Abstract
Experimental autoimmune encephalomyelitis (EAE) is a rodent model of multiple sclerosis that is executed in animals by immunization with myelin Ag in adjuvant. The SJL/J autoimmuneprone strain of mouse has been used to model relapsing–remitting multiple sclerosis. However, significant variations in peak scores, timing of onset, and incidence are observed among laboratories, with the postacute (relapse) phase of the disease exhibiting significant inconsistency. We characterized two substrains of SJL/J mice that exhibit profoundly different EAE disease parameters. Induction of EAE in the first SJL/J substrain resulted in many cases of chronic EAE that was dominated by an aggressive B cell response to the immunizing Ag and to endogenous CNS Ags. In contrast, the other SJL/J substrain exhibited a relapsing–remitting form of EAE concomitant with an elevated number of cytokine-producing CD4+ T cells in the CNS. Exploiting these interstrain differences, we performed a genome-wide copy number analysis on the two disparate SJL/J substrains and discovered numerous gene-dosage differences. In particular, one inflammation-associated gene, Naip1, was present at a higher copy number in the SJL/J substrain that exhibited relapsing–remitting EAE. These results demonstrate that substrain differences, perhaps at the level of genomic copy number, can account for variability in the postacute phase of EAE and may drive chronic versus relapsing disease.
Multiple sclerosis (MS) is an autoimmune disease that results in demyelination in the CNS accompanied by disability and paralysis. The etiology of the disease remains unclear, but it is likely a result of inheritance of risk allele(s) in combination with ill-defined environmental factors (1). Experimental autoimmune encephalomyelitis (EAE) is an inducible disease serving as the best-characterized animal model for human MS. EAE shares many important features with the human disease, including auto-reactive T cell activation and infiltration into the CNS, destruction of the protective myelin sheaths encasing nerve fibers, formation of perivascular inflammatory cell foci predominantly in the brain stem and spinal cord, and progression of these lesions toward demyelination (2). However, the spectrum of clinical manifestation of MS is variable, with the majority of patients presenting with relapsing–remitting MS (RRMS) and other patients presenting with primary progressive MS (PPMS) (3). Faithful recapitulation of different clinical aspects of MS in rodent models of EAE will help us to develop specific therapies for PPMS versus RRMS.
EAE can be induced in many species following immunization with myelin-derived peptides in adjuvant, with subtle or overt differences in symptoms among different animals/strains (4). In mice, EAE can be induced by components of myelin/oligodendrocyte glycoprotein (MOG) (5), and MOG35–55-induced chronic EAE is particularly useful in evaluating disease in the C57BL/6 strain of mice (6). This model requires the coinjection of pertussis toxin (PTx), a bacterially derived exotoxin that is thought to promote EAE by modulating blood brain barrier endothelial cells, thereby increasing the permeability of the blood brain barrier (7). Of note, PTx appears to have many additional consequences, including interruption of G protein-coupled receptor signaling (8), disruption of glucose regulation (9), and suppression of regulatory T cells (10, 11), all of which may have effects on EAE. In addition, the influence of some genetic loci that control EAE susceptibility is lost in the presence of PTx (12). Relapsing–remitting EAE (RR-EAE) has been studied in various strains, such as Biozzi/ABH mice immunized with MOG8–21 (5), and by immunization of SJL/J mice with proteolipid protein (PLP139–151). The SJL/J model is a widely studied RR-EAE model and has contributed critically to our understanding of RRMS as a result of its clinical and pathological similarities (13, 14). SJL/J mice are also well suited as a rodent MS model for uncovering disease-modifying genes, because they are susceptible to EAE induction without PTx coinjection.
Significant efforts have been made toward revealing genetic modifiers of disease in MS. Candidate gene association studies and genome-wide association studies revealed that the MHC region is the most important genetic region that has the capacity to confer disease risk (15). Approximately 16 non-MHC genes have also been identified that contribute to disease susceptibility but to a much lesser extent than the MHC (16). Furthermore, MS samples (peripheral blood and CNS) have also been subjected to intensive gene-expression analysis (17). However, because of the heterogeneity among MS patients, many investigators have taken advantage of animal models of MS to better assess genetic contributions to disease. These include studies using F1 crosses between susceptible and nonsusceptible rat strains (18) and similar approaches in mice (19, 20). From these studies, it is clear that susceptibility to EAE is under polygenetic control. More recently, efforts have been focused on identifying genetic modifiers of disease severity rather than disease susceptibility in EAE (21–23) and MS (24, 25). These approaches have great usefulness for understanding and developing therapeutics that are designed for RRMS versus PPMS.
Despite the well-characterized models of EAE and their widespread use, there remains considerable variability in terms of disease incidence and severity, and this has been noted in SJL/JEAE (26–28). To explain this variability, environmental contributions to disease have been documented, although not well understood. Age, sex, season, postnatal handling, diet and commensal composition are among the many environmental factors thought to influence rodent autoimmune diseases such as EAE (27, 28). To address these inconsistencies, we assessed several variables that may contribute to EAE incidence and severity, including the immunization dose of Mycobacterium tuberculosis adjuvant (myco) or the dose of PLP139–151. We also assessed whether there were differences in disease incidence or severity when we simultaneously immunized age-matched female SJL/J mice from four commercial vendors. To our surprise, the source of SJL/J (vendor) represented the biggest source of variability in disease severity. Taking advantage of the large degree of genetic similarity between SJL/J substrains, we performed a genome-wide analysis of gene copy number of the two in-bred substrains that displayed the most disparate clinical manifestation of EAE. We identified several intersubstrain gene copy number variations (CNVs), including consistently lower Naip1 gene copies in a substrain of mice that exhibited a chronic, rather than relapsing, EAE phenotype. Our data provide a new approach for isolating disease-modifying genes that may be useful for ultimately understanding the differences between different clinical forms of MS.
Materials and Methods
Mice
Wild-type SJL/J mice were obtained from Harlan Breeders (Indianapolis, IN) (SJL/JCrHsd), Taconic Farms (Germantown, NY) (SJL/JCrNTac), Charles River Laboratories (Wilmington, MA) (SJL/JOrlCrl), or The Jackson Laboratory (Bar Harbor, ME) (SJL/J). To simplify nomenclature, we abbreviated these strains as follows: SJL/JCrHsd = HAR, SJL/JCrNTac = TAC, SJL/JOrlCrl = CR, and SJL/J = JAX. All animals were housed in specific pathogen-free conditions. In all cases, 6-wk-old age-matched female mice were used for comparative studies. All experiments were performed according to the University of Toronto approved animal-use protocols.
Induction of disease
Unless indicated otherwise, mice were immunized with an emulsion containing 150 μl IFA (Difco, Detroit, MI, lot #8274870), supplemented with 100 μg PLP139–151 (HSLGKWLGHPDKF) and 200 μg Mycobacterium (Difco, lot #7199048; ground by mortar and pestle in PBS) in a total volume of 300 μl, with 100 μl injected s.c. in three spots on the back. No PTx was used.
Clinical evaluation
Mice were weighed and scored daily, as previously described (29). Scores were assigned as follows: 0 = asymptomatic, 1 = loss of tail tone, 2 = hind limb weakness, 3 = partial hind limb paralysis, 4 = complete hind limb paralysis, 5 = complete hind limb paralysis and partial forelimb paralysis, and 6 = moribund. Acute-phase disease was defined as a score ≥1, for at least two consecutive days. Remission was defined as a decrease in a peak acute-phase score ≥2.0 points, persisting for at least two consecutive days. Relapse was defined as a score ≥1.5 grades higher than the average remission-phase score for at least two consecutive days.
ELISA analysis
Serum from SJL/J mice was collected during the postacutephase (days 29–36). Serum was subjected to sandwich ELISA for PLP. Specifically, Nunc Maxi-Sorp plates (Roskilde, Denmark) were coated with 1 μg/well PLP139–151. Plates were blocked in PBS + 1% BSA and then washed. Serum was added, starting at 1/100 in blocking buffer, and incubated for 1 h at 37°C. Plates were washed three times, and HRP-labeled anti-IgM, anti-IgG1, or anti-IgG2a Abs were added. Plates were developed using ABTS substrate, and OD was measured at 405 nm. For MOG ELISA, we used the MOG1–125 kit from Anaspec (San Jose, CA), according to the manufacturer’s directions, using an anti-MOG standard for quantification.
Histopathology evaluation of CNS-resident infiltrates
Immune-mediated inflammation was analyzed at days 21 and 29 post-immunization, time points corresponding to the beginning and the peak of the postacute phase, respectively. Individual disease profiles for mice analyzed are shown in Supplemental Fig. 3. Briefly, SJL/J mice (HAR versus CR) were perfused with ice-cold PBS. Brain and spinal cord intended for histology were subsequently fixed in 10% formalin solution, embedded in paraffin, and stained with Luxol fast blue and H&E. Pathological scoring was performed on images acquired at ×100 magnification using the following scale: 0 = no infiltrates compared with unimmunized mice; 0.5 = any infiltration that is small in size, forming a perivascular cuff; 1 = several (> 3) small infiltrates or one large infiltrate; 1.5 = a few larger infiltrates accompanied by smaller infiltrates; and 2 = pervasive evidence of leukocyte infiltration. Demyelination was judged by the absence of Luxol fast blue staining compared with unimmunized control using the following scale: 0 = no demyelination, 0.5 = confined area of demyelination underlying small perivascular infiltrates, 1 = confined demyelinated area underlying several (> 3) small infiltrates or one large infiltrate, 1.5 = demyelination underlying several small and large infiltrates with diffuse spreading beyond area of infiltration, and 2 = several (>2) large areas of demyelination with diffuse spreading beyond the area of infiltration or in the absence of active infiltration. Scores for each mouse were averaged for the one cervical and two lumbar sections. For these quantifications, four independent blinded measurements were compared and averaged. B cell aggregates in the cerebellum/brain stem and in close proximity to the ventricles of the brain were evaluated by staining several serial frozen brain sections with B220-PE and CD4-FITC. The area occupied by B220-PE stain was calculated using Velocity software.
