Abstract
The relationships based on ITS sequences of 48 Hygrocybe s.l. specimens were studied and compared with previously described taxonomic groups. Our specimens formed two well separated genetic groups. The first one includes the species characterized by vivid yellow and red colours, while species belonging to other clades were pallid or pale brown, and in most cases with pink or olive tones. This separation is supported by the presence of muscaflavin pigments among some species referred to Hygrocybe (Bresinsky & Kronawitter 1986). The subgenera distinguished by morphological features can be relatively well recognized on phylogenetic trees, however, the majority of sections were not supported. Variability in the ITS region of Hygrocybe species is unusually high. In some cases sequences differed by more than 25 %, and the lengths of ITS regions also showed large differences. Taxa that were considered as closely related, e.g. the H. conica aggregate, were found to have identical or highly similar sequences. Our results seem to confirm the taxonomic concept of Bresinsky (2008) who proposed the division of the genus Hygrocybe. Hence H. calyptriformis and all examined members of subg. Gliophorus (H. irrigata, H. laeta, H. nitrata, H. psittacina) and subg. Cuphophyllus could be excluded from the genus Hygrocybe s.str. Based on these results further research using DNA markers at the intergeneric level is suggested to revaluate the taxonomy of former Hygrocybe species.
Keywords: Hygrocybe, ITS region, molecular taxonomy, muscaflavin pigments
INTRODUCTION
The term Hygrocybe originates from Fries (1821) who at first recognized the group as a member of the ‘tribus’ Clitocybe (‘subtribus’ Hygrocybe) and subsequently, not earlier than 1838, transferred them as a ‘tribus’ to the genus Hygrophorus. It was raised to the rank of a separate genus by Kummer (1871). According to the opinion of most mycologists, both Hygrocybe and Hygrophorus should be classified as separate genera in the family Hygrophoraceae. Currently about half of the researchers recognize multiple segregate genera while the remainder divides the genus Hygrocybe into three subgenera, namely subg. Hygrocybe s.str. Bon 1976, subg. Pseudohygrocybe Bon 1976 and subg. Cuphophyllus Donk 1962 (Boertmann 1995, Candusso 1997, Krieglsteiner 2001).
Some taxa of Hygrocybe can be determined unambiguously based on macroscopic characters, (e.g. H. citrinovirens, H. conica, H. conicoides, H. intermedia, H. ovina, H. pratensis, H. spadicea). Identification of other Hygrocybe species can be moderately improved by considering the results of detailed microscopical examination and macroscopic attributes simultaneously (e.g. determining H. aurantiosplendens, H. coccinea, H. constrictospora, H. marchii, H. phaeococcinea and H. reidii; separating the taxa in the groups H. punicea vs H. splendidissima, H. glutinipes vs H. vitellina, H. laeta var. flava vs H. helobia, H. miniata or H. ceracea vs H. insipida, and H. constrictospora vs H. mucronella.
The most important diagnostic features of the genus Hygrocybe on a macroscopic level are the thick, waxy, widely-spaced gills producing white spores, and the stipe without veil remnants. Additional significant microscopic markers common to most members of Hygrocybe are as follows: long, narrow basidia (6–9 times longer than its width) with smooth and inamyloid spores. All the taxa lack real pleurocystidia. However, to make a firm distinction between the three subgenera, structure and arrangement of the hyphae of the lamellar trama have been accepted as the most reliable microscopic characteristics (Boertmann 1995). The hyphae of the lamellar trama in the subgenus Hygrocybe are especially long (> 1 000 μm) and parallel to each other (regular structure), such as those of H. conica. Species of the second subgenus, Pseudohygrocybe, have a subregular arrangement of short hyphae that rarely exceed 150 μm. The exceptions in subg. Pseudohygrocybe that are classified by others in the genus Neohygrocybe, i.e., H. ingrata, H. nitrata and H. ovina; the trama hyphae of these species are 200–500 μm. The genera Neohygrocybe and Gliophorus were separated by Herink (1959) based in part on the absence of muscaflavin pigment. The lamellar trama in subg. Cuphophyllus (Camarophyllus) is composed of short hyphae < 150 μm, that are mostly cylindrically shaped and form a highly interwoven hyphal entanglement (irregular lamellar trama). Bas (1990) recategorized the genus Hygrocybe and classified it in the family Tricholomataceae together with the genus Camarophyllopsis as tribus Hygrocybeae. In his opinion genus Hygrophorus s.str. should also be placed in the family Tricholomataceae as a separate tribus. Candusso (1997) also removed the genus Hygrocybe from the family Hygrophoraceae, but classified it in the family Agaricaceae except for genus Camarophyllopsis. In contrast, Bon (1992) proposed to separate the Hygrophoraceae from the order Agaricales and treat it as a distinct order, o. Hygrophorales, due to its unique characters. The present authors consider the family Hygrophoraceae a distinct group within the Agaricales – a position supported by a multigene analysis by Matheny et al. (2006).
Currently, the number of Hygrocybe taxa recognized in Europe ranges between 60 and 133, depending on the authors and their various opinions on taxonomy. While Moser (1983), and Bon (1976, 1990, 1992) elevated certain taxa to higher taxonomic levels (giving species rank to varieties and forms), former separate species have occasionally been contracted by Boertmann (1995) thus reducing the number of Hygrocybe taxa. These two opposing processes are taking place simultaneously. While Bon (1976, 1990, 1992) often publishes new Hygrocybe species, Boertmann (1995) unites species as well as genera of Hygrocybe and Camarophyllus and he annuls certain sections inside the subgenera of Hygrocybe. At the same time, he still publishes and introduces new Hygrocybe taxa, despite the fact that these are usually constructed by the unification of former taxa. In some rare cases, however, Boertman differentiates, e.g. at H. laeta var. flava Boertm. var. nov., or at the aggregate of H. lacmus (Schumach.) P.D. Orton & Watling with three well separable species (H. lacmus, H. flavipes (Britzelm.) Arnolds, H. radiate Arnolds), even though others (e.g. Krieglsteiner 2000) join the species and refer to as H. lacmus. Until now, 35 taxa of genus Hygrocybe are known from Hungary on the basis of Boertmann’s taxonomy and nomenclature (Zagyva 2003). With respect to the genus Hygrocybe, the Őrség National Park is the best studied and explored area in Hungary, where 34 of the 35 taxa were found. The basiphyl H. conicoides is the only species that has not been found in this region yet (Zagyva et al. 2003). Hungary seems to be poor in Hygrocybe partly due to the dominating continental climate becoming increasingly arid, and partly due to the expansion of intensive agriculture in the past decades.
