Abstract
Background
Darier’s disease (keratosis follicularis) is known to have a genetic cause as evidenced by its autosomal dominant transmission in families. The gene causing this disease has not been discovered.
Objective
During an ongoing linkage study of schizophrenia, a family segregating Darier’s disease was found. This family is being studied in an attempt to locate prospective regions that may contain the Darier’s disease gene.
Methods
Two genetic strategies are being employed: (1) testing candidate genes for the disorder and (2) scanning the entire genome with polymerase chain reaction–based microsatellite markers.
Results
Thirty-nine marker systems located on chromosomes 1, 2, 4, 5, 6, 9, 11, 12, 16, 17, 22, X, and Y have been genotyped. Slightly positive lod scores were achieved between six markers and Darier’s disease. The remaining 33 markers were nonsegregating or indeterminate, or revealed an obligate recombinant.
Conclusion
Linkage analysis can lead to localization of the gene causing Darier’s disease. In these preliminary studies low positive lod scores were obtained, potentially pointing to the chromosomal location of the Darier’s disease gene.
Darier’s disease (Darier-White disease, keratosis follicularis) is characterized by warty papules and primarily affects the face, scalp, trunk, and groin.1 Involvement of the nails, palms, soles, and mucous membranes is also common.2, 3 The disease begins in late childhood or early adolescence. Affected persons are at risk of cutaneous viral and bacterial superinfection.4 Darier’s disease is inherited and has no racial or sexual predilection. Data from several multigenerational pedigrees indicate autosomal dominant transmission of the disease with complete penetrance.5–7 Expressivity of the disease is variable, ranging from mild or subtle changes such as palmar pits to severe involvement with extensive vegetative plaques3 (for review, see Berg and Bassett8), Although there are clues that Darier’s disease may be a disorder of keratinocyte differentiation9 or abnormal protease regulation,10 the cause of the cell dyshesion in this disease remains unknown. Treatment remains unsatisfactory although aromatic retinoids, (isotretinoin and etretinate) have benefited some patients.11
We have identified a large family segregating Darier’s disease and have begun a scan of all chromosomes with DNA markers in an effort to locate the causative gene. This report summarizes our strategy and the loci tested thus far.
METHODS
The family (Fig. 1) segregating Darier’s disease in this study was identified in an eastern province of Canada. Diagnosis was established by clinical examination and available medical records; at least one family member underwent skin biopsy. All subjects were examined by the same dermatologist (D. B), and diagnosis was made only in the presence of well-recognized physical findings of Darier’s disease.3 Seventeen family members were examined, lymphoblastoid cell lines were established,12 and DNA was extracted by high salt method. Nine members were affected, seven were unaffected, and one with nonspecific findings had an uncertain diagnosis.
Fig. 1.
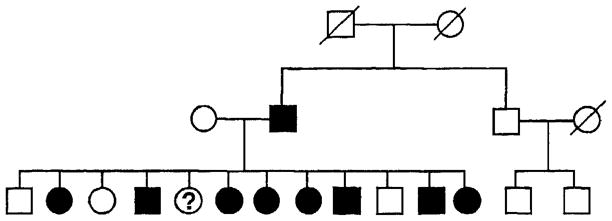
Eastern Canadian family with Darier’s disease. Black circles and squares represent female and male family members affected with definite or probable Darier’s disease. Question mark denotes person with possible Darier’s disease (see text) and blank circles and squares denote unaffected family members. Slash lines mark deceased persons. Represented gender of some subjects has been deliberately altered in this diagram, to protect the identity of the family.