Flow cytometric evaluation of CNS-resident infiltrates
Mice were sacrificed during the peak of the acute or postacute phase of disease (Supplemental Table I). Brain and spinal cord were homogenized in collagenase D buffer (10mM HEPES, 150mM NaCl, 1mM MgCl2, 5m MKCl, and 1.8 mM CaCl2 in HBSS), and tissue was digested following the addition of 1 mg/ml collagenase D and 60 μg/ml DNaseI and incubation for 45 min at 37°C. Lymphocytes were purified using a 30% Percoll solution and re-suspended in 10% media (10% FBS, L-glutamine, sodium pyruvate, penicillin G, streptomycin sulfate, and β-mercaptoethanol). Cells for intracellular cytokine analysis were restimulated with PMA and ionomycin in the presence of GolgiPlug and incubated at 37°C for 5 h. Cells were permeabilized and fixed using the BD Cytofix/Cytoperm kit and incubated with 1 μg/ml Fc-block in PBS + 2% FBS. Cells were then stained with CD4-PE-Cy5, TNF-α–FITC, IL-17–PE, or IFN-γ–APC. Purified CNS cells intended for extracellular cytokine staining were stained with CD8-FITC, CD11c-PE, CD80-PECy5, CD86-APC, CD11b-PE-Cy7, CD19-Biotin, CD45.1-Pacific Blue, or CD4-PE–Texas Red. Flow cytometry analysis was performed using the FACS-Canto, and fluorescence-minus-one controls were used to judge background stain (Supplemental Fig. 4).
Silver staining for axonal damage quantification
Silver stain was applied to paraffin-embedded spinal cord sections to quantify the degree of axonal loss. Slides were deparaffinized in xylene and hydrated in distilled water. Slides were then placed in 20% silver nitrate for 20 min in the dark, washed in water, and evaporated ammonia was added, drop by drop, and stirred until the precipitate cleared. Slides were kept in this solution for 20 min in the dark and then immersed in ammonia water (0.2% ammonium hydroxide). Slides were developed in an ammonia silver solution until nerve fibers appeared black. Slides were washed in water, placed in a sodium thiosulfate solution, washed again, dehydrated, and mounted. Axonal loss was quantified in a blinded manner by examining images at ×100 and using the following scale: 0 = normal/even silver stain throughout the white matter compared with unimmunized mice; 1 = small spurious areas in the white matter that lack silver stain; 1.5 = small, but frequent, areas in the white matter that lack silver stain; and 2 = extensive loss of silver stain throughout the white matter. For visualization of axonal swelling, the same region of the cervical section of the spinal cord white matter was imaged for each sample under high-power magnification (×400). For all quantifications, four independent blinded measurements were compared and averaged.
CNV analysis
Tail tissue was collected from CR and HAR female mice. DNA was isolated using standard procedures, and DNA copy analysis was performed according to the instructions for Oligonucleotide Array-Based CGH for Genomic DNA Analysis, Version 4.0. (Agilent, Santa Carla, CA). Briefly, 2 μg genomic DNA from tail clips was digested with AluI and RsaI and labeled using BioPrime Total for Agilent aCGH system (Invitrogen, Carlsbad, CA). Test (CR SJL/J mice) and reference DNA (HAR SJL/J mice) samples were labeled with cyanine 5- or cyanine 3-deoxyuridine triphosphate, according to the manufacturer’s recommendations. Cyanine 5- and cyanine 3-labeled DNA sample pairs were combined and mixed with mouse Cot-1 DNA, Agilent 10× Blocking Agent, and Agilent 2× Hybridization Buffer. Labeled target solution was hybridized to Agilent’s 244K Mouse Genome CGH Microarray (G4415A). After hybridization, microarrays were washed and dried according to the procedures described in Agilent’s protocol. Microarray slides were scanned using an Agilent microarray scanner. Data for individual features on microarray were extracted from the scan image, using Agilent’s Feature Extraction Software. Output files were imported into Agilent’s DNA Analytics 4.0 software (version 4.0.73) for DNA copy number analysis. The aberration detection method (ADM) was used to identify CNVs. The ADM algorithm identifies all aberrant intervals in a given sample with consistently high or low log ratios based on the Z statistical score implemented in the software. The algorithm searches for intervals in which a statistical score based on the average quality weighted log ratio of the sample and reference channels exceeds a user-specified threshold (ADM-1 uses a threshold of 6.0, and ADM-2 uses a threshold of 5.0). ADM-2 differs from ADM-1 by using probe-quality information to weight the log ratios before calculating the score for the interval. Array CGH and CNV analysis was performed by The Centre for Applied Genomics, The Hospital for Sick Children, Toronto, ON, Canada.
Validation of CNVs
Genes within the five regions identified by ADM-2, plus two potentially functionally relevant genes identified with ADM-1 (Il7 and Ccr2) were chosen for validation by real-time quantitative PCR (qPCR). qPCR CNV validation was performed on purified genomic DNA from 15 CR mice and 14 HAR mice using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on the AB Prism 7900HT sequence detection system (Applied Biosystems), as described previously (30). Genomic ratios were determined by comparing the absolute copy number of each gene of interest with that of the reference gene Sdha. Primers for test and control genes are described in Supplemental Table I.
Expression analysis
Total RNA was extracted from an appropriate tissue using TriReagent (Sigma-Aldrich Canada, Oakville, ON, Canada), following the manufacturer’s instructions. Following DNase treatment (Turbo DNA-free, Ambion, Austin, TX), 5 μg RNA was converted to cDNA using the SuperScript First-Strand Synthesis System (Invitrogen) and random hexamer primers. Samples were diluted 1/100 with sterile water and used directly in real-time assays using the Power SYBR Green PCR Master Mix and ABI Prism 7900HT sequence-detection system, as described previously (31). Each test gene was normalized to Hprt, and the results are presented as an expression ratio of the test gene to Hprt. Primers for test and control genes are described in Supplemental Table I. For each gene of interest, expression analysis was performed on five mice from each group (CR and HAR). The same 15 CR mice and 14 HAR mice used for the CNV validation were used for Naip1 expression analysis. Pair-wise comparison of CR Naip1 expression and HAR Naip1 expression was performed using a two-tailed Student t test in PAST (Paleontological STatistics) (http://folk.uio.no/ohammer/past/).
Preparation of cell lines, CNS cell cultures, and sorted CNS-resident leukocytes for Naip1 expression analysis
N2A cells (ATCC #CCL-131) were grown in high-glucose DMEM (Life Technologies, Carslbad, CA) with 10% FBS (Hyclone, Waltham, MA) and penicillin-streptomycin (Life Technologies) and cultured in a humidified incubator at 37°C with 5% CO2. N2A cells were differentiated to a neuronal phenotype by transferring to 2% FBS media and by adding retinoic acid (20 μM; Sigma-Aldrich Canada). Cells were harvested 72 h after treatment. Murine neurons were isolated from day-15 SJL/J mouse embryos. Briefly, the cerebral hemispheres were dissected out, and the meninges were removed. The cortices were minced and gently homogenized by stirring for 15 min at 37°C in supplemented (0.035% sodium bicarbonate, 1 mM sodium pyruvate, and 10 mM HEPES) HBSS, without calcium and magnesium, and containing 0.025% trypsin (Invitrogen). A single-cell suspension was then generated by crushing the tissue through a 130-μm nylon mesh using supplemented (0.035% sodium bicarbonate, 1 mM sodium pyruvate, and 10 mM HEPES) HBSS with calcium and magnesium. A total of 3 × 106 cells were plated on poly-ornithine (10 μg/ml; Sigma)-coated six-well plates in growth media (NEUROBASAL media, 2% B27 supplement, 2 mM L-glutamine, and 1% penicillin-streptomycin) and incubated at 37°C in 5% CO2. At day 2 following isolation, cells were treated with or without 0.5 μM staurosporine (Sigma) for 24 h and then harvested in 1 ml TRIzol reagent. Oligodendrocyte precursor cells were isolated by sequential immunopanning (32) from postnatal day-8 SJL/J pups. The brains, excluding the optic bulbs, were dissected out, diced, and dissociated in papain (Worthington, Lakewood, NJ) at 37°C for 90 min. A single-cell suspension was sequentially immunopanned on Thy1.2 (30 min), antigalactocerebrosidase (45 min), and O4 (45 min)-Ab coated plates to select GalC-O4+ oligodendrocyte precursor cells. Cells were removed from the O4 plate with 0.125% trypsin (Invitrogen), plated on poly-D-lysine (Sigma)-coated 6-cm petri dishes, grown in proliferation media (DMEM, BSA, putrescine, progesterone, sodium selenite, glutamine, N-acetyl-cysteine, sodium pyruvate, penicillin/streptavidin, trace elements B, d-biotin, insulin, B27, forskolin, ciliary neurotrophin factor, platelet-derived growth factor, and neurotrophin-3) for 4–5 d, and then switched into differentiation media (DMEM, BSA, putrescine, progesterone, sodium selenite, glutamine, N-acetyl-cysteine, sodium pyruvate, penicillin/streptavidin, trace elements B, d-biotin, insulin, B27, forskolin, ciliary neurotrophin factor, and triiodothyronine) for 2 d at 37°C and 10% CO2. In all cases, cells were harvested using 1 ml TRIzol reagent.
