Abstract
The Hedgehog (Hh) signaling pathway plays a conserved and essential role in regulating development and homeostasis of numerous tissues. Cytoplasmic signaling is initiated by Smoothened (Smo), a G-protein-coupled receptor (GPCR) family member, whose levels and activity are regulated by the Hh receptor Patched (Ptc). In response to Hh binding to Ptc, Ptc-mediated repression of Smo is relieved, leading to Smo activation, surface accumulation, and downstream signaling. We find that downregulation of Drosophila Smo protein in Hh-responding imaginal disc cells is dependent on the activity of G-protein-coupled receptor kinase 2 (Gprk2). By analyzing gain- and null loss-of-function phenotypes, we provide evidence that Gprk2 promotes Smo internalization subsequent to its activation, most likely by direct phosphorylation. Ptc-dependent regulation of Smo accumulation is normal in gprk2 mutants, indicating that Gprk2 and Ptc downregulate Smo by different mechanisms. Finally, we show that both Drosophila G-protein-coupled receptor kinase orthologues, Gprk1 and Gprk2, act in a partially redundant manner to promote Hh signaling. Our results suggest that Smo is regulated by distinct Ptc-dependent and Gprk2-dependent trafficking mechanisms in vivo, analogous to constitutive and activity-dependent regulation of GPCRs. G-protein-coupled receptor kinase activity is also important for efficient downstream signaling.
Keywords: Hedgehog signaling, G-protein-coupled receptor kinase, protein trafficking, Patched, Smoothened, Drosophila melanogaster
INTRODUCTION
Hedgehog (Hh) signaling regulates numerous cellular responses important for the growth and patterning of tissues in diverse species (Hooper and Scott, 2005; Jiang and Hui, 2008; Varjosalo and Taipale, 2008). It is also a critical regulator of stem cell maintenance and tissue homeostasis in adult animals (Beachy et al., 2004). Due to its central role in these processes, mis-regulation of Hh pathway activity is associated with congenital birth defects and cancer (McMahon et al., 2003; Nieuwenhuis and Hui, 2005).
Hh responsiveness is determined by activity of Patched (Ptc) and Smoothened (Smo) family transmembrane proteins. Smo belongs to a distantly-related subgroup of the G-protein-coupled receptor (GPCR) family, and serves as the signal-generating component of the pathway. In the absence of Hh, Ptc inhibits the activity of Smo. Hh binding inhibits Ptc, allowing Smo to become activated and to stimulate downstream signaling events that ultimately regulate Cubitus interruptus (Ci)/Gli family transcription factors. In Drosophila, the signal is transduced by a well-characterized pathway that includes the kinesin-like protein Costal-2 (Cos2) and the kinase Fused (Hooper and Scott, 2005; Jiang and Hui, 2008). Drosophila Smo can also signal as a classical GPCR, activating a heterotrimeric G-protein to modulate intracellular cAMP levels (Ogden et al., 2008).
Transcriptional and posttranslational regulation play an important role in controlling the activities of Ptc and Smo. ptc genes are transcriptionally activated by Hh signaling, providing an important mechanism for limiting the extent of Hh diffusion (Chen and Struhl, 1996; Goodrich et al., 1996; Hidalgo and Ingham, 1990). In the absence of Hh, a fraction of Ptc is found at the plasma membrane, where it undergoes ligand-independent internalization followed by lysosomal degradation (Capdevila et al., 1994b; Martin et al., 2001; Strutt et al., 2001; Torroja et al., 2004). Interaction of Hh with Ptc leads to co-internalization and degradation of the two proteins (Gallet and Therond, 2005; Incardona et al., 2000; Torroja et al., 2004).
Transcription of smo is not regulated in response to Hh signaling, but Smo protein accumulates in a complex pattern in tissues as a consequence of Ptc activity (Denef et al., 2000; Ingham et al., 2000). Ptc promotes endocytosis of Smo from the cell surface and its subsequent retention in an intracellular pool and/or degradation (Denef et al., 2000; Jia et al., 2004; Nakano et al., 2004; Zhu et al., 2003). Thus, cells in which Hh signaling is inactive accumulate low levels of Smo. In Drosophila, inhibition of Ptc by Hh leads to phosphorylation of the cytoplasmic C-terminal tail of Smo by Protein kinase A (PKA) and Casein kinase I (CKI) (Apionishev et al., 2005; Jia et al., 2004; Zhang et al., 2004). Phosphorylation activates Smo, which translocates to and accumulates at the cell surface in Drosophila cells (Denef et al., 2000; Nakano et al., 2004; Zhao et al., 2007; Zhu et al., 2003). A similar mechanism functions in mammals, where Hh controls membrane accumulation of Smo in primary cilia (Corbit et al., 2005; Rohatgi et al., 2007).