The presence of above mentioned muscaflavin pigments is a remarkable chemotaxonomical character. Bresinsky & Kronawitter (1986) detected muscaflavins in 42 of 53 studied Hygrocybe species. The authors distinguished six groups on the basis of pigment content, of which four comprise muscaflavin-free species. Cibula (1976) found that rhodohygrocybin pigments were present in Hygrocybe s.str. (except for H. andersonii; Cibula & Weber Smith 1996), but were absent from H. calyptriformis, Gliophorus, Neohygrocybe and Cuphophyllus species. Emerging or verifying taxonomic groups based on chemotaxonomical features is a widely applied method for various taxa (e.g. Agerer 1999, Binder & Besl 1999). Beisenherz (2002) used rDNA RFLP (Restriction Fragment Length Polymorphism) analysis and cytofluorometry to characterize the genus at a molecular level. Matheny et al. (2006) analysed several Hygrocybe s.l. species on the basis of multigene sequences; in their cladogram Gliophorus, Hygrophorus, Humidicutis and Camarophyllus (Cuphophyllus) are not close to Hygrocybe s.str. although still within the Hygrophoroid-clade. Recently, in spite of few results in molecular taxonomy, Bresinsky (2008) divided the European species of genus Hygrocybe s.l. into four genera: Hygrocybe s.str., Gliophorus, Neohygrocybe and Porpolomopsis (for H. calyptriformis). Brock et al. (2009) published a number of ITS sequences of Hygrocybe materials without taxonomic discussion. Binder et al. (2010) developed a six-locus nuclear dataset including two non-ribosomal protein coding genes, RPB1 and RPB2 plus the translation elongation factor (1-alpha tef1).
The goal of our studies was to give additional data for the identification, classification and distinction of ambiguously identified Hygrocybe species including a larger number of European species than previous analyses (Mattheny et al. 2006, Brock et al. 2009, Binder et al. 2010). We also aimed to find agreement between the phylogeny and previous studies based on chemotaxonomy. Sequence analyses of total ITS (internal transcribed spacer) regions supporting recent taxonomic investigations and a preliminary revision of the genus Hygrocybe s.l. were performed using materials from the Carpatho-Pannon region.
MATERIALS AND METHODS
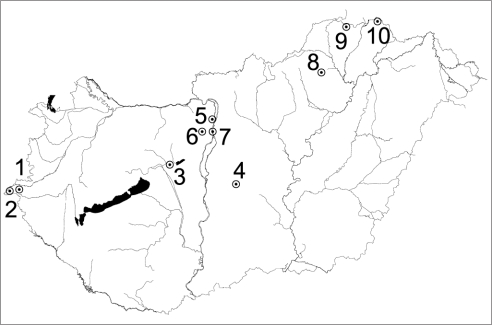
Fungal specimens for ITS sequence analyses were obtained from selected exsiccates of mainly Hungarian herbaria (Fig. 1). The majority of specimens originated from the area of the Őrség National Park (Apátistvánfalva, Farkasfa, Felsőszölnök, Kétvölgy). We sequenced several specimens of a species when possible.
Fig. 1.
Map of Hungary with collection locations: I. Transdanubian Hills: Őrség Hills: Farkasfa (1); Vendvidék Hills: Felsőszölnök (2), Kétvölgy (2), Apátistvánfalva (2); II. Great Hungarian Plain: Mezőföld: Székesfehérvár-Sóstó (3); Kiskunság: Kunbaracs (4); III. Transdanubian Medium Mountains: Pilis Hills: Budakalász (5); Buda Hills: Budakeszi (6), Budapest (7); IV. Northern Medium Mountains: Bükk Mountains: Bükkszentkereszt (8); Cserehát: Perecse (9); Zemplén Mountains: Lászlótanya (10). Further collection sites are: Austria, Niederösterreich: Puchberg; Steiermark: Feldbach.
For taxonomic determinations based on morphological characters, the keys of Boertmann (1995), Candusso (1997) and Krieglsteiner (2001) were used separately. EMBL (European Molecular Biology Laboratory) Nucleotide Sequence Database Accession Number and locality of the specimens used for molecular analysis are enumerated in Table 1. Basidiocarps were collected over the years 1997–2002, between June and October. EMBL Accession Numbers of other Hygrocybe sequences are represented below.
Table 1.
The investigated herbarium specimens. Species names are given according to distinct morphological keys of Boertmann (1995), Candusso (1997) and Krieglsteiner (2001). Based on recent molecular results H16 and H38 does not corresponds to H. cantharellus, which was described from the southern Appalachian Mountains in North Carolina, USA, therefore we treated them as H. lepida Arnolds (Deborah Jean Lodge, pers. comm.). (The specimen marked with * was identified by Prof. David Boertmann.)
