Abstract
Brain structure in familial schizophrenia was studied with computerized tomography in 42 individuals from six multigenerational families. Sulcal enlargement in the lateral temporal cortex, and ventricular and cisternal enlargement in the medial temporal region were observed in psychotic individuals compared to unaffected family members. Genetic factors in familial schizophrenia may exert part of their effect through determining or altering temporal lobe structure.
Keywords: Sylvian fissure, CT scan, schizophrenia genetics
Introduction
Study of familial schizophrenia offers the possibility of eventually deciphering the link between etiology and mechanism in this illness. Parallel with efforts to isolate genes contributing to schizophrenia, research using schizophrenia pedigrees is providing important information about phenotypic aspects of the illness, including phenomenology (Bassett et al 1993, 1994), neuropsychology (Roxborough et al 1993) and neurophysiology (Blackwood et al 1991; Waldo et al 1991). Neuroimaging studies of familial samples offer similar promise. Investigating family members has the considerable advantage of optimal matching of affected (psychotic) and unaffected (control) individuals. Brain imaging studies using sibling pairs and twins have indicated that ventricular enlargement and reduction in the size of temporal lobe structures occurs in the affected individuals (De-Lisi et al 1986; Reveley et al 1982, 1984; Suddath et al 1990; Weinberger et al 1981). A complementary strategy is to study multigenerational families in which two or more members have schizophrenia. This approach maximizes the possibility that all patients will have a genetic form of the illness. Investigation of brain morphology in affected and healthy members of such pedigrees may eventually contribute to the phenotypic characterization of specific genetic forms of schizophrenia. We have used computerized tomography (CT) scanning to assess brain morphology in subjects from six multigenerational families who were participating in a genetic linkage study of schizophrenia.
Methods
Subjects
Forty-two members of six families participated in the CT scan study. Details of the linkage study and subject recruitment are described elsewhere (Bassett et al 1993). Briefly, families were selected for large size, and an apparent autosomal-dominant like pattern of inheritance of psychosis. Families with bipolar disorder, or with assortative mating (illness from both sides of the family) were excluded. Each subject participated in a comprehensive diagnostic evaluation conducted by a psychiatrist (ASB), which included a Structured Clinical Interview (SCID) for DSM-III-R Axis I disorders (Spitzer et al 1990a), a SCID-II for Axis II disorders (Spitzer et al 1990b), the Positive and Negative Syndrome Scale (Kay et al 1987), and a mental status examination. Using data from this clinical assessment and from hospital records, two psychiatrists (ASB and WGH) made independent diagnoses according to Research Diagnostic and DSM-III-R criteria, using the Best Estimate Clinical Diagnosis method (Endicott 1988). On the basis of lifetime diagnoses, subjects were classified as either psychotic (schizophrenia n = 13, schizoaffective n = 8), or nonpsychotic (n = 21) for the purposes of data analysis. None of the psychotic individuals suffered from a neurological condition known to be associated with psychosis. None of the nonpsychotic individuals suffered from bipolar affective disorder, paranoid personality disorder, or schizotypal personality disorder.
CT Scans and Scoring
Axial CT scans were obtained using a General Electric 9800 HiLight scanner. Following a lateral scout film, 5 mm contiguous slices were obtained from the skull base to the vertex at an angle of approximately 12° to the canthomeatal line. The field of view was 25 cm and reconstruction used a 512×512 matrix.
A qualitative rating scale was constructed to assess the anatomy of brain regions relevant to schizophrenia. A similar approach to assessing regional brain anatomy on CT in neurological and psychiatric patients was described elsewhere (Benes et al 1983; LeMay et al 1986; McCarley et al 1989). Briefly, a 7-point scale was constructed for rating the frontal, temporal, and parietal lobes, as well as the ventricular system. For each of these brain areas, photographs were made using CT scan slices from the appropriate levels, which demonstrated ratings of 1, 3, 5, and 7. Larger scores denoted larger sulci or ventricular spaces.