Although it is clear that Smo subcellular localization is important for its activity, little is known about how this is controlled. In some systems, G-protein-coupled receptor kinases (GRKs) have been shown to participate in Hedgehog signaling. The principal function of GRKs is to regulate GPCR trafficking and activity (Moore et al., 2007; Reiter and Lefkowitz, 2006). GRKs specifically phosphorylate the agonist-bound, activated forms of GPCRs. This modification leads to recruitment of arrestins, which block receptor coupling to downstream G-proteins and promote clathrin-dependent receptor internalization, both of which serve to shut off signaling. Arrestins can also play a positive role by serving as scaffolds for the assembly of G-protein-independent signaling complexes (Reiter and Lefkowitz, 2006). In mammalian cells, GRK2 stimulated Smo phosphorylation and association with β-arrestin2, and promoted transcriptional responses induced by Smo activation (Chen et al., 2004; Kovacs et al., 2008; Meloni et al., 2006). β-arrestins were also required for Smo localization to cilia, suggesting a possible role for this system in regulating Smo trafficking (Kovacs et al., 2008). The role of GRKs in Hh signaling is conserved, as morpholino knockdown of the GRK2 or β-arrestin2 orthologues in zebrafish, or reduction of one of the two GRKs in Drosophila (called Gprk2) using double-stranded RNA (dsRNA) or a genomic deficiency also impaired Hh signaling in these organisms (Molnar et al., 2007; Philipp et al., 2008; Wilbanks et al., 2004). These observations suggest that GRKs are involved in regulating Smo activity and trafficking, although the precise mechanisms involved have not been defined.
In the present work, we examine the functions of the Drosophila GRKs during Drosophila wing imaginal disc development. Hh signaling plays a critical role in patterning the anterior-posterior axis of the wing. Hh produced by cells in the posterior compartment diffuses into the anterior compartment, forming a concentration gradient. In response, anterior cells in a broad stripe adjacent to the anterior/posterior (A/P) compartment boundary activate Hh target genes in a concentration-dependent manner (Strigini and Cohen, 1997). By analyzing gain- and null loss-of-function phenotypes, we provide evidence that Gprk2, the Drosophila orthologue of mammalian GRK4/5/6, promotes phosphorylation and downregulation of Smo subsequent to activation in Hh-responding wing disc cells. This mechanism of Smo downregulation is distinct from that mediated by Ptc, which prevents Smo accumulation and activity independent of Gprk2. We also show that Gprk1, the orthologue of mammalian GRK2 and GRK3, functions with Gprk2 in a partially redundant manner to promote efficient Hh signaling. Our results suggest that Smo is regulated by distinct Ptc-dependent and Gprk2-dependent trafficking mechanisms in vivo, similar to the constitutive and activity-dependent regulation typical of GPCRs.
METHODS
Fly strains and reagents
dpp10638 (dpp-LacZ), gprk206936 (gprk2-LacZ), hhts2, ptcS2, ap-GAL4, and hh-GAL4 are described in FlyBase. w;hs-I-SceI,hs-FLP, UAS-Hh (Ingham and Fietz, 1995), and UAS-Ptc flies were provided by S.M. Cohen. UAS-HAKrz flies (Mukherjee et al., 2005) were provided by S. Artavanis-Tsakonas. p{XP}d09952, pBac{WH}f00526, pBac{RB}e01955, and pBAC{WH}f06602 were from the Exelixis Collection at Harvard Medical School (Thibault et al., 2004). UAS-smo.RNAi flies were from the Vienna Drosophila RNAi Center. All other fly stocks were from the Bloomington Drosophila Stock Centre.
The gprk2del1 and gprk2del2 deletions were generated using FRT-site-bearing transposable elements as described (Parks et al., 2004). These deletions remove sequences located between pBac{RB}e01955 and pBAC{WH}f06602 (gprk2del1) or pBac{WH}f00526 and p{XP}d09952 (gprk2del2). The presence of the deletions was verified by genomic PCR and Southern blotting (not shown). The gprk2del1 deletion removes four genes (lox, CG11333, CG11334, and CG12063) in addition to gprk2, and is homozygous viable at 25°C and semi-lethal at 29°C. The gprk2del2 allele is identical to Df(3R)gprk2 (Molnar et al., 2007). This deletion likely disrupts the promoter of the neighbouring CG11337 gene, as it is homozygous lethal at 25°C, but viable in trans to other gprk2 null alleles.
