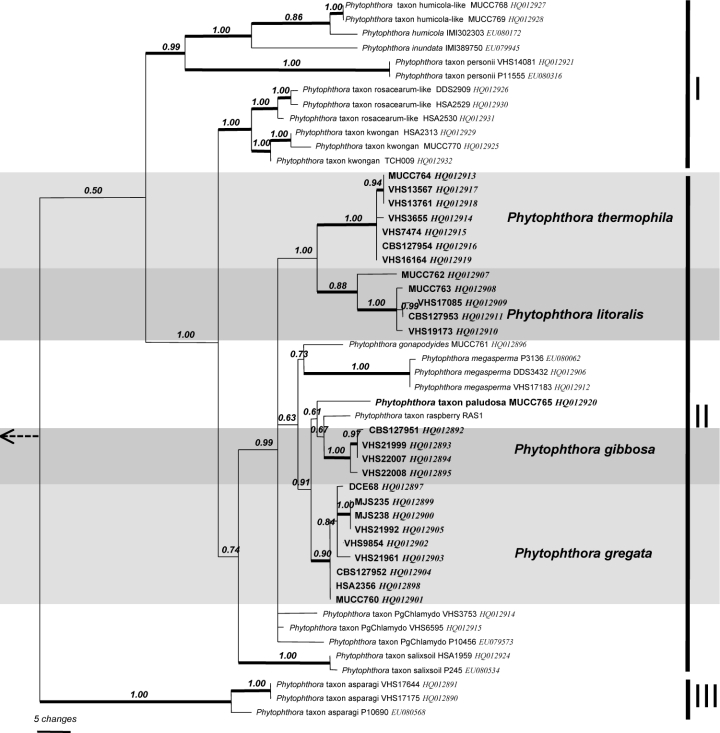
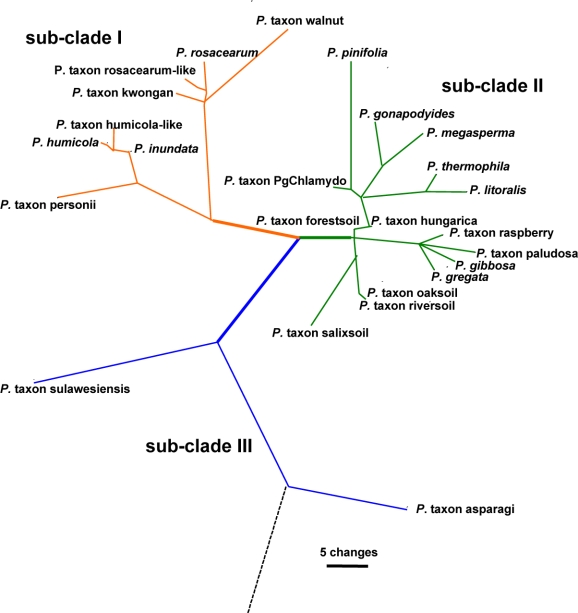
ITS characterisation of 10 species and 15 designated taxa — Including outgroups, the aligned ITS dataset contained 927 characters of which 195 were parsimony informative (158 or 18.67 % of sites are variable within Clade 6 alone) with significant (P < 0.01, g1 = −0.46) phylogenetic signal compared to 1 000 random trees. Heuristic searches resulted in 108 most parsimonious trees of 455 steps (CI = 0.61, RI = 0.89). The Bayesian analysis provided more support for deeper branches. Support for terminal clades and their clustering was equivalent in both analyses and the Bayesian analysis is presented here (Fig. 1, TreeBASE 10764). As previously reported (Brasier et al. 2003a), the analysis resolved three sub-clades. There are 59 variable sites in sub-clade I (7.13 %) and 83 in sub-clade II (10.03 %). Sub-clade I contained two clusters: the first included P. inundata, P. humicola, P. humicola-like and P. taxon personii, whilst the second cluster contained P. rosacearum, P. taxon rosacearum-like, P. taxon kwongan and P. taxon walnut. Sub-clade III is represented by P. taxon asparagi, and P. taxon sulawesiensis, for which only a single sequence is available on GenBank.
Fig. 1.
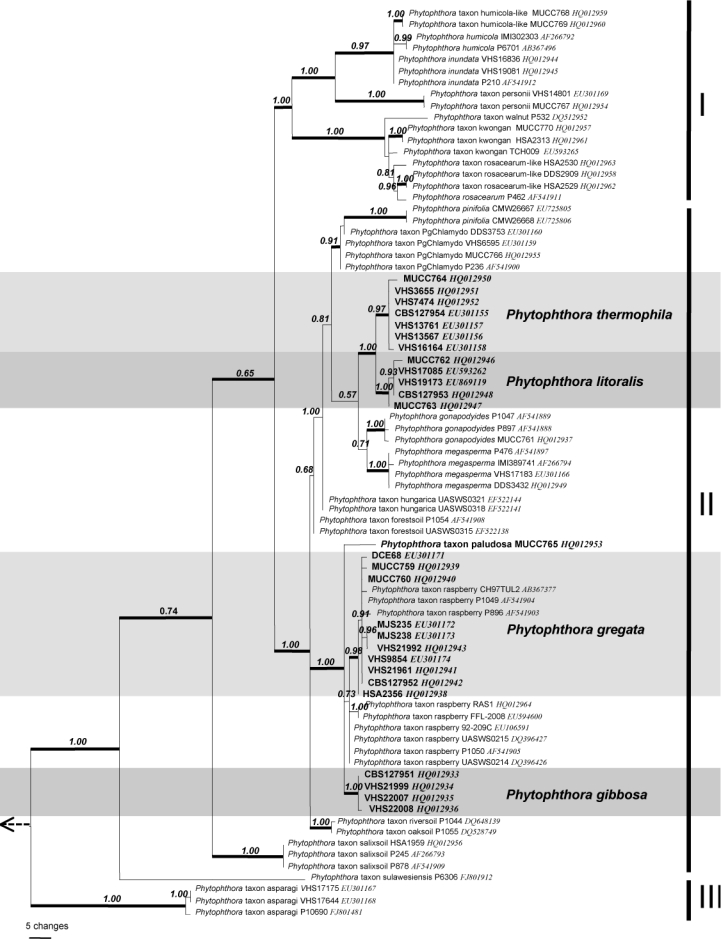
Bayesian inference tree based on rDNA ITS sequences showing phylogenetic relationships within Phytophthora ITS Clade 6. Numbers above the branches represent posterior probability values based on Bayesian analysis, thickened branches represent a bootstrap support of > 70 % based on parsimony analysis. Sub-clades I–III are indicated on right. Phytophthora cinnamomi, P. katsurae and P. palmivora were used as outgroup taxa (not shown).
Sub-clade II is larger and contains 14 discrete lineages corresponding to three described species (P. gonapodyides, P. megasperma, P. pinifolia), four new species (P. gibbosa, P. gregata, P. litoralis, P. thermophila) and seven designated phenotypic taxa (P. taxon forestsoil, P. taxon hungarica, P. taxon oaksoil, P. taxon paludosa, P. taxon PgChlamydo, P. taxon riversoil, P. taxon salixsoil). Phytophthora thermophila and P. litoralis are closely related but differ in the ITS region by 10 steps (changes). At a higher level they cluster with P. gonapodyides, P. megasperma and P. taxon PgChlamydo with distances between lineages of 10–24 steps. These five lineages share a common ancestor. Phytophthora pinifolia is loosely associated with this cluster of species. The other two new species, P. gibbosa and P. gregata, are also closely related to each other, but differ by 7–9 steps. Together with P. taxon raspberry and a single isolate designated here as P. taxon paludosa, they form a highly supported cluster within sub-clade II. Isolates obtained in Australia and previously designated as P. taxon raspberry (Brasier et al. 2003a) have identical ITS sequence to P. gregata. Additionally, a single isolate from Japan submitted to GenBank as P. citricola also resides within P. gregata. Phytophthora taxon raspberry isolates from Europe, available on GenBank, form a separate lineage 2–3 bp removed from P. gregata. Detailed morphological examination of European isolates is required to determine if they represent a sister species to P. gregata.
Phytophthora taxon salixsoil is basal to sub-clade II. Lineages corresponding to designated taxa from Europe, P. taxon forestsoil, P. taxon hungarica, P. taxon riversoil and P. taxon oaksoil, are excluded from the two species clusters described above.
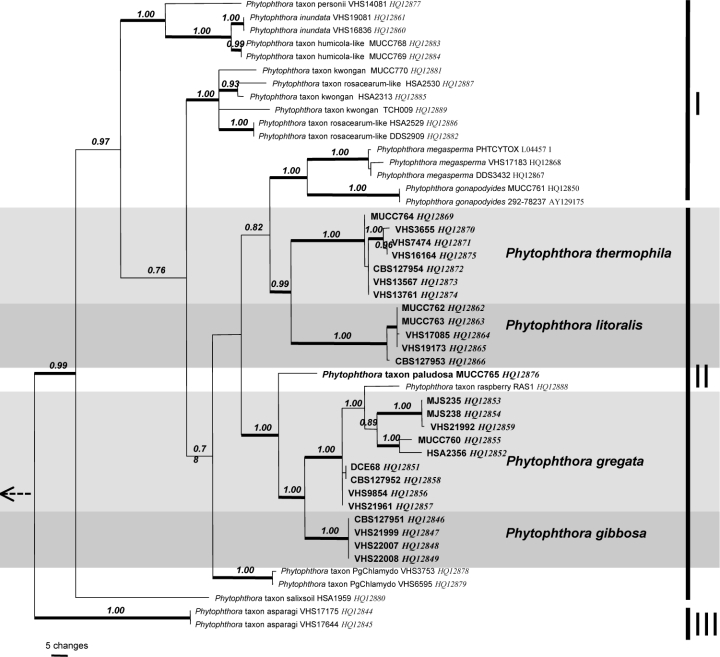
HSP90 characterisation of eight species and nine designated taxa — The HSP90 dataset contained 142 parsimony informative characters (127 within ITS Clade 6) and significant (P < 0.01, g1 = −0.51) phylogenetic signal compared to 1 000 random trees. Heuristic searches resulted in 32 most parsimonious trees of 328 steps (CI = 0.57, RI = 0.83). Bayesian analysis produced trees with similar topology and is presented here (Fig. 2, TreeBASE 10764). Sub-clade I was divided into the same two clusters observed in the ITS analysis, although in the HSP90 analysis these clusters are more distant. Sub-clade II comprises 10 discrete lineages in two main clusters. Phytophthora thermophila and P. litoralis group together as do P. gregata, P. gibbosa and single isolates each of P. taxon paludosa and P. taxon raspberry. As in the ITS analysis, P. megasperma and P. gonapodyides group together; however, due to the inclusion of only a single isolate of P. gonapodyides, this cluster is not fully resolved. Four sequences submitted to GenBank as P. gonapodyides were misidentified and actually correspond to the basal taxa, P. taxon salixsoil and P. taxon PgChlamydo. Sub-clade III is represented by P. taxon asparagi.
Fig. 2.
Bayesian inference tree based on HSP90 sequences showing phylogenetic relationships within Phytophthora ITS Clade 6. Numbers above the branches represent posterior probability values based on Bayesian analysis, thickened branches represent a bootstrap support of > 70 % based on parsimony analysis. Sub-clades I–III are indicated on right. Phytophthora cinnamomi, P. katsurae and P. palmivora were used as outgroup taxa (not shown).
cox1 characterisation of seven species and nine designated taxa — The cox1 dataset contained 187 parsimony informative characters (172 within ITS Clade 6) and significant (P < 0.01, g1 = −0.81) phylogenetic signal compared to 1 000 random trees. Heuristic searches resulted in four most parsimonious trees of 575 steps (CI = 0.49, RI = 0.81). Bayesian analysis produced trees with similar topology and is presented here (Fig. 3; TreeBASE 10764). Sub-clade I was divided into the same two clusters observed in the HSP90 analysis. Sub-clade II comprised 8 lineages. Phytophthora thermophila and P. litoralis resided in strongly supported terminal clades separated by 54–59 steps. There is greater intraspecific variability in the cox1 data than in the two nuclear genes with 8 steps variation among isolates of P. thermophila and four among isolates of P. litoralis. As in the ITS analysis they form a cluster with P. megasperma and P. gonapodyides and these four species appear to share a common ancestor.
Fig. 3.
Bayesian inference tree based on mitochondrial gene cox1 sequences showing phylogenetic relationships within Phytophthora ITS Clade 6. Numbers above the branches represent posterior probability values based on Bayesian analysis, thickened branches represent a bootstrap support of > 70 % based on parsimony analysis. Sub-clades I–III are indicated on right. Phytophthora infestans, P. multivora and P. nicotianae were used as outgroup taxa (not shown).
The cox1 sequence data of all P. gibbosa isolates is identical, however large intraspecific variability (22 steps) was observed among isolates designated as P. gregata based on nuclear gene sequence. A European isolate of P. taxon raspberry clustered with P. gregata in the cox1 analysis. As with the nuclear gene regions, P. taxon paludosa together with P. gibbosa and P. gregata formed a strongly supported cluster indicating a common ancestor. In this analysis, P. taxon PgChlamydo is basal to sub-clade II and P. taxon salixsoil is basal to sub-clades I and II. Sub-clade III is represented by P. taxon asparagi.
TAXONOMY
Morphological and physiological characters and measurements of the five new Phytophthora taxa and related species are given in the comprehensive Table 2.
Table 2.
Morphological characters, dimensions and temperature–growth relations of Phytophthora gibbosa, P. gregata, P. litoralis, P. thermophila, P. taxon paludosa, P. gonapodyides, P. megasperma and P. drechsleri. Characters decisive for species discrimination are highlighted in bold.
