Abstract
Mechanisms determining temporal lobe structural asymmetries may be involved in the pathogenesis of schizophrenia. To investigate the temporal lobes in familial schizophrenia, computed tomographic scans were obtained from 51 subjects (seven families). Enlargement of sylvian fissures and temporal lobe sulcal spaces was observed in family members with schizophrenia. The posterior one-third of the sylvian fissure was larger on the left side in subjects with schizophrenia, and larger on the right side in unaffected individuals. This disturbed pattern of posterior sylvian fissure asymmetry suggests that adjacent language regions may be affected in schizophrenia. An intermediate degree of disturbance in subjects who had schizophrenia-related illnesses or were obligate carriers suggests that genetic factors may be important determinants of temporal lobe asymmetries in familial schizophrenia.
Keywords: Schizophrenia, Genetics, Sylvian fissure, Cerebral asymmetry, Development
Introduction
Schizophrenia is a common, severe mental illness characterized by hallucinations, delusions, disordered thought processes and seriously compromised ability to function in everyday life. Schizophrenia appears to be unique to humans and has a significant genetic component.1 In this context, Crow2 proposed that processes involved in the development of cerebral lateralization (most highly evolved in language areas) might hold the key to understanding the genetics of schizophrenia. Based on neuropathological and brain imaging studies,3 the normal asymmetry of temporal lobe structures was hypothesized to be disturbed in schizophrenia. These structures include the planum temporale, located on the inferior bank of the posterior portion of the sylvian fissure, a region which is larger on the left than the right in normal individuals.4 The provocative hypothesis2 that disturbed temporal lobe asymmetries represent the structural expression of an abnormal cerebral dominance gene in schizophrenia has not yet been directly tested in families with the illness.
We previously used a quantitative rating scale to assess regional brain structure in multigenerational families with schizophrenia.5 Enlargement of cerebrospinal fluid (CSF) spaces adjacent to the temporal lobe was observed in psychotic individuals, relative to unaffected family members. Regional enlargement of CSF spaces is likely to reflect relatively smaller amounts of underlying brain tissue.6 We now report an extended sample, including additional families and allowing assignment of subjects to genetic risk classes. Quantitative image analysis techniques allowed us to test the hypothesis that as well as having enlarged temporal CSF spaces, individuals with schizophrenia in these multiply affected families would exhibit abnormal asymmetry of the portion of the sylvian fissure overlying the language regions.
Subjects and Methods
Fifty-one individuals were recruited from seven families in which schizophrenia was inherited in a pattern consistent with autosomal dominant transmission.7 All gave written, informed conset. Each subject was diagnosed using RDC and DSM-III-R criteria. For analyses, subjects were classified into three genetic risk-level groups based on family studies of schizophrenia and related ‘schizophrenia spectrum’ conditions.1 The first group comprised schizophrenia (n = 18) or schizoaffective disorder (n = 7); the second group (spectrum, n = 8) comprised subjects with conditions probably indicating increased genetic risk for schizophrenia: non-affective functional psychosis (n = 2), depression and psychosis (n = 1) and schizotypal personality disorder (n = 1). Four additional subjects in this group did not have schizophrenia spectrum illnesses, but were obligate carriers based on pedigree analysis. The final group (n = 18) comprised members from the affected side of each family, but with no schizophrenia spectrum-related condition. No subjects had substance abuse or dependence. There were 27 men and 24 women; distribution of the sexes between risk-level groups did not differ. The mean age was 45 years (s.d. 15, range 19–82). Mean age did not differ between groups.