Following review by a neuroradiologist, all scans were rated independently by WGH and GNS using the photographic reference anchors. Raters were blinded to diagnosis and family membership. To assess interrater reliability, intraclass correlation coefficients were calculated (Bartko and Carpenter 1976). The interrater reliability for the individual item scores (listed in Figure 1) was high, with intraclass correlation coefficients from 0.79 to 0.96. To assess the validity of the scale, the scores were compared to the neuroradiologists’s classification of the degree of diffuse atrophy (none, mild, or moderate). For each regional score, scans designated as having no atrophy had lower scores than those with mild atrophy, and scans with moderate atrophy had the highest scores.
Figure 1.
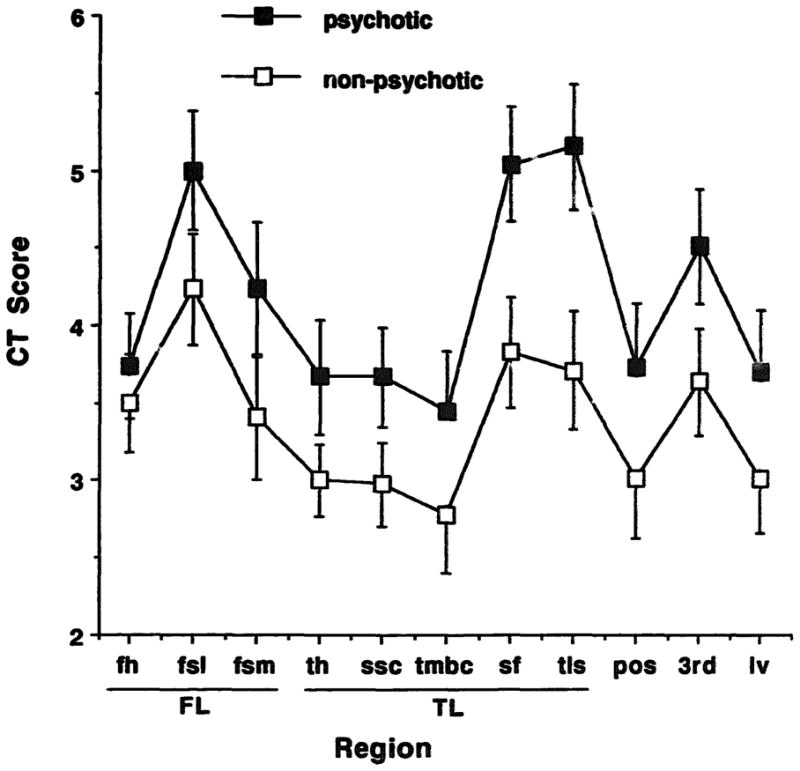
Regional scores on CT scans in familial schizophrenia. The cortical measures related to the temporal lobe were most affected in the psychotic individuals. Regions—FL: frontal lobe (fh: frontal hom, fsl: frontal sulci lateral; fsm: frontal sulci medial); TL: temporal lobe (th: temporal horn; ssc: suprasellar cistern; tmbs: temporal midbrain cistern; sf: sylvian fissure; tls: temporal lobe sulci); pos: parietal-occipital sulci; 3rd: third ventricle; Iv; lateral ventricle. Mean and SEM are illustrated.
The left and right sides were rated separately in scans in which skull and internal landmarks indicated no appreciable tilt of the head. If tilt was present, the side with the larger sulci or spaces was scored. In order to be able to include data from all subjects for overall analyses, where asymmetries existed the larger score was used. For assessment of possible differences in laterality, data from 31 scans with no appreciable head tilt were used (15 with psychosis, 16 unaffected). In addition to scoring brain regions with the rating scale, the widths of the frontal and occipital lobes were measured. Proceeding from the vertex, the first slice showing the pineal was chosen. A vertical line intersecting the most caudal point of the falx, the septum pellucidum, and the pineal was drawn. A line was constructed perpendicular to this, at the level of the posterior end of the falx for the measurement of the left and right frontal lobe widths. A perpendicular line was constructed at the posterior extent of the cerebellar cistern for the measurement of the left and right occipital lobe widths. The right frontal lobe is usually wider than the left, and the left occipital lobe is usually wider than the right, although changes in these relationships are reported in patients with schizophrenia (Falkai et al 1993).