Southern blotting was performed as previously described.13 The polymerase chain reaction (PCR) was employed to obtain genotypes for the following loci: tyrosine hydroxylase (HUMTH01 [AATG]6–12 primers14), DRD4,15 D22S156,16 CRYB2,17 IGF1,18 D6S87,19 D8S88,20 and D2S72.21 All PCR products except those from DRD4 were coupled to a radioactive detection system. Radioactive detection of PCR products was achieved by including 1 μCi [α-32P]-deoxycytidine 5′-triphosphate (dCTP) and the desired primer set in a standard PCR medium consisting of 50 ng genomic DNA template per 10 μl total volume, PCR buffer (Perkin-Elmer Cetus, Norwalk, Conn.), 100 μmol/L of each primer, 1 unit Taq polymerase, and 200 μmol/L of each nucleotide except dCTP, which was 50 μmol/L. Amplification was performed with an initial denaturing at 95° C for 3 minutes, then 25 cycles as follows: denaturing at 94° C for 45 seconds, annealing at 55° C for 45 seconds, and extension at 72° C for 45seconds (Perkin Elmer 9600 thermocycler). The products were subjected to electrophoresis through a 6.0% polyacrylamide/8.2 mol/L urea gel at 1800V, 27 mÅ, and 47 W for 2½ to 3 hours. Size standards were prepared by amplifying specific alleles from persons of known genotype. Gels were wrapped and exposed to x-ray film overnight. The standard medium for the nonradioactive detection of the DRD4 alleles is described in detail by Lichter et al.15 Autoradiographs or photographs of the gels were interpreted separately by at least two of us without knowledge of the diagnosis.
The genetic linkage model for Darier’s disease was defined as autosomal dominant, penetrance of homozygotes was set equal to heterozygotes at 0.95, and gene frequency was tested for prevalences in the range of one per 55,00022 to one per 100,000.6 Phenocopies (false positives) for the diagnosis were estimated at one per 1000.
RESULTS
The assessment of the family with informative markers revealed no genotype discrepancies that would suggest nonpaternity or mistyping. The average readability of the autoradiographs and photographs was 95% of DNA samples tested. To date, we have studied 17 family members with 39 marker systems for 24 loci and found six markers to be slightly positive in pairwise linkage analyses with Darier’s disease. The following markers were indeterminant: D5S39, HIOMT, DRD1, DRD3, DRD4, PBGD, 5HT1Dβ, D5S76, DXYS20, DXYS28, TH, D16S85, D22S156, and IGF1. These markers did not provide lod scores of sufficient magnitude to generate significant results. The following markers revealed an obligate recombinant that ruled out linkage: D5S39, DRD4, HRAS, D4S139, D2S72, D8S88, CRYB2, and HUMTHO1 [AATG]6–12.
The locus MIC2 had a slightly positive lod score (0.60), and this marker is located near several genes for another cutaneous disorder, keratosis follicularis spinulosa decalvans. This disorder maps to DXS41 (Xp22.1) and DXS28 (Xp21.3) in an extended Dutch pedigree.23, 24 The marker in our series with the most positive lod score (1.2) was D6S87, provisionally assigned to chromosome 6q.
DISCUSSION
During an ongoing linkage study of schizophrenia in large families from eastern Canada, one was found to have multiple members with Darier’s disease. Although some cosegregation occurs, Darier’s disease and schizophrenia are not always found together in this family. There is no known history of Darier’s disease before the parental generation, which suggests that the mutation is recent.
Markers that generated slightly positive lod scores in our study (D6S87, MIC2, D2S44, D17S444, and D1S7) may lead the way to the gene responsible for Darier’s disease. Currently we are investigating the low positive lod scores further by typing these markers in additional family members and in five other Canadian families segregating Darier’s disease (n = 55). Under the assumption of a linked marker, simulation analyses of the six families ascertained but not genotyped demonstrate a maximum lod score of 5 and an average lod score of 2, indicating that further analysis of these families may yield conclusive evidence of linkage to an informative marker.