Computerized tomographic (CT) scans were obtained using a standardized protocol for a GE9800 scanner, with 5 mm slices.5 While higher resolution magnetic resonance imaging (MRI) would be preferred, the medium to large pedigrees required for the present study were drawn largely from rural areas, and considerations of compliance, distance and cost rendered MRI impractical. Scans were imaged using a constant light source (Northern Light illuminator) and a Sony CCD video camera connected via a Quickcapture video interface board to a Macintosh Quadra 700 computer. Images were analysed blind to subject identity and risk status, using NIH Image software.8 Regions of interest (sylvian fissures, temporal lobe sulci, and temporal horns) were outlined on original scans using atlases.9,10 Proceeding from the skull base, the slice on which the frontal horns were seen first was identified. The two slices inferior to this were included, as were all superior slices to the rostral limit of the thalamus (Fig. 1). A thresholding procedure was applied to separate sulcal and ventricular spaces from parenchyma. These thresholded regions were used as guides for manually outlining regions of interest, and the linear scale on the scans used to adjust measurements of area to square millimetres. Intracranial volume was also measured within the limits defined above. Volumes in cubic millimetres were calculated by multiplying areas by slice thickness. During the study period, 10 scans were remeasured independently. Intraclass correlations for the two operators’ measurements (CC and WGH) were: sylvian fissure 0.96, temporal lobe sulci 0.91, and temporal horn 0.89.
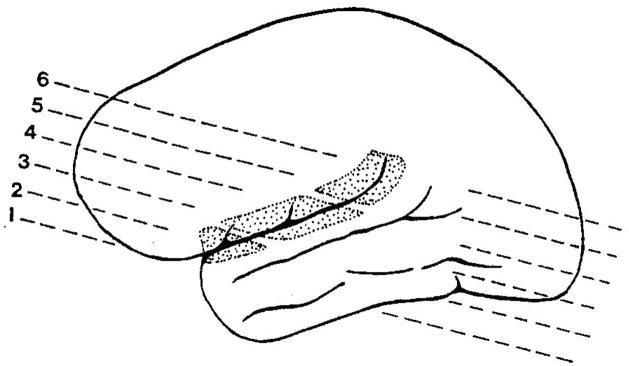
FIG. 1.
Slices used and anatomy of the sylvian fissure and lateral temporal sulci. Slices used to form anterior (1, 2), middle (3, 4) and posterior (5, 6) segments of the sylvian fissure are indicated. The posterior segment is bounded on the inferior aspect by the planum temporale.
Volumetric measurements were normalized to intracranial volume for analysis of covariance (ANCOVA). Putative effects of genetic risk level, family of origin, and sex on regional CSF volumes were analysed using age as a covariate, since CSF spaces are known to increase in volume with age. The risk level by sex interaction was also included. Temporal sulcal measures were transformed by calculating the square root prior to ANCOVA, in order to normalize the distribution. To analyse the asymmetry hypothesis, sylvian fissure volumes from slices 1 and 2 were combined to form left and right anterior segments, volumes from slices 3 and 4 to form left and right middle segments, and volumes from slices 5 to 6 to form left and right posterior segments. The latter segment overlies the planum temporale region.11 The asymmetry index for each segment was calculated as (left − right)/((left + right)/2).12 Repeated measures analysis of variance was carried out on the asymmetry index using risk level, family, sex, and risk level by sex as between subjects factors, age as a covariate and segment as a within-subject repeated measure. Interaction effects with segment were also calculated, and the Greenhouse-Geiser correction applied in reporting the significance level of the F-statistics.
Results
Absolute volumes of regions of interest appear in Table 1. From reported MRI studies,13,14 the left sylvian fissure was expected to be larger than the right, and the right temporal horn larger than the left. No prediction was possible regarding the temporal lobe sulci. Observation of the expected asymmetries on CT images in the overall sample supported the accuracy of the measurement techniques. The first series of comparisons concerned overall volumes of the sylvian fissures, temporal lobe sulci and temporal horns. Results are illustrated in Figure 2 according to risk level and age. Analysis of sylvian fissure data indicated significant effects of risk level (F = 4.34, p = 0.02), family (F = 3.66, p = 0.006), and age (F = 61.37, p = 0.0001), but not sex or risk level by sex interaction. Sylvian fissure volume was larger in both schizophrenic/schizoaffective and intermediate risk schizophrenia spectrum individuals, compared with unaffected family members (Tukey compromise test, p < 0.05). Temporal sulcal volumes also differed according to risk level (F = 8.39, p = 0.001), and a significant age effect (F = 50.59, p = 0.0001) was noted. Family and sex effects were not significant. Sulcal volumes were highest in the schizophrenia/schizoaffective group, which differed significantly from the unaffected group (Tukey’s compromise test, p < 0.05). The spectrum group was intermediate between the other two groups and did not differ significantly from either one. In contrast to lateral temporal measures, no effect of risk level was detected for temporal horns, however the family effect (F = 2.48, p = 0.04) was significant. Age and sex effects were not significant.