Statistical analysis
All diseased mice (score ≥1 for at least 2 consecutive days) were included in the statistical analyses of scores/weight loss. The statistical significance of clinical differences was first determined by Kruskal–Wallis statistical analysis for non-Gaussian distributions; a post hoc analysis was subsequently applied. The unpaired two-tailed Student t test was applied for immunopathological analyses. Differences were considered statistically significant when p < 0.05. For ELISA and FACS analysis, the Mann–Whitney U test and statistical significance was noted for p < 0.05.
Results
Clinical manifestation of acute EAE in SJL/J mice differs significantly between SJL/J substrains but is largely independent of immunization conditions
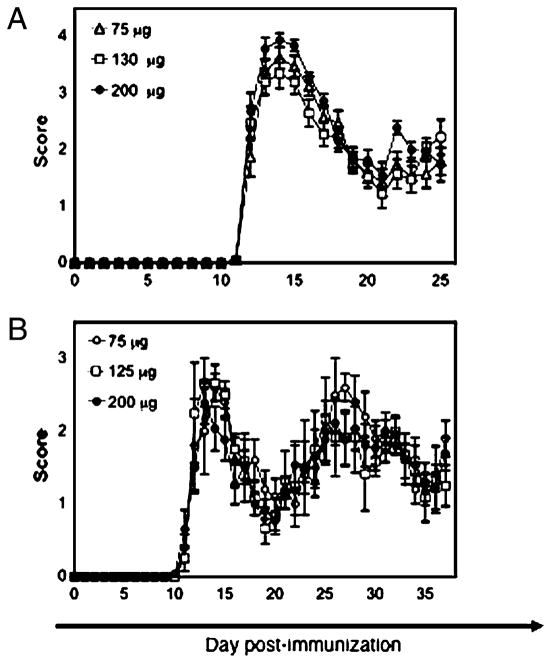
Several variables have been shown to affect the clinical manifestation of EAE in SJL/J mice (26–28). Two components are used to induce EAE in SJL/J mice: PLP139–151 and myco emulsified in oil. The consequence of different doses of immunogen/adjuvant was reported by titrating the amount of spinal cord homogenate and myco (13). The investigators reported considerable variation in disease incidence and severity in repeat experiments using the same titrated doses; they ultimately concluded that no dose combination predictably induced mild or severe disease. No purified PLP peptide dose titration has been reported. Therefore, we sought to determine whether levels of myco or PLP139–151 affected EAE in SJL/J mice. Accordingly, mice were immunized with an IFA-PBS emulsion containing 75, 130, or 200 μg myco. In addition, different doses of PLP139–151 (75, 125, or 200 μg) were tested. Mice immunized with any of the doses of myco had equivalent disease incidence, cumulative score, day of onset, peak acute-phase score, and average acute-phase score(Fig. 1A, Table I). Furthermore, no difference was found in mice immunized with increasing doses of PLP139–151 (Fig. 1B, Table I). Similar weight loss was also observed in all cases (Supplemental Fig. 1). The same results were observed when different doses of myco were tested against different doses of PLP139–151, suggesting that no combinatorial effect of the two immunization agents affected clinical the scores for EAE (data not shown). Therefore, the dose of myco and PLP139–151 does not represent a major source of variance in the clinical manifestation of EAE in SJL/J mice.
FIGURE 1.
Dose of myco or PLP peptide has little effect on EAE. A, Three doses of myco were used to induce EAE; no difference in clinical scores was observed. Mean clinical scores ± SE of 20 mice/myco dose are shown. Data are representative of two similar experiments. B, Differing doses of PLP peptide were used to induce EAE; no obvious change in clinical scores emerged. At least 8–15 mice were immunized for each PLP dose. Mean clinical scores ± SE are shown. The experiment was performed two times with similar results, and a representative experiment is shown.
Table I.
Impact of differing amounts of M. tuberculosis and PLP139–151 on EAE
| Dose | Incidence of Disease (%) | Cumulative Score | Day of Onset | Mean Acute-Phase Score | Peak Acute-Phase Score |
|---|---|---|---|---|---|
| 75 μg mycoa | 100 | 32.0 ± 2.3 | 12.3 ± 0.1 | 2.9 ± 0.2 | 3.8 ± 0.2 |
| 130 μg myco | 100 | 31.4 ± 1.9 | 12.3 ± 0.2 | 2.8 ± 0.2 | 3.7 ± 0.1 |
| 200 μg myco | 100 | 35.9 ± 1.7 | 12.0 ± 0.1 | 2.9 ± 0.1 | 4.2 ± 0.1 |
| Significance | NS | NS | NS | NS | |
| 75 μg PLP(139–151)b | 100 | 33.9 ± 6.1 | 13.9 ± 1.3 | 1.6 ± 0.2 | 2.7 ± 0.3 |
| 125 μg PLP(139–151) | 100 | 38.2 ± 6.1 | 12.5 ± 0.7 | 1.7 ± 0.3 | 2.8 ± 0.3 |
| 200 μg PLP(139–151) | 100 | 39.1 ± 5.0 | 12.1 ± 0.4 | 1.6 ± 0.2 | 2.5 ± 0.2 |
| Significance | NS | NS | NS | NS |
All values indicate mean ± SEM.
Myco titration covers days 9–25.
PLP titration covers days 0–38.
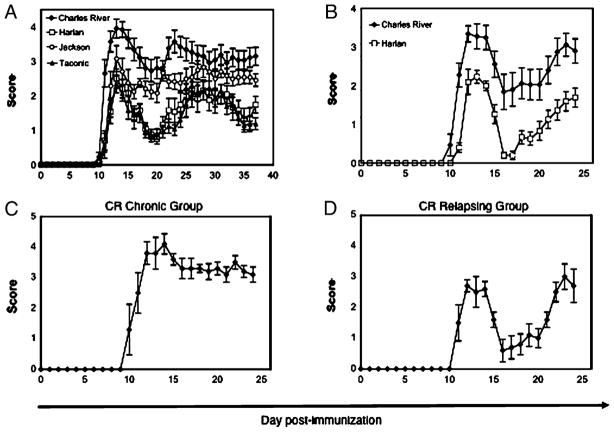
We next investigated whether potential differences in disease incidence or severity might be introduced by environmental or genetic variation between SJL/J mice from different suppliers. We simultaneously immunized female SJL/J mice from Charles River Laboratories, Harlan Breeders, The Jackson Laboratory, and Taconic Farms and compared the ensuing disease head to head. The onset of disease in SJL/J mice from all four suppliers was at approximately day 10–11, and disease incidence did not vary among strains (Fig. 2A). However, the source of SJL/J vendor contributed significantly to disease-severity parameters, including the cumulative score, the average acute-phase score, and the peak acute-phase score (p < 0.0001 for all parameters by Kruskal–Wallis test; Table II). A post hoc Dunn test revealed that CR mice exhibited the most severe disease, exhibiting significantly different clinical parameters compared with all three other strains (Table II), and this correlated with the most pronounced weight loss (Supplemental Fig. 1). HAR and TAC mice exhibited similar disease severity. JAX mice displayed an intermediate EAE clinical phenotype, as well as significant intra-cohort variability, with elevated cumulative disease scores compared with HAR and TAC mice; however, these differences were not statistically significant. Nevertheless, JAX mice remained significantly less sick than CR mice for all clinical parameters, with the exception of cumulative disease score (Table II). However, the difference in cumulative disease score between JAX and CR mice reached statistical significance when historical CR and JAX data were added to the cohort, thereby increasing the n (data not shown). Therefore, the statistical difference in cumulative disease between CR and JAX mice was likely influenced by the high degree of variability within the JAX cohort. Taken together, with respect to EAE, the SJL/J substrain is a significant source of variance, with the CR mice proving to be the outlier strain.
FIGURE 2.
SJL vendor has a significant effect on acute and postacute disease course. A, Clinical mean EAE scores in a head-to-head comparison of acute phase of EAE in SJL/J mice from four vendors. Mean clinical scores ± SE for 13–15 mice per substrain are shown. The experiment was performed two times with similar results, and a representative experiment is shown. B, Disease course of the two most disparate substrains: CR and HAR. Mean clinical scores ± SE of 19–20 mice per substrain are shown. The experiment was performed three times with similar results, and a representative experiment is shown. Distinct postacute disease course is observed when CR mice in B are subdivided into chronic (C) and relapsing (D) groups.
Table II.