| Boertmann (1995) | Candusso (1997) | Krieglsteiner (2001) | Locality | In Herbaria | Acc. number | |
|---|---|---|---|---|---|---|
| H1 | persistens var. persistens (Britzelm.) Singer (1940) | acutoconica (Clem.) Singer (1951) | persistens(Britzelm.) Singer (1940) | Kétvölgy | T. Zagyva | FM208852 |
| H2 | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | Felsőszölnök | T. Zagyva | FM208853 |
| H3 | calyptriformis (Berk.) Fayod (1889) | calyptriformis (Berk.) Fayod (1889) | calyptriformis (Berk.) Fayod (1889) | Kétvölgy | T. Zagyva | FM208854 |
| H4 | chlorophana (Fr.) Wünsche (1877) | chloraphana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | Felsőszölnök | T. Zagyva | FM208855 |
| H5 | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | Felsőszölnök | T. Zagyva | FM208856 |
| H6 | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | Kétvölgy | T. Zagyva | FM208857 |
| H7 | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | chlorophana (Fr.) Wünsche (1877) | Kétvölgy | T. Zagyva | FM208858 |
| H8 | coccinea (Schaeff.) P. Kumm. (1871) | coccinea var. coccinea (Schaeff.) P. Kumm. (1871) | coccinea var. coccinea (Schaeff.) P. Kumm. (1871) | Kétvölgy | T. Zagyva | FM208859 |
| H9 | conica var. conicoides (P.D. Orton) Boertm. (1995) | conicoides (P.D. Orton) P.D. Orton & Watling (1969) | conica var. conicoides (P.D. Orton) Boertm. (1995) | Székesfehérvár | T. Zagyva | FM208860 |
| H10 | conica var. conicoides (P.D. Orton) Boertm. (1995) | conicoides (P.D. Orton) P.D. Orton & Watling (1969) | conica var. conicoides (P.D. Orton) Boertm. (1995) | Székesfehérvár | T. Zagyva | FM208861 |
| H11 | conica var. conicoides (P.D. Orton) Boertm. (1995) | conicoides (P.D. Orton) P.D. Orton & Watling (1969) | conica var. conicoides (P.D. Orton) Boertm. (1995) | Székesfehérvár | T. Zagyva | FM208862 |
| H12 | coccinea (Schaeff.) P. Kumm. (1871) | coccinea var. coccinea (Schaeff.) P. Kumm. (1871) | coccinea var. coccinea (Schaeff.) P. Kumm. (1871) | Felsőszölnök | T. Zagyva | FM208863 |
| H13 | quieta (Kühner) Singer (1951) | obrussea (Fr.) Wünsche (1877) | obrussea (Fr.) Wünsche (1877) | Felsőszölnök | T. Zagyva | FM208864 |
| H16 | cantharellus (Schwein.) Murrill (1911) | cantharellus (Schwein.) Murrill (1911) | cantharellus (Schwein.) Murrill (1911) | Farkasfa | T. Zagyva | FM208865 |
| H17 | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | Pilisszentkereszt | T. Zagyva | FM208866 |
| H18 | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | Budakeszi | T. Zagyva | FM208867 |
| H19 | virginea var. fuscescens (Bres.) Arnolds (1986) | fuscescens (Bres.) P.D. Orton & Watling (1969) | virginea var. fuscencens (Bres.) Arnolds (1986) | Székesfehérvár | T. Zagyva | FM208868 |
| H20 | virginea var. virginea (Wulfen) P.D. Orton & Watling (1969) | virginea (Wulfen) P.D. Orton & Watling (1969) | virginea (Wulfen) P.D. Orton & Watling (1969) | Székesfehérvár | T. Zagyva | FM208869 |
| H21 | virginea var. ochraceopallida (P.D. Orton) Boertm. (1995) | virginea var. ochraceopallida (P.D. Orton) Boertm. (1995) | virginea var. fuscencens (Bres.) Arnolds (1986) | Budapest | T. Zagyva | FM208870 |
| H22 | persistens var. persistens (Britzelm.) Singer (1940) | acutoconica (Clem.) Singer (1951) | persistens (Britzelm.) Singer (1940) | Székesfehérvár | T. Zagyva | FM208871 |
| H23 | persistens var. persistens (Britzelm.) Singer (1940) | acutoconica (Clem.) Singer (1951) | persistens (Britzelm.) Singer (1940) | Kétvölgy | T. Zagyva | FM208872 |
| H24 | virginea var. ochraceopallida (P.D. Orton) Boertm. (1995) | virginea var. ochraceopallida (P.D. Orton) Boertm. (1995) | virginea var. fuscencens (Bres.) Arnolds (1986) | Kétvölgy | T. Zagyva | FM208873 |
| H26 | pratensis var. pratensis (Pers.) Bon (1976) | pratensis var. pratensis (Pers.) Bon (1976) | pratensis (Pers.) Bon (1976) | Kétvölgy | T. Zagyva | FM208874 |
| H27 | psittacina var. psittacina (Schaeff.) P. Kumm. (1871) | psittacina (Schaeff.) P. Kumm. (1871) | psittacina var. psittacina (Schaeff.) P. Kumm. (1871) | Kétvölgy | T. Zagyva | FM208875 |
| H29 | punicea (Fr.) P. Kumm. (1871) | punicea (Fr.) P. Kumm. (1871) | punicea var. punicea (Fr.) P. Kumm. (1871) | Apátistvánfalva | T. Zagyva | FM208876 |
| H30 | quieta (Kühner) Singer (1951) | obrussea (Fr.) Wünsche (1877) | obrussea (Fr.) Wünsche (1877) | Bükkszentkereszt | T. Zagyva | FM208877 |
| H31 | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | Székesfehérvár | T. Zagyva | FM208878 |
| H32 | spadicea var. spadicea (Scop.) P. Karst. (1879) | spadicea (Scop.) P. Karst. (1879) | spadicea (Scop.) P. Karst. (1879) | Kétvölgy | T. Zagyva | FM208879 |
| H34 | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | Kétvölgy | T. Zagyva | FM208880 |
| H35 | irrigata (Pers.) Bon (1976) | irrigata (Pers.) Bon (1976) | irrigata (Pers.) Bon (1976) | Kétvölgy | T. Zagyva | FM208881 |
| H36 | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | conica var. conica (Scop.) P. Kumm. (1871) | Székesfehérvár | T. Zagyva | FM208882 |
| H37 | ceracea (Wulfen) P. Kumm. (1871) | ceracea (Wulfen) P. Kumm. (1871) | ceracea (Wulfen) P. Kumm. (1871) | Budakalász | L. Albert | FM208883 |