Comparisons of continuous variables were made with analysis of covariance, using either diagnostic group (affected versus unaffected), gender, or alcohol history as between-subjects factors, and age as a covariate where appropriate. The x2 test was used for categorical variables.
Results
Description of Subjects
The demographic and clinical descriptions of the subjects appears in Table 1. Two generations were represented from each of the six families. The mean number of individuals scanned per family was seven (range 5–11), with at least two affected individuals scanned per family. A history of alcohol abuse or dependence in the year prior to the CT scan was present in two of the family members with psychosis, and two nonpsychotic subjects. In the other subjects with alcohol abuse, the disorder was in remission for longer than 1 year.
Table 1.
Demograpnic, Clinical and Radiological Data from Family Members
| Variables | Non- psychotic | Psychotic | Diagnostic effect (p-value) |
|---|---|---|---|
| Demographic and Clinical Data | |||
| Age (years) | 46(16) | 47(16) | NS |
| Sex (male/female) | 9/12 | 13/8 | NS |
| Alcohol abuse or dependence, lifetime (yes/no) | 8/13 | 11/10 | NS |
| CT scan data | |||
| Frontal lobe score | 11.1(4.5) | 13.0(4.8) | NS |
| Temporal lobe score | 16.3(6.2) | 21.0(7.2) | 0.006 |
| Temporal cortex score | 7.5(3.3) | 10.2(3.5) | 0.001 |
| Medial temporal score | 8.8(3.2) | 10.8(4.1) | 0.05 |
| Parietal-occipital score | 3.0(1.8) | 3.7(1.9) | NS |
| Lateral ventricle score | 3.0(1.7) | 3.7(1.8) | NS |
| Third ventricle score | 3.6(1.6) | 4.5(1.7) | 0.04a |
| Asymmetry indices | |||
| R/L Frontal lobe | 1.00 (.05) | 1.00 (.05) | NS |
| L/R Occipital lobe | 1.00 (.07) | 1.01 (.08) | NS |
The third ventricle score was significantly different between men and women, and the difference between the diagnostic groups was not significant when gender was taken into account.
Mean and SD are indicated. For CT scan data, the probability values are based on analysis of covariance with diagnosis as a between-subjects factor and age as a covariate. Frontal lobe score = frontal hom + frontal sulci lateral + frontal sulci medial. Temporal lobe score = temporal hom + suprasellar cistern + temporal midbrain cistern + sylvian fissure + temporal sulci lateral. Temporal cortex score = sylvian fissure + temporal sulci lateral. Medial temporal score = temporal horn + suprasellar cistern + temporal midbrain cistern.
Regional Brain Anatomy in Familial Schizophrenia
The mean ratings for each brain region according to diagnostic group are illustrated in Figure 1. The scores for all regions were larger in the psychotic family members. The score for each structure was significantly associated with age, therefore age was used as a covariate for subsequent analyses.
Temporal Lobe Anatomy in Familial Schizophrenia
The total temporal lobe score was significantly elevated in the psychotic family members compared to their unaffected relatives (Table 1). Both component scores, temporal cortex, and medial temporal lobe were elevated. Neither gender nor lifetime history of alcohol abuse/dependence showed a significant effect on the temporal lobe scores. The most prominent effect of diagnosis was found for the temporal cortex score. The relationship between the temporal cortex score, age, and diagnostic group is illustrated in Figure 2. Family members with psychosis who were in their 30’s and 40’s frequently exhibited temporal cortex findings comparable to nonpsychotic subjects in their 70’s. This observation is illustrated in Figure 3 for the trio of a parent and two offspring.
Figure 2.