| P. gibbosa | P. gregata | P. litoralis | P. thermophila | P. taxon paludosa | P. gonapodyides | P. megasperma 1 | P. drechsleri | |
|---|---|---|---|---|---|---|---|---|
| No. of isolates/source | 4 | 9 | 5 | 5 | 1 | Brasier et al. (1993) | 2 | Erwin & Ribeiro (1996) |
| Sporangia | Ovoid, ellipsoid, limoni-form, nonpapillate, some semipapillate | Ovoid, limoniform, obpyriform, nonpapillate | Ovoid, limoniform, nonpapillate | Ovoid, ellipsoid, limoni-form, nonpapillate, often with tapering base | Ovoid, limoniform, non-papillate or semi-papillate | Ellipsoid, obpyriform, ovoid, nonpapillate | Ovoid, obpyriform, nonpapillate | Obpyriform, ovoid or elongated, nonpapillate, often with tapering base |
| l × b mean (μm) | 48.8 ± 9.6 × 30.8 ± 5.4 | 51.0 ± 13.8 × 30.5 ± 5.9 | 43.6 ± 7.7 × 29.4 ± 5.4 | 44.8 ± 6.3 × 25.7 ± 3.9 | 54.7 ± 4.3 × 43.3 ± 3.7 | 53.7 × 34.3 2 | 59.3 ± 8.8 × 42.8 ± 4.5 | 52 × 28 |
| Total range (μm) | 24.8–71.1 × 17.4–48.0 | 25.7–102.3 × 14.8–50.7 | 27.8–76.9 × 16.0–40.4 | 29.0–64.8 × 15.6–39.3 | 43.1–64.6 × 31.4–51.7 | 48–64 × 25–40 | 37–84 × 35–56 | 40–71 × 22–34 |
| Isolate means (μm) | 44.8–52.2 × 27.9–33.0 | 37.3–72.7 × 25.6–35.0 | 39.7–53.4 × 27.1–33.0 | 44.2–46.8 × 24.1–26.6 | ||||
| l/b ratio | 1.58 ± 0.15 | 1.67 ± 0.32 | 1.51 ± 0.26 | 1.78 ± 0.26 | 1.27 ± 0.09 | 1.43 | 1.39 ± 0.2 | 1.9 ± 0.3 |
| Isolate means | 1.57–1.60 | 1.37–2.19 | 1.35–1.73 | 1.67–1.86 | − | 1.48–2.06 | 1.34–1.40 | |
| Exit pores | ||||||||
| Width (μm) | 12.7 ± 3.5 | 10.7 ± 2.7 | 11.9 ± 2.7 | 13.9 ± 2.9 | 10.6 ± 2.1 | na | 12.4 ± 1.2 | na |
| Isolate means (μm) | 10.0–14.6 | 8.4–14.1 | 10.0–14.4 | 9.7–16.4 | − | na | 11.8–12.4 | na |
| Proliferation | Internal extended and external, never nested | Internal extended and nested, never external, sporangiophore partly branching inside empty sporangium | Internal nested and extended and external, secondary lateral sporangia | Internal extended and nested, never external, sporangiophore may branch inside or outside empty sporangium | Internal extended and external, never nested | Internal nested and extended & external | Internal nested and extended, never external | Internal nested and extended |
| Hyphal swellings | Subglobose, elongated 1 , never catenulate | Globose, elongated, angular, partly catenulate | Globose, elongated, angular, partly catenulate | Globose or elongated, partly catenulate | Globose or elongated, partly catenulate | Globose or elongated, only in some isolates | Globose or angular, catenulate or clustered | Globose or angular, catenulate or clustered |
| Mean diam (μm) | 18.7 ± 5.0 | 14.8 ± 3.8 | 15.7 ± 4.7 | 12.6 ± 2.3 | 16.2 ± 3.1 | 25.4 ± 1.8 | na | |
| Hyphal aggregations | − | Abundant, up to 170 μm | − | Rare, small | − | − | − | − |
| Chlamydospores | − | − | Globose, radiating hyphae; in 1 isolate | Globose, radiating hyphae; in 3 isolates | − | − | − | Globose, only in some isolates |
| Mean diam (μm) | 34.3 ± 5.3 | 41.5 ± 14.7 | 7.9(4–11) | |||||
| Sexual system | Homothallic | Homothallic or partially or sporadically self fertile | Sterile or selfsterile silent A1 | Sterile or potentially self fertile (1 isolate selfing in soil filtrate) | Homothallic | Sterile, silent A1 | Homothallic | Heterothallic |
| Oogonia | c. 50 % ornamented | Smooth | Smooth | Smooth | Smooth | Smooth | ||
| Mean diam (μm) | 38.1 ± 5.4 | 36.8 ± 4.1 | − | 31.1 ± 2.5 | 33.3 ± 3.5 | − | 41.8 ± 2.4 3 | 33 |
| Total range (μm) | 27.0–49.9 | 23.9–50.9 | − | 27.2–38.0 | 24.4–40.7 | − | 27–52 3 | 28–38 |
| Isolate means (μm) | 36.6–39.7 | 34.0–39.8 | − | |||||
| Oospores | Always aplerotic | Usually aplerotic | − | Highly aplerotic | Always aplerotic | − | Usually aplerotic | Plerotic |
| Mean diam (μm) | 31.4 ± 4.6 | 31.6 ± 4.0 | − | 23.6 ± 2.2 | 28.1 ± 2.8 | − | 33.8 ± 2.4 3 | 28 |
| Total range (μm) | 18.9–39.4 | 21.4–45.3 | − | 20.4–29.7 | 21.7–34.3 | − | 23–42 3 | 16–37 |
| Isolate means (μm) | 30.0–33.0 | 27.8–35.5 | − | − | ||||
| Wall thickness (μm) | 3.17 ± 0.69 | 2.65 ± .0.81 | − | 2.3 ± 0.7 | 2.5 ± 0.4 | − | 3.31 ± 0.4 | 3.0 |
| Oospore wall index | 0.49 ± 0.06 | 0.42 ± 0.09 | − | 0.46 ± 0.09 | 0.44 ± 0.04 | − | 0.46 ± 0.06 | na |
| Abortion rate of isolates | 16–37 % | 52.5–99.8 % | − | < 10 % | < 10 % | < 10 % | na | |
| Antheridia | Amphigynous | Predominantly paragynous | Paragynous | Predominantly paragynous | Paragynous and amphigynous | Amphigynous | ||
| l × b mean (μm) | 13.6 ± 2.4 × 14.0 ± 2.0 | 17.1 ± 3.0 × 11.0 ± 1.8 | 15.5 ± 2.4 × 9.3 ± 0.9 | 16.6 ± 3.7 × 13.0 ± 1.5 | − | 13 ± 1.5 × 10.4 ± 1.3 | 14–15 × 13 | |
| Total range (μm) | 10.6–24.9 × 7.6–17.8 | 10.6–24.9 × 7.6–17.8 | 11.1–20.9 × 7.6–16.6 | 8.1–23.8 × 10.6–15.0 | − | 10.7–15.8 × 8.1–13 | na | |
| Maximum temperature (°C) | 32.5–< 35 | 32.5–< 35 | 32.5–< 35 | 35–< 37 | 32.5 | 30–< 35 | 32.5 | 35–37 |
| Optimum temperature (°C) | 30 | 25 (1 isolate 30) | 30 | 32.5 | 25 | 25–30 | 22.5–25 | 25–30 |
| Growth rate on V8A at optimum (mm/d) | 6.3 ± 0.3 | 6.5 ± 0.7 | 4.6 ± 0.3 | 4.8 ± 0.6 | 6.3 | na | 6.7 ± 0.1 | na |
| Growth rate on V8A at | 5.2 ± 0.1 | 5.2 ± 0.6 | 4.0 ± 0.3 | 3.2 ± 0.5 | 5.5 | na | 6.6 ± 0.1 | na |
1 measurements were made on isolates available in the current study and the ranges are in agreement with those reported from numerous studies by Erwin & Ribeiro (1996).
2 data of the British P. gonapodyides group.
3 data only from isolate VHS 17183 since isolate DDS 3432 did not produce oogonia in single culture.
Phytophthora gibbosa T. Jung, M.J.C. Stukely & T.I. Burgess, sp. nov. — MycoBank MB518763; Fig. 4
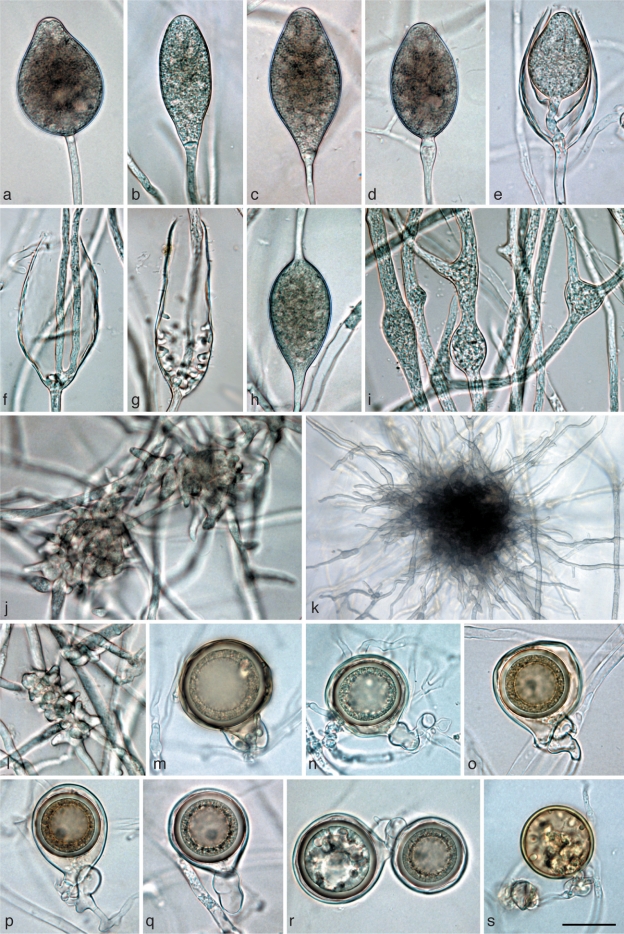
Fig. 4.
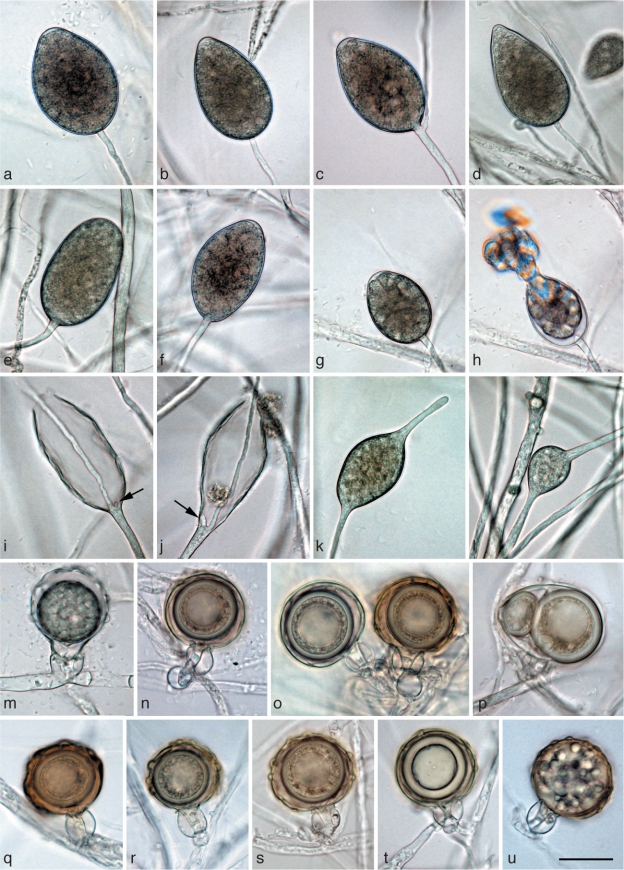
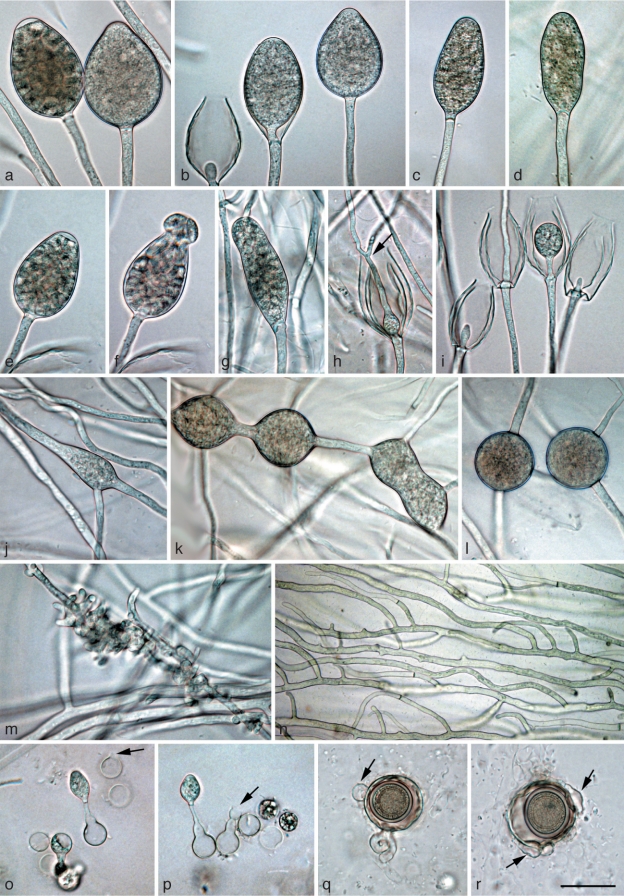
Morphological structures of Phytophthora gibbosa. a–l. Structures formed on V8 agar flooded with soil extract: a, b. ovoid semipapillate sporangia; c. ovoid semipapillate sporangium with external proliferation; d. obpyriform sporangium with nonpapillate pointed apex; e. nonpapillate ellipsoid sporangium; f. ovoid slightly excentric sporangium; g. ovoid sporangium with swollen apex shortly before release of the already differentiated zoospores; h. same sporangium as in g releasing zoospores; i, j. empty elongated ovoid and limoniform sporangium, respectively, showing both internal extended proliferation and formation of an additional basal undeveloped sporangiophore (arrows); k, l. intercalary hyphal swellings originating from undeveloped sporangia that did not form a basal septum and continued to grow at their apex; m. immature ornamented oogonium with aplerotic oospore and amphigynous intercalary antheridium; n–s. mature often bronze-brown oogonia with amphigynous antheridia and thick-walled aplerotic oospores each containing a large ooplast: n. smooth-walled; o, q–s. ornamented gibbose oogonia; p. excentric smooth-walled oogonium with two oospores; t. gibbose oogonium with thickwalled aborted oospore; u. gibbose golden-brown oogonium aborted before oospore formation. — Scale bar = 25 μm.
Systema sexus homothallica; oogonia terminalia vel lateralia, in medio 40 μm (28–48 μm), globosa, subglobosa vel rare excentrica, in medio 31 % abortiva, paries saepe gibbosi et maturitate frequenter pigmentati aureo-fusci ad aerei, rare cum duis oosporis. Oosporae apleroticae, in medio 32 μm (24–38 μm), paries in medio 3.2 μm (1.9–4.3 μm). Antheridia singulares, terminalia, lateralia vel interdum intercalaria, unicellularia, hyalina, globosa ad cylindrica, in medio 14 × 14 μm (11–16 × 11–17 μm), semper amphigynosa. Sporangiophora simplicia vel rare ramosa sympodiis laxis. Sporangia abundantia in cultura liquida, terminalia, nonpapillata vel interdum semipapillata, ovoidea, ellipsoidea vel limoniformia, in medio 52 × 33 μm (31–71 × 20–45 μm), ratio longitudo ad altitudinem in medio 1.6 (1.3–2.1). Proliferationes sporangiorum semper internae et extentae, numquam niduformes vel externae. Inflationes hypharum subglobosae ad elongatae, numquam catenulatae. Chlamydosporae non observatae. Temperaturae crescentiae in agaro ‘V8A’, optima 30 °C et maxima 33–< 35 °C. Coloniae in agaro ‘V8A’ uniformes et pubescentes. Regiones ‘rDNA ITS’, ‘cox1’ et ‘HSP90’ cum unica sequentia (GenBank HQ012933, HQ012846, HQ012892).
Etymology. Name refers to the gibbous ornamented surface of the oogonia (gibbosa Latin = gibbous, knaggy).
Sporangia and hyphal swellings (Fig. 4a–l) — Sporangia of P. gibbosa were not observed on solid agar but were produced abundantly in non-sterile soil extract. Sporangia were typically borne terminally on unbranched sporangiophores, less frequently in lax sympodia. Sporangia were non-caducous, semipapillate (Fig. 4a–c) or more often nonpapillate (Fig. 4d–f), usually with a flat apex (Fig. 4a–c, e–g), sometimes with a pointed apex (Fig. 4d). Sporangial shapes ranged from ovoid (59 %; Fig. 4a–c, f–h) to elongated ovoid (18 %; Fig. 4i), ellipsoid (11 %; Fig. 4e), limoniform (10 %; Fig. 4j) or less frequently pyriform or obpyriform (1 %; Fig. 4d). Sporangia proliferated either externally (Fig. 4c) or internally in an extended way, often with the formation of an additional basal sporangiophore initial which usually remained short (Fig. 4i, j). Nested proliferation was never observed. Zoospores of P. gibbosa were discharged directly through an exit pore 7.7–21.7 μm wide (av. 12.7 ± 3.5 μm) (Fig. 4h). They were limoniform to reniform whilst motile, becoming spherical (av. diam = 12.9 ± 1.6 μm) on encystment. Cysts usually germinated by forming a hypha (98 %). Diplanetism (= germination of cysts by release of a secondary zoospore) or formation of a microsporangium was rarely observed in one isolate (VHS22008). Sporangial dimensions of four isolates of P. gibbosa averaged 48.8 ± 9.6 × 30.8 ± 5.4 μm (overall range 24.8–71.1×17.4–48.0 μm) with a range of isolate means of 44.8–52.2 × 27.9–33.0 μm. The length/breadth ratio averaged 1.58 ± 0.15. In liquid culture, hyphal swellings were regularly formed which, according to their morphology, were most likely undeveloped sporangia which had failed to form a basal septum and continued to grow at their apex (Fig. 4k, l). Small, globose hyphal swellings along sporangiophores were only rarely observed.