There is little in the literature regarding the relation between Darier’s disease and psychiatric illness. One study suggests that suicidal ideation is a particular problem in patients with Darier’s disease.25 Emotional factors acting as nonspecific stressors are also known to precipitate or aggravate many skin diseases, such as psoriasis.26 Of interest is a study by Getzler and Flint5 of a family in which Darier’s disease and neuropsychiatric conditions overlapped in all but one case of psychosis. In that family three members had Darier’s disease and a psychiatric illness. Craddock et al. (personal communication) identified a family with multiple members affected with Darier’s disease and major depression. Also, Hailey-Hailey disease has been reported in a German family segregating psychiatric illness (P. Propping, personal communication). One possible explanation, other than spurious association, is that a gene mutation causing Darier’s disease may be an allelic variant of, or located near, a gene predisposing to psychiatric illness.
ADDENDUM
Since submission of this article, linkage of Darier’s disease to chromosome 12q has been established. Refinement of diagnostic criteria after submission of this report has resulted in the reassignment of one “probably affected” and two “probably unaffected” subjects from the family in this study to “uncertain” diagnostic status. The corrected totals are as follows: eight affected, five unaffected, and four uncertain. These diagnostic changes do not affect the overall results or conclusions of this study.
Table I.
Preliminary pairwise lod scores between Darier’s disease and markers in an eastern Canadian family
| Locus | Marker | Enzyme | Lod scores
|
||||
|---|---|---|---|---|---|---|---|
| 0.0 (θ) | 0.1 (θ) | 0.2 (θ) | 0.3 (θ) | 0.4 (θ) | |||
| D1S7 | MS1 | HaeIII | 0.30 | 0.21 | 0.13 | 0.06 | 0.01 |
| D17S444 | WH4 | MspI | 0.30 | 0.21 | 0.13 | 0.06 | 0.01 |
| D17S444 | WH4 | BgIII | 0.30 | 0.21 | 0.13 | 0.06 | 0.01 |
| D2S44 | YNH24 | HaeIII | 0.30 | 0.21 | 0.13 | 0.06 | 0.01 |
| MIC2 | pSG1 | MspI | 0.60 | 0.46 | 0.31 | 0.17 | 0.04 |
| D6S87 | MFD47 | — | 1.20 | 0.97 | 0.71 | 0.43 | 0.14 |
θ, Recombination fraction.
Acknowledgments
Supported by the National Alliance for Research in Schizophrenia and Depression, the Canadian Psychiatric Research Foundation, and the Ontario Mental Health Foundation, and Physicians’ Services Incorporated Foundation.
References
- 1.Baden HP. Darier-White disease (keratosis follicularis) and miscellaneous hyperkeratotic disorders. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in general medicine. 3. New York: McGraw-Hill; 1987. pp. 520–5. [Google Scholar]
- 2.Zaias N, Ackerman AB. The nail in Darier-White disease. Arch Dermatol. 1973;107:193–9. [PubMed] [Google Scholar]
- 3.Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40–50. doi: 10.1016/0190-9622(92)70154-8. [DOI] [PubMed] [Google Scholar]
- 4.Rand R, Baden HP. Commentary: Darier-White disease. Arch Dermatol. 1983;119:81–3. [PubMed] [Google Scholar]
- 5.Getzler NA, Flint A. Keratosis follicu1aris: a study of one family. Arch Dermatol. 1966;93:545–9. doi: 10.1001/archderm.93.5.545. [DOI] [PubMed] [Google Scholar]
- 6.Svendsen IB, Alberechtsen B. The prevalence of dyskeratosis follicularis (Darier’s disease) in Denmark. Acta Derm Venereol (Stockh) 1959;39:256–69. [PubMed] [Google Scholar]
- 7.Beck AL, Finocchio AF, White JP. Darier’s disease: a kindred with a large number of cases. Br J Dermatol. 1977;97:335–9. doi: 10.1111/j.1365-2133.1977.tb15192.x. [DOI] [PubMed] [Google Scholar]