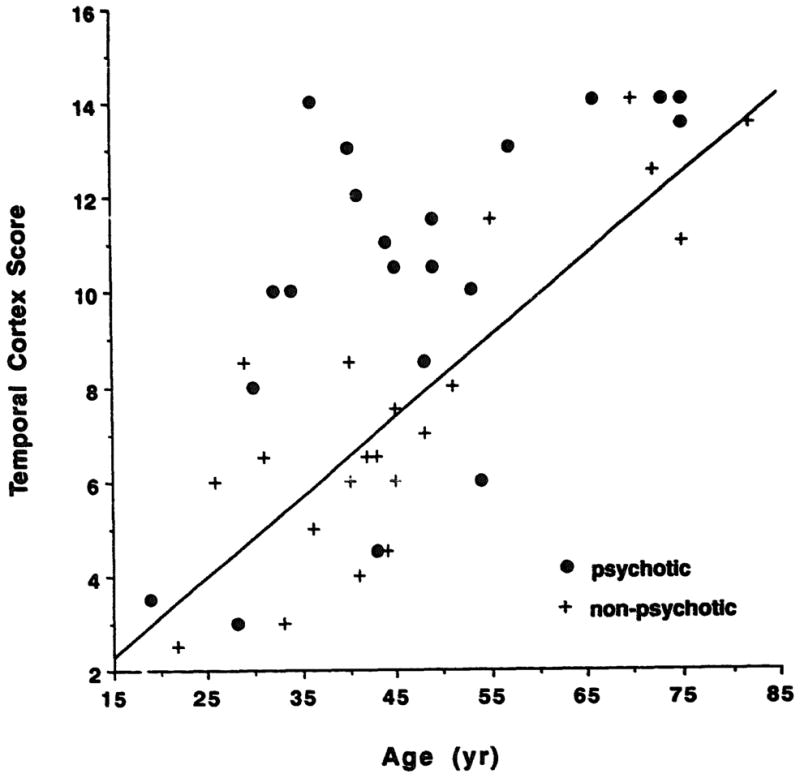
Temporal cortex score and age in familial schizophrenia. The effect of aging on temporal cortex score in controls is shown by the line (y = 0.17x – 0.24, r = 0.84, p = 0.0001). Family members with psychosis had significant evidence of changes in the temporal cortex compared to their unaffected relatives.
Figure 3.
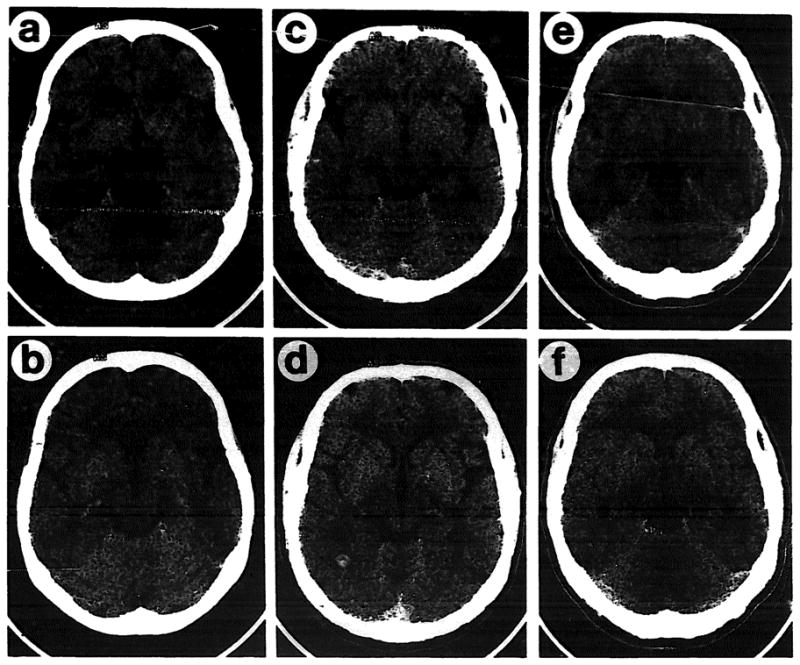
Temporal cortical changes in familial schizophrenia. CT images from two levels in each of three individuals are shown. (a,b): woman 75 years old, unaffected; (c,d): her 40-year-old son with schizophrenia: (e,f): her 43-year-old unaffected son. Enlargement of the sylvian fissure and temporal lobe sulci is apparent in the affected individual.