| H38 | cantharellus (Schwein.) Murrill (1911) | cantharellus (Schwein.) Murrill (1911) | cantharellus (Schwein.) Murrill (1911) | Lászlótanya | L. Albert | FM208884 |
| H39 | nitrata (Pers.) Wünsche (1877) | murinacea (Bull.) M.M. Moser (1967) | nitrata (Pers.) Wünsche (1877) | Lászlótanya | L. Albert | FM208885 |
| H40 | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | miniata (Fr.) P. Kumm. | Budakalász | L. Albert | FM208886 |
| H41 | laeta var. laeta (Pers.) P. Kumm. (1871) | laeta var. laeta (Pers.) P. Kumm. (1871) | laeta (Pers.) P. Kumm. (1871) | Budakalász | L. Albert | FM208887 |
| H43 | intermedia (Pass.) Fayod (1889) | intermedia (Pass.) Fayod (1889) | intermedia (Pass.) Fayod (1889) | Perecse | L. Albert | FM208888 |
| H44 | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | citrinovirens (J.E. Lange) Jul. Schäff. (1947) | Perecse | L. Albert | FM208889 |
| H47 | laeta var. laeta (Pers.) P. Kumm. (1871) | laeta var. laeta (Pers.) P. Kumm. (1871) | laeta (Pers.) P. Kumm. (1871) | Feldbach | L. Albert | FM208890 |
| H48 | lacmus (Schumach.) P.D. Orton & Watling (1969) | lacmus (Schumach.) P.D. Orton & Watling (1969) | lacmus (Schumach.) P.D. Orton & Watling (1969) | Feldbach | L. Albert | FM208891 |
| H49* | splendidissima (P.D. Orton) M.M. Moser (1967) | splendidissima (P.D. Orton) M.M. Moser (1967) | punicea var. splendidissima (P.D. Orton) Krieglst. (1992) | Felsőszölnök | L. Albert | FM208892 |
| H51 | persistens var. konradii (R. Haller Aar.) Boertm. (1995) | konradiiR. Haller Aar. (1955) | persistens (Britzelm.) Singer (1940) | Kunbaracs | K. Halász | FM208893 |
| H52 | pratensis var. pallida (Cooke) Arnolds (1985) | berkeleyiP.D. Orton & Watling (1969) | pratensis (Pers.) Bon (1976) | Kétvölgy | T. Zagyva | FM208894 |
| H53 | psittacina var. psittacina (Schaeff.) P. Kumm. (1871) | psittacina (Schaeff.) P. Kumm. (1871) | psittacina var. psittacina (Schaeff.) P. Kumm. (1871) | Apátistvánfalva | T. Zagyva | FM208895 |
| H54 | flavipes (Britzelm.) Arnolds (1989) | flavipes (Britzelm.) Arnolds (1989) | lacmus (Schumach.) P.D. Orton & Watling (1969) | Puchberg | H. Pidlich-Aigener | FM208896 |
| H55 | colemanniana (A. Bloxam) P.D. Orton & Watling (1969) | colemanniana (A. Bloxam) P.D. Orton & Watling (1969) | lacmus (Schumach.) P.D. Orton & Watling (1969) | Puchberg | H. Pidlich-Aigener | FM208897 |
| H57 | punicea (Fr.) P. Kumm. (1871) | punicea (Fr.) P. Kumm. (1871) | punicea var. punicea (Fr.) P. Kumm. (1871) | Felsőszölnök | T. Zagyva | FM208898 |
| H58 | turunda sensu Lange [Fl. Ag. Dan. 5: 27 & pl. 168H (1940)] | turunda sensu Lange [Fl. Ag. Dan. 5: 27 & pl. 168H (1940)] | coccineocrenata (P.D. Orton) M.M. Moser (1967) | Farkasfa | Á. Zöld-Balogh | FM208899 |
| Hygrocybe canescens (A.H. Sm. & Hesler) P.D. Orton | GenBank | DQ486685 | ||||
| Humidicutis marginata (Peck) Singer | GenBank | DQ490625 | ||||
| outg. | Lactarius semisanguifluus R. Heim & Leclair | GenBank | AF140268 | |||
| outg. | Lactarius scrobiculatus (Scop.) Fr. | GenBank | AF140263 | |||
| outg. | Lactarius quieticolor Romagn. | GenBank | AF140269 | |||
| outg. | Pleurotus ostreatus (Jacq.) P. Kumm. | GenBank | EF514248 | |||
DNA extractions and PCR reactions were carried out according to Gardes et al. (1991). The universal primers ITS1 and ITS4 (White et al. 1990) and the fungal specific ITS1F primer (Gardes & Bruns 1993) were used for amplifications. PCR products were cleaned using a Montage-PCR (Millipore) microfilter. Sequencing reactions were carried out using BigDye™ Terminator Cycle 3.1 Sequencing Kit (Applied Biosystems). PCR products were sequenced with ITS1 and ITS4 primers, on ABI PRISM 3100 Genetic Analyser.
CLUSTALW2 (Larkin et al. 2007) program was applied to generate alignments and phylogenetic and molecular evolutionary analyses were conducted using MEGA4 (Tamura et al. 2007).
RESULTS AND DISCUSSION
Total ITS1 + 5.8S rDNA + ITS2 regions were sequenced successfully in most cases. The number of nucleotides were between 348 (H. nitrate, but probably not a whole ITS sequence) and 665 (H. flavipes).
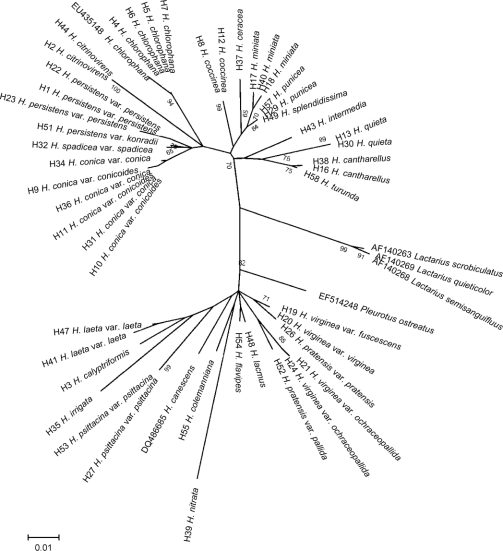
At first sight, numerous clades are distinguishable in the phylograms in Fig. 2, 3, 4. Large differences were noted among sequences, some with only 24 % sequence homology. Few nodes are marked with high bootstrap values, and the topology of the sections or aggregates was rather variable. Every model, however, showed two major clades (A and B) separated in the phylograms comprising all sequences (Fig. 2). The branch supporting the separation of the two major groups had a bootstrap value of 89 %.
Fig. 2.
Evolutionary relationships of Hygrocybe s.l. species. The evolutionary history was inferred using the Neighbour-joining method. The bootstrap consensus tree inferred from 1 000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50 % bootstrap replicates are collapsed. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). Phylogenetic analyses were conducted in MEGA4.
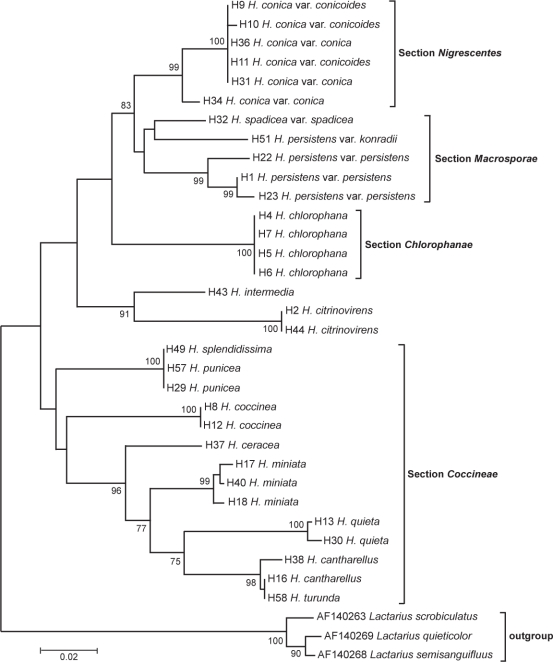
Fig. 3.
