Abstract
Port-wine stains (PWS) represent a group of vascular malformations that are usually accompanied by psychological distress for affected patients, often reflected in high treatment demand. Although the pulsed-dye laser (PDL) was established as standard therapy for PWS more than a decade ago, therapeutic outcome may be unsatisfactory. One of the main drawbacks to successful PDL therapy is PWS revascularization shortly after laser exposure. Therefore, inhibition of revascularization should improve therapeutic outcome of PDL therapy. In this study, we first evaluated the effects of various light energies on normal cutaneous vessels over a period of 14 days, particularly the proliferation and stem cell marker expression of dermal endothelial cells, which were found to be highest 8 days following laser exposure. We found that PDL exposure induced dose-dependent damage of dermal vessels up to energy densities of 6 J/cm2, above which no increase in PDL-induced effects were observed with the energies employed in this study. In dermal endothelial cells of PDL-exposed skin, we found strong expression of the proliferation marker Ki-67 as well as the stem cell marker nestin but not other stem cell markers such as CD133 and CD166. The influence of rapamycin (RPM), used as an adjuvant to PDL exposure, was also investigated. RPM administration reduced Ki-67 and nestin expression in dermal endothelial cells and increased PDL-induced destruction of dermal vessels, indicating that the use of RPM after PDL exposure may be an interesting new approach for prolonging and improving PWS laser therapeutic outcome.
Vascular lesions represent a heterogeneous group of diseases which can be divided into real neoplasms, characterized by proliferation of endothelial cells, and vascular malformations (VM) caused by embryological malformations.1 Lesions may occur anywhere on the human body and, according to their blood supply, can be divided into slow-flow and fast-flow malformations. Slow-flow anomalies include capillary, venous and lymphatic malformations and combinations thereof.
Nevus flammeus is the most common VM and is a descriptive term for lesions most commonly situated on the head and neck. Approximately 25 – 40% of newborns have such birthmarks with no racial predilection. Lesions are usually small, but have been given characteristic names, because of their appearances, such as salmon-patch (VM on the glabella) and stork-bite (nape of the neck).2 Histologically, nevus flammeus is characterized by increased numbers of dilated thin capillaries and venules. Most of the vessels are situated in the upper part of the reticular dermis although occasionally superficial areas of subcutaneous fat are also involved. Histologically, these lesions are formed by dilated, pre-existent blood vessels. Larger lesions are called port-wine stains (PWS) and occur in three of 1000 newborns.3 PWS are usually present at birth and may cover large areas of the newborn’s skin. Lesions are progressive and may thicken and become partially nodular over time. PWS may be isolated or occur as part of more complex syndromes like Sturge-Weber or Klippel-Trenaunay syndrome. Although PWS themselves do not affect overall life expectancy, they very often result in lifelong psychological distress.4 Until the 1970s when the argon laser was first used, no therapy was available to treat successfully PWS. The pulsed-dye laser (PDL) therapy became available in early 1990s and, to date, remains the standard of therapy for PWS treatment. Different therapeutic protocols have been evaluated, with wavelengths between 577 and 595 nm, and pulse durations between 0.45 and 1.5 ms producing the most effective results in terms of PWS fading.5,6 The therapeutic effect of PDL therapy is mediated by a mechanism called photothermolysis. The emitted laser energy is absorbed by hemoglobin within erythrocytes in the subepidermal tissue. Absorption of energy leads to erythrocyte coagulation and to consecutive thrombus formation and vessel destruction. Although initial reports predicted complete clearing of lesions treated using PDL, recent studies have reported that although most PWS fade in response to PDL therapy, complete clearance usually does not occur, particularly with large lesions and those located on the extremities.7,8 Furthermore, laser therapy for PWS is associated with a long-term risk of pigmentary abnormalities of the skin, fibrosis and scarring,7 with these dermal repair processes lessening the vulnerability of dermal vessels to subsequent PDL treatments.9 Although newer laser protocols have improved lesion fading, other strategies are urgently needed to improve therapeutic results especially on large PWS.10 Although regeneration of damaged blood vessels is extremely important in maintaining homeostasis for wound healing, such processes are highly undesirable after PDL therapy of PWS. Usually, vessel destruction first leads to activation of perivascular stromal cells, which produce angiogenic factors such as vascular endothelial growth factor (VEGF) as well as chemotactic substances to attract immune cells. These events subsequently induce neo-angiogenesis and re-growth of blood vessels, which may also be facilitated by the recruitment of endothelial stem cells into sites of destroyed blood vessels. Pluripotent stem cells residing locally in the dermis, namely in the follicular bulge region, identified by their expression of the neural stem cell marker nestin, have been identified recently.11,12 Upon appropriate stimulus, these cells may differentiate into endothelial cells and can lead to formation of new blood vessels.11,13 Other possible contributors to new vessel formation are circulating endothelial stem cells (characterized by surface expression of CD13314) and mesenchymal stem cells (characterized by expression of CD16615). Therefore, re-growth of destroyed vessels after PDL therapy may be a multifactorial process; however, irrespective of the exact mechanism, blocking endothelial cell proliferation and blood vessel growth should improve PWS therapeutic outcome in response to PDL therapy by inhibiting post-treatment blood vessel reformation.