Table 1.
Mean absolute volumes (±s.d.; mm3) of sulcal, ventricular and cisternal spaces in subjects grouped according to risk for schizophrenia/schizoaffective disorder (S/SA)
| Region | Side | Risk level
|
||
|---|---|---|---|---|
| Unaffected (n= 18) | Spectrum (n = 8) | S/SA (n = 25) | ||
| Sylvian fissure | Left | 4734 ± 3452 | 7689 ± 4604 | 7652 ± 3462 |
| Right | 4409 ± 2533 | 6282 ± 3688 | 6582 ± 3575 | |
| Total* | 9143 ± 5840 | 13 971 ± 8214 | 14 235 ± 6813 | |
| Temporal sulci | Left | 341 ± 834 | 593 ± 767 | 954 ± 976 |
| Right | 402 ± 540 | 539 ± 500 | 883 ± 981 | |
| Total** | 743 ± 1359 | 1132 ± 1018 | 1836 ± 1906 | |
| Temporal horn | Left | 297 ± 154 | 530 ± 430 | 465 ± 395 |
| Right | 415 ± 249 | 647 ± 612 | 472 ± 440 | |
| Total | 712 ± 780 | 1178 ± 1029 | 937 ± 780 | |
Spectrum individuals are at increased risk compared to unaffected. Significant differences between risk level groups:
p = 0.02
p = 0.001, with volumes normalized to intracranial volume and controlled for effects of family of origin, sex and age.
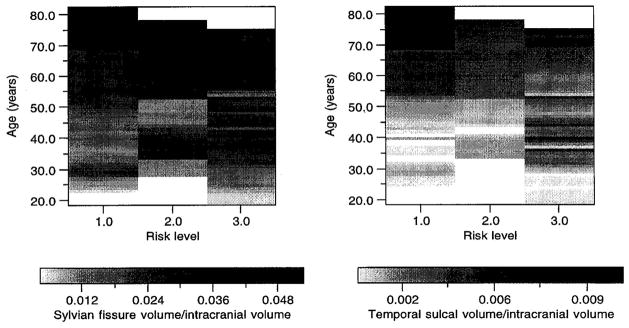
FIG. 2.
Raster plot indicating sylvian fissure (left) and temporal sulcal (right) volume/intracranial volume according to risk class (1 = unaffected family members, 2 = schizophrenia spectrum/obligate carrier, 3 = schizophrenia/schizoaffective) and age. Original data values were retained by the weighted fill procedure used in creating the image. Larger volume at a given age is indicated by a darker grey scale level.
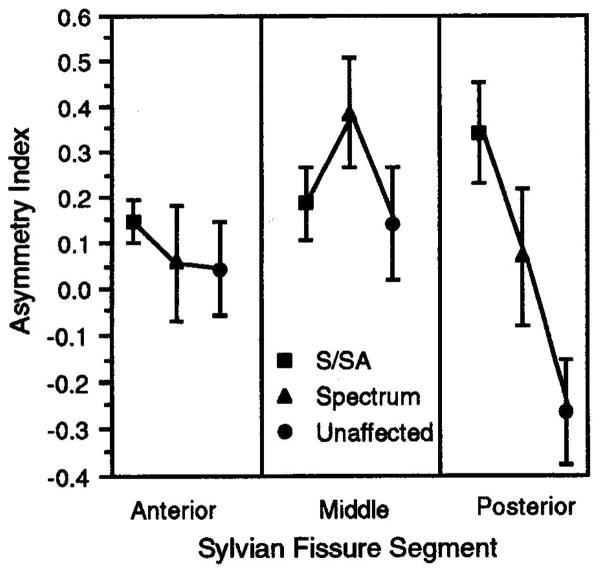
Sylvian fissure asymmetry assessments were carried out on anterior, middle and posterior segments. The results are presented in Figure 3 and indicate a significant effect of risk level (F = 5.18, p = 0.01) and a risk by segment interaction (F = 3.99, p = 0.007). In schizophrenic/schizoaffective subjects, the asymmetry of the sylvian fissure posterior segment observed in unaffected family members (left smaller than right) was reversed. Segment by sex, and segment by risk level by sex interactions were not significant. No significant family effects were observed.