Oogonia, oospores and antheridia (Fig. 4m–u) — Gametangia were readily produced in single culture by all isolates of P. gibbosa on V8A within 4 d. Oogonia were borne terminally or laterally, had either wavy edged to ornamented gibbous (32–68 %, on av. 53 %; Fig. 4m, q–u) or smooth walls (Fig. 4n–p) and were usually globose, subglobose or slightly excentric. In all isolates oogonial walls often turned golden-brown to bronze-brown (Fig. 4n, o, q–s, u) while ageing. Oogonial diameters averaged 38.1 ± 5.4 μm (overall range 27.0–49.9 μm and range of isolate means 36.6–39.7 μm). Oospores had a mean diameter of 31.4 ± 4.6 μm (total range 18.9–39.4 μm), were always aplerotic, usually globose and contained a large ooplast (Fig. 4m–t). The oospores were relatively thick-walled (3.17 ± 0.69 μm; total range 1.2–5.1 μm), with a mean oospore wall index of 0.49 ± 0.06. On average 30 % (16–37 %) of the oogonia aborted either before (Fig. 4u) or after oospore formation (Fig. 4t). Some oogonia contained two oospores of unequal sizes (Fig. 4p). The antheridia were exclusively amphigynous, averaging 13.6 ± 2.4×14.0 ± 2.0 μm, with shapes ranging from subglobose to cylindrical or irregular (Fig. 4m–u). They were usually formed terminally or laterally, and were rarely intercalary (Fig. 4m).
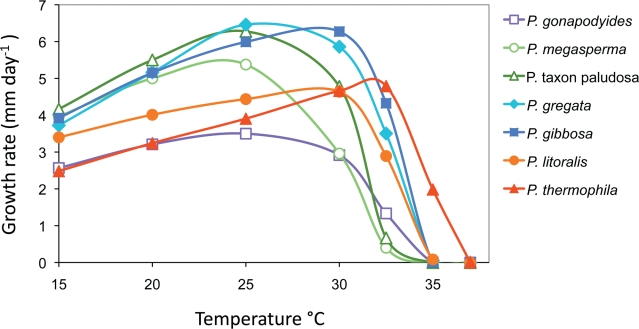
Colony morphology, growth rates and cardinal temperatures (Fig. 9, 11) — All four P. gibbosa isolates formed similar uniform colonies on the four different types of media (Fig. 9). Colonies on V8A and MEA had limited aerial mycelium, while colonies on PDA appeared woolly and growth on CMA was mostly submerged with very sparse aerial mycelium. All isolates had identical cardinal temperatures and similar growth rates at all temperatures. The temperature–growth relations are shown in Fig. 11. The maximum growth temperature was between 32.5 and 35 °C. All isolates were unable to grow at 35 °C, and isolates did not resume growth when plates incubated for 7 d at 35 °C were transferred to 20 °C. The average radial growth rate on V8A at the optimum temperature of 30 °C was 6.3 ± 0.3 mm/d. At 20 °C mean growth rates on V8A, MEA, CMA and PDA were 5.2, 5.9, 6.0 and 5.0 mm/d, respectively.
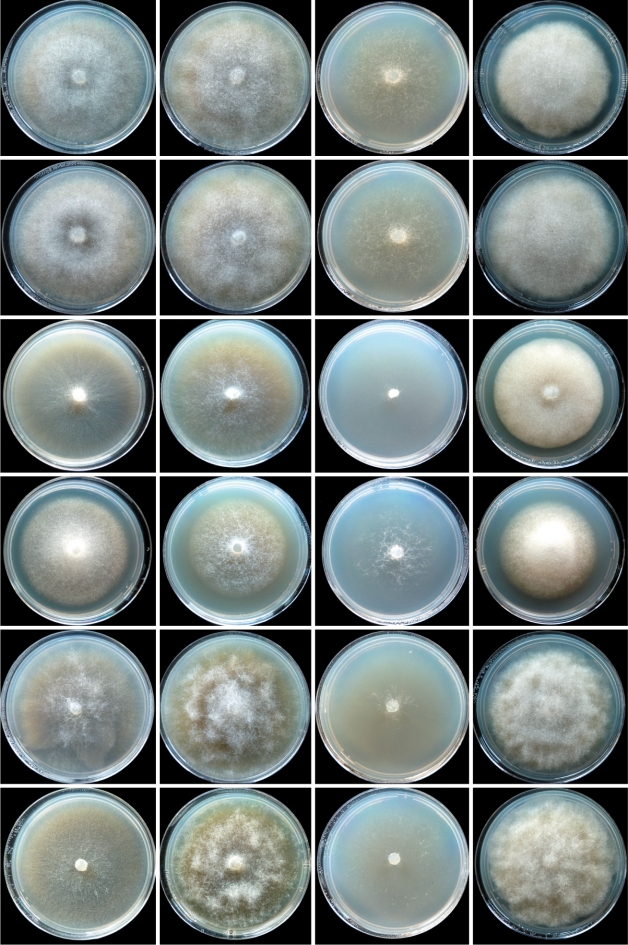
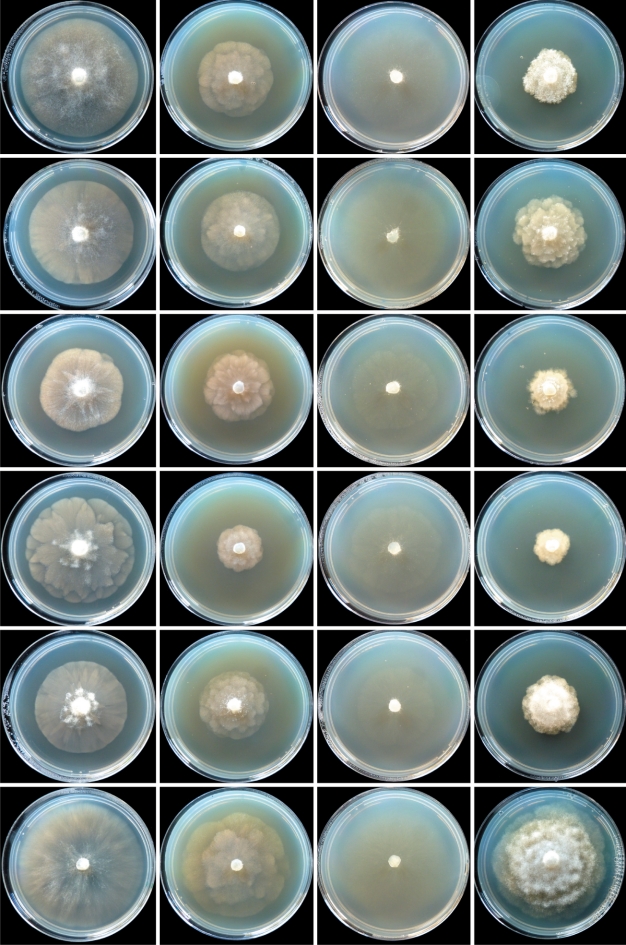
Fig. 9.
Colony morphology of Phytophthora gibbosa isolates CBS127951 (ex-type) and VHS22007, P. gregata isolates CBS127952 (ex-type), MJS235 and VHS9854, and P. taxon paludosa isolate MUCC765 (from top to bottom) after 7 d growth at 20 °C on V8 agar, malt extract agar, corn meal agar and potato-dextrose agar (from left to right).
Fig. 11.
Mean radial growth rates of Phytophthora gibbosa (four isolates), P. gregata (eight isolates), P. litoralis and P. thermophila (each four isolates), P. taxon paludosa, P. gonapodyides and P. megasperma (each one isolate) on V8 agar at different temperatures.
Specimens examined. Western Australia, Scott River ironstones, from rhizosphere soil of dying Acacia pycnantha, 2009, VHS, holotype MURU 461 (dried culture on V8A, Herbarium of Murdoch University, Western Australia), cultures ex-type CBS127951 and VHS21998; Scott River ironstones, from rhizosphere soil of dying Xanthorrhoea gracilis, 2009, VHS, VHS21999; Scott River ironstones, from rhizosphere soil of dying Acacia pycnantha, 2009, VHS, VHS22007; Scott River ironstones, from rhizosphere soil of dying Grevillea sp., 2009, VHS, VHS22008.
Phytophthora gregata T. Jung, M.J.C. Stukely & T.I. Burgess, sp. nov. — MycoBank MB518764; Fig. 5
Fig. 5.
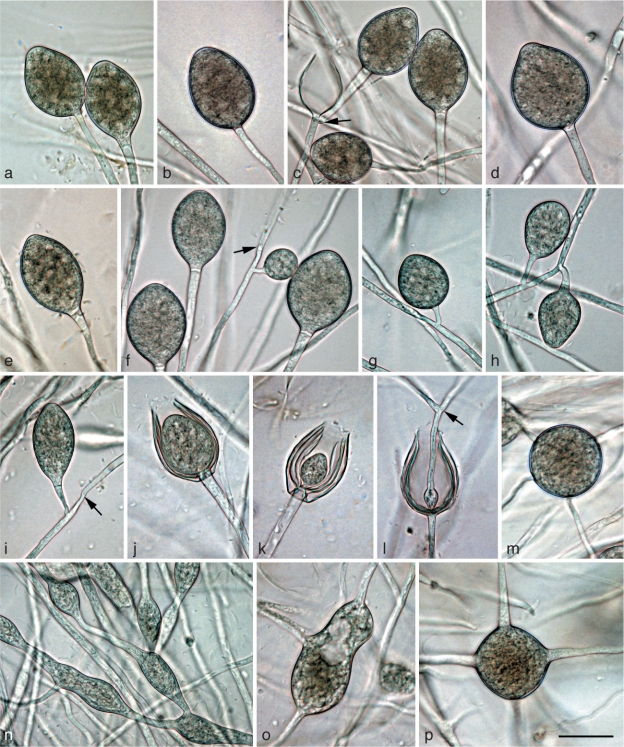
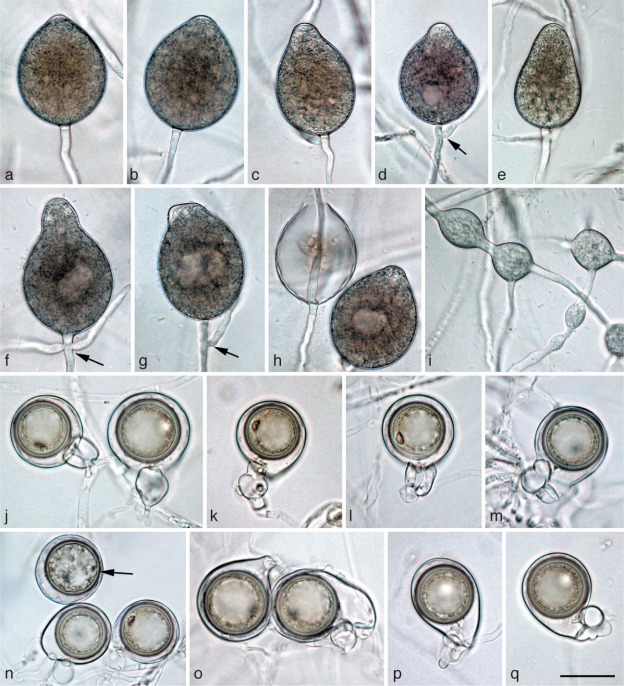
Morphological structures of Phytophthora gregata. a–l. Structures formed on V8 agar flooded with soil extract: a. obpyriform nonpapillate sporangium; b. elongated-ellipsoid nonpapillate sporangium with a tapering base; c. limoniform nonpapillate sporangium; d. ovoid nonpapillate sporangium with a conspicuous basal plug and a widening of the sporangiophore towards the sporangial base; e. ovoid empty sporangium with internal nested proliferation, and obpyriform young sporangium; f. ovoid empty sporangium with internal extended proliferation via two sporangiophores; g. elongated sporangium with internal extended proliferation and a multitude of wall ingrowths; h. hyphal swellings formed from undeveloped sporangium that did not form a basal septum and continued to grow at the apex; i. elongated and angular hyphal swellings; j–l. hyphal aggregations; m–q. mature oogonia containing a thick-walled oospore with a large ooplast and a nucleus: m, n. oogonia produced by selfing of P. gregata isolates MUCC760 (m) and CBS127952 (n) in paired cultures with P. cinnamomi mating type A2 isolate DCE60; m. with nearly plerotic oospore and amphigynous antheridium; n. with aplerotic oospore and paragynous antheridium; o, p. oogonia produced by selfing in paired cultures of P. gregata isolates MJS235 and MUCC760; o. excentric with tapering base, aplerotic oospore and paragynous antheridium; p. elongated with aplerotic oospore and paragynous antheridium; q. with nearly plerotic oospore and paragynous antheridium, produced by isolate VHS21961 in paired culture with Trichoderma reesei; r. mature oogonia with thick-walled aborted (left) and viable oospore (right) produced by isolate VHS21961 in paired culture with T. reesei; s. golden-brown aborted oogonium produced by selfing of isolate CBS127952 in paired culture with P. cinnamomi mating type A2 isolate DCE60. — Scale bar = 25 μm for all except (k) where scale bar = 50 μm.
Systema sexus homothallica, solum partim functionalis; oogonia in medio 95 % abortiva, terminalia vel lateralia, in medio 38 μm (27–45 μm), globosa, subglobosa vel rare excentrica, paries semper levigati. Oosporae apleroticae, in medio 32 μm (23–39 μm), paries in medio 3.0 μm (1.3–4.4 μm) maturitate frequenter pigmentati lutei ad luteifusci. Antheridia singulares, terminalia vel lateralia, unicellularia, hyalina, claviformes, subglobosa vel cylindrica, in medio 19×11 μm (14–25×9–13 μm), paragynosa vel interdum amphigynosa. Sporangiophora simplicia. Sporangia abundantia in cultura liquida, terminalia, nonpapillata cum apicibus applanatis, ovoidea, limoniformia vel obpyriformia, frequenter cum basim attenuato, interdum cum multis intrusionibus parierum, in medio 50×31 μm (31–68×17–43 μm), ratio longitudo ad altitudinem in medio 1.6 (1.3–2.2). Proliferationes sporangiorum semper internae, niduformes et extentae, numquam externae. Aggregationes hypharum frequenter in agaro ‘V8A’ et in cultura liquida, diameter 15–170 μm. Inflationes hypharum subglobosae, angulares vel elongatae, partim catenulatae. Chlamydosporae non observatae. Temperaturae crescentiae in agaro ‘V8A’, optima 25 °C et maxima 33–35 °C. Coloniae in agaro ‘V8A’ striatae cum mycelio aerio restricto. Regiones ‘rDNA ITS’, ‘cox1’ et ‘HSP’ cum unica sequentia (GenBank HQ012942, HQ012858, HQ012904).
Etymology. Name refers to the abundant hyphal aggregations regularly formed by all isolates (gregata Latin = aggregated, in clumps).
Sporangia, hyphal swellings and aggregations (Fig. 5a–l) — Sporangia of P. gregata were not observed on solid agar but were produced abundantly in non-sterile soil extract. Sporangia were usually borne terminally on unbranched sporangiophores, often in chains of internally proliferating sporangia, or much less frequently in lax sympodia. Sporangia were non-caducous, nonpapillate and usually with a flat apex (Fig. 5a–e). Sporangial shapes ranged from ovoid (81 %; Fig. 5d, e) to elongated ovoid (4 %; Fig. 5f, g), limoniform (10 %; Fig. 5c) or less frequently ellipsoid (Fig. 5b), pyriform or obpyriform (Fig. 5a, e). Sporangia usually proliferated internally in both a nested (Fig. 5e) and extended way (Fig. 5f, g), often with the formation of an additional basal sporangiophore initial, which usually remained short, but sometimes developed into a second sporangiophore (Fig. 5f). External proliferation was not observed. In all isolates some sporangia formed numerous wall ingrowths which became clearly visible after zoospore release (Fig. 5g). Zoospores were discharged through an exit pore 5.1–18.7 μm wide (av. 10.7 ± 2.7 μm) (Fig. 5e–g). They were limoniform to reniform whilst motile, becoming spherical (av. diam = 13.1 ± 1.6 μm) on encystment. Cysts usually germinated by forming a hypha, but both diplanetism and formation of a microsporangium was also observed in all isolates. Sporangial dimensions of nine isolates averaged 51.0 ± 13.8×30.5 ± 5.9 μm (overall range 25.7–102.3×14.8–50.7 μm) with a wide range of isolate means of 37.3–72.7×25.6–35.0 μm. The length/breadth ratio averaged 1.67 ± 0.32 with a wide range of isolate means of 1.37–2.19. As with P. gibbosa, hyphal swellings were formed, which according to their morphology were most likely undeveloped sporangia which, when failing to form a basal septum, continued to grow at their apex (Fig. 5h). In addition, globose, angular or irregular-elongated, often catenulate hyphal swellings were regularly formed (Fig. 5i). Inside the V8 agar and also in liquid culture, all isolates frequently produced dense hyphal aggregations formed from both clusters of lateral hyphae and the twisting of hyphae around each other (Fig. 5j–l). In rare cases such aggregations resulted from sporangia in the agar inside of which the zoospores had directly germinated (not shown). Aggregations had diameters of 15–170 μm.