The gprk1KO and gprk2KO alleles were generated by “ends-out” homologous recombination (Gong and Golic, 2003). To generate targeting constructs, upstream and downstream homology arm fragments (3.9 and 3.7 kb, respectively, for gprk1; 3.4 and 4.0 kb, respectively, for gprk2) were PCR amplified and cloned into the pW25 vector. The resulting targeting constructs were transformed into flies. After the crosses to generate potential recombinants with the targeting constructs mapping to the correct chromosome, homologous recombination events were identified either by Western blot analysis of adult fly head extracts with an anti-GRK2 antibody (reported to cross-react with Gprk1 (Lee et al., 2004)) or by genomic PCR across each arm of homology (for gprk2). Multiple independent targeting events were identified for each gene. The gprk1KO allele is homozygous viable at 25°C and 29°C. The gprk2KO allele is homozygous viable at 25°C and semi-lethal at 29°C.
UAS-Gprk2 was constructed by cloning the 3058 nucleotide EcoRI/XhoI insert fragment from EST LD21923 into pUAST. This fragment contains the full-length gprk2 coding sequence, plus 602 and 328 nucleotides of 5′ and 3′ untranslated sequences, respectively.
Preparation of anti-Gprk2 antibody
A portion of the sequence encoding the C-terminus of Gprk2 (amino acids 560–714) was PCR amplified (5′ primer: AATAAGGATCCAACGGTCGCATGGGCGGG; 3′ primer: TATATGAATTCTCAGCTTTCGACCGTCGTG). The product was digested with BamHI and EcoRI and cloned into BamHI/EcoRI-digested pGEX4T-1 (Invitrogen). Bacterially expressed GST-fusion protein was purified with glutathione agarose (Pierce) under native conditions according to the manufacturer s instructions. Guinea pigs were injected with 50–100 μg of purified fusion protein mixed with Sigma Adjuvant (Sigma), at 3-week intervals. For immunostainings, serum was affinity purified using the AminoLink® Plus Immobilization Kit as per manufacturer s instructions (Pierce).
Antibodies, immunostainings, and image analysis
Wing discs were dissected and immunostained as described (Hipfner et al., 2004). Primary antibodies were: mouse anti-α-Tubulin (12G10), mouse anti-En (4D9), mouse anti-Ptc (Apa I), and mouse anti-Smo (20C6) (Developmental Studies Hybridoma Bank), mouse anti-Col (a gift from M. Crozatier), rat anti-Smo (a gift from S. Cohen), rat anti-Ci155 (a gift from T. Kornberg), rabbit anti-Moesin (a gift from D. Kiehart), rabbit anti-β-galactosidase (#A-11132) (Molecular Probes), rabbit anti-HA (#Y-11) and rabbit anti-GRK2/3 (H-222) (Santa Cruz Biotechnology). All images were taken with a Zeiss LSM510 confocal microscope using a 40X oil immersion objective, and are depicted with an additional magnification of 1.2X (with the exception of Fig. 7D, which is shown magnified 5.33-fold). Image analysis was performed using ImageJ software. Quantification of fluorescence signals along the anterior-posterior axis of wing discs was performed on a rectangular region encompassing most of the central portion of the wing pouch using the “Plot Profile” function. Results for individual channels were normalized to zero by subtracting the lowest average pixel intensity from each measurement.
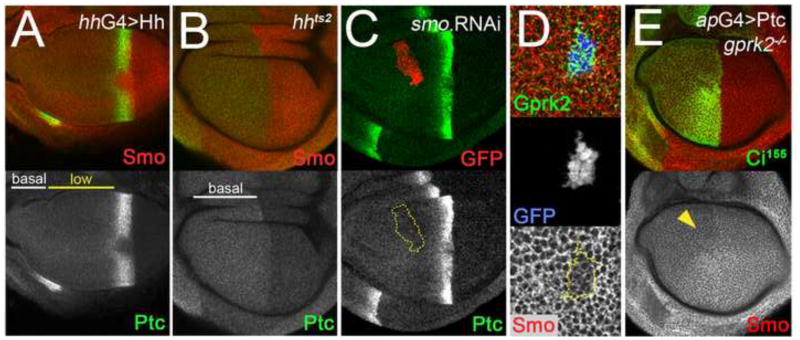
Fig. 7. Ptc protein reflects the Hh ligand gradient, and regulates Smo accumulation independently of Gprk2.