Head-to-head comparison of EAE parameters between two SJL/J substrains
| Substrain | Incidence (%)a | Cumulative Scoreb | Day of Onset | Mean Acute-Phase Score | Peak Acute-Phase Score |
|---|---|---|---|---|---|
| CR | 100 (13/13) | 86.6 ± 6.9 | 10.9 ± 0.2 | 3.3 ± 0.2 | 4.3 ± 0.2 |
| HAR | 100 (15/15) | 39.1 ± 5.0 | 12.1 ± 0.4 | 1.6 ± 0.2 | 2.5 ± 0.2 |
| JAX | 100 (14/14) | 60.6 ± 5.0 | 11.5 ± 0.4 | 2.3 ± 0.2 | 3.2 ± 0.2 |
| TAC | 100 (14/14) | 39.0 ± 2.9 | 11.7 ± 0.2 | 1.7 ± 0.1 | 2.8 ± 0.2 |
| Significance | NS | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 |
| Post hoc analysis | |||||
| CR versus HAR | NS | p < 0.001 | p < 0.05 | p < 0.001 | p < 0.001 |
| CR versus JAX | NS | NS | NS | p < 0.05 | p < 0.05 |
| CR versus TAC | NS | p < 0.001 | NS | p < 0.001 | p < 0.001 |
| HAR versus JAX | NS | NS | NS | NS | NS |
| HAR versus TAC | NS | NS | NS | NS | NS |
| JAX versus TAC | NS | NS | NS | NS | NS |
All values indicate mean ± SEM.
Incidence of acute phase.
Cumulative score days 0–37.
Because the SJL/J substrain proved to be a significant source of variance in the clinical parameters of EAE, we selected the two most disparate substrains (CR versus HAR) for subsequent head-to-head experiments, including all immunopathological readouts. Indeed, in addition to the differences in the other disease parameters, these two strainsdisplayedastatisticallysignificantdifferenceintermsofdayof disease onset, a parameter that was not changed between any other pairing of substrains. Analysis of the postacute phase for these two substrains confirmed the difference in disease severity noted with the four-strain experiment (Fig. 2B) and, importantly, revealed an interesting tendency for CR mice to develop chronic EAE (C-EAE). Specifically, the frequency of CR mice that exhibited C-EAE (absence of a remission, see Materials and Methods for clinical definitions) were 29%, 33%, and 25% in three separate experiments, whereas HAR mice always underwent a relapse and never exhibited C-EAE in any experiment. Based on clinical criteria, CR mice can be subdivided into C-EAE (Fig. 2C) and RR-EAE (Fig. 2D) subgroups.
Collectively, these results highlight significant differences between SJL/J mice from different suppliers, with a disease-severity hierarchy of CR > JAX > TAC = HAR, and identify CR mice as an outlier substrain that always demonstrates exacerbated, and sometimes chronic, disease compared with any other substrain.
Histopathological analysis of CNS tissue during the postacute phase in CR versus HAR SJL/J substrains
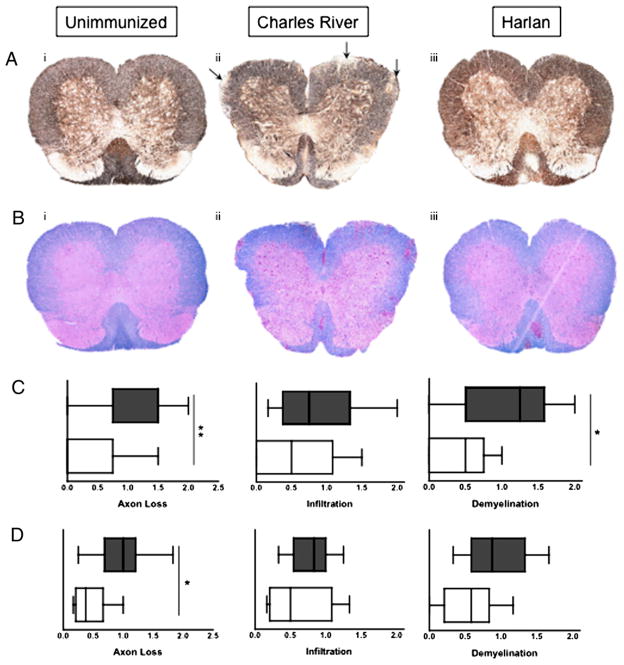
To further characterize possible differences between strains, we assessed various attributes of cellular infiltration and axonal damage during the postacute phase of EAE in the CNS of HAR versus CRSJL/J substrains: the two substrains with the most disparate clinical manifestations of disease. We first examined the extent of axonal damage in HAR versus CR spinal cord sections using silver staining. We found that, on average, CR mice exhibited greater levels of axonal loss at the beginning and at the peak of the postacute phase (days 21 and 29, respectively), as evidenced by dimmer/absent silver staining in the white matter of spinal cord sections (Fig. 3A, representative images of cervical spinal cord slices). All mice selected for quantification were experiencing a relapse or were chronically sick (see Supplemental Fig. 3 for individual disease profiles of a subset of mice). We quantified axonal loss at both time points during the postacute phase (Fig. 3C, 3D) and found this parameter to be significantly increased for CR mice. We also observed axon swelling in the CR spinal cords (Supplemental Fig. 2, representative images), suggestive of significant stress and damage to the CNS environment.
FIGURE 3.
Pathology of CNS immune infiltrates. A, Representative images of spinal cord sections stained with Bielschowsky’s Silver Stain from un-immunized (i), CR (ii), and HAR (iii) SJL/J mice obtained during the postacute phase of disease (original magnification ×100.) Arrows denote areas of axon damage/loss. B, Representative images of spinal cord sections from unimmunized (i), CR (ii), and HAR (iii) mice obtained during the postacute phase of disease (Luxol fast blue with H&E counterstain; original magnification ×100). C and D, Histological scores evaluating extent of axonal loss, infiltration, and de-myelination in the postacute phase [day 21 (C) and day 29 (D)] of EAE. Shaded boxes represent CR mice, and empty boxes represent HAR mice. Results of 8–10 representative mice are shown. *p < 0.05; **p < 0.01; Student t test.
We next examined the size of infiltrates and the extent of de-myelination in the spinal cord by H&E staining of multiple spinal cord sections. In terms of infiltrating leukocytes, we did not observe a consistent difference in the size of infiltrate in HAR versus CR substrains (Fig. 3B for representative images; Fig. 3C, 3D for composite scoring of the entire spinal cord). Areas with infiltrating leukocytes were always associated with demyelination, with the exception of examples of demyelination in the absence of infiltrates that occurred exclusively in the CR mice that exhibited chronic disease (approximately two thirds of C-EAE mice showed evidence of demyelinated plaques; see Supplemental Fig. 2 for an example). These demyelinated plaques likely account for the increased de-myelination we observed in the CR cohort, which was significantly higher at day 21 and modestly increased at day 29 (Fig. 2C). Taken together, CR mice exhibit exacerbated axonal damage/swelling and demyelination, but the size of infiltrates in the spinal cords of the two strains was equivalent.
Analysis of infiltrating T cells and dendritic cells by flow cytometry
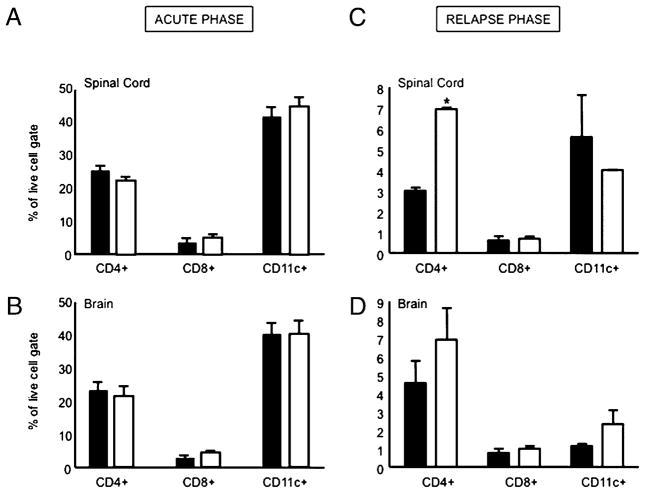
To further assess the composition of infiltrating leukocytes in the CNS of the SJL/J substrains, we homogenized CNS tissues and analyzed leukocytes by flow cytometry (controls and examples shown in Supplemental Fig. 4; clinical parameters of mice analyzed are provided in Supplemental Table I). We did not observe differences in the frequency of CD4+ T cells, CD8+ T cells, or CD11c+ dendritic cells (DCs) during the acute phase of disease (Fig. 4A, 4B). However, during the postacute phase, we noticed an increase in the frequency of CD4+ T cells in the spinal cord and brain of HAR mice (Fig. 4C, 4D); this was also true for the absolute numbers of these cells in both CNS compartments of CR versus HAR mice (0.75 × 105 ± 3.5 × 104 versus 4.5 × 105 ± 1.1 × 105 in the brain, 1.3 × 105 ± 8.6 × 104 versus 4.7 × 105 ± 3.0 × 105 in the spinal cord; data not shown).
FIGURE 4.
Composition of CNS immune infiltrates. Mean frequency (mean and SE) of CD4+ T cells, CD8+ T cells, and DCs (CD11c+) in the spinal cord and brain of the acute and postacute phase of CR (black bars) versus HAR (white bars) SJL/J substrains. Results are representative of six mice/group with three mice pooled for each FACS sample. *p < 0.05; Student t test.