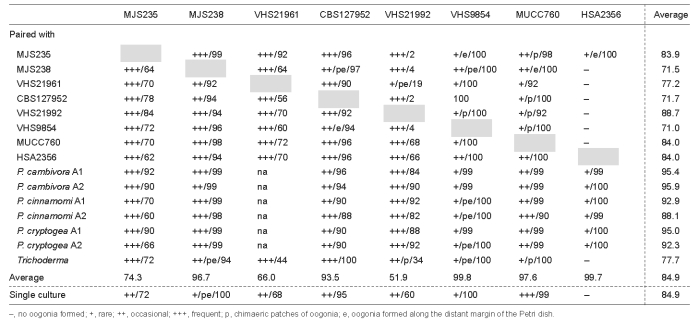
Oogonia, oospores and antheridia (Fig. 5m–s) — With the exception of HSA2356, all isolates of P. gregata produced oogonia in single culture on V8A. In paired cultures with A1 and A2 tester strains of P. cambivora, P. cinnamomi and P. cryptogea, other P. gregata isolates, and T. reesei, all isolates including HSA2356 produced oogonia within three to four weeks (Table 3). Interestingly, in most isolates, higher numbers of oogonia were produced in paired cultures than in single culture (Table 3). In most pairings of MUCC760 and VHS9854, in single culture and in one pairing of MJS238, and in a few pairing combinations of CBS127952 and VHS21992, oogonia were predominantly produced in chimaeric patches and/or along the distant margin within the P. gregata colonies (Table 3). The average abortion rate was very high with 84.9 % in both single culture and over all pairing combinations (Table 3). However, the abortion rate varied considerably between isolates, and in some isolates also between different pairing combinations. While VHS21992, VHS21961 and MJS235 showed average abortion rates of 60.0–72.0 % in single culture and 51.9–74.3 % over all pairings, the other five isolates produced hardly any viable oospores (Table 3). Within-isolate variation was highest in VHS21992 which had mean abortion rates of 2 and 4 % in paired cultures with MJS235 or CBS127952 and MJS238 or VHS9854, respectively, 60 % in single culture and 82–92 % in pairings with the A1 and A2 tester strains. None of the P. gregata isolates induced oogonia formation in the A1 and A2 tester isolates. In conclusion, the breeding system of P. gregata is homothallic or partially or sporadically self fertile. Oogonia were borne terminally or laterally and had globose, subglobose to slightly excentric or elongated shapes, often with a tapering base (Fig. 5m, o–q). Oogonial walls sometimes turned golden-brown (Fig. 5m, o–p, s) while ageing. Oogonial diameters averaged 36.8 ± 4.1 μm (overall range 23.9–50.9 μm and range of isolate means 34.0–39.8 μm). Oospores were usually aplerotic (Fig. 5n–r) although some plerotic oospores could be observed in all isolates (Fig. 5m). Oospores were globose and contained a large ooplast (Fig. 5m–s). They had a mean diameter of 31.6 ± 4.0 μm (total range 21.4–45.3 μm), thick walls (av. 2.65 ± 0.81 μm, total range 1.0–5.3 μm) and a mean oospore wall index of 0.42 ± 0.09. In all isolates, the majority of oogonia aborted, either prior to (Fig. 5s) or after forming an oospore (Fig. 5r). Antheridia were formed terminally or laterally (Fig. 5m) and were predominantly paragynous (Fig. 5n–r), averaging 17.1 ± 3.0×11.0 ± 1.8 μm, with shapes ranging from clavate, subglobose to irregular. Amphigynous antheridia (Fig. 5m) could also be observed in most isolates.
Table 3.
Abundance and spatial distribution of oogonial formation and oogonial abortion rates (%) of Phytophthora gregata isolates in single culture and in pairings with other P. gregata isolates, A1 and A2 tester strains of P. cambivora, P. cinnamomi and P. cryptogea, and Trichoderma reesei.
 |
Colony morphology, growth rates and cardinal temperatures (Fig. 9, 11) — Colony growth patterns of different isolates of P. gregata showed some variation. On V8A and MEA faintly striate, stellate or uniform colonies with sparse to limited aerial mycelium were formed. Colonies on CMA had no distinctive growth pattern and were mostly submerged with no, or very sparse, aerial mycelium while colonies on PDA appeared woolly, sometimes dome-shaped, with a uniform or faintly stellate pattern. Temperature-growth relations are shown in Fig. 11. With the exception of HSA2356, which was consistently slower growing at all temperatures, all eight isolates included in the growth test had similar growth rates at their optimum temperature of 25 °C. All isolates except MUCC760 were unable to grow at 35 °C, and isolates did not resume growth when plates incubated for 7 d at 35 °C were transferred to 20 °C. The maximum growth temperature was between 32.5 and 35 °C. The average radial growth rate at the optimum temperature of 25 °C was 6.5 ± 0.7 mm/d. With mean radial growth rates at 20 °C of 5.2, 5.9, 5.6 and 5.1 mm/d on V8A, MEA, CMA and PDA, respectively, P. gregata showed almost no agar media preferences.
Specimens examined. Western Australia, Busselton, from rhizosphere soil of dying Patersonia sp., 2009, VHS, holotype MURU 462 (dried culture on V8A, Herbarium of Murdoch University, Western Australia), cultures ex-type CBS127952 and VHS21962; Scott River ironstones, from rhizosphere soil of dying plants in native forest, 2009, VHS, VHS21992; Lancelin, from rhizosphere soil of dying X. preissii, 2001, VHS, VHS9854; Busselton, from rhizosphere soil of dying Hakea sp., 2009, VHS, VHS21961; Busselton, from rhizosphere soil of dying Patersonia sp., 2009, VHS, VHS21962; Nannup, from rhizosphere soil of dying Pinus radiata, 1982, M.J.C. Stukely, MJS235; Nannup, from root collar of dying P. radiata, 1981, M.J.C. Stukely, MJS238; Byford, from soil, 1965, not known, DCE68; Cataby, from root of B. prionotes, 1996, R. Hart, HSA 2356. – Victoria, Devlin’ s Bridge, soil from pasture, 2008, W.A. Dunstan, MUCC760.
Phytophthora litoralis T. Jung, M.J.C. Stukely & T.I. Burgess, sp. nov. — MycoBank MB518765; Fig. 6
Fig. 6.
Morphological structures of Phytophthora litoralis formed on V8 agar flooded with soil extract. a–l. Nonpapillate sporangia: a. ovoid (left) and limoniform (right); b. ovoid; c. ovoid sporangia and external proliferation (arrow); d. broadly-ovoid; e. limoniform with a conspicuous basal plug; f. ovoid to limoniform terminal sporangia and secondary lateral sporangium formed with cytoplasm remaining after the formation of the terminal primary sporangium (arrow); g. secondary, lateral ovoid sporangium; h. secondary, lateral, obovoid (left) and ovoid (right) sporangia; i. secondary, lateral limoniform sporangium formed just below the empty upper section of the sporangiophore (arrow); j, k. empty sporangia with internal nested proliferation; l. empty sporangium with internal nested and extended proliferation and branching of the sporangiophore outside the sporangium (arrow); m. chlamydospore with radiating hyphae; n. irregular catenulate hyphal swellings; o. irregular hyphal swelling with radiating hyphae; p. globose hyphal swelling with radiating hyphae. — Scale bar = 25 μm.
Systema sexus sterilis. Sporangiophora simplicia vel ramosa in sympodiis laxis. Sporangia abundantia in cultura liquida, terminalia vel lateralia, nonpapillata, ovoidea vel limoniformia, in medio 41×27 μm (30–49×16–34 μm), ratio longitudo ad altitudinem in medio 1.5 (1.2–2.2). Proliferationes sporangiorum internae, niduformes et extentae, et externae. Inflationes hypharum globosae, angulares, irregulares vel elongatae, partim catenulatae et partim cum hyphis radiatis. Chlamydosporae globosae, partim cum hyphis radiatis observatae. Temperaturae crescentiae in agaro ‘V8A’, optima 30 °C et maxima 33–35 °C. Coloniae in agaro ‘V8A’ stellatae cum mycelio aerio restricto. Regiones ‘rDNA ITS’, ‘cox1’ et ‘HSP’ cum unica sequentia (GenBank HQ012948, HQ012866, HQ012911).
Etymology. Name refers to the frequent association of this species with coastal and riparian vegetation and the littoral zone of water bodies (litus Latin = coast and bank).
Sporangia and hyphal swellings (Fig. 6a–l, n–p) — Sporangia of P. litoralis were not formed on solid agar but were produced abundantly in non-sterile soil extract. Sporangia were non-caducous and nonpapillate (Fig. 6a–k). Sporangial shapes ranged from ovoid and elongated ovoid (85.6 %; Fig. 6a–d, g, h, j–l), limoniform (10 %; Fig. 6a, c, e, f, i) to less frequently ellipsoid, pyriform, obpyriform, obovoid (Fig. 6h) or ampulliform. Mean sporangial dimensions of five isolates were 43.6 ± 7.7×29.4 ± 5.4 μm (overall range 27.8–76.9×16.0–40.4 μm) with a range of isolate means of 39.7–53.4×27.1–33.0 μm. The length/breadth ratio averaged 1.51 ± 0.26 with a range of isolate means of 1.35–1.73. Sporangia were borne terminally on unbranched sporangiophores, often in chains of internally proliferating sporangia, or in lax sympodia. In addition, secondary lateral sporangia were sometimes formed from the cytoplasm remaining in the sporangiophore after the formation of the primary terminal sporangium (Fig. 6f–i). These lateral sporangia were usually considerably smaller than the primary terminal sporangia (Fig. 6f–h). Sporangia usually proliferated internally in both a nested (Fig. 6j–l) and extended way (Fig. 6l). Sometimes, internally proliferating sporangiophores branched just after having passed through the exit pore of the empty primary sporangium (Fig. 6l). The formation of a second basal sporangiophore initial inside internally proliferating sporangia was much less frequent than in P. gregata, and these initials always remained short. External proliferation was also common (Fig. 6c). Zoospores of P. litoralis were discharged through an exit pore 6.2–19.5 μm wide (av. 11.9 ± 2.7 μm). They were limoniform to reniform whilst motile, becoming spherical (av. diam = 10.8 ± 1.3 μm) on encystment. Cysts usually germinated by forming a hypha but diplanetism or formation of a microsporangium was also common in all isolates. Only one of the five isolates (VHS17085) produced globose chlamydospores, which averaged 34.3 ± 5.3 μm diam and sometimes had radiating hyphae (Fig. 6m). Globose, angular or irregular-elongated, often catenulate hyphal swellings with an average diameter of 15.7 ± 4.7 μm were regularly formed (Fig. 6n–p); often forming branching points (Fig. 6n–o), sometimes with radiating hyphae (Fig. 6p).
Oogonia, oospores and antheridia — None of the five P. litoralis isolates tested produced gametangia in single culture or when paired with other P. litoralis isolates, with A1 and A2 tester strains of P. cambivora, P. cinnamomi and P. cryptogea or with T. reesei. However, two of the isolates (MUCC762 and VHS17085) stimulated the formation of oogonia in the A2 isolate of P. cinnamomi. These oogonia were clearly formed by selfing of P. cinnamomi because they were found within the P. cinnamomi colony close to the distant margin of the petridish and sometimes had 2–7 paragynous antheridia attached (Hüberli et al. 2001). In conclusion, the sexual system of P. litoralis can be either fully sterile or self-sterile silent A1.
Colony morphology, growth rates and cardinal temperatures (Fig. 10, 11) — All four P. litoralis isolates examined formed stellate colonies with sparse aerial mycelium on V8A; petaloid, adpressed to submerged colonies on MEA and submerged uniform colonies on CMA (Fig. 10). Colonies on PDA were either petaloid with limited aerial mycelium or irregular and dense-felty (Fig. 10). The temperature–growth relations are shown in Fig. 11. All isolates had similar growth rates at all temperatures except MUCC763 which was markedly slower at 32.5 °C than the other isolates, and MUCC762 which showed some growth at 35 °C while all other isolates failed to grow at 35 °C. All isolates resumed growth when plates incubated for 7 d at 35 °C were transferred to 20 °C. However, 37 °C was lethal to all isolates when tested in the same way. Thus, the maximum growth temperature was between 32.5 and 35 °C. The average radial growth rate at the optimum temperature of 30 °C was 4.6 ± 0.3 mm/d. At 20 °C mean radial growth rates on V8A, MEA, CMA and PDA were 4.0, 3.1, 4.1 and 2.4 mm/d, respectively.
Fig. 10.
Colony morphology of Phytophthora litoralis isolates CBS127953 (ex-type) and MUCC762, P. thermophila isolates CBS127954 (ex-type) and MUCC764, P. gonapodyides isolate MUCC761 and P. megasperma isolate DDS3432 (from top to bottom) after 7 d growth at 20 °C on V8 agar, malt extract agar, corn meal agar and potato-dextrose agar (from left to right).
Specimens examined. Western Australia, Ravensthorpe, from rhizosphere soil of dying Banksia sp., 2008, VHS, holotype MURU 463 (dried culture on V8A, Herbarium of Murdoch University, Western Australia), cultures ex-type CBS127953 and VHS20763; Hopetoun, from rhizosphere soil of dying Banksia sp., 2007, VHS, VHS17085; Wilga, from rhizosphere soil of dying X. preissii, 2008, VHS, VHS19173; Borden, stream baiting, 2008, D. Hüberli, MUCC762; Borden, stream baiting, 2008, D. Hüberli, MUCC763.
Phytophthora thermophila T. Jung, M.J.C. Stukely & T.I. Burgess, sp. nov. — MycoBank MB518766; Fig. 7
Fig. 7.
Morphological structures of Phytophthora thermophila formed on V8 agar flooded with soil extract. a–g. Nonpapillate mature sporangia: a. ovoid, the left one with swollen apex shortly before release of the already differentiated zoospores; b. ovoid (right), ellipsoid formed by nested proliferation (centre), and ovoid empty with internal nested proliferation beginning (left); c. elongated-ovoid with a tapering base and a conspicuous basal plug; d. elongated-ellipsoid with a tapering base; e. ovoid sporangium with swollen apex shortly before release of the already differentiated zoospores; f. same sporangium as in e releasing zoospores; g. elongated, cigar-like with a conspicuous basal plug; h. empty sporangium with nested and extended proliferation, and branching of the sporan-giophore (arrow) after leaving the sporangium; i. empty sporangia with internal nested and extended proliferation; j. elongated hyphal swelling at a branching point; k. catenulate globose and irregular hyphal swellings; the right one forming a branching point; l. intercalary globose chlamydospores, distinguished from hyphal swellings by having thicker walls and septa to the bearing hyphae; m. hyphal aggregation; n. undulating main hyphae; o, p. encysted zoospores having geminated by forming a secondary microsporangium or by having released a secondary zoospore (diplanetism; arrows); q. mature oogonium with a twisted oogonial stalk, a paragynous antheridium (arrow) and an aplerotic oospore containing a large ooplast; r. mature oogonium with two paragynous antheridia (arrows) and an aplerotic oospore containing a large ooplast. — Scale bar = 25 μm.