Other Brain Regions in Familial Schizophrenia
The lateral ventricle, frontal, and parietal-occipital lobe scores showed no significant difference between psychotic and unaffected family members. The psychotic family members appeared to have evidence of enlargement of the third ventricle. The third ventricle was larger in men than in women, however. When gender was introduced as an additional covariate the effect of diagnosis was rendered nonsignificant.
Brain Laterality and Familial Schizophrenia
The analysis of possible differences in lateralization in familial schizophrenia was performed on those scans without appreciable head tilt. The ratios of left-to-right-scores for temporal horn, sylvian fissure, temporal lobe sulci, frontal horn, lateral ventricle, frontal and parietal lobe sulci did not show any differences between subjects with psychosis and unaffected family members. The assessments of frontal and occipital lobe widths appear in Table 1; no statistically significant differences were observed.
Discussion
Our results indicate that familial schizophrenia is associated with altered brain structure in the affected individuals from multigenerational pedigrees. Specifically, we observed substantially larger sulcal, ventricular, and cisternal spaces in the temporal lobe region of individuals with psychosis compared to their unaffected family members. The temporal cortex appeared to be more affected than the medial structures. The findings in the lateral temporal region are consistent with increasing numbers of neuroimaging and postmortem studies implicating the superior temporal gyrus and planum temporale in schizophrenia (Barta et al 1990; Falkai et al 1992; Shenton et al 1992). The CT scan methodology may be less sensitive to altered structure in the medial temporal lobe, and the magnitude of the differences in this brain region could be underestimated in the present study.
In contrast to the temporal lobe findings, differences in the frontal lobe and in the ventricular system did not reach statistical significance. Several studies reporting a comprehensive assessment of regional brain anatomy in schizophrenia indicate that changes in the region of the sylvian fissure are of greater magnitude than frontal or ventricular changes (Bogerts et al 1987; Dauphinais et al 1990; Kaiya et al 1989; McCarley et al 1989; Pandurangi et al 1984; Schwartz et al 1992; Suddath et al 1990). The present sample was large enough to demonstrate differences in the temporal region, but may have lacked sufficient numbers to demonstrate changes elsewhere in the brain. Alternatively, the schizophrenia in the families studied could be more specifically associated with temporal lobe abnormalities. Affected and unaffected family members were compared in our analyses. In this regard, investigations of lateral ventricular size in nontwin sibling pairs have not consistently indicated increased size in the psychotic sibling (DeLisi et al 1986; Weinberger et al 1981). In the sample available here, the expected frontal and occipital lobe asymmetries did not appear to be present in psychotic or unaffected individuals. Additional studies are required to fully assess the possibility of alterations in brain laterality in families with schizophrenia.
The participating subjects were recruited from families with probable autosomal dominant, unilineal inheritance of nonaffective psychosis. Extensive geneological and psychiatric records were available. The ascertainment procedure maximized the likelihood that all patients would have a genetic form of schizophrenia, and because of the nature of the population, quite possibly a homogeneous form of the illness (Bassett et al 1993). This approach complements the high-risk studies that assessed genetic contributions to brain structure in schizophrenia (Cannon et al 1989,1993). In the high-risk sample, one or both parents were affected with schizophrenia or a related disorder. Brain structure was studied in a large sample of offspring, only a small proportion of whom had schizophrenia themselves. The high-risk studies indicated that ventricular and cortical structural changes were related to the genetic predisposition for schizophrenia, rather than to the expression of a schizophrenia genotype. The comparison of affected and unaffected individuals in the present sample of families with schizophrenia indicates that the temporal lobes are an important site at which genetic effects in schizophrenia appear to be manifest.