Although various systemic anti-angiogenic substances are currently being tested for their clinical usage, the topical administration of such agents for PWS treatment would be ideal. One such topical preparation is the streptomyces-derived macrolide rapamycin (RPM), which is an FDA approved drug for the inhibition of organ rejection after renal transplantation.16,17 RPM works by blocking the mammalian target of rapamycin (mTOR) pathway,18 which is crucial to cell cycle regulation and cell growth control. Additionally, RPM exhibits strong anti-angiogenic effects by inhibiting VEGF synthesis and downstream signaling of VEGF in endothelial cells.19 – 21 Although RPM structurally resembles the related streptomyces-derived macrolide tacrolimus (which is also used as an immunosuppressive drug), RPM exhibits more potent anti-angiogenic effects; however, unlike tacrolimus, RPM is not FDA approved for topical use.
In this report, we evaluated the effects of different laser energies on the expression of proliferation and stem cell markers by endothelial cells following PDL exposure. Additionally, we evaluated the effects of topical RPM on these parameters.
Materials and methods
Preparation of topical RPM
RPM powder was obtained from LC Laboratories (Woburn, MA, USA). To prepare a 2% topical RPM cream, 0.1 gm of powder was solubilized in 100 μl of DMSO and thoroughly mixed using a mortar and pestle with 5 g of Hydrocerin® cream (an unscented water in oil emulsion consisting of petrolatum, mineral oil, mineral wax, ceresin alcohol, DMDM hydantoin, methyl and propyl parabens; Geritrex Corp., Mt Vernon, NY, USA). The mixture was stored in polypropylene plastic tubes, tightly capped and kept at 4°C. The RPM cream was applied daily after laser exposure as a thin layer to the skin surface and kept under occlusion using a Tegoderm® bandage. Site-matched laser-exposed control skin was kept under occlusion without topical RPM application.
Laser exposure
Laser exposure of in situ normal human skin was performed on test sites located on the volar surface of the forearm using a Candela C-Beam® pulsed-dye laser (Candela, Wayland, MA, USA) operating at a wavelength of 585 nm and pulse duration of 0.45 ms. The laser beam was focused onto a spot size of 10 mm diameter. Sites were exposed to energy densities of 2, 4, 6 or 8 J/cm2. All test sites were exposed to laser irradiation on the same day. Cryogen spray cooling was not used to ensure that maximal blood vessel heating would occur in blood vessels in close proximity to the skin surface.
