Abstract
Darier disease is an autosomal dominant skin disorder characterized by abnormal keratinocyte adhesion. Recent data have provided evidence for linkage of the Darier disease locus to 12q23-24.1 in British families. We have carried out linkage analysis using the 12q markers D12S58, D12S84, D12S79, D12S86, PLA2, and D12S63 in 6 Canadian families. Pairwise linkage analysis generated positive lod scores at all 6 markers at various recombination fractions, and each family showed positive lod scores with more than one marker. The peak lod score in the multipoint analysis (Zmax) was 5.5 in the interval between markers D12S58 and D12S84. These positive lod scores in North American families of varied European ancestry confirm the location of the Darier disease gene, and suggest genetic homogeneity. The future identification and sequencing of the gene responsible for Darier disease should lead to improved understanding of the disease and of keratinocyte adhesion in general.
Keywords: Darier disease, keratosis follicularis, linkage, chromosome 12
INTRODUCTION
Darier disease (keratosis follicularis) is an autosomal dominant genodermatosis with complete penetrance and variable expressivity [Beck et al., 1977; Munro, 1992; Burge and Wilkinson, 1992]. It is worldwide in distribution, with a prevalence ranging from 1 in 55,000 [Wilkinson et al., 1977] to 1 in 100,000 [Svendsen and Albrectsen, 1959]. The disease is characterized by the presence of warty papules and plaques primarily affecting the so-called seborrhoeic areas of the skin on the face, scalp, trunk, and groin [Baden, 1987]. Involvement of the nails, palms, soles and mucous membranes is common [Zaias and Ackerman, 1973; Burge and Wilkinson, 1992]. In some patients, disfigurement or complications such as superinfection of skin lesions is a major source of morbidity. Approximately 75% of patients have signs or symptoms of the disease by age 20 [Burge and Wilkinson, 1992]. Histologically there is evidence of abnormal adhesion of keratinocytes [Lever and Schaumberg-Lever, 1990]. Although there has been demonstration of abnormal distribution of keratins and desmosomal proteins [Burge and Garrod, 1991; Burge et al., 1988] as well as abnormal protease regulation [Burge et al., 1989], the cause of abnormal cell adhesion in this disease remains unknown.
Darier disease has been associated with a variety of neurologic or psychiatric conditions, including schizophrenia in one large Canadian family included in this study [Berg and Bassett, 1993]. Evidence for a link between Darier disease and mood disorder in one family was presented recently [Craddock et al., 1993]. The presence of epilepsy or salivary gland abnormalities in certain families [Burge and Wilkinson, 1992] and the variable presence of neuropsychiatric abnormalities have suggested the possibility of heterogeneity [Munro, 1992], allelic heterogeneity, or pleiotropic effects.
Candidate genes for linkage analysis of Darier disease have included the keratin genes on chromosomes 12 and 17 as well as various desmosomal proteins with known chromosomal locations [Arnemann et al., 1991]. However, markers tested from candidate regions including these genes have not been reported to show linkage to Darier disease [Bashir et al., 1993; Sidenberg et al., 1994; Goldsmith et al., 1993].
Recently, Craddock et al. [1993] and Bashir et al. [1993] have reported evidence of linkage between markers on chromosome 12q and Darier disease. The families evaluated in both studies were limited to the British population. The Craddock et al. [1993] study, using age-adjusted penetrances, found a peak lod score of 5.54 in their multipoint analysis at zero recombination with D12S84. Bashir et al. [1993] reported two families showing positive pairwise lod scores with the marker D12S79, located 6 cM telomeric to D12S84.
The current study reports the findings from analysis of 6 highly informative markers on chromosome 12q in 6 Canadian families. We confirm linkage of Darier disease to 12q, add support to the hypothesis of genetic homogeneity, and provide additional information regarding the location of the disease gene.