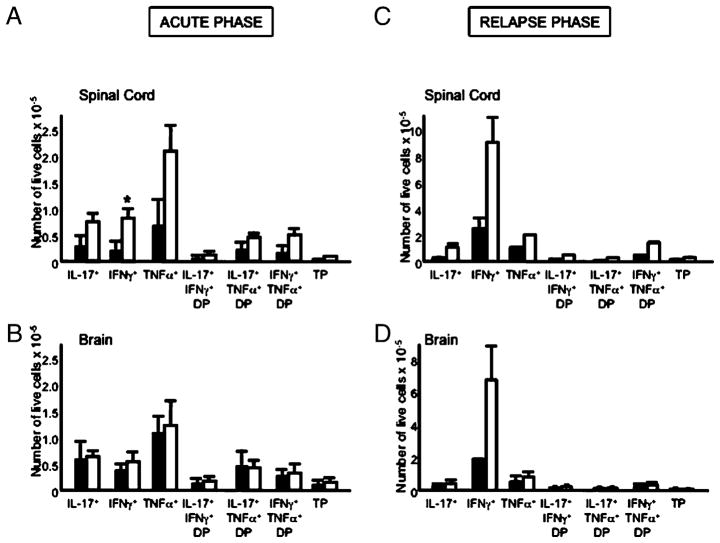
The increased number and frequency of CD4+ T cells in the HAR SJL/J mice prompted us to examine whether there were also differences in cytokine-secreting CD4+ T cells in the CNS of these two different substrains. In terms of the frequency of cytokine-secreting cells as a proportion of the CNS-infiltrating CD4+ T cells, we did not observe differences between the two SJL/J substrains in the acute or the postacute phase of disease (data not shown). During the acute phase of disease, we observed increased numbers of IL-17–, IFN-γ–, and TNF-α–secreting CD4+ T cells in the spinal cord of HAR mice; however, there were no observed differences in the brain (Fig. 5A, 5B). During the postacute phase, the differences in cytokine-secreting CD4+ T cells between the two strains in the spinal cord were largely normalized; however, there was a notable increase in IFN-γ–secreting cells in the brains of HAR SJL/J mice (Fig. 5C, 5D). Taken together, the HAR mice, which exhibit RR-EAE, seem to have a greater number of CD4+ T cell infiltrates in the CNS; this is particularly true for CD4+ IFN-γ–secreting T cells in the brain during the postacute phase.
FIGURE 5.
Enumeration of cytokine-secreting CNS-resident CD4+ T cells. Mean numbers (mean and SE) of cytokine-secreting CD4+ T cells in the spinal cord and brain of the acute and postacute phase of CR (black bars) and HAR (white bars) SJL/J substrains. Results are representative of six mice/group with three mice pooled for each FACS sample. TP, triple positive (IFN-γ+IL-17+TNF-α+). *p < 0.05; Student t test.
Analysis of B cell aggregates and PLP- and MOG-specific Abs in CR versus HAR SJL/J substrains
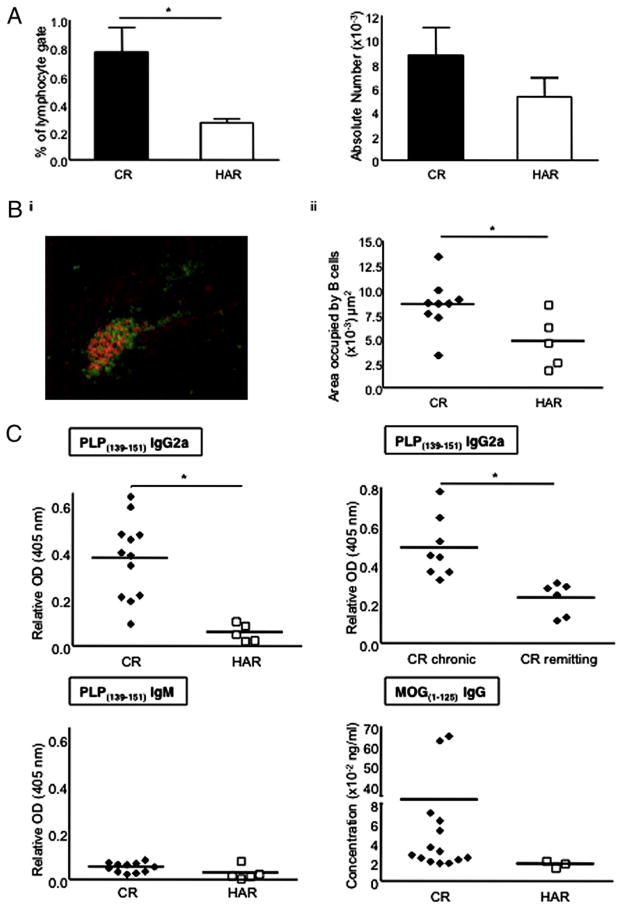
B cells and Abs play a significant role in the pathogenic process of EAE and MS (33–37). We evaluated the number of B cells in the spinal cords of postacute EAE mice and found that there was a statistically significant increase in B cell frequency in the CR mice and a corresponding increase in B cell numbers (Fig. 6A). B cell aggregates can form in the brain stem/cerebellum of EAE mice, and these aggregates are thought to be proinflammatory mediators of disease, in particular because pharmacological neutralization of these aggregates is accompanied by therapeutic efficacy (34). B cell aggregates are also found in secondary progressive MS (SPMS) and, to a lesser extent, in PPMS but not RRMS; thus, it is hypothesized that these aggregates drive chronic rather than relapsing disease (38, 39). Using fluorescence microscopy, we quantified the size of B220+ aggregates (red stain) in the brain stem/cerebellum and near the ventricles of postacute-phase EAE mice. These ectopic follicles were typically dominated by B220+ cells, with some CD4+ T cells interspersed within the follicles (Fig. 6Bi). Using morphometric software, the average area of B cell follicles in the CNS was found to be greater for the CR substrain compared with the HAR substrain (Fig. 6Bii). The fewer data points for the HAR mice is indicative of the reduced number of follicles that could be enumerated. Because these structures can develop functional germinal centers capable of driving the production of high-affinity class-switched Ab (39, 40), we evaluated the level of anti-PLP Abs in the serum of each SJL/J substrain during the postacute phase of EAE. Although titers of PLP-specific IgM Abs were found to be quite low and comparable between the two SJL/J strains, we observed a significant increase in PLP-specific IgG2a Ab for the CR mice versus HAR mice (Fig. 6C). Comparing chronic CR mice (C-EAE) with relapsing-remitting CR mice (RR-EAE), we noticed that anti-PLP IgG2a Abs were significantly greater in the C-EAE subgroup of CR mice (Fig. 6D). No significant levels of PLP-specific IgG1 were detected in either strain (data not shown). Therefore, CR mice with C-EAE exhibited the most robust PLP-specific humoral response, largely consisting of class-switched Abs, and an overall hierarchy of anti-PLP titers of CR (C-EAE) > CR > HAR was noted.
FIGURE 6.
Evaluation of CNS-resident B cells and CNS Ag-specific Ab responses during the postacute phase of EAE. A, Mean frequency and numbers (mean and SE) of B cells in the spinal cords of postacute-phase EAE mice. Results are representative of four to six mice per substrain. B, i, A representative image of a B cell follicle in the brain meninges taken from a CR SJL/J mouse during the postacute phase of EAE (red, B220 stain; green, CD4 stain; ×200 magnification). ii, Quantification of the B cell areas in CNS meningeal follicles from postacute-phase mice (day 21). C, Relative α-PLP139–151 and α-MOG1–125 Ab titers in serum of mice (CR versus HAR or CR chronic versus CR remitting) during the postacute phase of disease. Serum titers from both postacute time points (days 21 and 29) were similar. Five to 15 mice were tested in each group. *p < 0.05.
We next assessed whether CR mice would also develop titers to CNS-resident Ags other than the immunizing PLPAg. Therefore, we measured IgG titers directed toward MOG, which is expressed in the CNS. Interestingly, CR mice exhibited striking levels of anti-MOG Abs, ~100-fold higher than the HAR mice (Fig. 6C). Furthermore, the other two substrains of SJL/J mice (TAC and JAX) did not exhibit significant anti-MOG Abs, and titers were similar to those observed for HAR mice (data not shown). Specifically, 15 of 18 CR mice exhibited anti-MOG titers compared with 4 of 18 HAR mice, 5 of 16 JAX mice, and 1 of 14 TAC mice. Collectively, CR mice exhibited an aggressive humoral immune response directed at the immunizing myelin Ag as well as endogenous CNS Ags.
Gene CNVs exist between CR and HAR SJL/J substrains, resulting in altered expression levels of Naip1
Given that SJL/J mice from Harlan Breeders versus Charles River Laboratories had distinct clinical and immunological features when induced for EAE, we reasoned that there had been genetic drift between the two substrains. To quantify possible differences in gene expression, the ADM was used to identify regions that showed CNV between the CR and HAR SJL/J substrains. ADM-2, the more stringent method, reported five contiguous aberrant genomic regions that overlapped coding exons, whereas the less stringent ADM-1 reported an additional 56 regions overlapping coding exons (see Materials and Methods for further information on ADM-1 versus ADM-2). The regions were annotated according to the National Center for Biotechnology Information Build 36 of the mouse genome (UCSC mm8, February 2006). The five regions from the stringent analysis, plus two other potentially functionally relevant genes from the less stringent analysis (Il7 and Ccr2) were chosen for further analysis. These regions are shown in Table III. CNV data were deposited in the ArrayExpress database (www.ebi.ac.uk/microarray/) under accession number E-MEXP-2534.