Biopsy protocol
Punch biopsies from both PDL + RPM and PDL-only exposed sites were taken 4, 8 and 14 days after laser exposure. Control biopsies (no laser exposure and no RPM) were also obtained (n = 3). Biopsy tissue was fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA, USA) and processed for permanent paraffin embedding on an ASP 300 tissue processor (Leica Microsystems, Bannockburn, IL, USA). About 5-μm thick paraffin sections were stained with hematoxylin and eosin (H&E) using a Leica Autostainer XL. Immunohistochemical staining for Ki-67 (clone 30 – 9, Ventana Medical Systems, Inc., Tucson, AZ, USA) was performed on the ES automated immunohistochemistry instrument (Ventana) using an indirect biotin avidin diaminobenzidine (DAB) detection system on formalin-fixed, paraffin-embedded, 4-μm thick sections. For nestin, CD133 and CD166 immunohistochemical staining, paraffin sections were deparaffinized according to routine procedures and incubated in citrate buffer (pH 6.0; Dako Denmark A/S, Glostrup, Denmark) overnight at 80°C. Primary antibodies included a monoclonal mouse antihuman nestin antibody (clone 10C2, Chemicon, Nuernberg, Germany), a polyclonal rabbit antibody to human CD133 (Abcam, Cambridge, MA, USA) and a monoclonal mouse antibody to human CD166 (clone MOG/07, Abcam). Sections were incubated overnight at 4°C with appropriate dilutions of primary antibodies. Appropriate positive controls were used. Bound primary antibodies were detected using the DAKO Envision Kit (Dako, Glostrup, Denmark). Stained slides were examined on an AH3-RFCA microscope (Olympus, Vienna, Austria) using an AxioCam MRc5 device (Zeiss, Vienna, Austria). Images were analyzed with AxioVision Rel.4.4 Software (Zeiss).
Results
PDL-induced vessel damage and induction of endothelial cell proliferation are both time-dependent and dose-dependent.
Biopsies of test sites taken 4 days after exposure with PDL at an energy density of 6 J/cm2 primarily showed a sparse perivascular lymphocytic infiltrate, as well as some vessel destruction and erythrocyte extravasation, when compared to unexposed control skin (Fig. 1A). Eight days after laser exposure, the observed destruction of vessels in both the papillary and reticular dermis had increased in intensity relative to biopsies obtained 4 days after PDL-exposure (Fig. 1A). Additionally, some vessels showed activation of endothelial cells (epithelioid morphology with hobnailing) and signs of regeneration (new vessel formation).
Fig. 1.
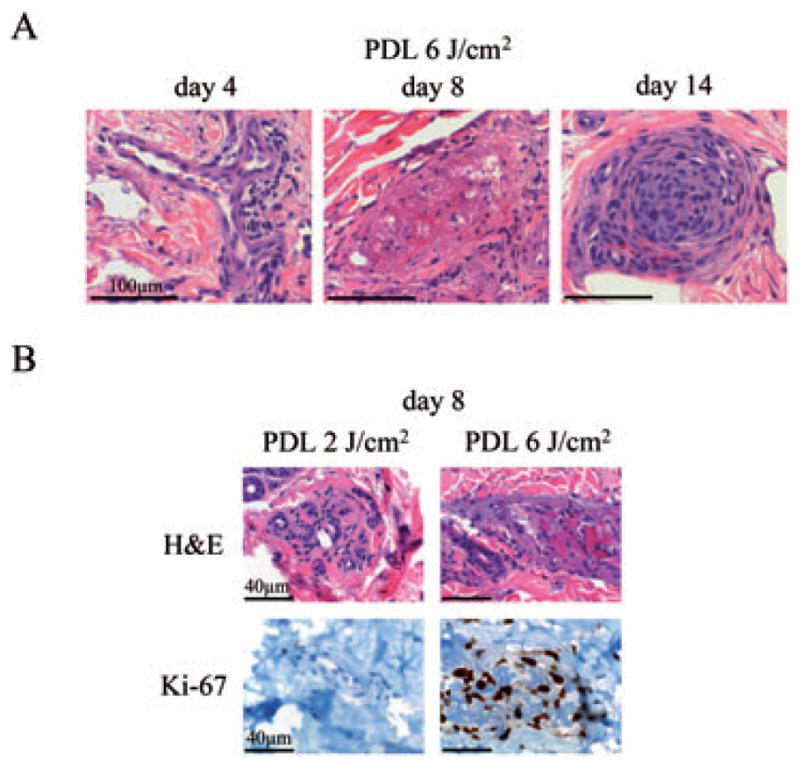
PDL-induced vascular pathology depends on PDL energy density and is time-dependent. A) H&E stained sections of skin biopsies before and after PDL-exposure with 6 J/cm2 at indicated time points. Bar: 1 mm. B) Biopsies of skin taken at day 8 after PDL-exposure with 2 or 6 J/cm2 PDL energy density were examined. Sections were stained with H&E and immunostained with an antibody against Ki-67 and analyzed for vessel damage and cell proliferation, respectively. Bar: 40 μm.