METHODS
Subjects
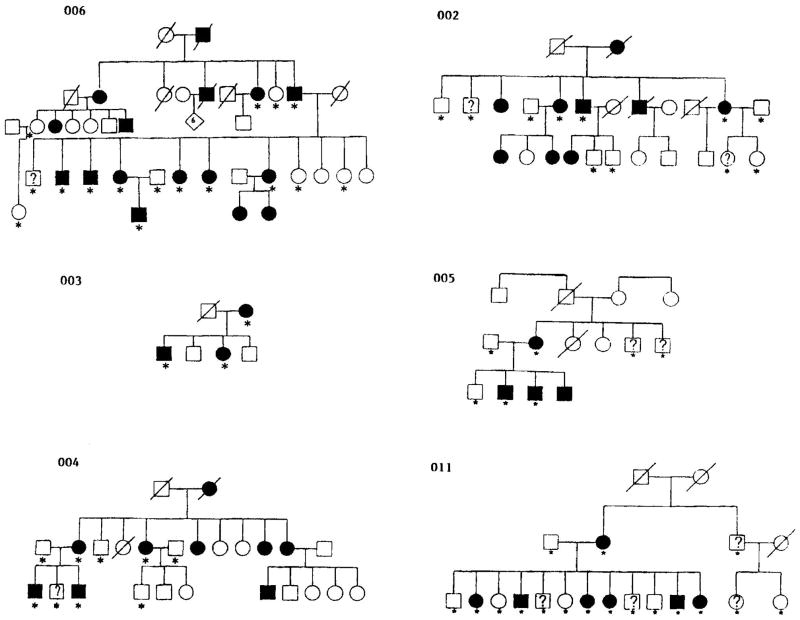
Six Canadian families of varied ancestry (English, Irish, Italian, and German) were ascertained primarily through referrals from clinical dermatologists for Darier’s disease (Fig. 1). Informed consent was obtained and available relatives were interviewed and examined by the same dermatologist (DB). Standardized diagnostic criteria were used [Berg et al., 1993] to obtain a diagnosis of affected (N = 30), unaffected (N = 24), or uncertain diagnosis (N = 10). The diagnosis was considered uncertain if the clinical examination yielded nonspecific findings. For example, a patient with no specific physical findings but a history consistent with a previous “flare” of skin disease would be considered uncertain. Skin biopsy confirming Darier disease was available on at least one member of each family.
Fig. 1.
Pedigrees ascertained. An open square denotes an unaffected male; an open circle an unaffected female; a solid square or circle denotes, respectively, a male or female affected with Darier disease. A question mark (?) indicates uncertain affection status. A slash (/) through the symbol denotes the individual is deceased. An asterisk (*) denotes the subjects for whom DNA was obtained.
DNA Studies
Venous blood was obtained on all available relatives (64), and DNA extracted using standard techniques. Genotypes of relatives were determined with dinucleotide repeat markers D12S58 and D12S63 from the database of J. Weber, and D12S76, D12S79, D12S84, and D12S86 from Genethon [Weissenbach et al., 1992]. Marker typings were performed as described elsewhere [Weber and May, 1989]. The trinucleotide repeat for phospholipase A2 (PLA2) was typed as described by Polymeropoulos et al. [1990]. Autoradiography were interpreted separately by at least two of us, blind to diagnostic status. Marker allele frequencies were obtained from 20 unrelated individuals from eastern Canada.
Linkage Analysis
Lod scores for pairwise linkage analysis used the program LIPED, and for multipoint analysis the LINKAGE package version 5.1 was used. Subjects with an uncertain diagnosis were genotyped and included in the linkage analysis to help establish phase, but were classified as unknown in terms of disease status. We employed two different age-adjusted penetrances (AAP1 and AAP2). AAP1 was as used by Craddock et al. [1993]: 0.98 for ages 61 years and above, 0.96 for ages 51–60, 0.94 for 41–50, 0.88 for 31–40, 0.78 for 21–30, and 0.36 for less than 20 years of age. AAP2 was based on the clinical data from our Canadian families supplemented by the epidemiologic data of Burge and Wilkinson [1992] as follows: 0.98 for age 51 or above, 0.97 for 41–50, 0.95 for 31–40, 0.93 for 21–30, 0.73 for 13–20, and 0.40 for less than age 13. For the pairwise analyses using the LIPED program, a linear age adjustment of penetrance was used with the following lower and upper values, respectively: for AAP1, 0.36 at age 20, 0.98 at age 61; and for AAP2, 0.40 for age 13, 0.93 for age 30. Other parameters for the genetic linkage model were autosomal dominant inheritance, Darier disease gene frequency 0.000005, and phenocopies at 0.0001. The genetic map used for the multipoint analysis, using sex-averaged distances, was as follows: cen—//—D12S58-5.2 cM—D12S84—6.0 cM—D12S79—//—qter.