Systema sexus sterilis. Sporangiophora simplicia. Sporangia abundantia in cultura liquida, terminalia, nonpapillata, ovoidea, ellipsoidea vel limoniformia, in medio 46×25 μm (33–65×17–39 μm), ratio longitudo ad altitudinem in medio 1.9 (1.4–2.9). Proliferationes sporangiorum solum internae, niduformes et extentae. Cystae plerumque germinantes cum una zoospora secundaria vel una microsporangia. Hyphae saepe undulatae. Aggregationes hypharum parvae et solum rare observatae. Inflationes hypharum globosae, angulares, irregulares vel elongatae, partim catenulatae et partim cum hyphis radiatis. Chlamydosporae globosae, partim cum hyphis radiatis, in medio 33 (24–33 μm). Temperaturae crescentiae in agaro ‘V8A’, optima 33 °C et maxima 35 °C. Coloniae in agaro ‘V8A’ stellatae cum mycelio aerio restricto. Regiones ‘rDNA ITS’, ‘cox1’ et ‘HSP’ cum unica sequentia (GenBank EU301155, HQ012872, HQ012916).
Etymology. Name refers to the high optimum and maximum temperatures for growth (thermophila Latin = thermophilic).
Sporangia, hyphal swellings and chlamydospores (Fig. 7a–l) — Sporangia of P. thermophila were not observed on solid agar but were produced abundantly in non-sterile soil extract. Sporangia were borne terminally on unbranched sporangiophores, often in chains of internally proliferating sporangia. Due to the lack of external proliferation no sympodia are formed. Sporangia were non-caducous and nonpapillate (Fig. 7a–g). Sporangial shapes ranged from ovoid and elongated ovoid (72.8 %; Fig. 7a–c, e, f, h, i) to limoniform (13.2 %), ellipsoid (12 %; Fig. 7b, d) and, less frequently, pyriform, obpyriform or cylindrical. Features such as a tapering base or a conspicuous basal plug were quite common (Fig. 7c, d, g). Sporangia usually proliferated internally in both a nested and extended way (Fig. 7b, h, i). Branching of internally proliferating sporangiophores just after having passed through the exit pore of the empty primary sporangium occurred regularly (Fig. 7h). The formation of a second basal sporangiophore initial inside internally proliferating sporangia was much less common than in P. gregata, and these initials always remained short. External proliferation was never observed. The formation of secondary lateral sporangia was very rare and much less frequent than in P. litoralis. Sporangial dimensions of five isolates of P. thermophila averaged 44.8 ± 6.3×25.7 ± 3.9 μm (overall range 29.0–64.8×15.6–39.3 μm) with rather similar isolate means (44.2–46.8×24.1–26.6 μm). The length/breadth ratio of the sporangia averaged 1.78 ± 0.26 with a range of isolate means of 1.67–1.86. Zoospores were discharged through an exit pore 5.5–20.9 μm wide (av. 13.9 ± 2.9 μm) (Fig. 7f). They were limoniform to reniform whilst motile, becoming spherical (av. diam = 10.9 ± 1.1 μm) on encystment. Cysts germinated more often indirectly by releasing a secondary zoospore (diplanetism) or by forming a microsporangium (Fig. 7o, p) than directly by forming a hypha. In liquid culture globose, angular or irregular-elongated hyphal swellings, sometimes catenulate and with radiating hyphae or forming branching points, were regularly formed (Fig. 7j, k). Hyphal swellings had a mean diameter of 12.6 ± 2.3 μm. Globose, intercalary or terminal chlamydospores (Fig. 7l) with a mean diameter of 41.5 ± 14.7 μm were produced in liquid culture by three of the five isolates. Some isolates produced hyphal aggregations much smaller and much less frequent than those of P. gregata (Fig. 7m). Main hyphae often showed undulating growth (Fig. 7n).
Oogonia, oospores and antheridia — None of the five P. thermophila isolates tested produced gametangia in single culture or when paired with other P. thermophila isolates, A1 and A2 tester strains of P. cambivora, P. cinnamomi and P. cryptogea or with T. reesei. Also, none of the isolates stimulated the formation of oogonia in the A1 or A2 tester strains. Interestingly, VHS3655 formed oogonia abundantly in single culture when flooded with non-sterile soil filtrate. Shapes of oogonia ranged from globose and subglobose to excentric (Fig. 7q, r). Oogonial stalks were sometimes twisted (Fig. 7q). Oogonial diameters averaged 31.1 ± 2.5 μm with a total range of 27.2–38.0 μm. Most oogonia looked viable, containing oospores with a large ooplast (abortion rate = 8 %) (Fig. 7q, r). Oospores were highly aplerotic and averaged 23.6 ± 2.2 μm diam with relatively thick oospore walls (on av. 2.3 ± 0.7 μm) and a high mean oospore wall index of 0.46 ± 0.09. The antheridia were exclusively paragynous (Fig. 7q, r) and measured 15.5 ± 2.4×9.3 ± 0.9 μm. Sometimes two or three antheridia were attached to one oogonium (Fig. 7r). In conclusion, the sexual system of P. thermophila is sterile or potentially self fertile when induced by certain stimuli.
Colony morphology, growth rates and cardinal temperatures (Fig. 10, 11) — All four P. thermophila isolates examined formed stellate to petaloid colonies with sparse to limited aerial mycelium on V8A and MEA, and uniform submerged colonies on CMA (Fig. 10). Colonies on PDA were irregular and dense-felty (Fig. 10). Phytophthora thermophila proved to be a high temperature species with an optimum temperature for growth of 32.5 °C and a maximum temperature for growth of 35–< 37 °C (Fig. 11). All isolates failed to grow at 37 °C, but isolate MUCC764 resumed growth when plates incubated for 7 d at 37 °C were transferred to 20 °C. Of the five newly described species and taxa, P. thermophila showed the slowest growth on all four agar media. The average radial growth rate on V8A at the optimum temperature of 32.5 °C was 4.8 ± 0.6 mm/d. With a mean radial growth rate of 3.2 mm/d P. thermophila showed markedly faster growth at 20 °C on V8A and CMA than on MEA (2.0 mm/d) and PDA (1.3 mm/d).
Specimens examined. Western Australia, Dwellingup, from rhizosphere soil of dying Eucalyptus marginata, 2004, VHS, holotype MURU 464 (dried culture on V8A, Herbarium of Murdoch University, Western Australia), cultures ex-type CBS127954 and VHS13530; Quinninup, from rhizosphere soil of dying plants in native forest, 1998, VHS, VHS3655; Manjimup, from rhizosphere soil of dying plants in native forest, 2000, VHS, VHS7474; Pemberton, from rhizosphere soil of dying Banksia grandis, 2006, VHS, VHS16164; Brunswick, baiting from Lunenburg river, 2008, G.E.St.J. Hardy, MUCC764.
Phytophthora taxon paludosa — Fig. 8
Fig. 8.
Morphological structures of Phytophthora taxon paludosa formed on V8 agar. a–h. Mature sporangia produced in soil extract: a, b. ovoid semipapillate; c. ovoid nonpapillate with external proliferation; d. ovoid semipapillate with a large vacuole and external proliferation (arrow); e. nonpapillate obpyriform with external monochasial proliferation; f. semipapillate obpyriform with a large vacuole and external dichasial proliferation (arrow); g. semipapillate ovoid with a large vacuole, laterally displaced apex and external monochasial proliferation (arrow); h. two ovoid sporangia with internal extended proliferation (left) and a large vacuole (right); i. intercalary catenulate hyphal swellings produced in soil extract; j–q. mature smooth-walled oogonia with thickwalled aplerotic oospores containing a large ooplast and a nucleus: j. two globose oogonia with amphigynous (left) and paragynous antheridium (right); k. subglobose with paragynous antheridium; l. globose with amphigynous antheridium; m. subglobose with tapering elongated base and paragynous antheridium; n. two subglobose to elongated oogonia with paragynous antheridia and viable oospores and a subglobose oogonium with aborted oospore (arrow); o. subglobose tapering (left) and elongated with curved tapering base (right); p. elongated excentric with paragynous antheridium; q. subglobose with curved tapering base and paragynous antheridium with finger-like hyphal projection. — Scale bar = 25 μm.
Etymology. The provisional epithet relates to the occurrence of the only known isolate SLPA 166 in a swamp in the Sugarloaf Reservoir Reserve in Victoria (paludosa Latin = swampy).
Sporangia and hyphal swellings (Fig. 8a–h) — Sporangia of P. taxon paludosa were not formed on solid agar but were produced abundantly in non-sterile soil extract. Sporangia were typically borne terminally on unbranched sporangiophores or in lax sympodia. Sporangia were non-caducous, semipapillate (Fig. 8a, d, f, g) or nonpapillate (Fig. 8b, c, e, h). Sporangial shapes were usually ovoid to broad-ovoid (90 %; Fig. 8a–d, g, h), and less frequently limoniform (8 %) or obpyriform (2 %, Fig. 8e, f). In semipapillate sporangia the apex was sometimes laterally displaced (Fig. 8g). Sporangia were quite large, averaging 54.7 ± 4.3×43.3 ± 3.7 μm (overall range 43.1–64.6×31.4–51.7 μm) and often contained a large vacuole (Fig. 8d, f–h). Their mostly broad-ovoid shape is reflected by the low mean length/breadth ratio of 1.27 ± 0.09. Sporangia proliferated externally (Fig. 8c–g) with one (Fig. 8c, d, g) or sometimes two sporangiophores (Fig. 8f), and internally in an extended way (Fig. 8h). Nested proliferation was never observed. Zoospores were discharged through an exit pore 7.5–14.8 μm wide (av. 10.6 ± 2.1 μm) (Fig. 8h). They were limoniform to reniform whilst motile, becoming spherical (av. diam = 11.7 ± 0.4 μm) on encystment. Cysts germinated by forming a hypha. Diplanetism was never observed. In liquid culture catenulate, globose, ellipsoid or angular hyphal swellings (av. diam = 16.2 ± 3.1 μm) were abundant (Fig. 8i).
Oogonia, oospores and antheridia (Fig. 8j–q) — Gametangia were readily produced by P. taxon paludosa in single culture on V8A within 4 d. Oogonia were borne terminally or laterally, had smooth walls and were globose to subglobose (Fig. 8j–o, q) or elongated (Fig. 8n–p), often with a tapering base (Fig. 8m, o–q). Oogonial diameters averaged 33.3 ± 3.5 μm (overall range 24.4–40.7 μm). Oospores were always aplerotic, had a mean diameter of 28.1 ± 2.8 μm (range 21.7–34.3 μm) and were usually globose, containing a large ooplast and a nucleus (Fig. 8j–q). With a mean thickness of 2.5 ± 0.4 μm (total range 1.6–3.4 μm) and a mean oospore wall index of 0.44 ± 0.04 the oospore walls were relatively thick. The abortion rate of oospores was less than 10 %. The antheridia were predominantly paragynous (Fig. 8j, k, m–q) but some amphigynous antheridia were also observed (Fig. 8j, l). Antheridia averaged 16.6 ± 3.7×13.0 ± 1.5 μm, with shapes ranging from club-shaped, subglobose to cylindrical or irregular (Fig. 8j–q). They were usually formed terminally or laterally, and were rarely intercalary.
Colony morphology, growth rates and cardinal temperatures (Fig. 9, 11) — Phythophthora taxon paludosa produced uniform, mostly submerged colonies on V8A and CMA, and faintly stellate colonies on MEA and PDA with limited and fluffy aerial mycelium, respectively (Fig. 9). Growth was fast (6.3 mm/d) at the optimum temperature of 25 °C, and there was only slow growth at 32.5 °C (Fig. 11); 35 °C was lethal since cultures did not resume growth when plates incubated for 7 d at 35 °C were transferred to 20 °C. With mean radial growth rates at 20 °C of 5.5, 5.7, 5.5 and 5.4 mm/d on V8A, MEA, CMA and PDA, respectively, P. taxon paludosa showed no agar media preferences.
Specimen examined. Victoria, Sugarloaf Reservoir Reserve, baiting from an artificial swamp in pasture, 2008, W.A. Dunstan, MUCC765.
Notes — Phytophthora gibbosa, P. gregata, P. litoralis, P. thermophila and P. taxon paludosa can easily be separated from each other and from other species of ITS Clade 6 and from morphologically similar Phytophthora species by their ITS, cox1 and HSP90 sequences, and by a combination of morphological and physiological characters of which the most decisive are highlighted in bold in Table 2. In all gene trees, P. thermophila and P. litoralis are sister species which share a common ancestor with P. gonapodyides and P. megasperma, while P. gibbosa, P. gregata, P. taxon paludosa and P. taxon raspberry are also sister taxa.
Phytophthora gibbosa can be easily differentiated from all other species from ITS Clade 6 by the production of ornamented (gibbous) oogonia in single culture. In addition, it can be separated from P. gregata and P. taxon raspberry by the lack of nested proliferation of sporangia. More distantly related species, which due to the production of ornamented oogonia are morphologically similar to P. gibbosa, can also be easily differentiated by a combination of morphological and physiological features: P. cambivora from ITS Clade 7 is heterothallic, has larger two-celled antheridia and shows nested sporangial proliferation (Erwin & Ribeiro 1996). Both, P. alni ssp. alni and P. alni ssp. multiformis from ITS Clade 7 have a varying proportion of distorted, often comma-shaped oogonia, usually larger two-celled antheridia and show nested sporangial proliferation (Brasier et al. 2004). Phytophthora katsurae from ITS Clade 5 has smaller oogonia with funnel-shaped stalks, smaller papillate sporangia, forms chlamydospores and does not produce hyphal swellings (Erwin & Ribeiro 1996). Phytophthora cyperibulbosi produces paragynous antheridia and caducous sporangia, and is not culturable (Erwin & Ribeiro 1996). Phytophthora verrucosa produces both amphigynous and paragynous antheridia, shows nested sporangial proliferation and is not culturable (Stamps et al. 1990, Erwin & Ribeiro 1996).
In previous studies P. gregata is referred to as P. taxon raspberry (Brasier et al. 2003a) and P. sp. 7 (Burgess et al. 2009). It has been previously misidentified in WA as P. megasperma or P. megasperma var. sojae (Burgess et al. 2009). Phytophthora gregata can be separated from P. megasperma by the high rate of oogonial abortion, markedly smaller oogonia sizes, the production of hyphal aggregations and different colony growth patterns on MEA; from P. gibbosa by the absence of ornamented oogonia, the production of hyphal aggregations and catenulate hyphal swellings and by having nested sporangial proliferation; from P. taxon paludosa by high oogonial abortion rates, the production of hyphal aggregations and by nested sporangial proliferation. Phytophthora gregata also comprises Australian isolates designated as P. taxon raspberry. Brasier et al. (2003a) examined one European isolate (P1050) of P. taxon raspberry in detail. It had slightly higher maximum temperature for growth and markedly higher oogonial diameters than P. gregata in our study, and an even ratio of amphigynous and paragynous antheridia while P. gregata has predominantly paragynous antheridia. However, more European isolates have to be investigated to clarify whether or not these differences are consistent.
Phytophthora litoralis is referred to as P. sp. 11 (Burgess et al. 2009). It is distinguished from P. thermophila by being partly silent A1, by the occurrence of external proliferation and secondary lateral sporangia, by having lower optimum and maximum temperatures for growth, and by faster growth on PDA. Characters present in P. litoralis, that differentiate it from P. gonapodyides include the frequent production of hyphal swellings which were often catenulate and sometimes had radiating hyphae, the production of secondary lateral sporangia, the occurrence of chlamydospores in some isolates, and the predominance of ovoid sporangia (P. gonapodyides has mostly obpyriform to ellipsoid sporangia). In addition, most isolates of P. litoralis were sterile while all isolates of P. gonapodyides are silent A1 (Brasier et al. 1993, 2003a, Erwin & Ribeiro 1996). Phytophthora litoralis can be easily separated from P. megasperma by the absence of oogonia, the occurrence of external sporangial proliferation and secondary lateral sporangia, the production of chlamydospores by some isolates and higher optimum temperature for growth.