(A) Wing disc in which the range of Hh ligand diffusion was increased by overexpressing Hh in the posterior compartment (driven by hh-Gal4). Ptc is in green, Smo in red. The “low-Ptc” region extends through most of the anterior compartment. (B) hhts2 mutant wing disc in which functional Hh ligand was eliminated after culture for 16 h at the restrictive temperature (29°C). Ptc is in green, Smo in red. The “low-Ptc” region is absent. (C) Wing disc with a clone expressing a dsRNA targeting Smo situated in the “low-Ptc” region. The clone is marked by co-expression of GFP (red channel). The levels of Ptc (green channel) in the clone are unchanged. (D) Closeup view of a posterior compartment clone of ptcS2 homozygous mutant cells co-expressing GFP (blue channel) and Gprk2 (green channel), generated using the MARCM system. Smo levels (red channel) are lower in Gprk2-expressing ptc mutant cells than in surrounding wild-type cells. (E) gprk2KO/gprk2KO mutant wing disc in which Ptc was overexpressed in the dorsal compartment with ap-GAL4. Hh signaling was inhibited by Ptc expression, as evidenced by the loss of Ci155 stabilization in dorsal cells (green channel). Smo levels (red channel) were reduced throughout the dorsal compartment, most notably in anterior compartment cells that would otherwise show ectopic accumulation of the protein (yellow arrowhead).
Imaginal disc lysates and λ–phosphatase treatment
Wing/haltere/leg imaginal disc complexes were dissected, lysed, and fractionated by SDS-PAGE as described (Hipfner et al., 2004). For some experiments, a portion of the lysate was first incubated with 400U λ–phosphatase (New England Biolabs) in a 30 μl reaction volume containing 1X reaction buffer and 2 mM MnCl2.
Cell culture
S2 cells were cultured as described (Hipfner et al., 2004). For Hh treatments, S2 cells stably transfected with a puromycin-selectable pMT vector containing sequences encoding an active N-terminal fragment of Drosophila Hh (HhN) (kindly provided by S. Cohen) were selected in 10 μg/ml puromycin. Hh-conditioned medium was prepared by culturing these cells in the absence of puromycin and inducing HhN expression with 0.5 mM CuSO4. After the cells reached confluence, the medium was harvested, clarified by centrifugation for 5 min at 1000 × g, and sterile filtered. Control conditioned medium was obtained by treating wild-type S2 cells the same way.
dsRNA and Hh treatment of Drosophila S2 cells and Western blotting
Double stranded RNA was prepared as described (Hipfner et al., 2004). The Gprk2 template was prepared by PCR amplification of nucleotides 372 to 870 of Gprk2 cDNA. As a control, dsRNA was prepared from a template containing nucleotides 64 to 559 of the EGFP coding sequence. S2 cells were treated with dsRNAs and lysates prepared essentially as described (Hipfner et al., 2004). In some experiments, cells were treated for 2 h with Hh-conditioned or S2-conditioned medium prior to lysis.
Cell surface biotinylation
Cells were treated for 2 hours in control- or Hh-conditioned medium, washed, and cell surface proteins labelled with 1 mg/ml Sulfo-NHS-SS-Biotin (Pierce) as described (Denef et al., 2000). After purification, strepatavidin-agarose-bound proteins were recovered by suspension in 1x SDS–PAGE sample buffer and incubation at 50°C for 10 min to cleave the disulfide bond, followed by denaturation for 5 min at 75°C prior to SDS-PAGE.
Wing mounting and measurement
Flies were collected in 50% ethanol/50% glycerol. After rinsing with water, wings were transferred into a drop of Faure’s solution on glass slides and coverslipped. Wing images were analyzed using the polygon selection tool in ImageJ to outline and measure the areas bounded by L3 and L4 veins and total wing areas for at least five wings of each genotype. Statistical significance was tested using the Student’s t-Test.