Evolutionary relationships of 35 taxa of clade B. Neighbour-joining consensus tree inferred from 1 000 replicates. Branches corresponding to partitions reproduced in less than 70 % bootstrap replicates are collapsed. Bootstrap values (% of 1 000 replications) are given for selected nodes. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). Outgroup was three species of genus Lactarius.
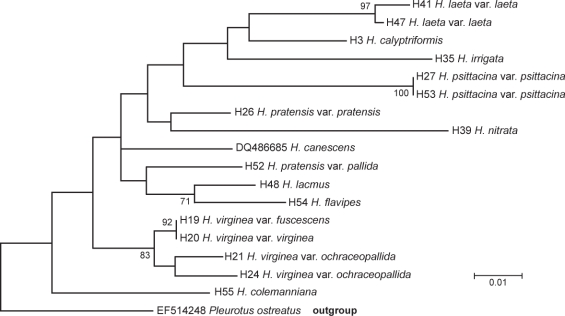
Fig. 4.
Evolutionary relationships of 17 taxa of clade A. Neighbour-joining consensus tree constructed using MEGA4. The scale bar indicates the number of base substitutions per site. Bootstrap support values from 1 000 replicates are shown at the nodes. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). The tree was rooted to Pleurotus ostreatus (GenBank EF514248).
The species of clade B (Fig. 3) are characterizable by the presence of vivid yellow, orange and red colours, whereas clade A (Fig. 4) is represented by dull or pale coloured basidiocarps, some with pink, purple or olive tints. Since our phylograms lacked well circumscribed additional groups, our remarks are discussed by sections according to Boertmann’s (1995) and Candusso’s (1997) taxonomic system. Clade A contains Cuphophyllus species, plus species of Gliophorus (G. irrigatus, G. laetus and G. psittacinus) and Neohygrocybe (N. nitrata) presented here as genus Cuphophyllus, sections Neohygrocybe and Glutinosae, and the species H. calyptriformis. In a comprehensive multilocus analysis of Agaricales, Matheny et. al (2006) demonstrated a monophyletic origin of Hygrophoraceae if certain genera traditionally treated in Hygrophoraceae (e.g., Camarophyllopsis and Neohygrophorus) were excluded, while other genera previously considered to belong to the Tricholomataceae were included (e.g., Pseudoarmillariella). They suggested the rehabilitation of genus names Camarophyllus, Gliophorus and Humidicutis. Clade B includes section Coccineae and subgenus Hygrocybe except for H. calyptriformis.
Especially in the taxa of subg. Hygrocybe, differences in the microscopic properties alone cannot be considered as sufficient for the distinction among the taxa. Moreover, attempts that were based solely on microscopic features of dried sporophores (e.g. between H. ceracea, H. constrictospora and H. insipida) often failed. The only hope for unambiguous identification in these cases is if certain features of the fresh sporophores had already been fixed, noted, recorded, or registered at the habitat. There are many taxa at the same time, that could be safely separated based on their macro- and/or microscopic features after decades of storage. They were questioned as separate species, or taxa only in the last decade, primarily due to Boertmann’s examinations (Boertmann 1995).
OVERVIEW OF PROBLEMS OF CLASSIFICATION
While there are distinct circumscriptions of the genus Hygrocybe (subgenera and sections), some species are difficult to define and often not determined unambiguously. This is caused not only by weather related variations (changes in colour), but also by the lack of molecular taxonomical investigations (Bresinsky 2008). Considering the multidimensional taxonomic problems typical of the genus, below we compare and analyze the results based on the previous taxonomic notions, within the genus, by taxa.
Subgenus Cuphophyllus Donk
Section Cuphophyllus
-
Resolving clade A, section Cuphophyllus proves to be a mono- or polyphyletic group, depending on the applied method. The two subsections within section Cuphophyllus do not seem to segregate, and bootstrap support for basal branches was weak. Differentiation of subsections Cuphophyllus and Virginei on the basis of morphological features often raises difficulties.
Subsection Cuphophyllus
Although subsection Cuphophyllus has a dry pileus surface and shorter spores (5–6 μm) than in subsection Virginei, misidentifications occur in numerous cases, as among H. virginea and certain specimens of H. pratensis var. pallida.
Subsection Virginei Bataille
Flavipes aggregate: flavipes, lacmus
Hygrocybe lacmus, H. flavipes and H. colemanniana do not vary much microscopically, but DNA sequence comparisons reveal large sequence differences among them. According to Boertmann (1995), they should appear as separate species in the future, primarily based on macromorphological features, and secondly due to their minimal and inconstant spore size differences. Krieglsteiner (2000) claimed that neither macro- nor microscopic properties can be used for the reliable differentiation among the three above mentioned taxa. On our phylogenetic trees H. lacmus and H. flavipes constitute a tight monophyletic group (bootstrap value, bt = 74%), which may be characterized by blue tints in the sporophore.
Virginei aggregate: C. colemanniana, C. fuscescens, C. ochraceopallida, C. virginea
While the branch containing Cuphophyllus species is rather homogeneous, the C. pratensis and C. virginea groups are separate, but with low bootstrap values. The distal group in this clade (H19, H20, H21, H24; Fig. 4) comprises three taxa (C. virginea, and two taxa identified as C. ochraceopallida), which together form a well-supported clade (bt = 77 %). Cuphophyllus colemanniana fell in the Virginei clade, but without bootstrap support. Boertmann (1995) recommended taxonomic revision of this group (H. colemanniana, H. fuscescens, H. ochraceopallida, H. russocoriacea, H. virginea). Candusso (1997) discussed three sections within subg. Cuphophyllus. Sect. Virginei comprises H. virginea and its varieties with H. canescens and H. berkeleyi, sect. Cuphophyllus incorporates H. pratensis and Flavipes aggregate among others. Though bootstrap support was lacking (Fig. 4), the C. flavipes aggregate does not appear to belong to the same clade as C. pratensis, nor does C. canescens fall within the Virginei clade. None of the examined species belongs to the third section Oreocybe.
Subgenus Pseudohygrocybe Bon
Section Neohygrocybe Herink
The uniquely short ITS region of H. nitrata significantly separates this species from any other taxa, but additional sequences would be needed to confirm this result and for group definition. Bresinsky & Kronawitter (1986) revealed the correspondence of sect. Neohygrocybe with their pigment group 3.0, which lacks muscaflavins. This result seems to confirm the separation of Neohygrocybe genus by Bresinsky (2008).