FIG. 3.
Sylvian fissure volume asymmetry index (mean ± s.e.) plotted according to segment of fissure and risk class. Disturbed asymmetry was observed in posterior regions in schizophrenia/schizoaffective cases.
Discussion
The primary findings were increased sylvian fissure and temporal sulcal volumes in schizophrenic and schizoaffective members of multiply affected families, and disturbed patterns of posterior sylvian fissure asymmetry in the same subjects. Regarding overall sylvian fissure and sulcal changes, these results confirm previous findings using samples of schizophrenic patients unselected for familial forms of the illness.13 In one study of adult offspring of schizophrenic women (‘high-risk’ individuals), overall brain sulcal CSF was increased in subjects with schizophrenia and in those with schizophrenia spectrum conditions, compared with unaffected but at-risk subjects.15 The present finding of increased sylvian fissure volume in schizophrenic/schizoaffective subjects, as well as subjects with schizophrenia spectrum conditions or obligate carriers, supports the relationship between genetic risk for schizophrenia and sulcal enlargement. The temporal sulcal measure was increased only in the schizophrenia/schizoaffective group compared with the unaffected group. This measure may be less sensitive as a genetic risk indicator, or these sulcal changes may be associated with illness-related factors such as age of onset, duration or severity. The absence of an effect of risk level on temporal horn volume may indicate that the medial temporal lobe is less affected in familial schizophrenia. The finding of significant family effects on both sylvian fissure and temporal horn volumes suggest that considerable variability in studies of unrelated subjects could be attributed to undetected interfamilial differences, as predicted by studies of lateral ventricle size in monozygotic twins.16,17
The observation of altered asymmetry of the posterior segment of the sylvian fissure in schizophrenic and schizoaffective family members replicates a previous finding in unrelated schizophrenics and controls.18 A disturbed pattern of sylvian fissure volume asymmetry related to genetic risk supports Crow’s hypothesis2,3 that a gene or genes related to the lateralization of temporal lobe development may contribute to schizophrenia. However, an investigation of monozygotic twins discordant for schizophrenia failed to demonstrate disturbed asymmetry of sylvian fissure length or angle.19 The latter measures may be related to developmental features different from those indicated by measures of volume. Alternatively, the genetic component of schizophrenia in these twin pairs may be different than the families studied here. The findings of abnormal asymmetry in posterior rather than anterior portions of the sylvian fissure may support studies indicating planum temporale abnormalities in schizophrenia.20,21 However, because our measurements were of CSF spaces, changes located in the parietal operculum forming the superior and posterior aspects of the sylvian fissure cannot be excluded. It is also possible that posterior asymmetry reflects a difference in fissure orientation, since the in-plane resolution of CT exceeds the slice thickness. A range of sylvian fissure anatomical patterns is present in normal individuals,11,22 and is proposed to reflect different patterns of temporal and parietal lobe development. Differences in sylvian fissure length and decreased left/right ratio in schizophrenia were reported in a postmortem study.23 The overall temporal gyral pattern was also reported to be abnormal in schizophrenia.24
Conclusion
The present findings indicate that sylvian fissure and temporal sulcal spaces are increased in familial schizophrenia, and increased genetic risk for schizophrenia predisposes to enlargement of the sylvian fissure. A disturbance of the normal pattern of sylvian fissure asymmetry is observed in schizophrenia and also appears to be related to genetic risk level. Localization of this abnormality to the posterior one-third of the sylvian fissure suggests that adjacent temporal regions related to language function could be affected by a gene or genes predisposing to schizophrenia.