After 14 days laser exposure, most of the destroyed and damaged vessels had largely disappeared and been replaced by newly formed vessels or showed evidence of thrombus organization and vessel recanalization (Fig. 1A). Moreover, at this later time point, more prominent perivascular sclerosis was also observed.
After establishing the time-course of vascular damage and regeneration, we next tested the effects of different laser energies on vascular damage at the day 8 time-point. PDL-exposed skin, biopsied at this point, showed an increasing extent of blood vessel destruction with escalating laser energies (Fig. 1B). Low exposure energy densities (2 J/cm2) did not induce visible damages to cutaneous vessels studied by routine H&E morphology. In contrast, energy densities of 6 J/cm2 had a profound effect on superficial and deep blood vessels. As described above, thrombosed vessels were observed in the papillary dermis, reticular dermis and even within the deep vascular plexus, situated at the border between the reticular dermis and subcutis (Fig. 1B). Exposure to an energy density of 4 J/cm2 revealed inconsistent results, intermediate to those of the 2 and 6 J/cm2 groups with scattered damaged vessels (data not shown). A further increase in energy density (8 J/cm2) had no additional effect compared with the effects observed with 6 J/cm2 PDL energy density (data not shown).
Analysis of cell proliferation in PDL-exposed skin samples as examined by Ki-67 immunostaining showed no increase in Ki-67 positivity in skin exposed to a PDL energy density of 2 J/cm2 (Fig. 1B). In contrast, at the same time point (8 days after PDL-exposure), skin exposed to 6 J/cm2 showed strong induction of Ki-67 staining in endothelial cells. An intermediate level of Ki-67 expression was seen with the administration of 4 J/cm2 laser energy, while no appreciable increase in Ki-67 staining was seen by increasing the dose of laser energy from 6 to 8 J/cm2. These data showed an apparent threshold value of 6 J/cm2 of laser energy, with maximal vessel destruction seen at the 8-day time point.
PDL-exposure induces nestin but not CD133 or CD166 expression in cutaneous vessels
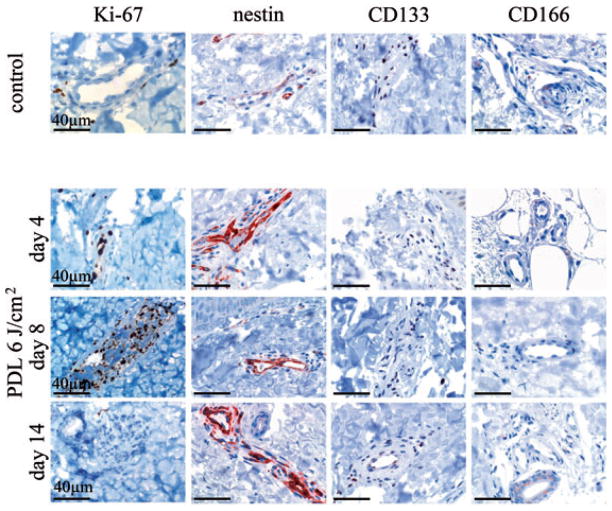
Next, the question of whether stem cell recruitment contributes to vascular repair following PDL-exposure was addressed. For this purpose, immunohistochemical analysis to identify CD133-expressing, circulation-derived stem cells as well as CD166-expressing and nestin-expressing skin-derived (hair bulge) stem cells was performed. Sections of skin 4, 8 and 14 days after 6 J/cm2 PDL-exposure were evaluated and results correlated with Ki-67 immunoreactivity (Fig. 2). Scattered nestin positivity of endothelial cells was observed in control skin. However, nestin expression was markedly upregulated following PDL-exposure. Increased nestin expression was seen at day 4, preceding the major Ki-67 induction at day 8. Nestin upregulation persisted until day 14, at which point Ki-67 immunoreactivity was already markedly diminished from its peak activity at day 8. In contrast, staining for stem cell markers CD133 and CD166 was absent in endothelial cells in control skin and at all time points examined after PDL-exposure (Fig. 2).