Section Glutinosae Kühner
All three examined species (Gliophorus laetus, Gliophorus unguinosus and G. psittacinus) are strictly monophyletic with high bootstrap values. In contrast, nucleotide homology among the species is relatively low. These three taxa can be differentiated morphologically without difficulties. The common features of the taxa are the glutinosity of the sporophore and the medallion clamp connections at the base of the basidia. Matheny et al. (2006), based on their phylogenetic study, proposed to resurrect the Gliophorus laetus (Pers.: Fr.) Herink as the valid name of this taxon instead of H. laeta. Hygrocybe psittacina was recombined in Gliophorus by Herink (G. psittacinus (Schaeff.) Herink). Recently, H. irrigata was also proposed to be transferred to Gliophorus (Bresinsky 2008), though H. unguinosa, which Boertmann considers a synonym, has already been combined in Gliophorus. The dilemma with the combination Gliophorus unguinosus (Fr.) Kovalenko is that there are several species in Europe, according to their ITS sequences, they are nearly identical morphologically, and we don’t know which ones correspond to which name (or don’t correspond to any name).
Psittacina aggregate: laeta, psittacina
Greenish colour appears near the apex of the stem of both taxa. Hygrocybe psittacina has green, yellowish green colouring, H. laeta var. laeta can show somewhat olive green shade at the same part sometimes (Candusso 1997, Beisenherz 2002). Beisenherz (2002: 53) claimed that red pigment masks the blue and green pigments. Bresinsky & Kronawitter (1986) demonstrated that H. psittacina, H. laeta, moreover H. sciophana (Fr.) Wünsche do not contain any muscaflavin pigments. They are believed to have caretenoid pigments, readily apparent in dried specimens. Accordingly, all our examined species of sect. Glutinosae are integrated in clade A, among the muscaflavin-free species. The feature of H. psittacina is a disappearing green colour affected by solar radiation or drying. Moreover, Boertmann (1995) described a variety without any green colour on page 81. He affirms that the wide colour palette may have evolved either due to the effect of edaphic factors, like scrubby type on the fixed sand dunes, or by unique variations. Inside the section, only H. laeta has decurrent trama and ixocheilocystidia, as well as pale rose-coloured exsiccata. The others have adnexed gills and turn bright orange when dried. Although H. laeta samples H41 and H47 represent very distant populations, they showed a minimal difference in ITS regions. Hygrocybe laeta f. pseudopsittacina (H41) differing in few morphological features from H. laeta f. laeta, has an ITS region practically identical with that of the type variety. Candusso’s sect. Glutinosae incorporates H. insipida, while Boertmann does not. Our molecular phylogeny is in agreement with Boertmann on this point. Hygrocybe psittacina, H. sciophana and H. sciophanoides cannot be distinguished based on their microscopical properties; Boertmann (1995) accepted only three valid variants of H. psittacina.
Section Coccineae Fayod
-
This section seems to be monophyletic according to our molecular examinations. The species of the three subsections (Coccineae, Siccae and Squamulosae) do not form a separated genetic group. Matheny et al. (2006) as well as Bresinsky (2008) discussed all species of this section among Hygrocybe s.str.
Subsection Coccineae (Bataille) Singer
This subsection is consistent with Boertmann’s classification, though it represents a grade rather than a clade (Fig. 3). The two H. coccinea samples had identical sequences (bt = 100 %). Hygrocybe punicea and H. splendidissima proved to be identical based on ITS sequences. According to Krieglsteiner (2001), H. splendidissima seems to be a variety of H. punicea. Hygrocybe ceracea was a well-supported species, having higher ITS homology with H. miniata than with other species.
Subsection Siccae Boertm.
Subsection Siccae proved to be a polyphyletic group, although, only H. splendidissima and H. quieta were examined. Two samples of H. quieta constitute a branch (bt = 80 %) together with species with decurrent lamellae. According to Moser (1983), H. konradii and H. obrusseus ss. Cooke, Konrad and Maubl. are synonyms, whereas, Candusso (1997) and Krieglsteiner (2001) considered H. quieta to be a synonym of H. obrussea. However, our H. splendidissima (H49) ITS sequence was identical to H. punicea (H29 and H57).
Subsection Squamulosae (Bataille) Singer
In the phylogenetic trees, the two aggregates separate into distant clades. One of them incorporates the species H. cantharellus and H. turunda with decurrent lamellae, the other contains H. miniata with adnexed lamellae.
Two controversial aggregates are discussed:
1. Lepida aggregate: H. coccineocrenata, H. lepida, H. turunda
Hygrocybe lepida (H16 and H38) and H. turunda (H58) formed a well-supported clade characterized by decurrent lamellae and a minutely squamulose pileus. Macroscopically, H. coccineocrenata and H. turunda caps are covered with dense black scale, while H. lepida is not more scaled than H. calciphila, H. lepida and H. turunda, have been distinguished by macroscopic characters, and they remain distinguishable in storage. However, nobody succeeded in observing significant microscopic difference among them.
2. Miniata aggregate: miniata (adnate or free lamellae)
Hygrocybe miniata samples (H17, H18 and H40) were almost identical genetically and form a well-separable isolated branch (bt = 98).
Subgenus Hygrocybe
Subgenus Hygrocybe proved to be a monophyletic group based on our molecular examinations. A well-supported clade (bt = 82 %) unites all samples of sections Hygrocybe and Chlorophanae, though H. intermedia appears with H. citrinovirens on a separate, basal branch within clade B (bt = 89 %). Hygrocybe calyptriformis appeared in sect. Glutinosae in clade A rather than clade B where it has traditionally been placed because of the long lamellar trama hyphae exceeding 1 000 μm and the conical pileus shape. Bresinsky (2008) showed that this species and other members of sect. Glutinosae lack the water soluble vivid coloured muscaflavin pigments of Hygrocybe s.str. in clade A, so he established a new genus, Porpolomopsis, for H. calyptriformis.
Section Microsporae Boertm.
Two samples of H. citrinovirens form a well supported branch (bt = 100 %) that is far separated from all other species except H. intermedia in clade B. All four H. chlorophana samples were genetically identical and formed a well-separable supported branch (bt = 100 %). In the course of our macroscopic and microscopic examinations sample H7 can be determined as H. chlorophana var. aurantiaca. It was identical with other H. chlorophana samples.