RESULTS
Positive pairwise linkage results at various recombination fractions were determined for each of the 6 markers studied when all families were pooled (Table I). Three loci, D12S58, D12S79, and D12S84, were significantly linked (lod > 3.0) to Darier disease, with the highest pairwise score (lod = 4.6) occurring at a recombination fraction of 0.05 from D12S58. However, the maximum lod score would probably have occurred at D12S84 if this marker had been informative in all families. Data shown are the results obtained using the higher penetrance values of AAP2; similar but slightly lower lod score values were obtained using AAP1. Table II reveals that all families showed positive lod scores within θ = 0.05 of D12S84 or D12S58, and none showed consistently negative scores, thus there was no evidence for heterogeneity.
TABLE I.
Two-point Lod Scores for Five Loci on Chromosome 12q*
| Locus | Number typed (n) | Recombination fraction (θ)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.001 | 0.01 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | ||
| D12S58 | 58 | 3.48 | 3.81 | 4.47 | 4.61 | 4.26 | 3.24 | 2.00 | 0.71 |
| D12S84 | 62 | 3.26 | 3.56 | 4.21 | 4.39 | 4.05 | 3.01 | 1.80 | 0.65 |
| D12S79 | 61 | 1.42 | 1.84 | 2.97 | 3.87 | 3.94 | 3.32 | 2.24 | 0.96 |
| D12S86 | 56 | −inf | −2.52 | 0.43 | 2.24 | 2.67 | 2.43 | 1.66 | 0.70 |
| PLA-2 | 62 | −inf | −3.34 | 1.17 | 2.14 | 2.68 | 2.51 | 1.74 | 0.73 |
| D12S63 | 62 | −8.11 | −7.71 | −5.49 | −1.89 | −0.36 | 0.51 | 0.65 | 0.34 |
Markers are in order from most centromeric (D12S58) to most telomeric (D12S63). These LIPED analyses used AAP2 (see text).
TABLE II.
Maximum Two-Point Lod Scores for Individual Pedigrees
| Pedigree |
Zmax(θ)
|
||
|---|---|---|---|
| D12S58 | D12S84 | D12S79 | |
| 002 | 1.32 (0.00) | 1.32 (0.00) | −0.03 (0.04) |
| 003 | 0.30 (0.00) | 0.30 (0.00) | 0.30 (0.00) |
| 004 | 0.02 (0.28) | 1.03 (0.00) | −0.01 (0.40) |
| 005 | −0.13 (0.40) | 0.73 (0.00) | 0.73 (0.00) |
| 006 | 2.00 (0.00) | 1.42 (0.10) | 2.61 (0.00) |
| 011 | 2.71 (0.00) | 0.00 (0.00) | 2.41 (0.00) |
Marker-to-marker studies of PLA2 with the other loci showed that PLA2 maps near D12S86, with a peak lod of 9.8 at θ = 0.052 (sex-averaged; data not shown); however, the odds ratios for location were not significant enough to place PLA2 definitively in the multipoint map. An approximate map of the region is as follows: cen—//—D12S58—5.2 cM—D12S84—6.0 cM—D12S79—(7.2 cM)—PLA2—(5.2 cM)—D12S86—//—qter. In multipoint linkage analyses of Darier disease across D12S58, D12S84, and D12S79, with all 6 families using AAP2, the Zmax was 5.5 at θ = 0.01 from D12S58 in the telomeric direction toward D12S84 (Fig. 2). The D12S58–D12S84 distance was obtained from Dawson et al. [1993], and the D12S84-D12S79 distance was from the Genethon map [Weissenbach et al., 1992]. Using AAP1, multipoint linkage analyses produced a peak lod score of 4.0. Overall, these analyses placed the Darier disease locus in the interval between D12S58 and D12S84. The odds ratio in favor of locating the Darier gene in the D12S84-D12S58 interval compared to the D12S84-D12S79 interval was 33:1, and compared to the centromeric side of D12S58 was 3:1.
Fig. 2.