Fig. 2.
PDL-exposure induces expression of the stem cell marker nestin but not CD133 or CD166. Biopsies of skin initially exposed to 6 J/cm2 PDL energy density were taken at indicated time points. Immunochemistry for detection of stem cell markers (nestin, CD133 and CD166) and the proliferation marker Ki-67 was performed. Representative photomicrographs of skin samples taken before and 4, 8 and 14 days after PDL-exposure are shown. Bar: 40 μm.
Topically applied RPM impairs nestin upregulation and increases PDL-induced damage of skin vessels
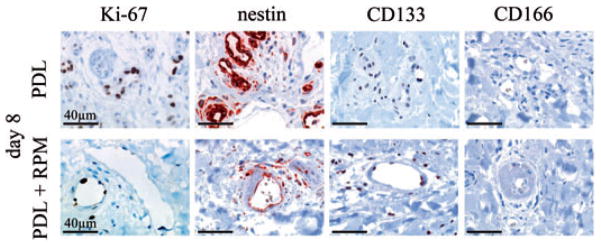
Next we investigated whether topically applied RPM had an effect on the expression of proliferation marker Ki-67 and vascular stem cell markers (Fig. 3) in normal human skin. For this purpose, skin was exposed to a PDL energy density of 8 J/cm2 (to ensure that a suprathreshold dose of light was applied) and RPM was administered topically or skin left untreated after PDL-exposure. Paired biopsies were harvested after 4, 8 and 14 days. Consistent with RPM’s role in reducing the viability of PDL-exposed endothelium, RPM abrogated Ki-67 upregulation by damaged endothelium (Fig. 3), which we showed previously.22 Although CD133 and CD166 were undetectable in the endothelium of PDL-exposed skin, with or without adjuvant RPM, RPM administration almost entirely abolished endothelial nestin upregulation following PDL-exposure, akin to RPM’s effect on Ki-67 expression (Fig. 3). This observed abrogation of the ‘regenerative program’, as evidenced by the blunted nestin upregulation following RPM administration, correlated with the depth and persistence of vascular damage observed at the different study time-points (Fig. 4A,B). Four days after laser exposure, the extent of the observed vessel damage did not differ between skin exposed to PDL alone or with PDL + RPM. Both samples showed destroyed vessels in the papillary dermis and mid reticular dermis as deep as 900 μm below the overlying epidermis. The depth of vascular damage decreased continuously with time in skin treated with PDL only (315 μm below epidermis after 8 days; 175 μm after 14 days). In contrast, skin exposed to PDL irradiation followed by topical RPM administration showed a markedly reduced vascular regeneration. Vascular damage could still be observed at depths of 950 and 620 μm below epidermis after 8 and 14 days, respectively. Topical administration of RPM had no influence on skin thickness. There were no significant differences in epidermal or dermal thickness during the observation period and between control skin and RPM administered skin (PDL alone: epidermis 61 μm, SD ± 8 μm; dermis: 1482 ± 79 μm. PDL + RPM: epidermis 69 μm, SD ± 19 μm; dermis: 1296 ± 158 μm).
Fig. 3.
RPM inhibits induction of the proliferation marker Ki-67 and the stem cell marker nestin in cutaneous endothelial cells after PDL therapy. Immunohistochemical detection of Ki-67, nestin, CD133 and CD166 expression within endothelial cells in skin exposed to 8 J/cm2 PDL alone or skin exposed to an energy density of 8 J/cm2 PDL followed by topical RPM administration. Representative photomicrographs are shown. Bar: 40 μm.
Fig. 4.