The present study is also complementary to investigations of monozygotic twins discordant for schizophrenia, where the ascertainment method emphasizes forms of schizophrenia that may be nongenetic, or may be related to genetic factors of reduced penetrance. The twin studies consistently report lateral ventricular enlargement as a difference between affected and nonaffected twins (Reveley et al 1982,1984; Suddath et al 1990). Similar to our findings, the magnetic resonance imaging (MRI) twin study noted differences in the temporal lobes (Suddath et al 1990).
Studies of multigenerational pedigrees with schizophrenia provide a method to relate the genotype and the phenotype of the illness (Honer et al 1990; Rieder and Gershon 1978). Structural brain-imaging evidence from the present study indicates that abnormalities of temporal lobe structure occur in familial schizophrenia. Other work suggests that increased latency of the P300 evoked potential is related to lateral temporal structural abnormalities (McCarley et al 1989) and that P300 abnormalities occur in schizophrenia pedigrees (Blackwood et al 1991). These converging lines of evidence support the possibility that abnormalities of the temporal lobes may represent important aspects of the phenotypic manifestations of the genetic abnormalities in schizophrenia.
Acknowledgments
The authors acknowledge the support of the Schizophrenia Small Grants Program of the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York. Additional support was provided by the B.C. Health Research Foundation, the Canadian Psychiatric Research Foundation, the Scottish Rite Schizophrenia Research Program and the Ontario Mental Health Foundation.
We acknowledge me support of Drs. H. Pardes, R.O. Rieder, I. Prohovnik, and D. Toms. J. McAlduff, RN, S. Dalton, RT, and D. MacIsaac, RT provided invaluable assistance.
References
- Barta PE, Pearlson GD, Powers RE, et al. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bartko JJ, Carpenter WT. On the methods and theory of reliability. J Nerv Ment Dis. 1976;163:307–317. doi: 10.1097/00005053-197611000-00003. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Collins EJ, Nuttall S, et al. Positive and negative symptoms in families with schizophrenia. Schizophr Res. 1993;11:9–19. doi: 10.1016/0920-9964(93)90033-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Bury A, Honer WG. Testing Liddle’s three syndrome model in families with schizophrenia. Schizophr Res. 1994;12:213–221. doi: 10.1016/0920-9964(94)90031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Swigar ME, Rothman SLG, et al. CT scan studies of superficial cerebral regions: frequency and distribution of abnormalities in elderly psychiatric patients. Neurobiol Aging. 1983;4:289–295. doi: 10.1016/0197-4580(83)90005-2. [DOI] [PubMed] [Google Scholar]
- Blackwood DHR, St Clair DM, Muir WM, et al. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry. 1991;48:899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Wurthmann C, Piroth HD. Himsubstanzdefizit mit paralimbischem und limbischem Schwerpunkt im CT Schizophrener. Der Nervenarzt. 1987;58:97–106. [PubMed] [Google Scholar]
- Cannon TD, Mednick SA, Parnas J. Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry. 1989;46:883–889. doi: 10.1001/archpsyc.1989.01810100025005. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Mednick SA, Parnas J, et al. Developmental brain abnormalities in the offspring of schizophrenic mothers. Arch Gen Psychiatry. 1993;50:551–564. doi: 10.1001/archpsyc.1993.01820190053006. [DOI] [PubMed] [Google Scholar]
- Dauphinais I, DeLisi L, Crow T, et al. Reduction in temporal lobe size in siblings with schizophrenia: a magnetic resonance imaging study. Psychiatry Res Neuroimaging. 1990;35:137–147. doi: 10.1016/0165-1781(90)90156-y. [DOI] [PubMed] [Google Scholar]