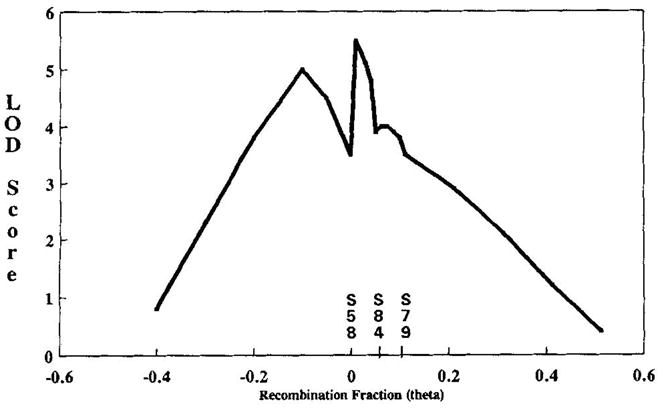
Multipoint of Darier disease across D12S58, D12S84, and D12S79, showing lod scores obtained when the Darier disease locus was moved across a fixed map of D12S58 set arbitrarily at θ = 0.0, D12S84 set at θ = 0.052, and D12S79 at θ = 0.112 (sex-averaged).
DISCUSSION
The current study provides additional evidence that the gene for Darier disease is in the 12q23-24.1 region. The data suggest that the most likely position of the Darier disease locus is a few centimorgans centromeric of the marker locus D12S84; however, location on the telomeric side of D12S84 cannot be ruled out. This is consistent with linkage data from 7 British families [Craddock et al., 1993; Bashir et al., 1993], although our data peak at a different location, approximately 5 cM centromeric to the peak of Craddock et al. [1993]. The individual recombinant with marker D12S84 in family 006 is a Darier case according to clinical criteria, and typing of new markers in this family may be useful in localizing the disease gene. The AAP2 data, that had a wider age range specified and a generally higher penetrance than AAP1, produced a maximum lod score 1.5 units higher than the maximum using AAP1. Thus, further evaluation and refinement of age-adjusted penetrances may be helpful. Families in the current study, which were derived from three different European ethnic groups, all showed evidence of linkage to 12q markers, as did the two published studies on British families, indicating homogeneity of Darier disease. Additional markers from the 12q23-q24.1 region, particularly in the interval between D12S58 and D12S84, will need to be typed in extended Darier disease pedigrees to further localize and ultimately identify the disease gene. The reported association with mood disorders and psychosis merits further study.
The keratin genes known to be on chromosome 12 are located at a relatively large distance proximal to the Darier disease locus. It is possible that the Darier disease gene produces a hitherto unlocalized keratin or desmosomal protein, or is a novel gene involved in dermatologic structure or function. Identifying disease- causing mutations may shed light on issues pertaining to pathophysiology and diagnostic assessments, particularly in the case of relatives with subthreshold skin conditions.
Acknowledgments
The authors thank the families for their participation in the study, the dermatologists who helped us, and Dr. Nicholas Voudouris. The work was supported by grants from the Physicians’ Services Incorporated Foundation, Canadian Psychiatric Research Foundation, Ontario Mental Health Foundation, and the National Alliance for Research on Schizophrenia and Depression.