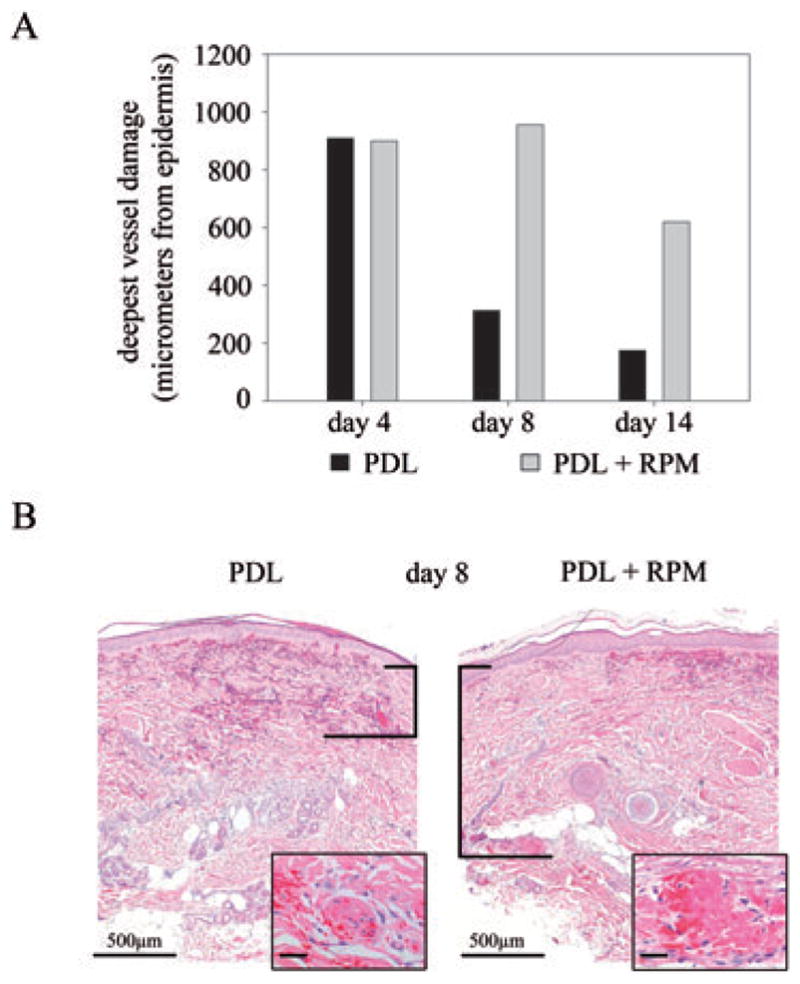
Topical RPM increases PDL-induced endothelial damage. Skin was exposed to PDL alone (8 J/cm2 energy density) or to a combination of PDL (8 J/cm2 energy density) and topical RPM. Punch biopsies of exposed skin areas were taken after 4, 8 and 14 days. A) Deepest measurable vascular damage in both treatment groups displayed in bar graph format, in micrometer from the base of the epidermis. Results 4, 8 and 14 days post-PDL-exposures are shown. B) Representative H&E stained sections from each treatment protocol are shown 8 days post-PDL. The vertical bars indicate the distance from the base of the epidermis to the deepest damaged vessel (inset shows high power view of destroyed vessels). Bar: main image: 500 μm; inset: 100 μm.
Discussion
The PDL has been successfully used in the treatment of PWS and, although other devices have been tested within the last decade, it remains the single most effective form of therapy. Nevertheless, it has been generally accepted that this treatment is unable to achieve complete clearance of the majority of these lesions. We have now shown that increasing the energy density over a range of 2 – 6 J/cm2 PDL induces more profound changes on the vasculature but at the same time induces a dose-dependent expression of the proliferation marker Ki-67 in dermal endothelial cells. This may indirectly point to an induction of endothelial cell proliferation, which partially abolishes the therapeutic effect. Improvement of therapeutic efficacy might be achieved by inhibiting regenerative processes while maintaining the destructive effects of PDL on PWS blood vessels.
It has been shown recently that topical administration of RPM enhances the effect of PDL-induced blood vessel photothermolysis.22 We have now extended this observation by analyzing the effects of RPM on the PDL-induced expression of stem cell markers in dermal endothelial cells. Current reports postulate that regeneration of blood vessels or neo-angiogenesis rely, at least partially, on the recruitment of stem cells from either the circulation (bone marrow-derived circulating endothelial stem cells), the connective tissue or from the dermal follicular bulge region.13,15,23 – 25 We found profound expression of the neural stem cell marker nestin in the cytoplasm of endothelial cells throughout the whole dermis in PDL-exposed skin. Interestingly, the stem cell markers CD166 (characteristic for mesenchymal derived stem cells) and CD133 (expressed by circulating endothelial stem cells) could not be identified in regenerating vessels.