RESULTS
Temperature-dependent impairment of Hh signaling in gprk2 mutants
GRKs have been implicated in Hh signaling and Smo regulation in several systems, including Drosophila. To examine the function of gprk2 in flies, we generated deletions of all or part of the gene. In one approach, pairs of engineered piggyBAC transposable elements flanking gprk2 were used to generate defined chromosomal deletions removing most (gprk2del1) or all of the gene (gprk2del2) (Fig. 1A) (Parks et al., 2004). We also generated an allele with most of the exons encoding the kinase domain replaced with a white marker transgene, by homologous recombination (gprk2KO; Fig. 1A) (Gong and Golic, 2003). Although gprk2del1 and gprk2del2 remove all or parts of neighbouring genes, the deleted segment these alleles share in common contains only gprk2 gene sequences. Thus by using trans-heterozygous combinations of these three alleles, we were able to analyze clean gprk2 null phenotypes.
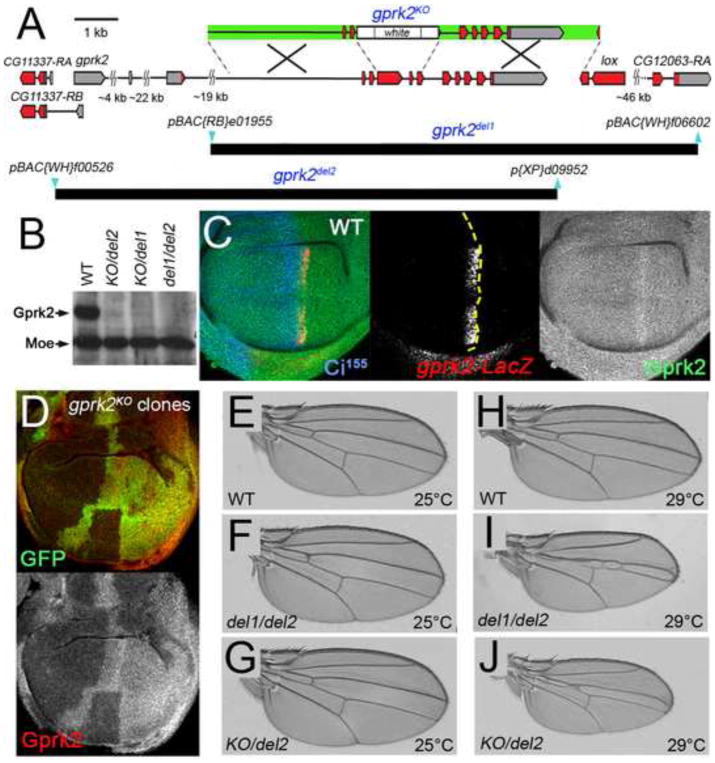
Fig. 1. Generation and characterization of gprk2 null alleles.
(A) Map of the gprk2 locus and defined deletions. The relative positions of non-coding (grey) and coding (red) exons of gprk2, the upstream neighbouring gene CG11337, and two downstream genes (lox and CG12063) are indicated. Two additional genes (CG11333 and CG11334) situated between lox and CG12063 are not shown. A schematic of the targeting construct used to generate the gprk2KO allele is shown above the map (arms of homology in green). The positions of the piggyBac insertions (blue) used to make the gprk2del1 and gprk2del2 alleles and extents of the resulting deletions (black) are shown below. (B) Western blot analysis of wing disc lysates prepared from wild-type, grpk2KO/gprk2del2, grpk2KO/gprk2del1, and grpk2del1/gprk2del2 third-instar larvae. Gprk2 protein is absent from the mutants. The blot was also probed with an anti-Moesin antibody as a loading control. (C) Immunofluorescence staining of a wild-type wing disc to detect Gprk2 (green), nuclear β-galactosidase expressed from a gprk2-LacZ enhancer trap (red), and Ci155 (blue). In this and all subsequent figures, discs are oriented with dorsal up and posterior to the right. Gprk2 protein is detected throughout the disc. The levels of both gprk2 enhancer trap activity and Gprk2 protein are upregulated in anterior cells abutting the A/P compartment boundary (yellow dotted line, as determined by the boundary of Ci immunostaining). (D) Immunofluorescence staining of a wing disc with homozygous gprk2KO clones, marked by the absence of GFP (green). Gprk2 staining (red) is strongly reduced in the clones. (E to J) Wings of flies raised at 25°C (E-G) or 29°C (H–J). Genotypes are: (E,H) wild-type (w1118); (F,I) gprk2del1/gprk2del2; (G,J) gprk2KO/gprk2del2.