Section Nigrescentes (Bat.) Candusso
-
Hygrocybe conica (Matheny et al. 2006), then later all other members (Bresinsky 2008) were discussed among Hygrocybe s.s. Boertmann (1995) uses a wider species concept than Bon (1990) in the black-staining group, so he does not recognize H. riparia, H. tristis and H. veselskyi as valid taxa.
Conica aggregate: H. conica, H. conicoides, H. olivaceonigra, H. riparia, H. tristis, H. veselskyi
Three samples each of H. conica var. conica, and var. conicoides formed a monophyletic group. Five of the six ITS sequences were identical. This corresponds with Boertmann’s (1995) findings, who considered blackening wax caps as types of one species without microscopical differences. The sequence of H34, identified as H. conica var. conica, however, represents a separate species. Except H. olivaceonigra, Boertmann acknowledges only H. conica as a separate species, primarily on the basis of the characteristic of spores, considering the rest as synonyms or forms of H. conica.
Section Macrosporae Haller ex Bon
H. acutoconica, H. aurantiolutescens, H. konradii, H. persistens, H. subglobispora
According to Boertmann the first two taxa may be only synonyms, and he treats H. konradii as a variant of H. persistens and H. subglobispora as a form of H. persistens var. konradii under the name H. persistens var. konradii f. subglobispora. Three of our H. persistens samples formed a monophyletic group (bt = 93 %), while H. spadicea and H. konradii proved to be separated taxa. The appearance of H. spadicia among the non-staining species was somewhat surprising. Arnolds (1980), Bon (1990) (H. aurantiolutescens var. parapersistens, H. persistens) and Moser (1983) (H. persistens) recognize H. persistens as a collective species, which includes at least two species and several varieties and forms.
Summarizing our results, it can be stated that the subgenera based on morphological differences can be well distinguished in the phylogenetic tree. It is especially relevant to subg. Hygrocybe. Identity or high level similarity were demonstrated in cases of species that were considered as closely related taxa, e.g. H. conica aggregate.
Our clearly separable groups based on full ITS sequences support Bresinsky’s (2008) view that genus Hygrocybe s.l. should be narrowed, and that H. calyptriformis, all examined members of subg. Gliophorus (H. irrigata, H. laeta, H. nitrata, H. psittacina) and subg. Cuphophyllus should be excluded from the genus Hygrocybe. Based on these results we suggest further research using additional DNA markers that are useful at the intergeneric level to re-evaluate the taxonomy of former Hygrocybe species due to the limitations of ITS at higher taxonomic levels. A second marker with much less variation among the species also needs the corrobation regarding Bresinsky’s concept of the reclassification of the genus.
In the future it would be necessary to extend the molecular examinations to as many species as possible so that the relationships among Hygrocybe taxa can be more precisely described. As the environmental burden on Hygrocybe habitat is gradually becoming heavier and heavier, the chance is diminishing to gain enough samples to conduct comprehensive surveys covering the whole range of diversity with satisfying number of samples. Several authors (Boertmann & Rald 1991, Boertman 2000) consider Hygrocybe species as indicator organisms whose abundance and diversity indicate undisturbed habitats. The improved taxonomic knowledge of the genus Hygrocybe could considerably contribute to conservation biology research on these natural grasslands of great importance.
Acknowledgments
We express our gratitude to L. Albert, P. Finy and H. Pidlich-Aigener for submitting samples of their collections for us to study. We thank D. Boertmann for providing additional information and critically reviewing the manuscript. This work has been partly financed by grant PHARE HU001503-40.
REFERENCES
- Agerer R.1999. Never change a functionally successful principle: The evolution of Boletales s.l. (Hymenomycetes, Basidiomycota) as seen from below-ground features. Sendtnera 6: 5 – 91 [Google Scholar]
- Arnolds E.1980. De oecologie en sociologie van Wasplaten. Hygrophorus subgenus Hygrocybe sensu lato. Natura 77: 17 – 44 [Google Scholar]
- Arnolds E.1985. Notes on Hygrophorus – IV. New species and new combinations in Hygrophoraceae. Persoonia 12: 475 – 478 [Google Scholar]
- Arnolds E.1986. Notes on Hygrophoraceae – VIII. Taxonomic and nomenclatural notes on some taxa of Hygrocybe. Persoonia 13: 137 – 160 [Google Scholar]
- Arnolds E.1989. Notes on Hygrophoraceae – XI. Observations on some species of Hygrocybesubgenus Cuphophyllus. Persoonia 14: 43 – 46 [Google Scholar]
- Bas C.1990. Tricholomataceae R. Heim ex Pouz. In: Bas C, Kuyper ThW, Noordeloos ME, Vellinga EC. (eds), Flora Agaricina Neerlandica 2: 65 – 70 Balkema, Rotterdam, Brookfield: [Google Scholar]
- Beisenherz M.2002. Zur Ökologie und Taxonomie der Saftlinge und Ellerlinge. Hygrocybe, Agaricales. Regensburger Mycologische Schriften 10: 3 – 65 [Google Scholar]
- Binder M, Besl H.1999. 28S rDNA sequence data and chemotaxonomical analyses on the generic concept of Leccinum. Boletales. A.M.B., Italy. Centro Studi Micologici, Micologia 2000: 71 – 82 [Google Scholar]
- Binder M, Larsson K-H, Matheny PB.2010. Amylocorticiales or. nov. and Jaapiales ord. nov.: Early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102, 4: 865 – 880 [DOI] [PubMed] [Google Scholar]
- Boertmann D.1995. The genus Hygrocybe. Fungi of Northern Europe. Vol. 1 Greve, Denmark: [Google Scholar]
- Boertmann D, Rald E.1991. Notater om de danske vokshattes udbredelse, økologi og fænologi. Svampe 23: 30 – 40 [Google Scholar]