- DeLisi L, Goldin L, Hamovit J, et al. A family study of the association of increased ventricular size with schizophrenia. Arch Gen Psychiatry. 1986;43:148–153. doi: 10.1001/archpsyc.1986.01800020058007. [DOI] [PubMed] [Google Scholar]
- Endicott J. Best Estimate Clinical Evaluation and Diagnosis Form (BECED) New York: Department of Research Assessment and Training, New York State Psychiatric Institute; 1988. [Google Scholar]
- Falkai P, Bogerts B, Greve B, et al. Loss of sylvian fissure asymmetry in schizophrenia. Schizophr Res. 1992;7:23–32. doi: 10.1016/0920-9964(92)90070-l. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Schneider T, et al. Reduced frontal and occipital lobe asymmetry on the CT-scans of schizophrenic patients. Schizophr Res. 1993;9:198. doi: 10.1007/BF01271470. [DOI] [PubMed] [Google Scholar]
- Honer WG, Bassett AS, Kopala L, et al. A genotype-phenotype research strategy for schizophrenia. Can J Psychiatry. 1990;35:776–783. doi: 10.1177/070674379003500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiya H, Uematsu M, Ofuji M, et al. Computerised tomography in schizophrenia: familial versus non-familial forms of illness. Br J Psychiatry. 1989;155:444–450. doi: 10.1192/bjp.155.4.444. [DOI] [PubMed] [Google Scholar]
- Kay S, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- LeMay M, Stafford J, Sandor T, et al. Statistical assessment of perceptual CT scan ratings in patients with Alzheimer type dementia. J Comput Assist Tomogr. 1986;10:802–809. doi: 10.1097/00004728-198609000-00018. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Faux SF, Shenton M, et al. CT abnormalities in schizophrenia. Arch Gen Psychiatry. 1989;46:698–708. doi: 10.1001/archpsyc.1989.01810080028004. [DOI] [PubMed] [Google Scholar]
- Pandurangi AK, Dewan MJ, Lee SH, et al. The ventricular system in chronic schizophrenic patients: a controlled computed tomography study. Br J Psychiatry. 1984;144:172–176. doi: 10.1192/bjp.144.2.172. [DOI] [PubMed] [Google Scholar]
- Reveley A, Reveley M, Clifford C, et al. Cerebral ventricular size in twins discordant for schizophrenia. Lancet. 1982;ii:540–541. doi: 10.1016/s0140-6736(82)92047-5. [DOI] [PubMed] [Google Scholar]
- Reveley A, Reveley M, Murray R. Cerebral ventricular enlargement in non-genetic schizophrenia: a controlled twin study. Br J Psychiatry. 1984;144:89–93. doi: 10.1192/bjp.144.1.89. [DOI] [PubMed] [Google Scholar]
- Rieder R, Gershon E. Genetic strategies in biological psychiatry. Arch Gen Psychiatry. 1978;35:866–873. doi: 10.1001/archpsyc.1978.01770310072005. [DOI] [PubMed] [Google Scholar]
- Roxborough H, Muir WJ, Blackwood DHR, et al. Neuropsychological and P300 abnormalities in schizophrenics and their relatives. Psychol Med. 1993;23:305–314. doi: 10.1017/s0033291700028385. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Aylward E, Barta PE, et al. Sylvian fissure size in schizophrenia measured with the magnetic resonance imaging rating protocol of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1992;149:1195–1198. doi: 10.1176/ajp.149.9.1195. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, First MB. Structured Clinical Interview for DSM-III-R-Patient Edition (SCID-P, Version 1.0) Washington, DC: American Psychiatric Press; 1990a. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, et al. Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II, Version 1.0) Washington, DC: American Psychiatric Press; 1990b. [Google Scholar]
- Suddath R, Christison G, Torrey E, et al. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Carey G, Myies-Worsley M, et al. Codistribution of a sensory gating deficit and schizophrenia in multi-affected families. Psychiatry Res. 1991;39:257–268. doi: 10.1016/0165-1781(91)90092-4. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, DeLisi LE, Neophytides AN, et al. Familial aspects of CT scan abnormalities in chronic schizophrenic patients. Psychiatry Res. 1981;4:65–71. doi: 10.1016/0165-1781(81)90009-3. [DOI] [PubMed] [Google Scholar]