Using an antiserum raised against the C-terminus of Gprk2, we observed abundant levels of the kinase in wing disc lysates from wild-type third instar larvae in Western blots (Fig. 1B). Gprk2 was undetectable in animals trans-heterozygous for each of the three combinations of gprk2 alleles (Fig. 1B). In immunofluorescence stainings, Gprk2 was detected at a uniform level throughout wing discs, with a stripe of upregulation near the centre of the disc (Fig. 1C). This stripe corresponded to the first rows of Hh-responding, Ci-expressing anterior cells at the A/P boundary, and co-localized with the expression domain of a gprk2-LacZ enhancer trap (Fig. 1C) (Schneider and Spradling, 1997). This pattern is also similar to that of gprk2 mRNA, and is consistent with the identification of gprk2 as a Hh target gene (Molnar et al., 2007). Gprk2 staining was lost in gprk2KO mutant clones (Fig. 1D, red), confirming the specificity of staining. We conclude that all three alleles disrupt Gprk2 production, as expected. In all subsequent experiments, we did not observe significant phenotypic differences between the different trans-heterozygous genotypes, and we have used these interchangeably for our analyses.
Under standard culture conditions (25°C), loss of gprk2 had little effect. Progeny of all three trans-heterozygous allelic combinations were recovered at near-expected ratios. The only obvious phenotype was in the wings, which were slightly ruffled (data not shown). There was no apparent reduction of the area between wing veins 3 and 4 (L3 and L4), indicative of Hh signaling defects (Fig. 1E-G and data not shown). To see if the mutants showed more subtle defects, we measured and calculated the L3-L4 intervein area as a fraction of total wing area for wild-type and gprk2 mutants. Of the three mutant genotypes, one (gprk2KO/gprk2del2) showed a statistically significant reduction, which was minimal (less than 3%; see Fig. S1A in supplementary material). gprk2 mutant adult flies survived, although females were sterile. gprk2 thus appears to be dispensable for Hh signaling and for viability under standard culture conditions.
To look at Hh signaling at a molecular level, we compared Hh-dependent transcriptional responses in wild-type and gprk2 mutant wing discs by immunofluorescent staining of target gene products. In wild-type discs, strong activation of the pathway induced expression of high threshold targets in the first few rows of anterior cells abutting the A/P compartment boundary (except in some cases for cells near the dorsal ventral compartment boundary, where Hh signaling is attenuated by Notch and Wingless signaling) (Glise et al., 2002). These include anterior Engrailed (En), which is also expressed throughout the posterior compartment in a Hh-independent manner (see Fig. S2 in supplementary material); Collier (Col) (Fig. S2); and Ptc, which is also expressed throughout the rest of the anterior compartment at a basal level, independent of Hh (Fig. 2A, green channel) (Capdevila and Guerrero, 1994; Phillips et al., 1990). Expression of the low threshold Hh target dpp (detected using a dpp-LacZ reporter) was high in a wider stripe of anterior cells, with low-level expression extending farther towards the anterior (Fig 2A, blue channel). Consistent with the absence of an adult wing phenotype, transcriptional responses looked normal in gprk2 mutants at 25°C. Col and anterior En were activated like in wild-type discs (Fig. S2), as were Ptc and dpp-LacZ (Fig. 2B).
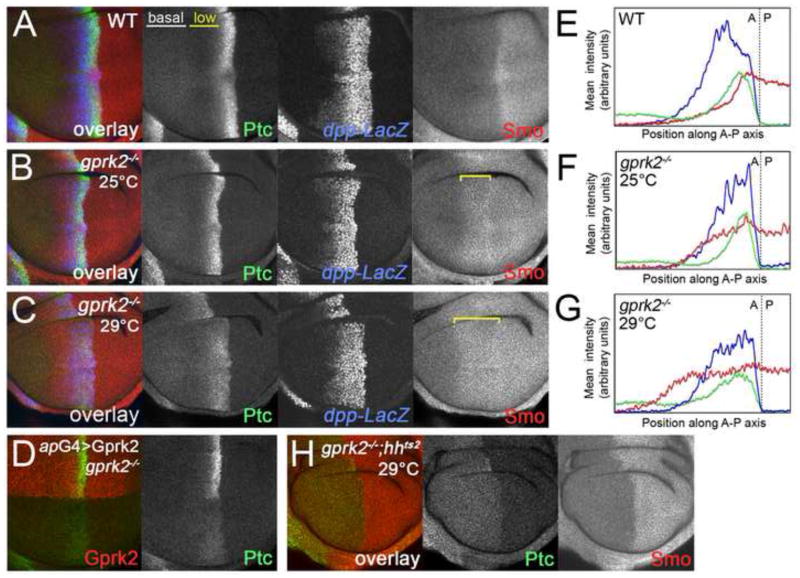
Fig. 2. Impaired Hh signaling and ectopic accumulation of Smo in gprk2-mutant Hh-responding cells.