- Bon M.1976. Clé monographique des Hygrophoraceae Roz. Documents Mycologiques 7, 25: 1 – 24 [Google Scholar]
- Bon M.1990. Flore mycologique d’Europe 1. Les Hygrophores. Documents Mycologiques Mémoire Hors Série No. 1: 35 – 37 [Google Scholar]
- Bon M.1992. Die Grosspilzflora von Europa. 1. Hygrophoraceae. Übersetzt und bearbeitet von A. Einhellinger. IHW-Verlag, Eching: [Google Scholar]
- Bresinsky A.2008. Die Gattungen Hydropus bis Hypsizygus mit Angaben zur Ökologie und Verbreitung der Arten, Porpolomopsis: 145 – 146 Regensburger Mykologische Schriften 15. [Google Scholar]
- Bresinsky A, Kronawitter I.1986. Zur Kenntnis der Hygrocybenpigment. Zeitschrift für Mykologie 52, 2: 321 – 334 [Google Scholar]
- Brock PM, Döring H, Bidartondo MI.2009. How to know unknown fungi: the role of a herbariu. New Phytologist 181, 3: 719 – 724 [DOI] [PubMed] [Google Scholar]
- Candusso M.1997. Hygrophorus s.l. Fungi Europaei 6: 401 – 422, 529,, 784 Alassio, Italy: [Google Scholar]
- Cibula WG.1976. The pigments of Hygrophorus section Hygrocybe and their significance in taxonomy and phylogeny. Electronic Doctoral Dissertations for UMass Amherst. Paper AAI7706390. [Google Scholar]
- Cibula WG, Weber Smith N.1996. Hygrocybe andersonii, a new species of psammophilous Hygrocybe from Horn Island, a Mississippi barrier island. Mycologia 88: 514 – 518 [Google Scholar]
- Donk MA.1962. The generic names proposed for the Agaricaceaea. Beihefte Nova Hedwigia 5 S: 1 – 320 [Google Scholar]
- Fayod V.1889. Prodrome d’une histoire naturelle des Agaricinés. Annales des Sciences Naturelles, Botanique, sér. 7, 9: 181 – 411, pl. 6, 7. [Google Scholar]
- Fries EM.1821. Systema mycologicum 1. Lund, Greifswald: [Google Scholar]
- Fries EM.1838. Epicrisis Systematis Mycologi, seu Synopsis Hymenomycetum. Typographia Academica, Uppsala: [Google Scholar]
- Gardes M, Bruns TD.1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113 – 118 [DOI] [PubMed] [Google Scholar]
- Gardes M, White TJ, Fortin JA, Bruns TD, Taylor JW.1991. Identification of indigenous and introduced symbiotic fungi in ectomycorrhizae by amplification of nuclear and mitochondrial ribosomal DNA. Canadian Journal of Botany 69: 180 – 190 [Google Scholar]
- Haller R.1955. Contribution à l’étude du genre Hygrocybe. Hygrocybe konradii nom.nov. Schweizerische Zeitschrift für Pilzkunde 33: 169 – 172 [Google Scholar]
- Herink J.1959. Species familiae Hygrophoracearum, collem “Velká Horka” dictum prope Mnichovo Hradiště habitantes. Acta Musei et Horti Botanici Bohemiae borealis 1: 53 – 86 [Google Scholar]
- Karsten PA.1879. Rysslands, Finlands och den Skandinaviska halföns Hattsvampar. Förra Delen: Skifsvampar. Bidrag till Kännedom of Finlands Natur Folk 32: 1 – 571 [Google Scholar]
- Krieglsteiner GJ.1992. Anmerkungen, Korrekturen und Nachträge zum Verbreitungsatlas des Grosspilze Deutschlands (West), Band 1, Teilbände A und B. Beiträge zur Kenntnis der Pilze Mitteleuropas 8: 173 – 204 [Google Scholar]
- Krieglsteiner GJ.2000. Hygrocybe lacmus in Baden-Württemberg, Germany. Beiträge zur Kenntnis der Pilze Mitteleuropas 13: 19 – 23 [Google Scholar]
- Krieglsteiner GJ. (ed). 2001. Die Grosspilze Baden-Württembergs Band 3: 33 – 77 Verlag Eugen Ulmer GmbH & Co., Stuttgart, Germany: [Google Scholar]
- Kummer P.1871. Der Führer in die Pilzkunde. Luppe, Zerbst: [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG.2007. ClustalW2 and ClustalX version. Bioinformatics 23, 21: 2947 – 2948 [DOI] [PubMed] [Google Scholar]
- Matheny PB, Curtis JM, Hoffstetter V, Aime MC, Moncalvo JM, Ge ZW, Yang ZL, Slot JC, Ammirati JF, Baroni TJ, Bougher NL, Hughes KW, Lodge DJ, Kerrigan RW, Seidl MT, Aanen DK, DeNitis M, Daniele GM, Desjardin DE, Kropp BR, Norvell LL, Parker A, Vellinga EC, Vilgalys R, Hibbett DS.2006. Major clades of Agaricales: a multi-locus phylogenetic overview. Mycologia 98: 982 – 995 [DOI] [PubMed] [Google Scholar]
- Moser M.1967. Kleine Kryptogamenflora von Mitteleuropa – Die Blätter- und Baupilze (Agaricales und Gastromycetes). Volume: IIb/2, Edition 3 Fischer Verlag, Stuttgart: [Google Scholar]
- Moser M.1983. Die Röhrlinge und Blätterpilze. Band IIb/2. Basidiomyceten. 2. Teil: 83–90. Gustav Fischer Verlag, Stuttgart: [Google Scholar]
- Murrill WA.1911. The Agaricaceae of tropical North America II. Mycologia 3, 4: 189 – 199 [Google Scholar]
- Orton PD, Watling R.1969. A reconsideration of the classification of the Hygrophoracea. Notes from the Royal Botanic Garden, Edinburgh 29, 1: 129 – 138 [Google Scholar]
- Schäffer J.1947. Beobachtungen an oberbayerischen Blätterpilzen. Berichte der Bayerischen Botanischen Gesellschaft 27: 201 – 225 [Google Scholar]
- Singer R.1940. Notes sur quelques Basidiomycètes. Revue de Mycologie, Paris 5: 3 – 13 [Google Scholar]
- Singer R.1951 (‘1949’). The Agaricales in modern taxonomy. Lilloa 22, 2: 1 – 832 [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S.2007. MEGA4: Molecular Evolutionary Genetics Analysi. MEGA version 4.0. Molecular Biology and Evolution 24: 1596 – 1599 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, Taylor JW.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols – A guide to methods and applications. Part 3: 315 – 322 Academic Press, Inc; [Google Scholar]
- Wünsche O.1877. Die Pilze. Eine Anleitung zur Kenntniss derselben. Teubner, Leipzig: [Google Scholar]
- Zagyva T.2003. Die Grosspilzflora der Magerwiesen im Nationalpark Őrség–Vendvidék und Vorschläge für Erhaltung und Management. Burgenländische Forschungen. Landesarchiv Eisenstadt 87: 41 – 49 [Google Scholar]
- Zagyva T, Halász K, Albert L, Bratek Z.2003. Taxonomische Probleme innerhalb der Gattung Hygrocybe. Fritschiana 42: 71 – 74 Karl-Franzens Universität Graz; [Google Scholar]