Acknowledgments
Supported by the Medical Research Council of Canada (W.G.H., A.S.B.), the Schizophrenia Small Grants Program of Columbia University (W.G.H., A.S.B.) and the Theodore and Vada Stanley Foundation Scholars Program (C.C., W.G.H.). Many thanks to Dr D. Toms, J. McAduff, RN, S. Dalton, RT and D. Maclsaac, RT.
References
- 1.Gottesman . Schizophrenia Genesis: The Origins of Madness. New York: Freeman; 1991. [Google Scholar]
- 2.Crow TJ, Ball J, Bloom SR, et al. Arch Gen Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 3.Crow TJ. Schizophrenia Bull. 1990;16:433–444. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind N, Levitsky W. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 5.Honer WG, Bassett AS, Smith GN, et al. Biol Psychiatry. 1994;36:737–743. doi: 10.1016/0006-3223(94)90084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasen NC, Flashman L, Flaum M, et al. J Am Med Assoc. 1994;272:1763–1769. [PubMed] [Google Scholar]
- 7.Bassett AS, Honer WG. Am J Hum Genet. 1994;54:864–870. [PMC free article] [PubMed] [Google Scholar]
- 8.Rasband W. NIH Image. Bethesda, MD: NIMH; 1992. [Google Scholar]
- 9.Matsui T, Hirano A. An Atlas of the Brain for Computerized Tomography. Tokyo: Igaku-Shoin; 1978. [Google Scholar]
- 10.Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. New York: Thieme; 1990. [Google Scholar]
- 11.Habib M, Renucci RL, Vanier M, et al. J Comput Assist Tomogr. 1984;8:922–927. doi: 10.1097/00004728-198410000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Galaburda AM, Corsiglia J, Rosen GD, et al. Neuropsychologia. 1987;25:853–868. [Google Scholar]
- 13.Harvey I, Persaud R, Ron MA, et al. Psychol Med. 1994;24:689–699. doi: 10.1017/s0033291700027847. [DOI] [PubMed] [Google Scholar]
- 14.DeCarli C, Murphy DGM, Gillette JA, et al. Am J Neuroradiol. 1994;15:689–696. [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon TD, Mednick SA, Parnas J, et al. Arch Gen Psychiatry. 1994;51:955–962. doi: 10.1001/archpsyc.1994.03950120027006. [DOI] [PubMed] [Google Scholar]
- 16.Reveley A, Reveley M, Clifford C, et al. Lancet. 1982;ii:540–541. doi: 10.1016/s0140-6736(82)92047-5. [DOI] [PubMed] [Google Scholar]
- 17.Suddath R, Christison G, Torrey E, et al. New Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 18.Bogerts B, Wurthmann C, Piroth HD. Nervenarzt. 1987;58:97–106. [PubMed] [Google Scholar]
- 19.Bartley AJ, Jones DW, Torrey EF, et al. Biol Psychiatry. 1993;34:853–863. doi: 10.1016/0006-3223(93)90053-g. [DOI] [PubMed] [Google Scholar]
- 20.Falkai P, Bogerts B, Schneider T, et al. Schizophrenia Res. 1995;14:161–176. doi: 10.1016/0920-9964(94)00035-7. [DOI] [PubMed] [Google Scholar]
- 21.Petty RG, Barta PE, Pearlson GD, et al. Am J Psychiatry. 1995;152:715–721. doi: 10.1176/ajp.152.5.715. [DOI] [PubMed] [Google Scholar]
- 22.Witelson SF, Kigar DL. J Comp Neurol. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- 23.Falkai P, Bogerts B, Greve B, et al. Schizophrenia Res. 1992;7:23–32. doi: 10.1016/0920-9964(92)90070-l. [DOI] [PubMed] [Google Scholar]
- 24.Jakob H, Beckmann H. J R Soc Med. 1989;82:466–469. doi: 10.1177/014107688908200808. [DOI] [PMC free article] [PubMed] [Google Scholar]