(A) Immunofluorescence staining of a wild-type late third instar wing disc with antibodies recognizing Ptc (green), Smo (red) and nuclear β-galactosidase expressed from a dpp-LacZ enhancer trap (blue). (B) Wing disc from a gprk2del1/gprk2KO mutant animal cultured at 25°C, immunostained as in (A). Target gene expression is normal. Ectopic Smo is indicated by a yellow bracket. (C) Wing disc from a gprk2del1/gprk2KO mutant animal cultured at 29°C, immunostained as in (A). Hh-dependent Ptc expression is strongly reduced. Ectopic Smo is indicated by a yellow bracket. (D) Immunofluorescence staining of a wing disc from a gprk2del2/gprk2KO mutant animal raised at 29°C, in which transgenic expression of Gprk2 (red) in the dorsal compartment was driven with ap-GAL4. Re-expression of Gprk2 specifically rescued Ptc expression (green). (E–G) Quantification of the fluorescence signals along the anterior-posterior axes from rectangular regions encompassing much of the wing pouches of discs shown in (A), (B), and (C), respectively. The green traces represent Ptc, the red traces Smo, and the blue traces dpp-LacZ.. The location of the anterior-posterior compartment boundary, as determined by the boundary of Ptc upregulation, is indicated by a dotted line. (H) hhts2,gprk2del1/hhts2,gprk2del2 double mutant wing disc cultured for 16 h at the restrictive temperature (29°C). Ptc is in green, Smo in red.
The lack of phenotype in gprk2 mutants was surprising in light of the previously reported impairment of Hh signaling upon double-stranded RNA (dsRNA)-mediated knockdown of gprk2 in wing discs (Molnar et al., 2007). We note that these knockdown experiments were performed by culturing animals at 29°C, where GAL4-dependent expression of the dsRNA transgene would be expected to be higher. An alternative interpretation is that the effects of gprk2 reduction/mutation could be temperature-dependent. To test this, we examined the phenotype of null mutants raised at higher temperature. The consequences of disrupting gprk2 were much more severe when animals were raised at 29°C. Under these conditions, most gprk2 mutants died before eclosion. Those that survived to adulthood had an average reduction in the L3-L4 intervein:total wing area ratio of between 19 and 30% (p<.001) (Fig. 1H-J and Fig. S1B), which is characteristic of reduced Hh signaling. Hh target gene induction was obviously impaired in gprk2 mutant discs at 29°C. Expression of high threshold targets was either lost (Col and En) (see Fig. S3 in supplementary material) or strongly reduced (Ptc) (Fig. 2C). To confirm that these effects were specific, we tested whether target gene expression could be rescued in the mutants by transgenic expression of Gprk2. When Gprk2 was expressed throughout the dorsal compartment of gprk2del2/gprk2KO mutant wing discs (using the apterous-GAL4 (ap-GAL4) driver), ptc induction was restored in dorsal but not ventral A/P boundary cells (Fig. 2D). We conclude that elimination of gprk2 affects Hh signaling in a temperature-dependent manner.
Gprk1 acts in a partially redundant manner with Gprk2 in Hh signaling
The reason for the temperature-dependent effects of the gprk2 deletions is not clear. We considered the possibility that another kinase acts partially redundantly with Gprk2 to regulate Hh signaling. A likely candidate is Gprk1, the second GRK orthologue in flies. Gprk1 is expressed in the adult fly retina, where it may participate in regulating the GPCR Rhodopsin (Lee et al., 2004). Nothing is known about gprk1 expression in other tissues, and no mutations in the gene have been isolated. gprk1 expression was readily detectable in wing imaginal discs by RT-PCR (see Fig. S4A in supplementary material). We mutated gprk1 by replacing exons three to five with a white marker transgene by homologous recombination (gprk1KO; Fig. 3A) (Gong and Golic, 2003). This allele has most of the highly conserved amino-terminal RGS domain deleted, and is predicted to produce a frame shift and formation of a severely truncated protein in the event that the resulting transcript can still be spliced. In Western blot analyses of head extracts using a commercial anti-human GRK2/3 antibody (reported to cross-react with Gprk1 (Lee et al., 2004)), we saw the disappearance of a band of the expected size for Gprk1 in gprk1KO homozygous flies (Fig. 3B). This protein was also absent in S2 cells treated with gprk1-specific dsRNA, confirming that it is Gprk1 and that our targeting strategy worked.