Phytophthora thermophila has previously been misidentified as P. drechsleri and is referred to as P. sp. 3 (Burgess et al. 2009). This species can be distinguished from P. drechsleri mainly by being either sterile or sporadically self fertile with paragynous antheridia, but also by the branching of internally proliferating sporangiophores outside the empty primary sporangium and the regular occurrence of diplanetism; from P. gonapodyides by its markedly higher optimum temperature for growth, a higher length/breadth ratio of the sporangia, the absence of external sporangial proliferation, the frequent production of hyphal swellings which were often catenulate and sometimes had radiating hyphae, the occurrence of chlamydospores in some isolates and the inability to induce selfing in A2 isolates; from P. megasperma by the sterility of most isolates, the production of chlamydospores by some isolates and a markedly higher optimum and a slightly higher maximum temperature for growth.
Phytophthora gibbosa is known only from a very limited area in the southwest of WA around Scott River where it has been found associated with dying native vegetation on seasonally wet sites. Phytophthora gregata has a scattered distribution in WA. It was recovered from native vegetation in the Northern sandplains around Lancelin, from native vegetation on seasonally wet sites further south around Busselton, Nannup and Scott River (together with P. gibbosa), and from 1 yr old dying Pinus radiata on seasonally wet sites in the Donnybrook Sunklands plantations. Phytophthora gregata also occurs in southeastern Australia in wet native Eucalyptus forests and in swamps in Victoria, and was associated with dying alpine heathland vegetation in Tasmania. The known distribution of P. litoralis comprises areas along the south coast of WA and isolated sites c. 60–80 km inland around Wilga and Borden where it was recovered from river systems and dying native vegetation on wet sites. The distribution of P. thermophila in WA is quite scattered, ranging from the northern Sandplains, the Perth area and rehabilitated mine-sites in the northern Jarrah forest around Dwellingup, to the southwest around Nannup, Boyup Brook, Manjimup and Pemberton in the Southern Jarrah Forest and further east to Tambellup in the Great Southern. It was most often associated with dying native vegetation on both low and high disease impact sites. In addition it was also found widespread in river systems across the southwest of WA (http://www.fishingforphytophthora.murdoch.edu.au).
DISCUSSION
Four new Phytophthora species and one informally designated taxon within ITS Clade 6, P. thermophila, P. litoralis, P. gregata and P. gibbosa and P. taxon paludosa, have been described from natural ecosystems in Australia based on differences in morphology and sequence data of three gene regions. The phylogenies of the ITS and HSP90 gene regions are congruent, with each species residing in highly supported terminal clades. The mitochondrial cox1 gene shows considerable intraspecific variation, and while isolates from a single species form a single lineage, the support is not as strong as in the nuclear gene regions. In their overview of ITS Clade 6, Brasier et al. (2003a) recognised three sub-clades comprising three described species (P. gonapodyides, P. megasperma, P. humicola) and 10 phenotypic taxa. Now, in total, there are 10 species and 15 designated taxa, which is getting closer to the estimation of Brasier (2009) that the number of extant species in Clade 6 is between 28 and 84.
Radiation within Clade 6
Brasier et al. (2003a) introduced several new taxa in sub-clade II; P. taxon PgChlamydo and P. taxon salixsoil, previously included in P. gonapodyides, P. taxon forestsoil, P. taxon riversoil and P. taxon oaksoil from natural ecosystems in Europe, and P. taxon raspberry from Australia and Sweden. To date none of these taxa has been described; however, based on ITS sequence, the P. taxon raspberry isolates from Australia are now considered as P. gregata. With the addition of P. pinifolia (Durán et al. 2008), P. taxon hungarica and the four new species and one taxon from the current study, sub-clade II has expanded significantly (Fig. 12). However, while individual taxa reside in strongly supported terminal clades, sub-clade II is still predominantly characterised by multiple short branches with weak bootstrap and Bayesian support for higher level clustering. This tree topology supports recent radiation from an ancestral type and average genetic distance across the sub-clade is only 1.9 %. Phytophthora pinifolia is an exception within sub-clade II as it is 14 bp different from the closest taxon, P. taxon PgChlamydo, and differs from other taxa by a genetic distance of 2.2–3.3 % (Fig. 12). The inclusion of the new taxa has increased the structure within sub-clade II and two distinct clusters can be distinguished. Phytophthora thermophila and P. litoralis are sister species to each other and more distantly to P. gonapodyides and P. megasperma (Fig. 12). These four species share a common ancestor. Similarly, P. gregata, P. gibbosa and P. taxon paludosa, and isolates of P. taxon raspberry from Europe, form a new cluster within sub-clade II which also share a common ancestor (Fig. 12). Phytophthora taxon salixsoil differs from other taxa by a genetic distance of 1.8–3.5 % in ITS region and is consistently placed in a basal position in all parsimony, distance and Bayesian analyses (also in HSP90 and cox1 phylogenies). The other European taxa are poorly resolved and differ from each other by 2–7 bp.
Fig. 12.
Radial phylogenetic tree generated after Bayesian analysis of ITS rDNA sequences, showing relationships between species and designated taxa in Clade 6 of Phytophthora. The branches corresponding to the sub-clades are coloured accordingly.
Although there are relatively small differences between many sub-clade II taxa, including the newly described species, ITS sequences presented in this study were singular with the exception of 2–3 ambiguous sites in P. litoralis and P. taxon PgChlamydo. These data concur with previous observations where no evidence for reticulation was found, even though radiation appeared to be recent and the taxa shared niches (Brasier et al. 2003a). However, this is an extreme over-simplification of the observations from Australian surveys in native vegetation and waterways. We have described here several discernable species from among a huge array of putative hybrids all containing numerous single base pair polymorphisms (unpubl. data). In phylogenetic analyses these putative hybrids fall within the P. thermophila/P. litoralis cluster and characterisation studies are currently underway.
In 2003, sub-clade I consisted of P. humicola and three undescribed taxa: P. sp. O-group (later described as P. inundata by Brasier et al. 2003b), P. sp. apple-cherry (later described as P. rosacearum by Hansen et al. 2009b) and P. taxon walnut. Several new taxa have now been recognised within sub-clade I: P. taxon personii, P. taxon humicola-like, P. taxon kwongan and P. taxon rosacearum-like. Brasier et al. (2003a) characterised this group as having relatively long branch lengths thought to be indicative of ancient divergence; and indeed there is 18–28 bp difference (up to 4.5 %) between taxa in the two main branches (Fig. 12). However, within the two branches there are now additional taxa separated by smaller genetic distances representing more recent divergence. Sub-clade III is represented by P. taxon asparagi (Saude et al. 2008) and P. taxon sulawesiensis, which are distantly related to each other and the other taxa within ITS Clade 6.
When discussing the role of geographic isolation in speciation we are hampered by uncertainty about the origin of taxa due to considerable anthropogenic movement. Often the same taxa can be observed across several continents in both hemispheres and the true origin of the taxa is obscured. Phytophthora gonapodyides, P. taxon salixsoil and P. taxon PgChlamydo are ubiquitious in water bodies in Europe and the northwestern USA (Jung et al. 1996, Brasier et al. 2003a, Nechwatal & Mendgen 2006, Hansen et al. 2009a, Reeser et al. 2011) and appear to have a global distribution, excluding the tropics (Zeng et al. 2009). However, there are some distribution limits; P. taxon PgChlamydo was not found in Alaska (Reeser et al. 2011) and in WA, P. gonapodyides has never been isolated and P. taxon PgChlamydo and P. taxon salixsoil were only rarely recovered from native flora, but not from streams. Other species have a more limited current distribution: P. thermophila and P. litoralis are only known from Australia where they occupy the same niche as P. gonapodyides and P. taxon salixsoil in the Northern Hemisphere. Phytophthora taxon forestsoil and P. taxon riversoil are only recorded from Europe (Brasier et al. 2003a, Bakonyi et al. 2009) and P. taxon hungarica has been recorded from Alaska and Europe (Adams et al. 2009, Bakonyi et al. 2009). Phytophthora taxon oaksoil has been found in Europe and in mountain stream surveys in Oregon (Reeser et al. 2011). New taxa close to P. gonapodyides, P. taxon salixsoil and P. taxon PgChlamydo have been recently found in North American surveys (Hulvey et al. 2010, Reeser et al. 2011). As more sequence data become available and new species are described, a clearer picture of natural species distribution will emerge.
Seven of the nine taxa in sub-clade I are found in WA (P. humicola was only reported from Taiwan and P. taxon walnut only from California). Phytophthora inundata is widely distributed, commonly isolated in stream surveys and infrequently associated with dying vegetation (Stukely et al. 2007); and many unique isolates related to P. rosacearum have been isolated from dry kwongan heathlands. Until more information becomes available from environmental surveys world-wide, the current distribution and variation suggests either an Australian origin or substantial post-introduction radiation within Western Australia.
Evolutionary and ecological implications
Based on data from 12 taxa in ITS Clade 6, Brasier et al. (2003a, b) postulated a tendency towards inbreeding or breakdown of sexual reproduction. Only seven years later and another 13 taxa richer, this hypothesis is reinforced. Of the 15 known taxa in sub-clade II, seven including P. gibbosa, P. gregata and P. taxon paludosa are homothallic, three are self-sterile and silent A1s, one is self-sterile and partially silent A1 (P. litoralis), one is self-sterile or sporadically self fertile (P. thermophila) and three are fully sterile (Table 4). Of the eight known taxa in sub-clade I, five are homothallic, one is heterothallic (P. inundata) and two are fully sterile (Table 4). Also P. taxon asparagi from sub-clade III is homothallic; no data are available for P. taxon sulawesiensis and hence it is excluded from further discussion. Overall, 13 taxa (54 %) are homothallic and 10 taxa (42 %) are sterile while only one species, P. inundata, is heterothallic (Table 4). This is in contrast to the 89 Phytophthora species described so far from the other nine ITS clades of which 72 % are homothallic, 25 % are heterothallic and only 3 % are sterile.
Table 4.
Habitats and phenotypic characters of Phytophthora taxa from ITS Clade 6.
| Taxon and source of data | Habitats 1 | Maximum temp. (°C) | Optimum temp. (°C) | Breeding behaviour 2 | Mean oogonia diam (μm) | Antheridia type 3 | Chlamydospores 4 | Hyphal swellings 4 | Sporangial proliferation 5 |
|---|---|---|---|---|---|---|---|---|---|
| Sub-clade I | |||||||||
| P. inundata 6 , 7 , 8 (P. sp. O-group) | Aq, Rip, Hor | 35–38 | 28–30 | HET A1 csf, HET A2 SS, PARHET A2 csf or SS | 40.1 | A | ++, g, [rad] 8 | ++, g, ang, el, [cat, rad] 8 | Int ne 8 |
| P. humicola 6 , 9 | Hor | 38 | 28 | HO | 43.8 6 , 39 9 | P/a | − | +++, g, cat, rad | Int e, [ext] |
| P. taxon humicola-like 8 | Hea | na | na | HO | 41.0 | P | ++, g, [ irr, rad] | ++, g, el, cat [rad] | Int ne, ext |
| P. taxon personii 8 | Aq, Rip, Hor | na | na | Sterile | +++, g, [ irr, rad] | +++, g, el, [cat], rad | Int ne, [ext] | ||
| P. taxon walnut 6 | Hor | 38 | 28 | Sterile | − | na | na | ||
| P. rosacearum 6 , 10 (P. sp. apple-cherry) | Hor | 36 10 , 38 6 | 28 6 , 30 10 | HO | 37.5 6 , 29–34 10 | P/A | − 10 | na | Int ne, ext |
| P. taxon rosacearum-like 8 | Hea | na | na | HO | na | na | na | na | na |
| P. taxon kwongan 8 | Hea | na | na | HO | na | na | na | na | na |
| Sub-clade II | |||||||||
| P. taxon salixsoil 6 , 11 , 12 | Aq, Rip | 37 11 , 38 6 , | 30 11 , 33 6 | SS silent A1 | − | (+), g, [cat] | Int ne, [ext] | ||
| P. taxon PgChlamydo 6 , 7 , 13 | Aq, Rip, For, Am, Nur | 36–37 | 25–30 6 | SS, silent A1 | +++, g, irr | +++, g, cat | Int ne, [ext] | ||
| P. taxon forestsoil 6 , 14 | For 6 , Rip 14 | 33 6 , < 33 14 | 25–28 | HO | 42.3 6 , 37.7 14 | P/a | − | +, g, [rad] | Int ne, ext |
| P. taxon hungarica 14 | Rip | < 33 | 25 | HO | 41.4 | P/a | − | - | Int ne, ext |
| P. megasperma 6 , 9 , 12 | Rip, For, Hor, Nur, Ag | 25–34 | 20–25 | HO | 43.5–60.5 6 | P, P/aor P/A | − | +++, g, ang, el, cat | Int ne |
| P. gonapodyides 6 , 7 | Aq, Rip, For, Am, Nur | 34–37 | 25–28 | SS, silent A1 | − | (+), g | Int ne, [ext] | ||
| P. thermophila 12 | Aq, Rip, For | 35–< 37 | 32.5 | Sterile or partially or sporadically self fertile | 31.1 | P, mul | (++), g (rad) | +++, g, ang, el, cat, [rad] | Int ne |
| P. litoralis 12 | Aq, Rip, Hea | 32.5–< 35 | 30 | Sterile or SS silent A1 | (++), g [rad] | +++, g, ang, el, cat, [rad, agg] | Int ne, ext, sec | ||
| P. pinifolia 15 , 16 | Pla, Can | 30 | 25 | Sterile | − | ++, g, rad | −, [cad] | ||
| P. taxon oaksoil 6 , 14 | Aq, Rip | 33 | 25 | Sterile | − | − | na | ||
| P. taxon riversoil 6 | Rip | na | na | Sterile? | − | na | na | ||
| P. taxon paludosa 12 | Rip | 32.5 | 25 | HO | 33.3 | P/a | − | ++, g, el, cat | Int e, ext |
| P. gibbosa 12 | For, Pla | 32.5–< 35 | 30 | HO | 38.1 | A | − | ++, g, el | Int e, ext |
| P. gregata 12 | Rip, For, Hea | 32.5–< 35 | 25 [30] | HO or potentially self fertile, csf, AB | 36.8 | P/a | − | +++, g, ang, el, cat, agg | Int ne |
| P. taxon raspberry (Europe) 6 | Hor | 35 | 28 | HO | 46.8 | P/A | − | na | na |
| Sub-clade III | |||||||||
| P. taxon asparagi 17 | Ag | < 30 | 25 | HO | 25–30 | A | − | na | Int e, ext |
na, not available; ( ), feature is only present in some isolates of a taxon; [ ], feature is rare.
1 Aq, aquatic; Rip, riparian or wetland soils; Hea, heathland soils; For, forest soils; Pla, forest plantations; Can, canopy; Hor, woody horticultural crops; Am, amenity trees; Nur, nurseries; Ag, agricultural crops.
2 HO, homothallic; HET, normally A1 or A2 heterothallic; PARHET, partially A1 or A2 heterothallic, fails to mate in many combinations; SS, self sterile in single culture; csf, chimaerically self fertile in single culture. SS Silent A1, self sterile but able to induce gametangial formation in an A2 type of another species (see Brasier et al. 2003b); sterile, no oogonia produced in single culture, or in pairings with other isolates of its own or A1 and A2 tester strains of other taxa; AB, high abortion rate (> 80 %).
3 P, paragynous; A, amphigynous; P/A, equal paragynous and amphigynous; P/a, predominantly paragynous; p/A, predominantly amphigynous; mul, some oogonia with 2-3 antheridia.
4 −, none; (+) rare in some isolates; ++, occasional; +++, frequent; g, globose to subglobose; irr, irregular; ang, angular; el, elongated; cat, catenulate; rad, radiating hyphae; agg, hyphal aggregations.
5 Int, internal proliferation; n, nested; e, extended; ext, external proliferation; -, no proliferation; sec, secondary production of lateral sporangia; cad, caducous.