Table III.
CNV between CR and HAR SJL/J mice detected by array CGH
| Chromosome | Gene | Coordinatesa | CGH Probes (n) | CNVb | Real-Time PCR Validation | Expression Validation |
|---|---|---|---|---|---|---|
| 5 | Fras1 | 96890471-96890871 | 3 | Decrease | No | No |
| 13 | Naip1 | 101471001-101521051 | 8 | Decrease | Yes | Yes |
| 17 | Znrf4 | 56143112-56150761 | 3 | Decrease | Yesc | Nod |
| 19 | Olfr143l | 12246162-12301154 | 9 | Increase | Yes | No |
| X | Mid1 | 165319559-165323474 | 2 | Increase | Yes | Noe |
| 3 | Il7 | 7536219-7587317 | 7 | Decrease | Yesf | No |
| 9 | Ccr2 | 123951533-123956516 | 2 | Decrease | Yesf | No |
According to National Center for Biotechnology Information Build 36 of the mouse genome (UCSC mm8, February 2006).
Seen in SJL/J mice purchased from Charles River Laboratories compared with SJL/J mice purchased from Harlan Breeders.
Znrf4 did not amplify from the genomic DNA of SJL/J mice purchased from Charles River Laboratories.
Znrf4 is only expressed during spermatogenesis.
Mid1 was not detected in adult tissues.
Copy number was variable within each supplier group.
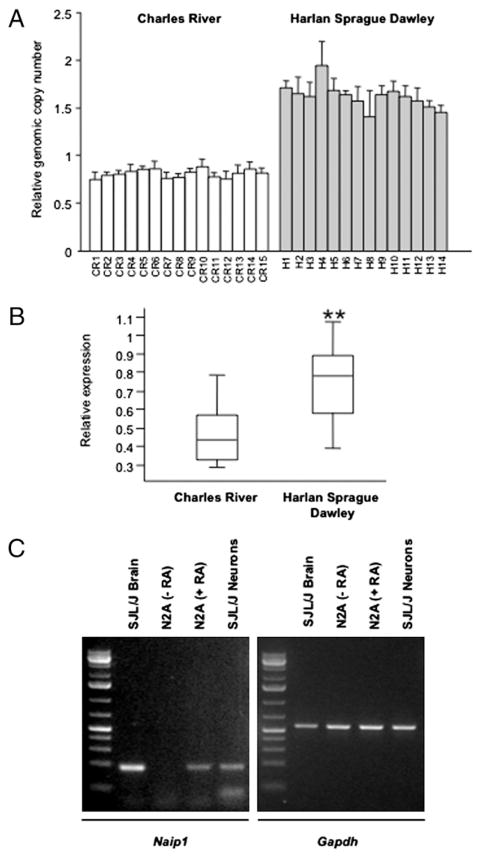
CNV validation confirmed changes in genomic copy number for six of the seven regions identified by array CGH. These regions encompassed multiple probes on the array and included both gain and loss of copy number in the CR mice compared with the HAR mice (Table III). For some regions, the CNV was consistent between mice within the same group (Naip1 shown in Fig. 7A, Znrf4, Olfr1431, and Mid1), whereas for some genes, copy number was variable, regardless of group (Il7 and Ccr2). In the case of one gene, Znrf4, no signal from CR genomic DNA was detected on the array, and we were subsequently unable to amplify the sequence from the CR mice.
FIGURE 7.
Naip1 genomic copy number and expression are different between CR and HAR substrains of SJL/J mice. A, Relative genomic copy number of Naip1 was determined using real-time qPCR in CR and HAR SJL/J substrains. Copy number is presented relative to the control gene Sdha. The data are presented as the mean ± SE of triplicate qPCR reactions. B, Box-plot showing expression of Naip1 in the small intestine of CR (n = 15) and HAR (n = 14) SJL/J mice. Expression is shown relative to the control gene Hprt. Naip1 expression was significantly different between the groups. **p < 0.001; Student t test. C, Left panel: PCR amplification of Naip1 in diseased SJL/J EAE brain (lane 1), N2A neuroblastoma cell line undifferentiated (lane 2), retinoic acid differentiated (lane 3), and cultured SJL/J neurons (lane 4). Right panel: expression of Gapdh as a loading control.
For all except one of the genes that overlapped with confirmed CNVs, we were unable to confirm different gene expression between the CR and HAR groups using the highly sensitive method of real-time qPCR. Two of the genes, Znrf4 and Mid1, were not expressed in the adult tissues available to us, whereas the remaining genes showed variable expression that did not correlate with copy number within groups or among groups. However, Naip1 showed a strong correlation between genomic copy number and gene expression (Fig. 7B). Expression was 2-fold higher in small intestine from HAR mice than in small intestine from CR mice (p < 0.001; Student t test), exactly mirroring the difference in relative genomic copy number. Therefore, there are considerable differences in the copy numbers of genes between CR mice and HAR SJL/J mice, and consistent differences were noted for Naip1. Interestingly Naip1 copy numbers in the JAX and TAC strain were similar to the HAR mice, designating the CR mice as the outlier substrain (data not shown). Relevant to EAE, we were able to detect Naip1 mRNA expression in SJL/J brain, in SJL/J neurons, and in a neuronal cell line differentiated into neurons by retinoic acid addition (Fig. 7C). Naip1 was not detected in infiltrating leukocytes (B cells, DCs, or macrophages) or in microglia or oligodendrocytes in the brains of SJL/J mice, although it is possible that the level of Naip1 mRNA in these samples is below the level of detection using our methods (data not shown). Taken together, CR SJL/J mice have half the number of Naip1 gene copies as HAR SJL/J mice, and Naip1 mRNA is expressed in neurons.
Discussion
Immunization of SJL/J mice with PLP139–151 in myco induces RR-EAE (14). However, the incidence of relapses in these mice is seldom 100%, and several protocol variations have been reported to affect EAE, such as the season of disease induction, the age of the mice, gender, mouse handling, and housing conditions (26, 27). We varied the dose of myco and PLP139–151, as well as the source of SJL/J mice, and found that the most significant source of variability in the incidence and intensity of relapses in RR-EAE was the SJL/J vendor. We kept the sex of our mice constant (always female) to avoid any possible influence of emulsification of myco + PLP139–151, as has been observed in male SJL/J mice (41). Furthermore, our head-to-head experiment with different vendors was performed three times during different seasons with the same results, thus eliminating the season as a confounding factor in our analysis (data not shown). We also avoided the use of PTx in our model because this agent can change the contribution of EAE-modifying loci (12). In our study, we found that CR mice exhibited significantly exacerbated disease compared with any other substrain tested. Moreover, HAR mice exhibited the most reliable relapse rates, whereas ~30% of CR mice did not undergo remission, but rather exhibited a form of C-EAE. Because the postacute phase of disease is the necessary target for therapeutic intervention in MS, it is important to understand which environmental and genetic variables result in C-EAE versus RR-EAE.
Given the significant differences in clinical phenotype of SJL/J substrains, we focused on the two most disparate strains (HAR and CR) and asked whether clinical disparity could be accounted for by underlying differences in CNS pathology and immune infiltration. Infiltrate size in the spinal cord did not seem to correlate with clinical disease severity, whereas axonal damage was increased in the more diseased CR mice. Therefore, infiltrates appear to be a common denominator to both forms of EAE, whereas the additional axonal damage, which may be induced by soluble mediators, could be more important for manifestation of chronic disease.
In addition to these histological differences, to our surprise we saw differences in cytokine-secreting CD4+ T cells between the HAR and CR substrains. Specifically, the HAR cohort tended to have a more dominant T cell-mediated immune response characterized by a marked increase in IFN-γ+ cells in the brain during the postacute phase. It is possible that the secretion of IFN-γ is required to counterbalance other cytokines, such as IL-17, and in the HAR mice, the IFN-γ–secreting cells are particularly elevated in the brain. The elevated production of IFN-γ in the HAR mice may have encouraged remission in these mice, as well as their propensity to develop RR-EAE rather than C-EAE. In support of this concept, enhanced IFN-γ signaling in the brain was shown to be associated with reduced brain inflammation (42).