References
- Arnemann J, Spurr NK, Wheeler GN, Parker AE, Buxton RS. Chromosomal assignment of the human genes coding for the major proteins of the desmosome junction, desmoglein DGI (DSG), desmocollins DGII/I1I (DSC), desmoplakins DPI/II (DSP), and plakoglobin DPIII (JUP) Genomics. 1991;10:640–645. doi: 10.1016/0888-7543(91)90446-l. [DOI] [PubMed] [Google Scholar]
- Baden HP. Darier-White disease (keratosis follicularis) and miscellaneous hyperkeratotic disorders. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in General Medicine. 3. New York: McGraw-Hill; 1987. pp. 520–525. [Google Scholar]
- Bashir R, Munro CS, Mason S, Stephenson A, Rees JL, Strachan T. Localisation of a gene for Darier’s disease. Hum Mol Genet. 1993;2:1937–1939. doi: 10.1093/hmg/2.11.1937. [DOI] [PubMed] [Google Scholar]
- Beck AL, Finocchio AF, White JP. Darier’s disease: A kindred with a large number of cases. Br J Dermatol. 1977;97:335–339. doi: 10.1111/j.1365-2133.1977.tb15192.x. [DOI] [PubMed] [Google Scholar]
- Berg D, Bassett AS. Darier’s disease: Current understanding of pathogenesis and future role of genetic studies. Int J Dermatol. 1993;32:397–400. doi: 10.1111/j.1365-4362.1993.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Sidenberg DG, Bassett AS, Kennedy JL. Genetic linkage studies of Darier’s disease, (abstract) Society for Investigative Dermatology Annual Meeting; November; Washington, DC. 1993. [Google Scholar]
- Burge SM, Fenton DA, Dawber RPR, Leigh IM. Darier’s disease: An immunohistochemical study using monoclonal antibodies to human cytokeratins. Br J Dermatol. 1988;118:629–640. doi: 10.1111/j.1365-2133.1988.tb02563.x. [DOI] [PubMed] [Google Scholar]
- Burge SM, Ryan TJ, Cederholm-Williams SA. Darier’s disease: An immunohistochemical study using antibodies to proteases. Br J Dermatol. 1989;121:613–621. doi: 10.1111/j.1365-2133.1989.tb08193.x. [DOI] [PubMed] [Google Scholar]
- Burge SM, Garrod DR. An immunohistochemical study of desmosomes in Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 1991;124:242–251. doi: 10.1111/j.1365-2133.1991.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Burge SM, Wilkinson VD. Darier-White disease: A review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40–50. doi: 10.1016/0190-9622(92)70154-8. [DOI] [PubMed] [Google Scholar]
- Craddock N, Dawson E, Burge S, Parfitt L, Mant B, Roberts Q, Daniels J, Gill M, McGuffin P, Powell J, Owen M. The gene for Darier’s disease maps to chromosome 12q23-q24.1. Hum Mol Genet. 1993;2:1941–1943. doi: 10.1093/hmg/2.11.1941. [DOI] [PubMed] [Google Scholar]
- Dawson E, Shaikh S, Weber JL, Wang Z, Weissenbach J, Powell JF, Gill M. A continuous linkage map of 22 short tandem repeat polymorphisms on human chromosome 12. Genomics. 1993;17:245–248. doi: 10.1006/geno.1993.1314. [DOI] [PubMed] [Google Scholar]
- Goldsmith LA, Wakem P, Polakowska R, Haake A, et al. Exclusion of candidate genes in Darier’s disease using positional cloning (abstract) J Invest Dermatol. 1993;101(3):458. [Google Scholar]
- Lever WF, Schaumberg-Lever G. Histopathology of the Skin. New York: J. B. Lipincott; 1990. [Google Scholar]
- Munro CS. The phenotype of Darier’s disease: Penetrance and expressivity in adults and children. Br J Dermatol. 1992;127:126–130. doi: 10.1111/j.1365-2133.1992.tb08044.x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Rath DS, Xiao H, Merril CR. Trinucleotide repeat polymorphism at the human pancreatic phospholipase A2 gene (PLA2) Nucl Acids Res. 1990;18:7468. [PMC free article] [PubMed] [Google Scholar]
- Sidenberg DG, Berg D, Bassett AS, King N, Petronis A, Kamble AB, Kennedy JL. Genetic linkage evaluation of twenty-four loci in an eastern Canadian family segregating Darier’s disease (keratosis follicularis): A preliminary study. J Am Acad Dermatol. 1994;31 (1):27–30. doi: 10.1016/s0190-9622(94)70130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen IB, Albrechtsen B. The prevalence of dyskeratosis follicularis (Darier’s disease) in Denmark. Acta Derm Venereol. 1959;39:256–269. [PubMed] [Google Scholar]
- Weber J, May P. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989;44:388–396. [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J, Gyapay G, Dib C, Vignal A, Morisette J, Millasseau P, Vaysseix G, Lathrop M. A second generation map of the human genome. Nature (London) 1992;359:794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson JD, Marsden RA, Dawber RPR. Review of Darier’s disease in the Oxford region. Br J Dermatol suppl. 1977;15:15–16. [Google Scholar]
- Zaias N, Ackerman AB. The nail in Darier-White disease. Arch Dermatol. 1973;107:193–199. [PubMed] [Google Scholar]