8 Jung & Stukely unpubl.;
12 this study;
14 József Bakonyi & Zoltán Á. Nagy, pers. comm.;
When considering the influence of natural selection on sexual reproduction in Phytophthora one has to be conscious of oospores not only providing new genotypes via recombination, but also acting as the most enduring resting structures for survival in unfavourable environmental conditions, such as drought and hot or cold temperatures. Homothallism provides resting structures, but limits a species’ ability for recombination and hence its adaptability to changing environmental conditions. Sterile taxa are essentially evolutionary dead-ends, having abandoned both the potential for sexual recombination and the potential of forming long-term resting structures. For Clade 6 Phytophthoras to follow this evolutionary path there must have been an advantage in this life strategy within their ecological niche.
In general, one of the most important selective influences on Phytophthora pathogens is the host plant (Brasier & Hansen 1992). In the tropical rainforests, high spatial diversity of the flora requires, from any pathogen, the potential for rapid adaptation to new host genotypes or even new host species via genetic recombination. In order to avoid falling back in the perpetual arms race with new host genotypes rapid adaptation is required, and this is usually best achieved by sexual recombination (Lane 2009). Therefore, heterothallism with its intrinsic tendency towards outcrossing is the most successful sexual strategy for tropical Phytophthoras, e.g. P. botryosa, P. capsici, P. cinnamomi, P. colocasiae, P. megakarya, P. nicotianae and P. palmivora. Due to the rare occurrence of both mating types simultaneously infecting the same host tissue, oospore formation in nature for heterothallic species is certainly much less common than for homothallic species. However, in the constantly warm and wet tropical environment, the conditions for asexual multiplication and spread via zoospores are continuously favourable and oospores are not required as long-term resting structures. The occasional mating will guarantee sexual recombination and adaptability. In contrast, in temperate and Mediterranean climates, Phytophthora species must be able to survive alternating low winter temperatures and long dry periods in summer. Here, homothallism, a compromise between the need for adaptability via sexual recombination and the regular and abundant production of resting structures for long-term survival in a dormant state, is by far the most common sexual breeding system, as reflected by P. austrocedrae, P. cactorum, P. citricola, P. ilicis, P. multivora, P. nemorosa, P. plurivora, P. pseudosyringae and P. quercina. In accordance with this simplified macroclimatic distribution of breeding systems, none of the 13 homothallic ITS Clade 6 taxa, except P. humicola which was recovered from Citrus orchards in Taiwan (Ko & Ann 1985), and none of the 10 sterile Clade 6 taxa, have ever been recorded from wet tropical climates (Erwin & Ribeiro 1996, Brasier et al. 2003a, Zeng et al. 2009).
Brasier et al. (2003a, b) proposed the sexual degeneration in Clade 6 may be connected to their aquatic habitat. Although sporadically recorded from forest soils, nine of the 10 sterile Clade 6 taxa are commonly found in rivers, lakes or water-logged soils and swamps (Table 4) which provide constantly wet conditions enabling continuous asexual multiplication and spread via zoospores. Within the freshwater aquatic environment, multiple Phytophthora, Pythium, Achlya and Saprolegnia species with high inoculum levels (Hallett & Dick 1981, Yamak et al. 2002, Nechwatal et al. 2008, Hwang et al. 2009, Reeser et al. 2011) are competing for the same substrate, i.e. freshly fallen green leaves and/or fine or coarse roots and root collars of riparian woody species (Brasier et al. 2003b, Jung & Blaschke 2004). The predominantly aquatic sterile taxa of Clade 6 have undergone an intense selection towards a life strategy focused on rapid and abundant asexual multiplication via zoospores in order to compete with the multitude of other oomycetes in freshwater ecosystems. Accordingly, oospores are not required as long-term resting structures and an allocation of resources into their production would be wasteful. Interestingly, in mountainous streams in Hainan sterile isolates of the homothallic P. insolita from ITS Clade 9 are commonly found (Zeng et al. 2009).
Without sexual recombination these sterile aquatic Clade 6 Phytophthoras also lose the ability to generate new genotypes. Significant genetic variation of phenotypic characters such as virulence, host range and growth rates in F1 progenies is observed for heterothallic outcrossing species and has also been shown for predominantly inbreeding homothallic species like P. cactorum, P. medicaginis, P. sojae or P. syringae (Boccas 1972, Erwin & Ribeiro 1996, MacIntyre & Elliott 1974). Many of the F1 progenies were less fit than the parents, showing poor growth and sporulation or low pathogenicity. The higher variation of behavioural characters provided by sexual as compared to asexual reproduction would lead to progenies less adapted to the specific and stable environmental conditions of fresh-water ecosystems than the parental genotype. Since sporangial production and aggressiveness are highly negatively correlated characters, as shown for P. infestans (Caten 1970), the aquatic Clade 6 taxa have presumably quit the arms race and co-evolution with potential host plants in favour of outnumbering the waterborne inoculum of competitors. In P. litoralis and P. thermophila this is achieved by chains of nested and extended proliferating sporangia, and the frequent germination of cysts by releasing secondary zoospores (diplanetism) or by forming microsporangia. In addition, P. litoralis is using the cytoplasm remaining in the sporangiophore after the formation of the primary terminal sporangia for the production of secondary lateral sporangia.
Ephemeral streams and some habitats, particularly in the shallow water zone along the banks of bigger rivers, lakes and swamps, periodically dry out. Thus, taxa within this niche must form short-term resting structures to survive until the water returns or they will become locally extinct. Four of the six self-sterile Clade 6 taxa commonly associated with streams, i.e. P. litoralis, P. thermophila, P. taxon PgChlamydo and P. taxon personii, the part-heterothallic P. inundata and the homothallic P. taxon humicola-like produce chlamydospores after several days at 15–20 °C in liquid culture (Table 4). Stimulation of chlamydospore production in water at temperatures too high or too low for growth or infection is known for P. cactorum, P. citrophthora, P. lateralis, P. nicotianae and P. palmivora and seems to be a stress-related phenomenon associated with high CO2 concentrations at sufficiently high oxygen concentrations (Blackwell 1943, Englander & Roth 1980, Darmono & Parke 1990, Erwin & Ribeiro 1996). Interestingly, in Europe the recently described P. gallica from ITS Clade 10 also occurs in seasonally flooded natural ecosystems, is sterile and forms chlamydospores (Jung & Nechwatal 2008). These examples of convergent evolution of the same life strategy under similar ecological conditions, in taxa from phylogenetically distant clades, illustrate the adoption of such a life strategy for survival in aquatic habitats. Since, as a trade-off, these taxa have relinquished the potential for sexual reproduction, adaptation to changing environmental conditions can only be achieved by spontaneous mutations or parasexuality, i.e. heterokaryon formation, hyphal anastomosis and zoospore fusion within or between species (somatic hybridisation). Mutations create new variation, but their occurrence is rare and most often deleterious. In the absence of sexual reproduction the combination of different beneficial mutations in the same individual of a sterile population can only occur via somatic fusion if vegetative compatibility permits. In Phytophthora the extent of parasexual processes and their role in generating phenotypic variation on which natural selection can work is still poorly understood (Kuhn 1981, Brasier 1992).
Within P. inundata, the only heterothallic Clade 6 species known to date, a notable proportion of intraspecific A1×A2 pairings failed to produce gametangia, indicative of a degenerating compatibility system (Brasier et al. 2003b). As with other introduced heterothallic Phytophthora species, it is thought this disruption is caused by the anthropogenic spread of this species beyond its native geographical distribution leading to reproductive isolation of the A1 and A2 mating types (Brasier 1992, Erwin & Ribeiro 1996). Taxa within Clade 6 that are self-sterile, may have evolved silent A1 mating types in a similar manner. However, homothallic species, i.e. P. cactorum, P. heveae, P. katsurae and P. sojae, are also able to stimulate oogonia formation in A1 and/or A2 isolates of the heterothallic species P. nicotianae and P. palmivora, respectively (Brasier 1972, Ko 1980). Hence, it can only be speculated whether the self-sterile silent A1 taxa have kept the genetic ‘memory’ of a heterothallic A1 ancestor or of a homothallic ancestor able to stimulate A2 strains of heterothallic Phytophthora species. Since the self-sterile and partially silent A1 species P. gonapodyides and P. litoralis most likely share an ancestor with the homothallic P. megasperma and the self-sterile or inconsistently homothallic P. thermophila, a homothallic ancestor of this cluster within sub-clade II is most plausible. This is supported by the finding of sterile isolates of the homothallic P. insolita in Taiwan which were able to induce selfing in A2 isolates of P. nicotianae (Ann & Ko 1994). In a later study, Ho et al. (2002) demonstrated occasional production of oogonia in aged cultures of some sterile isolates of P. insolita.
The disrupted mating system observed for P. gregata may reflect ‘evolution in action’ with the end point being sterility. While in the closely related P. gibbosa, abortion rates between 16 and 37 % were following simple Mendelian ratios, the observed average abortion rate of 85 % for P. gregata was far beyond Mendelian segregation of homozygous recessive lethals. Five of the eight isolates studied produced hardly any viable oospores, and some isolates produced oospores only in chimaeric patches or in paired cultures with A1 and A2 tester strains. Most likely, the extensive breakdown of the sexual system is caused by the accumulation of lethal mutations, repulsion linkages or chromosomal aberrations (Rutherford & Ward 1985, Brasier 1992). High abortion rates of oogonia are also known from other homothallic Phytophthora species. In some races of P. megasperma f. sp. glycinea oospore abortion rates reach 90 % (Rutherford & Ward 1985). Also the majority of oospores aborted within 14–21 d in all isolates of P. pseudosyringae and P. psychrophila examined (Jung et al. 2002, 2003). Despite the disruption of its breeding system, P. gregata is a successful species with a high genetic variability and a wide distribution across Australia. Since most isolations of P. gregata have come from native vegetation on permanently or seasonally wet sites the apparent evolution of this species towards sterility may follow similar rules of natural selection as in predominantly aquatic Clade 6 taxa. The role of the largely non-functional oospores as survival structures for adverse environmental conditions has presumably been taken over by the abundantly formed, dense and often quite large hyphal aggregations. These resemble stromata formed by P. ramorum beneath the cuticle of infected leaves (Moralejo et al. 2006). Recently, Brasier et al. (2010) also reported the formation of stromata in agar cultures of P. lateralis isolates from Taiwan.
Most Clade 6 taxa including those commonly occurring in waterways in boreal climates such as P. gonapodyides and P. taxon salixsoil (Reeser et al. 2011) have high optimum and maximum temperatures for growth (Brasier et al. 2003a, b). Also the four new species described in this study are ‘high temperature’ taxa, with P. thermophila having an optimum and maximum of 33 and 35 °C, respectively. Brasier et al. (2003a) suggested either a tropical origin of the ancestors of Clade 6 or an eco-physiological adaptation to litter breakdown as possible explanations. Due to the almost complete absence of heterothallism within Clade 6 and a lack of records of Clade 6 taxa from the tropics, a tropical origin seems unlikely. We favour the hypothesis of the high temperature optimum and tolerance being an adaptation to a warm microhabitat such as the littoral zone of water bodies where temperatures particularly in summer often exceed 30 °C due to a combination of factors like shallow depth and slow flow of the water, a dark colour of the muddy ground and intense litter breakdown.
Host range and pathogenicity
Phytophthora gibbosa, P. gregata, P. litoralis and P. thermophila have been recovered from rhizosphere soil of dying plants from four native species (Banksia grandis, Eucalyptus marginata, Xanthorrhoea gracilis and X. preissii), Acacia pycnantha (endemic to eastern Australia), from several unidentified species of the genera Banksia, Eucalyptus, Grevillea, Hakea and Patersonia, and also from the exotic plantation timber species Pinus radiata. Disease expression is often low impact or only associated with scattered individual plant deaths. In WA, all four new Phytophthora species appear to be opportunistic pathogens associated with sporadic but severe mortality on wet sites or following episodic favourable conditions such as unseasonably heavy rain or flooding. Similarly, P. gonapodyides and P. inundata episodically cause root and collar rot and aerial cankers in various tree species in Europe during extremely wet periods; Fagus sylvatica, Q. robur and Alnus glutinosa (P. gonapodyides; Jung et al. 1996, Jung & Blaschke 2004, Brown & Brasier 2007) and Aesculus, Salix and olive trees (P. inundata; Sanchez-Hernandez et al. 2001, Brasier et al. 2003b). However, the pathogenicity of all four new Phytophthora species and P. taxon paludosa is untested and their host ranges are unknown.
Implications for management
Significant public and private resources have been applied to the investigation and management of Phytophthora Dieback (caused by P. cinnamomi) in natural ecosystems in WA for more than three decades (Shearer & Tippett 1989, Shearer & Smith 2000). Extensive vegetation health survey has been in continuous operation in the WA jarrah (E. marginata) forest since 1978. Mapping of the extent of the disease, based on shadowless colour aerial photography, is verified by the testing of soil and root samples for the presence of Phytophthora (Strelein et al. 2006). Dieback-free areas are then quarantined to minimise the risk of spread of the pathogen into priority areas.
Recent re-examination of a large number of these Phytophthora isolates of species other than P. cinnamomi, and sequencing of their ITS gene regions to confirm identity, has revealed a surprisingly diverse number of new records and undescribed taxa (Burgess et al. 2009; this study). The majority of the soil and root samples from which these Phytophthoras were isolated were associated with dying native vegetation. It will be prudent, therefore, for land managers to apply the precautionary principle in managing all of these soilborne Phytophthora taxa in natural ecosystems, regardless of their present known impact.
While a particular Phytophthora taxon may not now be causing visible damage at a site where it is long-established (or endemic), it may cause very significant damage when first introduced to a new site – as a result of a different suite of plant species being present, and of subtle differences in the interaction of environmental and site factors. The devastating effect of the introduction of P. cinnamomi on WA native vegetation has been well documented. The implications of climate change must also be considered, whereby conditions more conducive to the pathogens and to disease expression may be created (e.g. increasingly frequent summer rainfall events). The presence of a swarm of Clade 6 hybrids in WA natural ecosystems (unpubl. data) adds a new dimension of urgency to the need to prevent the further transfer of Phytophthoras between sites.
Collectively, all of these Phytophthora taxa should now be regarded as a threat to native ecosystems, and they need to be managed in the same way as P. cinnamomi in terms of minimising their further spread. This can be achieved simply by implementing proven local dieback hygiene practices such as restricting activities to dry soil conditions, and ensuring that vehicles and footwear are thoroughly clean when moving from an infested to a clean area. This may be especially important for the Phytophthora taxa that are not yet widespread or common.
Conclusions
Clade 6 is arguably the most unusual and distinctive major clade in Phytophthora. Predominantly aquatic Clade 6 species have developed a highly successful ecological strategy as rapid colonisers of leaf litter and opportunistic pathogens of woody plants under flooded conditions while other species are aggressive soilborne pathogens of agricultural and horticultural crops with either wide (e.g. P. megasperma), moderately wide (e.g. P. rosacearum) or narrow (e.g. P. taxon asparagi) host ranges. Most extraordinarily for Clade 6 Phytophthoras, the recently described P. pinifolia has evolved towards an aerial lifestyle, producing caducous non-proliferating sporangia and causing an epidemic needle blight in Pinus radiata plantations in Chile (Durán et al. 2008). However, since P. pinifolia is a clonal introduction in Chile (Durán et al. 2010) its ecological niche in its unknown centre of origin may be different. Within Clade 6, the high number of taxa, their wide variation of habitats, lifestyle and breeding systems (heterothallic, homothallic, silent A1, potentially or sporadically self fertile, and fully sterile) and their tendency to hybridise may necessitate a rethinking of species boundaries and species recognition for this clade.
Acknowledgments
Collection of many of the samples in the field was carried out by staff of the WA Department of Environment and Conservation (and its predecessors, the Department of Conservation and Land Management, and the Forests Department). Others were collected by Daniel Hüberli, Tom Hill and Roz Hart. We thank Janet Webster and Juanita Ciampini (DEC) for technical assistance and Robert Gardiner (Yea, Victoria) for assistance with field work. The ongoing project is funded by DEC and through Research Capacity Funding provided by Murdoch University Sustainable Ecosystems Research Institute. Sugarloaf Pipeline Alliance/Melbourne Water funded the survey in Victoria.