Despite the clear distinction in clinical disease course, relatively little is known about how inflammatory patterns contribute to the pathology of RRMS versus progressive MS (PPMS/SPMS). The relapsing–remitting disease course is attributed to the balance of damage and repair following influx and subsequent resolution of immune infiltrates. In progressive MS, there is reduced immune infiltration but greater loss of oligodendrocytes and ongoing axonal damage (43). We hypothesize that this axonal pathology may be modulated by B cell responses (i.e., Ab/complement-mediated inflammation), and recent evidence suggests that B cell activation may be an important driver in CNS pathology (33–37). In patients with progressive MS (PPMS and SPMS), plasma B cells were found to accumulate and persist in the perivascular spaces and meninges, independent of T cell and B cell infiltration patterns (44). Given that autoantibodies in MS can target myelin-derived peptides, oligodendrocyte glycoprotein, axonal neurofilament protein, and neuronal ganglioside Ags (45), these plasma cell niches in progressive MS may be providing a continual source of long-lived, diffusible agents that directly contribute to ongoing axonal injury. Interestingly, compared with the HAR cohort, the CR cohort had larger B cell aggregates in the brain stem/cerebellum, increased spinal cord B cells, and an accompanying increase in PLP-specific IgG2a titers, indicative of a more robust humoral immune response. Furthermore, the CR mice had extraordinarily high levels of anti-MOG titers, indicating a vigorous humoral response to endogenous CNS Ags.
Our data corroborate other studies in SJL/J mice that highlighted a role for B cells in CNS damage. For example, CNS B cell follicles were shown to be important for disease propagation (34). Furthermore, Pöllinger et al. (37) showed that endogenous B cells appear to contribute to the pathology in SJL/J mice that contain T cells specific for MOG Ag. Not unlike our CR SJL/J mice, these investigators observed myelin-specific Abs that correlated with clinical severity, and the transfer of serum could induce disease in recipient mice. Intriguingly, in SJL/J mice with myelin-specific B cells, remission rates decrease, and a more chronic form of EAE is observed. Of note, these investigators used CR SJL/J mice for back-crossing. Therefore, a growing amount of data suggest that the Ab products of B cells may play a role in dictating a chronic versus a relapsing form of MS/EAE.
EAE-modifying loci have been identified in the SJL/J strain, with female-specific quantitative trait loci located on chromosomes 2, 9, and 11 (22). In addition, male-specific quantitative trait loci have been identified on chromosomes 10, 11, 12, 16, and 19 (22), and the use of consomic SJL/J mice revealed that polymorphisms in the Y chromosome also result in age-dependent disease exacerbation in male SJL/J mice (46). In addition to the HLA locus, other risk alleles have been identified for human MS (47). More recent work has identified genes involved in disease progression rather than disease susceptibility in EAE (21–23) and MS (24, 25). Given the genetic association with disease, we reasoned that genetic drift between SJL/J substrains from the vendors tested could account for differences in disease severity. At the molecular level, CNVs (deletions or duplications of submicroscopic DNA segments) and single nucleotide polymorphisms are the two major sources of genetic variation (48). We used CNV analysis to assess genetic drift between what should be identical mouse strains. To our surprise, we identified numerous genes with CNV between SJL/J substrains. Some of the genes that we found were subject to CNV have been identified as modulators of autoimmunity. For example, Ccr2 copy number was quite variable among SJL/J mice, and CCR2 deficiency has been associated with EAE resistance in some (49), but not all, genetic backgrounds (44), suggesting that the involvement of CCR2 in EAE acts in concert with other EAE-modifier genes. Il7 gene copy number also proved variable within SJL/J cohorts, and IL-7R (CD127) was identified as a risk allele in MS (47).
Although we observed variability within substrains for many of these genes, Naip1 copy numbers were consistently elevated 2-fold throughout the HAR SJL/J cohort. Furthermore, we validated that the copy number variability of Naip1 was represented at the mRNA transcript level and, that in the brain, Naip1 was predominantly expressed in neurons. Interestingly, Naip1 copy number in the other two substrains (JAX and TAC) was similar to the HAR strain, making the CR mice the outlier substrain (data not shown). This is consistent with CR mice exhibiting significantly exacerbated disease, as well as 100-fold elevated anti-MOG titers, compared with the other three SJL/J substrains. In terms of their pedigree, the CR mice are also the unique outlier, having been introduced to Centre National de la Recherche Scientifique in Orléans, France from The Jackson Laboratory in 1978 and ultimately acquired by Charles River Laboratories in 1997 at the 114th generation. In contrast, the TAC and HAR SJL/J mice were received from the National Institutes of Health in 1999 and 1987, respectively, from stock that originated from The Jackson Laboratory. Therefore, the CR mice were bred outside The Jackson Laboratory for a greater period of time compared with the HAR and TAC mice. Nevertheless, although reduced Naip1 copy number seems to correlate with greater disease severity and exacerbated humoral immune responses to CNS Ags in CR mice, it is important to note that Naip1 is likely not the only modifier gene affecting differences in EAE pathogenesis between SJL/J substrains. In agreement with this possibility, although JAX SJL/J mice were significantly less sick than CR mice, JAX mice exhibited an intermediate phenotype that, although not statistically significantly different from TAC/HAR mice, did trend toward greater disease than the TAC/HAR substrains. However, JAX mice harbor the same number of Naip1 gene copies as HAR and TAC mice. A CNV (or single nucleotide polymorphism) analysis between other SJL/J substrains may prove to be a useful approach for identifying additional genetic modifiers of EAE because the disease is clearly polygenic.
The involvement of Naip1 in EAE remains to be fully elucidated. Naip1, the most similar of six murine homologs to human NAIP (50), is a neuronal-expressed inhibitor of apoptosis protein family member that is induced in response to local CNS injury (51, 52). A protective role for Naip was shown repeatedly in rodent models of CNS injury [stroke and temporal lobe epilepsy (51, 52)], as well as human neurodegenerative diseases, spinal muscular atrophy, and Alzheimer’s disease (53, 54). Relevant to our study, oligodendrocyte-specific transgenic expression of p35, another inhibitor of apoptosis protein member, protects these cells from death (55). Furthermore, these transgenic mice have attenuated EAE progression. With these studies in mind, the most plausible explanation for our findings is that increased neuronal apoptosis (due to reduced Naip1 gene copies) could account for enhanced disease in CR mice. However, we also observed a heightened B cell response in the CR mice that correlated with reduced Naip1 expression; it remains unclear whether a reduction of Naip1 is linked to enhanced B cell responses. One possibility for such a connection is that heightened apoptosis of CR neurons during EAE results in the accumulation of apoptotic cell material that is not adequately cleared. This material may be presented to B cells to provoke a strong anti-myelin response. Thus, a pathogenic feedback loop would be initiated with more neuronal apoptosis resulting in enhanced priming and activation of myelin-specific B cells that, in turn, secrete Abs to trigger further neuronal pathology. We hypothesize that this feed-forward mechanism would drive chronic progressive EAE, independent of the waxing and waning of the encephalitogenic T cell response.
Recurrent CNVs have been identified among substrains of C57BL/6 mice (56), although our study is the first to examine this type of genetic variability in the SJL/J strain in the context of an autoimmune disease model. Given that the triggers that drive disease relapses in SJL/J mice may be influenced by CNV differences, the genomic dissection of SJL/J substrains should provide valuable genetic information for understanding the etiology of MS. Furthermore, it is possible that understanding immunological events leading to C-EAE may help us to model progressive MS, and the substrain differences that our study uncovered may provide a means for testing MS therapeutics in the context of chronic versus relapsing disease.
Supplementary Material
Acknowledgments
Tissue sections were processed by the Toronto Centre for Modeling Human Disease. We thank the Faculty of Medicine flow cytometry core facility and Dionne White for support. We also thank Dr. Shannon Dunn for critical reading of the manuscript.
This work was supported by an operating grant from the Multiple Sclerosis Society of Canada (to J.L.G.), a Canadian Institutes of Health Research grant (to L.R.O.), an operating grant from the Alberta Heritage Foundation for Medical Research (to S.S.O.), and a Canadian Institutes of Health Research Doctoral Research Award: Canada Graduate Scholarship (to L.S.d.L.).
Abbreviations used in this paper
- ADM
aberration detection method
- C-EAE
chronic experimental autoimmune encephalomyelitis
- CNV
copy number variation
- CR
SJL/JOrlCrl
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- HAR
SJL/JCrHsd
- JAX
SJL/J
- MOG
myelin/oligodendrocyte glycoprotein
- MS
multiple sclerosis
- myco
Mycobacterium tuberculosis adjuvant
- PLP
proteolipid protein
- PPMS
primary progressive multiple sclerosis
- PTx
pertussis toxin
- qPCR
quantitative PCR
- RR-EAE
relapsing–remitting experimental autoimmune encephalomyelitis
- RRMS
relapsing–remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- TAC
SJL/JCrNTac
- TP
triple positive (IFN-γ+IL-17+ TNF-α+)
Footnotes
The online version of this article contains supplemental material.