The extent and uniform pattern of nestin upregulation together with the failure to detect CD133 and CD166 immunostaining in PDL-exposed dermal vessels does not support the previously hypothesized recruitment of stem cells in vascular regeneration following laser damage. Rather, these findings support the concept that certain ‘stem cell’ markers, such as nestin, may be upregulated in terminally differentiated cells which become activated or de-differentiated as part of a ‘reparative program’. In support of this view, current reports describe expression of nestin in proliferating endothelial cells in human tumors and in activated endothelial cells in inflammation.26 – 29 Nevertheless, another possibility is that the recruitment and proliferation of stem cells occurs very rapidly, and that by 4 days post laser exposure (our first assay period), CD133 and CD166 are no longer expressed, and/or nestin-expressing stem cells and resultant progeny cells have already re-surfaced the damaged vessels. Additional work involving more sensitive kinetic studies will be needed to further investigate these possibilities.
Similar to earlier studies, the data presented herein showed that RPM augments PDL-induced damage to the dermal vasculature and, as seen previously, RPM hindered the re-growth of blood vessels as assayed by Ki-67 immunostaining.22 Similarly, we showed that topical RPM application largely abrogates the upregulation of certain ‘stem cell’ antigens. The ability of adjuvant RPM to mitigate expression of Ki-67 and nestin undoubtedly represents an effect of RPM on modulating the proliferation and repair of damaged endothelium, which thereby interrupts important mechanisms by which vascular lesions can repair themselves. Such an effect probably works by both limiting the survival and hyperplasia of damaged endothelial cells in the superficial dermis after a lower energy insult, as well as precluding the proliferation and repopulation of the skin with abnormal vessels from deeper tissues after successfully treated superficial disease. Future study aims should involve whether pre-treatment of PWS with topical RPM may further sensitize PDL-treated lesions, perhaps resulting in recognizable treatment differences at early time points (4 days). A more thorough investigation of endothelial activation and cytokine release will be necessary to shed light on the functional importance of nestin expression in regenerating blood vessels. Although additional studies will be necessary to further test the therapeutic efficacy of combination of PDL and topical RPM therapy, we believe that this combined ‘photochemotherapy’ approach may represent an important improvement in the treatment of vascular birthmarks such as PWS.
Acknowledgments
We would like to thank Sandra Kirley and Silvia Steele for their excellent technical assistance. This project was supported by research grants awarded from the National Institutes of Health (AR47551 and EB002495) to JSN.
Footnotes
This work was presented in part at the 29th Annual Conference of the American Society for Laser Medicine and Surgery in National Harbor, MD, on April 3, 2009.
Conflicts of interest
None.