REFERENCES
- Adams G, Catal M, Trimmer L.2009. Distribution and severity of Alder Phytophthora in Alaska. Proceedings of the Sudden Oak Death Fourth Science Symposium June 15–18, 2009, Santa Cruz, California USA http://www.fs.fed.us/psw/publications/documents/psw_gtr229/psw_gtr229_029.pdf [Google Scholar]
- Andjic V, Barber PA, Carnegie AJ, Hardy GEStJ, Wingfield MJ, Burgess TI.2007. Phylogenetic reassessment supports accommodation of Phaeophleospora and Colletogloeopsis from eucalypts in Kirramyces. Mycological Research 111: 1184 – 1198 [DOI] [PubMed] [Google Scholar]
- Ann PJ, Ko WH.1994. An asexual variant of Phytophthora insolita. Canadian Journal of Microbiology 40: 810 – 815 [Google Scholar]
- Bakonyi J, Nagy ZÁ, Belbahri L, Józsa A, Nechwatal J, Koltay A, Cooke DEL, Brasier C, Woodward S.2009. Identification and molecular characterization of Phytophthora taxa collected from woody hosts in Hungary. In: Book of Abstracts of the COST Action FP0801 “Established and Emerging Phytophthora: Increasing Threats to Woodland and Forest Ecosystems in Europe” 2nd Working Groups Meeting, WG3. Diagnostics and WG4. Management and Control, 10–12 September 2009: 16 University of Algarve, Faro, Portugal: [Google Scholar]
- Blackwell E.1943. The life history of Phytophthora cactorum (Leb. and Cohn) Schroet. Transactions of the British Mycological Society 26: 71 – 89 [Google Scholar]
- Blair JE, Coffey MD, Park S-Y, Geiser DM, Kang S.2008. A multi-locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology 45: 266 – 277 [DOI] [PubMed] [Google Scholar]
- Boccas B.1972. Contribution à l’ étude du cycle chez les Phytophthora. Les Comptes Rendus de l’Académie des Sciences 275D: 663 – 666 [Google Scholar]
- Brasier CM.1972. Observations on the sexual mechanism in Phytophthora palmivora and related species. Transactions of the British Mycological Society 58: 237 – 251 [Google Scholar]
- Brasier CM.1992. Evolutionary biology of Phytophthora. Part I: Genetic system, sexuality and the generation of variation. Annual Review of Phytopathology 30: 153 – 171 [Google Scholar]
- Brasier CM.2009. Phytophthora Biodiversity: How many Phytophthora species are there? In: Goheen EM, Frankel SJ. (eds), Phytophthoras in Forests and Natural Ecosystems Albany, CA: 101 – 115 USDA Forest Service General Technical Report PSW-GTR-221. [Google Scholar]
- Brasier CM, Cooke DEL, Duncan JM, Hansen EM.2003a. Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides–. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycological Research 107: 277 – 290 [DOI] [PubMed] [Google Scholar]
- Brasier CM, Hamm PB, Hansen EM.1993. Cultural characters, protein patterns and unusual mating behaviour of Phytophthora gonapodyides isolates from Britain and North America. Mycological Research 9: 1287 – 1298 [Google Scholar]
- Brasier CM, Hansen EM.1992. Evolutionary biology of Phytophthora. Part II: Phylogeny, speciation, and population structure. Annual Review of Phytopathology 30: 173 – 200 [Google Scholar]
- Brasier CM, Kirk SA, Delcan J, Cooke DEL, Jung T, Man in ’t Veld WA.2004. Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees. Mycological Research 108: 1172 – 1184 [DOI] [PubMed] [Google Scholar]
- Brasier CM, Sanchez-Hernandez E, Kirk SA.2003b. Phytophthora inundata sp. nov., a part heterothallic pathogen of trees and shrubs in wet or flooded soils. Mycological Research 107: 477 – 484 [DOI] [PubMed] [Google Scholar]
- Brasier CM, Vettraino AM, Chang TT, Vannini A.2010. Phytophthora lateralis discovered in an old growth Chamaecyparis forest in Taiwan. Plant Pathology 59: 595 – 603 [Google Scholar]
- Brown AV, Brasier CM.2007. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Phytopathology 56: 227 – 241 [Google Scholar]
- Burgess TI, Webster JL, Ciampini JA, White DW, Hardy GEStJ, Stukely MJC.2009. Re-evaluation of Phytophthora species isolated during 30 years of vegetation health surveys in Western Australia using molecular techniques. Plant Disease 93: 215 – 223 [DOI] [PubMed] [Google Scholar]
- Caten CE.1970. Spontaneous variability of single isolates of Phytophthora infestans. II. Pathogenic variation. Canadian Journal of Botany 48: 897 – 905 [Google Scholar]
- Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM.2000. A molecular phylogeny of Phytophthora and related Oomycetes. Fungal Genetics and Biology 30: 17 – 32 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Darmono TW, Parke JL.1990. Chlamydospores of Phytophthora cactorum: Their production, structure, and infectivity. Canadian Journal of Botany 68: 640 – 645 [Google Scholar]
- Dick MW.1990. Keys to Pythium. University of Reading Press, Reading, UK: [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Heled J, Kearse M, Moir R, Stones-Havas S, Sturrock S, Thierer T, Wilson A.2010. Geneious v5.1, Available from http://www.geneious.com [DOI] [PMC free article] [PubMed]
- Durán A, Gryzenhout M, Drenth A, Slippers B, Ahumada R, Wingfield BD, Wingfield MJ.2010. AFLP analysis reveals a clonal population of Phytophthora pinifolia in Chile. Fungal Biology 114: 746 – 752 [DOI] [PubMed] [Google Scholar]
- Durán A, Gryzenhout M, Slippers B, Ahumada R, Rotella A, Flores F, Wingfield BD, Wingfield MJ.2008. Phytophthora pinifolia sp. nov. associated with a serious needle disease of Pinus radiata in Chile. Plant Pathology 57: 715 – 727 [Google Scholar]
- Englander L, Roth LF.1980. Interaction of light and sterol on sporangium and chlamydospore production of Phytophthora lateralis. Phytopathology 70: 650 – 654 [Google Scholar]
- Erwin DC, Ribeiro OK.1996. Phytophthora diseases worldwide. APS Press, St. Paul, Minnesota: [Google Scholar]
- Hall G.1993. An integrated approach to the analysis of variation in Phytophthora nicotianae and a redescription of the species. Mycological Research 97: 559 – 574 [Google Scholar]
- Hallett IC, Dick MW.1981. Seasonal and diurnal fluctuations of oomycete propagule numbers in the free water of a freshwater lake. The Journal of Ecology 69: 671 – 692 [Google Scholar]
- Hansen EM, Reeser PW, Sutton W.2009a. The Phytophthora species known as “PgChlamydo”. In: Goheen EM, Frankel SJ. (tech. coords.), Proceedings of the fourth meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09: Phytophthoras in forests and natural ecosystems. Gen. Tech. Rep. PSW-GTR-221: 284–287 U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Albany, CA, USA: [Google Scholar]
- Hansen EM, Wilcox WF, Reeser PW, Sutton W.2009b. Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma “complex”. Mycologia 101: 129 – 135 [DOI] [PubMed] [Google Scholar]
- Ho HH, Zeng HC, Zheng FC.2002. Phytophthora insolita on Hainan island. Botanical Bulletin of Academia Sinica 43: 227 – 230 [Google Scholar]
- Hüberli D, Tommerup IC, Dobrowolski MP, Calver MC, Hardy GEStJ.2001. Phenotypic variation in a clonal lineage of two Phytophthora cinnamomi populations from Western Australia. Mycological Research 105: 1053 – 1064 [Google Scholar]
- Hüberli D, Tommerup IC, Hardy GEStJ.2000. False-negative isolations or absence of lesions may cause mis-diagnosis of diseased plants infected with Phytophthora cinnamomi. Australasian Plant Pathology 29: 164 – 169 [Google Scholar]
- Hulvey J, Gobena D, Finley L, Lamour K.2010. Co-occurrence and genotypic distribution of Phytophthora species recovered from watersheds and plant nurseries of eastern Tennessee. Mycologia 102: 1127 – 1133 [DOI] [PubMed] [Google Scholar]
- Hwang J, Oak SW, Jeffers SN.2009. Monitoring occurrence and distribution of Phytophthora species in forest streams in North Carolina using baiting and filtration methods. In: Goheen EM, Frankel SJ. (tech. coords.), Proceedings of the fourth meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09: Phytophthoras in forests and natural ecosystems. Gen. Tech. Rep. PSW-GTR-221: 91–95 U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Albany, CA, USA: [Google Scholar]
- Jung T.2009. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. Forest Pathology 39: 73 – 84 [Google Scholar]
- Jung T, Blaschke H, Neumann P.1996. Isolation, identification and pathogenicity of Phytophthora species from declining oak stands. European Journal of Forest Pathology 26: 253 – 272 [Google Scholar]
- Jung T, Blaschke M.2004. Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies. Plant Pathology 53: 197 – 208 [Google Scholar]
- Jung T, Burgess TI.2009. Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 22: 95 – 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T, Cooke DEL, Blaschke H, Duncan JM, Oßwald W.1999. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycological Research 103: 785 – 798 [Google Scholar]
- Jung T, Hansen EM, Winton L, Oßwald W, Delatour C.2002. Three new species of Phytophthora from European oak forests. Mycological Research 106: 397 – 411 [Google Scholar]
- Jung T, Nechwatal J.2008. Phytophthora gallica sp. nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycological Research 112: 1195 – 1205 [DOI] [PubMed] [Google Scholar]
- Jung T, Nechwatal J, Cooke DEL, Hartmann G, Blaschke H, Oßwald W, Duncan JM, Delatour C.2003. Phytophthora pseudosyringae sp. nov., a new species causing root and collar rot of deciduous tree species in Europe. Mycological Research 103: 785 – 798 [DOI] [PubMed] [Google Scholar]
- Ko WH.1980. Hormonal regulation of sexual reproduction in Phytophthora. Journal of General Mycology 116: 459 – 463 [Google Scholar]
- Ko WH, Ann PJ.1985. Phytophthora humicola, a new species from soil of a citrus orchard in Taiwan. Mycologia 77: 631 – 636 [Google Scholar]
- Kuhn DN.1981. Parasexuality in Phytophthora? In: Lucas JA, Shattock RC, Shaw DS, Cooke LR. (eds), Phytophthora: 242 – 255 Cambridge University Press, Cambridge, UK: [Google Scholar]
- Lane N.2009. Life ascending: The ten great inventions of evolution. Profile Books Ltd, London, UK: [Google Scholar]
- MacIntyre D, Elliott CG.1974. Selection for growth-rate during asexual and sexual propagation in Phytophthora cactorum. Genetics Research 24: 295 – 309 [Google Scholar]
- Marks GC, Kassaby FY.1974. Detection of Phytophthora cinnamomi in soils. Australian Forestry 36: 198 – 203 [Google Scholar]
- Martin FN, Tooley PW.2003. Phylogenetic relationships among Phytophthora species inferred from sequence analysis of mitochondrially encoded cytochrome oxidase I and II genes. Mycologia 95: 269 – 284 [PubMed] [Google Scholar]
- Moralejo E, Puig M, García JA, Descals E.2006. Stromata, sporangiomata and chlamydosori of Phytophthora ramorum on inoculated Mediterranean woody plants. Mycological Research 110: 1323 – 1332 [DOI] [PubMed] [Google Scholar]
- Nechwatal J, Mendgen K.2006. Widespread detection of Phytophthora taxon Salixsoil in the littoral zone of Lake Constance, Germany. European Journal of Plant Pathology 114: 261 – 264 [Google Scholar]
- Nechwatal J, Wielgoss A, Mendgen K.2008. Diversity, host, and habitat specificity of oomycete communities in declining reed stands (Phragmites australis) of a large freshwater lake. Mycological Research 112: 689 – 696 [DOI] [PubMed] [Google Scholar]
- Reeser PW, Sutton W, Hansen EM, Remigi P, Adams GC.2011. Phytophthora species in forest streams in Oregon and Alaska. Mycologia 103: 22 – 35 [DOI] [PubMed] [Google Scholar]
- Remigi P, Sutton W, Reeser PW, Hansen EM.2009. Characterizing the community of Phytophthora species in an Oregon forest stream. In: Goheen EM, Frankel SJ. (tech. coords.), Proceedings of the fourth meeting of the International Union of Forest Research Organizations (IUFRO) Working Party S07.02.09: Phytophthoras in forests and natural ecosystems. Gen. Tech. Rep. PSW-GTR-221: 311–314 U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Albany, CA, USA: [Google Scholar]
- Ronquist F, Huelsenbeck JP.2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Rutherford FS, Ward EWB.1985. Evidence for genetic control of oospore abortion in Phytophthora megasperma f. sp. glycinea. Canadian Journal of Botany 63: 1671 – 1673 [Google Scholar]
- Sanchez-Hernandez ME, Munoz-Garcia M, Brasier CM, Trapero-Casas A.2001. Identity and pathogenicity of two Phytophthora taxa associated with a new root disease of olive trees. Plant Disease 85: 411 – 416 [DOI] [PubMed] [Google Scholar]
- Saude C, Hurtado-Gonzales OP, Lamour KH, Hausbeck MK.2008. Occurrence and characterization of a Phytophthora sp. pathogenic to asparagus (Asparagus officinalis) in Michigan. Phytopathology 98: 1075 – 1083 [DOI] [PubMed] [Google Scholar]
- Shearer BL, Smith IW.2000. Diseases of Eucalyptus caused by soilborne species of Phytophthora and Pythium. In: Keane PJ, Kile GA, Podger FD, Brown BN. (eds), Diseases and Pathogens of Eucalypts: 259 – 291 CSIRO Publishing, Melbourne, Australia: [Google Scholar]
- Shearer BL, Tippett JT.1989. Jarrah dieback: the dynamics and management of Phytophthora cinnamomi in the jarrah (Eucalyptus marginata) forest of South-western Australia. Research Bulletin No. 3. Department of Conservation and Land Management, Como, Western Australia: [Google Scholar]
- Stamps DJ, Waterhouse GM, Newhook FJ, Hall GS.1990. Revised tabular key to the species of Phytophthora. Mycological Papers 162. CAB International Mycological Institute, Kew, Surrey: [Google Scholar]
- Strelein GJ, Sage LW, Blankendaal PA.2006. Rates of disease extension of Phytophthora cinnamomi in the jarrah forest bioregion of southwestern Australia. In: Brasier C, Jung T, Oßwald W. (eds), Proceedings of the third International IUFRO Working Party S07.02.09 Meeting at Freising, Germany, 11–18 September 2004 Progress in Research on Phytophthora Diseases of Forest Trees: 49–52. Forest Research, Farnham, UK: [Google Scholar]
- Stukely MJC, Webster JL, Ciampini JA, Brown E, Dunstan WA, Hardy GEStJ, Woodman GJ, Davison EM, Tay FCS.2007. Phytophthora inundata from native vegetation in Western Australia. Australasian Plant Pathology 36: 606 – 608 [Google Scholar]
- Swofford DL.2003. PAUP 4.0b10*: Phylogenetic Analysis Using Parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts: [Google Scholar]
- Tsao PH, Guy SO.1977. Inhibition of Mortierella and Pythium in a Phytophthora-isolation medium containing hymexazol. Phytopathology 67: 796 – 801 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: A Guide to Methods and Applications: 315 – 322 Academic Press, San Diego, California, USA: [Google Scholar]
- Yamak F, Peever TL, Grove GG, Boal RJ.2002. Occurrence and identification of Phytophthora spp. pathogenic to pear fruit in irrigation water in the Wenatchee River Valley of Washington State. Phytopathology 92: 1210 – 1217 [DOI] [PubMed] [Google Scholar]
- Zeng H-C, Ho H-H, Zheng F-C.2009. A survey of Phytophthora species on Hainan Island of South China. Journal of Phytopathology 157: 33 – 39 [Google Scholar]