- 8.Berg D, Bassett AS. Darier’s disease: current understanding of pathogenesis and the future role of genetic studies. Int J Dermatol. 1993;32:397–400. doi: 10.1111/j.1365-4362.1993.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lever WF, Schaumberg-Lever G. Histopathology of the skin. Philadelphia: JB Lippincott; 1990. [Google Scholar]
- 10.Burge SM, Ryan TJ, Cederholm-Williams SA. Darier’s disease: an immunohistochemical study using antibodies to proteases. Br J Dermatol. 1989;121:613–21. doi: 10.1111/j.1365-2133.1989.tb08193.x. [DOI] [PubMed] [Google Scholar]
- 11.Burge SM. Darier’s disease and other dyskeratoses: response to retinoids. Pharmacol Ther. 1989;40:75–90. doi: 10.1016/0163-7258(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 12.Anderson MA, Gusella JF. Use of cyclosporin A in establishing Epstein-Barr transformed lymphoblastoid cell lines. In Vitro. 1984;20:857. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- 13.Sidenberg DG, Demchyshyn L, Niznik HB, et al. New polymorphism for the human serotonin ID receptor variant (5HT1Dβ) not linked to schizophrenia in five Canadian pedigrees. Hum Hered. 1993;43:315–8. doi: 10.1159/000154150. [DOI] [PubMed] [Google Scholar]
- 14.Edwards AL, Civitello A, Hammond HA, et al. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991;49:746–56. [PMC free article] [PubMed] [Google Scholar]
- 15.Lichter JB, Barr CL, Kennedy JL, et al. A hypervariable segment in the human dopamine receptor D4 (DRD4) gene. Hum Mol Gen. 1993;2:767–73. doi: 10.1093/hmg/2.6.767. [DOI] [PubMed] [Google Scholar]
- 16.Weber JL, May PE. Dinucleotide repeat polymorphism at the D22S156 locus. Nucleic Acids Res. 1990;18:4639. [PMC free article] [PubMed] [Google Scholar]
- 17.Marineau C, Rouleau GA. Dinucleotide repeat polymorphism at the human CRYB2 gene locus (22q11. 2) Nucleic Acids Res. 1992;20:1430. [PMC free article] [PubMed] [Google Scholar]
- 18.Weber J, May P. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989;44:388–96. [PMC free article] [PubMed] [Google Scholar]
- 19.Weber JL, Kwitek AE, May PE. Dinucleotide polymorphism at the D6S87 locus. Nucleic Acids Res. 1990;18:4636. [PMC free article] [PubMed] [Google Scholar]
- 20.Weber JL, Kwitek AE, May PE, et al. Dinucleotide repeat polymorphisms at the D8S85, D8S87, and D8S88 loci. Nucleic Acids Res. 1990;18:4038. doi: 10.1093/nar/18.13.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber JL, May PE. Dinucleotide repeat polymorphism at the D2S72 locus. Nucleic Acids Res. 1990;18:2200. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson JD, Marsden RA, Dawber RPR. Review of Darier’s disease in the Oxford region. Br J Dermatol. 1977;15(suppl):15–6. [Google Scholar]
- 23.Oosterwijk JC, Nelen M, Van Zanvoort PM, et al. Confirmation of x-linked inheritance and provisional mapping of the keratosis follicularis spinulosa decalvans gene on Xp in a large Dutch family. Ophthalmic Paediatr Genet. 1992a;13:27–30. doi: 10.3109/13816819209070050. [DOI] [PubMed] [Google Scholar]
- 24.Oosterwijk JC, Nelen M, Van Zanvoort PM, et al. Linkage analysis of keratosis follicularis spinulosa decalvans, and regional assignment to human chromosome Xp21.2-p22.2. Am J Hum Genet. 1992;50:801–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Denicoff KD, Lehman ZA, Rubinow DR, et al. Suicidal ideation in Darier’s disease. J Am Acad Dermatol. 1990;22:196–8. doi: 10.1016/0190-9622(90)70022-a. [DOI] [PubMed] [Google Scholar]
- 26.Whitlock FA. Psychophysiological aspects of skin disease. London: WB Saunders; 1976. [Google Scholar]