The sequences presented in this article have been submitted to the Array Express database under accession number E-MEXP-2534.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- 3.Lublin FD, Reingold SC National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 4.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 5.von Büdingen HC, Tanuma N, Villoslada P, Ouallet JC, Hauser SL, Genain CP. Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination. J Clin Immunol. 2001;21:155–170. doi: 10.1023/a:1011031014433. [DOI] [PubMed] [Google Scholar]
- 6.Endoh M, Kunishita T, Nihei J, Nishizawa M, Tabira T. Susceptibility to proteolipid apoprotein and its encephalitogenic determinants in mice. Int Arch Allergy Appl Immunol. 1990;92:433–438. doi: 10.1159/000235176. [DOI] [PubMed] [Google Scholar]
- 7.Lu C, Pelech S, Zhang H, Bond J, Spach K, Noubade R, Blankenhorn EP, Teuscher C. Pertussis toxin induces angiogenesis in brain microvascular endothelial cells. J Neurosci Res. 2008;86:2624–2640. doi: 10.1002/jnr.21716. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro-Neto FA, Rodbell M. Pertussis toxin induces structural changes in G alpha proteins independently of ADP-ribosylation. Proc Natl Acad Sci USA. 1989;86:2577–2581. doi: 10.1073/pnas.86.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honnor RC, Naghshineh S, Cushman SW, Wolff J, Simpson IA, Londos C. Cholera and pertussis toxins modify regulation of glucose transport activity in rat adipose cells: evidence for mediation of a cAMP-independent process by G-proteins. Cell Signal. 1992;4:87–98. doi: 10.1016/0898-6568(92)90010-6. [DOI] [PubMed] [Google Scholar]
- 10.Cassan C, Piaggio E, Zappulla JP, Mars LT, Couturier N, Bucciarelli F, Desbois S, Bauer J, Gonzalez-Dunia D, Liblau RS. Pertussis toxin reduces the number of splenic Foxp3+ regulatory T cells. J Immunol. 2006;177:1552–1560. doi: 10.4049/jimmunol.177.3.1552. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Winkler-Pickett RT, Carbonetti NH, Ortaldo JR, Oppenheim JJ, Howard OM. Pertussis toxin as an adjuvant suppresses the number and function of CD4+CD25+ T regulatory cells. Eur J Immunol. 2006;36:671–680. doi: 10.1002/eji.200535353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankenhorn EP, Butterfield RJ, Rigby R, Cort L, Giambrone D, McDermott P, McEntee K, Solowski N, Meeker ND, Zachary JF, et al. Genetic analysis of the influence of pertussis toxin on experimental allergic encephalomyelitis susceptibility: an environmental agent can override genetic checkpoints. J Immunol. 2000;164:3420–3425. doi: 10.4049/jimmunol.164.6.3420. [DOI] [PubMed] [Google Scholar]
- 13.Brown AM, McFarlin DE. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981;45:278–284. [PubMed] [Google Scholar]
- 14.McRae BL, Kennedy MK, Tan LJ, Dal Canto MC, Picha KS, Miller SD. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J Neuroimmunol. 1992;38:229–240. doi: 10.1016/0165-5728(92)90016-e. [DOI] [PubMed] [Google Scholar]
- 15.Oksenberg JR, Baranzini SE, Sawcer S, Hauser SL. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat Rev Genet. 2008;9:516–526. doi: 10.1038/nrg2395. [DOI] [PubMed] [Google Scholar]
- 16.Fugger L, Friese MA, Bell JI. From genes to function: the next challenge to understanding multiple sclerosis. Nat Rev Immunol. 2009;9:408–417. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- 17.Comabella M, Martin R. Genomics in multiple sclerosis—current state and future directions. J Neuroimmunol. 2007;187:1–8. doi: 10.1016/j.jneuroim.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Olsson T, Dahlman I, Wallström E, Weissert R, Piehl F. Genetics of rat neuroinflammation. J Neuroimmunol. 2000;107:191–200. doi: 10.1016/s0165-5728(00)00224-1. [DOI] [PubMed] [Google Scholar]
- 19.Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 20.Sundvall M, Jirholt J, Yang HT, Jansson L, Engström A, Pettersson U, Holmdahl R. Identification of murine loci associated with susceptibility to chronic experimental autoimmune encephalomyelitis. Nat Genet. 1995;10:313–317. doi: 10.1038/ng0795-313. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- 22.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabelko-Downes KA, Gimenez MT, Suvannavejh GC, Miller SD, Russell JH. Genetic control of pathogenic mechanisms in autoimmune demyelinating disease. J Neuroimmunol. 2000;110:168–176. doi: 10.1016/s0165-5728(00)00350-7. [DOI] [PubMed] [Google Scholar]
- 24.Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, Kawano Y, Hasuo K, Tobimatsu S, Kobayashi T. Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol. 1996;40:569–574. doi: 10.1002/ana.410400405. [DOI] [PubMed] [Google Scholar]
- 25.Olerup O, Hillert J, Fredrikson S, Olsson T, Kam-Hansen S, Möller E, Carlsson B, Wallin J. Primarily chronic progressive and relapsing/remitting multiple sclerosis: two immunogenetically distinct disease entities. Proc Natl Acad Sci USA. 1989;86:7113–7117. doi: 10.1073/pnas.86.18.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Columba-Cabezas S, Iaffaldano G, Chiarotti F, Alleva E, Cirulli F. Early handling increases susceptibility to experimental autoimmune encephalomyelitis (EAE) in C57BL/6 male mice. J Neuroimmunol. 2009;212:10–16. doi: 10.1016/j.jneuroim.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Teuscher C, Bunn JY, Fillmore PD, Butterfield RJ, Zachary JF, Blankenhorn EP. Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am J Pathol. 2004;165:1593–1602. doi: 10.1016/S0002-9440(10)63416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westall FC. Molecular mimicry revisited: gut bacteria and multiple sclerosis. J Clin Microbiol. 2006;44:2099–2104. doi: 10.1128/JCM.02532-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J Exp Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall CR, Young EJ, Pani AM, Freckmann ML, Lacassie Y, Howald C, Fitzgerald KK, Peippo M, Morris CA, Shane K, et al. Infantile spasms is associated with deletion of the MAGI2 gene on chromosome 7q11.23-q21.11. Am J Hum Genet. 2008;83:106–111. doi: 10.1016/j.ajhg.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somerville MJ, Mervis CB, Young EJ, Seo EJ, del Campo M, Bamforth S, Peregrine E, Loo W, Lilley M, Pérez-Jurado LA, et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. N Engl J Med. 2005;353:1694–1701. doi: 10.1056/NEJMoa051962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli E, Baeten D, Jäger A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Columba-Cabezas S, Griguoli M, Rosicarelli B, Magliozzi R, Ria F, Serafini B, Aloisi F. Suppression of established experimental auto-immune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin β receptor-Ig fusion protein. J Neuroimmunol. 2006;179:76–86. doi: 10.1016/j.jneuroim.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. HERMES Trial Group. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pöllinger B, Krishnamoorthy G, Berer K, Lassmann H, Bösl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 41.Fillmore PD, Brace M, Troutman SA, Blankenhorn EP, Diehl S, Rincon M, Teuscher C. Genetic analysis of the influence of neuroantigen-complete Freund’s adjuvant emulsion structures on the sexual dimorphism and susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 2003;163:1623–1632. doi: 10.1016/S0002-9440(10)63519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-γ determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brück W, Lucchinetti C, Lassmann H. The pathology of primary progressive multiple sclerosis. Mult Scler. 2002;8:93–97. doi: 10.1191/1352458502ms785rr. [DOI] [PubMed] [Google Scholar]
- 44.Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol. 2000;47:694–706. [PubMed] [Google Scholar]
- 46.Spach KM, Blake M, Bunn JY, McElvany B, Noubade R, Blankenhorn EP, Teuscher C. Cutting edge: the Y chromosome controls the age-dependent experimental allergic encephalomyelitis sexual dimorphism in SJL/J mice. J Immunol. 2009;182:1789–1793. doi: 10.4049/jimmunol.0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, et al. International Multiple Sclerosis Genetics Consortium. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 48.Beckmann JS, Estivill X, Antonarakis SE. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat Rev Genet. 2007;8:639–646. doi: 10.1038/nrg2149. [DOI] [PubMed] [Google Scholar]
- 49.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaraghi Z, Korneluk RG, MacKenzie A. Cloning and characterization of the multiple murine homologues of NAIP (neuronal apoptosis inhibitory protein) Genomics. 1998;51:107–113. doi: 10.1006/geno.1998.5378. [DOI] [PubMed] [Google Scholar]
- 51.Holcik M, Thompson CS, Yaraghi Z, Lefebvre CA, MacKenzie AE, Korneluk RG. The hippocampal neurons of neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice display increased vulnerability to kainic acid-induced injury. Proc Natl Acad Sci USA. 2000;97:2286–2290. doi: 10.1073/pnas.040469797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu DG, Crocker SJ, Doucet JP, St-Jean M, Tamai K, Hakim AM, Ikeda JE, Liston P, Thompson CS, Korneluk RG, et al. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nat Med. 1997;3:997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- 53.Christie LA, Su JH, Tu CH, Dick MC, Zhou J, Cotman CW. Differential regulation of inhibitors of apoptosis proteins in Alzheimer’s disease brains. Neurobiol Dis. 2007;26:165–173. doi: 10.1016/j.nbd.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, et al. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell. 1995;80:167–178. doi: 10.1016/0092-8674(95)90461-1. [DOI] [PubMed] [Google Scholar]
- 55.Hisahara S, Araki T, Sugiyama F, Yagami K, Suzuki M, Abe K, Yamamura K, Miyazaki J, Momoi T, Saruta T, et al. Targeted expression of baculovirus p35 caspase inhibitor in oligodendrocytes protects mice against autoimmune-mediated demyelination. EMBO J. 2000;19:341–348. doi: 10.1093/emboj/19.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Egan CM, Sridhar S, Wigler M, Hall IM. Recurrent DNA copy number variation in the laboratory mouse. Nat Genet. 2007;39:1384–1389. doi: 10.1038/ng.2007.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.