References
- 1.Requena L, Sangueza OP. Cutaneous vascular anomalies. Part I. Hamartomas, malformations, and dilatation of preexisting vessels. J Am Acad Dermatol. 1997;37:523. doi: 10.1016/s0190-9622(97)70169-5. [DOI] [PubMed] [Google Scholar]
- 2.Enjolras O. Vascular malformations. In: Bolognia J, editor. Dermatology. 2. New York: Mosby; 2003. p. 1615. [Google Scholar]
- 3.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Miller AC, Pit-ten Cate IM, Watson HS, Geronemus RG. Stress and family satisfaction in parents of children with facial port-wine stains. Pediatr Dermatol. 1999;16:190. doi: 10.1046/j.1525-1470.1999.00051.x. [DOI] [PubMed] [Google Scholar]
- 5.Yu HY, Chen DF, Wang Q, Cheng H. Effects of lower fluence pulsed dye laser irradiation on production of collagen and the mRNA expression of collagen relative gene in cultured fibroblasts in vitro. Chin Med J (Engl) 2006;119:1543. [PubMed] [Google Scholar]
- 6.Sivarajan V, Maclaren WM, Mackay IR. The effect of varying pulse duration, wavelength, spot size, and fluence on the response of previously treated capillary vascular malformations to pulsed-dye laser treatment. Ann Plast Surg. 2006;57:25. doi: 10.1097/01.sap.0000208942.15897.15. [DOI] [PubMed] [Google Scholar]
- 7.Jasim ZF, Handley JM. Treatment of pulsed dye laser-resistant port wine stain birthmarks. J Am Acad Dermatol. 2007;57:677. doi: 10.1016/j.jaad.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Woo WK, Handley JM. Does fluence matter in the laser treatment of port-wine stains? Clin Exp Dermatol. 2003;28:556. doi: 10.1046/j.1365-2230.2003.01327.x. [DOI] [PubMed] [Google Scholar]
- 9.Levine VJ, Geronemus RG. Adverse effects associated with the 577- and 585-nanometer pulsed dye laser in the treatment of cutaneous vascular lesions: a study of 500 patients. J Am Acad Dermatol. 1995;32:613. doi: 10.1016/0190-9622(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 10.Lanigan SW, Taibjee SM. Recent advances in laser treatment of port-wine stains. Br J Dermatol. 2004;151:527. doi: 10.1111/j.1365-2133.2004.06163.x. [DOI] [PubMed] [Google Scholar]
- 11.Amoh Y, Li L, Yang M, et al. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA. 2004;101:13291. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman RM. The pluripotency of hair follicle stem cells. Cell Cycle. 2006;5:232. doi: 10.4161/cc.5.3.2397. [DOI] [PubMed] [Google Scholar]
- 13.Amoh Y, Li L, Yang M, et al. Hair follicle-derived blood vessels vascularize tumors in skin and are inhibited by doxorubicin. Cancer Res. 2005;65:2337. doi: 10.1158/0008-5472.CAN-04-3857. [DOI] [PubMed] [Google Scholar]
- 14.Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 2004;8:294. doi: 10.1111/j.1582-4934.2004.tb00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 16.Miller JL. Sirolimus approved with renal transplant indication. Am J Health Syst Pharm. 1999;56:2177. doi: 10.1093/ajhp/56.21.2177. [DOI] [PubMed] [Google Scholar]
- 17.Morelon E, Mamzer-Bruneel MF, Peraldi MN, Kreis H. Sirolimus: a new promising immunosuppressive drug. Towards a rationale for its use in renal transplantation. Nephrol Dial Transplant. 2001;16:18. doi: 10.1093/ndt/16.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and – independent pathways. Cancer Cell. 2003;4:147. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 20.Guba M, Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangio-genesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Petrimpol M, Molle KD, et al. Hypoxia-induced endothelial proliferation requires both mTORC1 and mTORC2. Circ Res. 2007;100:79. doi: 10.1161/01.RES.0000253094.03023.3f. [DOI] [PubMed] [Google Scholar]
- 22.Phung TL, Oble DA, Jia W, Benjamin LE, Mihm MC, Jr, Nelson JS. Can the wound healing response of human skin be modulated after laser treatment and the effects of exposure extended? Implications on the combined use of the pulsed dye laser and a topical angiogenesis inhibitor for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40:1. doi: 10.1002/lsm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amoh Y, Yang M, Li L, et al. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005;65:5352. doi: 10.1158/0008-5472.CAN-05-0821. [DOI] [PubMed] [Google Scholar]
- 24.Hilbe W, Dirnhofer S, Oberwasserlechner F, et al. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57:965. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagley RG, Walter-Yohrling J, Cao X, et al. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866. [PubMed] [Google Scholar]
- 26.Ishiwata T, Kudo M, Onda M, et al. Defined localization of nestin-expressing cells in L-arginine-induced acute pancreatitis. Pancreas. 2006;32:360. doi: 10.1097/01.mpa.0000220860.01120.21. [DOI] [PubMed] [Google Scholar]
- 27.Teranishi N, Naito Z, Ishiwata T, et al. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593. [PubMed] [Google Scholar]
- 28.Mokry J, Cizkova D, Filip S, et al. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004;13:658. doi: 10.1089/scd.2004.13.658. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara K, Kurihara H, Negishi M, et al. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]