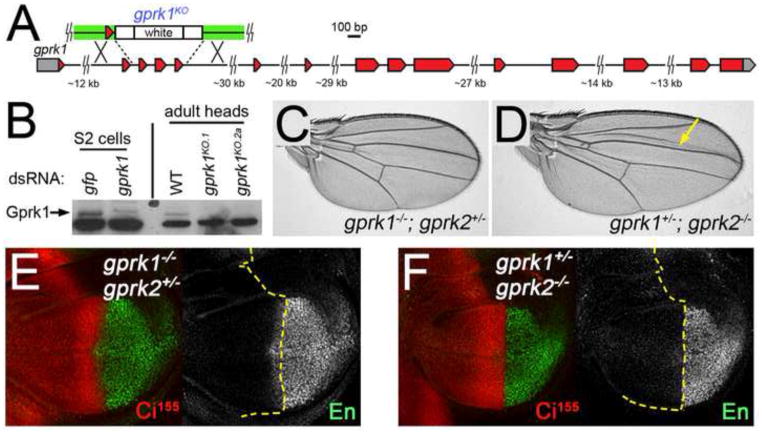
Fig. 3. Gprk1 partially compensates for the loss of Gprk2 in Hh signaling.
(A) Map of the gprk1 locus. The relative positions of non-coding (grey) and coding (red) exons of gprk1 are shown. There are no annotated genes in the immediate vicinity of gprk1. A schematic of the targeting construct used to generate the gprk1KO allele is shown above the map (arms of homology in green). (B) Western blot analysis using an anti-human GRK2/3 antibody. This antibody cross-reacts with Gprk1, as indicated by loss of a band of the appropriate size (~80 kDa) in lysates from S2 cells treated with gprk1-specific dsRNA relative to control gfp dsRNA-treated cells (left). Gprk2 is present in lysates from wild-type adult fly heads and absent in flies homozygous for two independently-isolated targeted alleles (right). The faster-migrating, major cross-reacting protein band serves as a loading control. (C) Wing from a gprk1KO/gprk1KO; gprk2KO/+ fly raised at 25°C. The wing is short but otherwise wild-type in appearance. (D) Wing from a gprk1KO/+; gprk2KO/gprk2KO fly raised at 25°C. Wing vein L3-L4 spacing is reduced, indicating insufficient Hh signaling. (E,F) Wing discs from gprk1KO/gprk1KO; gprk2KO/+ (E) and gprk1KO/+; gprk2KO/gprk2KO (F) animals raised at 25°C. Discs are stained with antibodies detecting En (green) and Ci155 (red). Yellow dotted lines mark the anterior-posterior compartment boundary, based on the boundary of Ci immunostaining. Anterior Engrailed is lost in gprk1KO/+; gprk2KO/gprk2KO discs.
gprk1KO homozygotes were viable at 25°C and 29°C. Wings from these flies were moderately shorter than normal, but showed no reduction in L3-L4 intervein area (Fig. S1C and data not shown). In crosses of doubly heterozygous parents at 25°C, we never recovered gprk1/gprk2 double mutant adults. This indicates that these kinases have some redundant and essential activity. We were able to recover flies that were heterozygous for one of these genes and homozygous mutant for the other. gprk1−/−,gprk2+/− animals were indistinguishable from gprk1 mutants alone, and showed no reduction in L3-L4 spacing in the wing or in Hh target gene activation (Fig. 3C,E and Fig. S1C). In contrast, the L3-L4 intervein:total wing area in gprk1+/−,gprk2−/− animals was significantly reduced by 8.9% compared to wild-type and 8.4% compared to gprk2 mutants at 25°C (p<.001 for both comparisons) (Fig. 3D and Fig. S1C). Impaired Hh signaling was apparent in gprk1+/−,gprk2−/− wing discs at 25°C, with anterior en expression being completely lost (Fig. 3F). Together, these results suggest that Gprk2 is the primary kinase involved in Hh signaling in flies. However, Gprk1 can substitute for the loss of Gprk2 at 25°C, but does so less efficiently. This could explain the temperature-dependent effects of eliminating Gprk2, for example if Gprk1 is unable to stably interact with the relevant substrate(s) at higher temperatures.
Gprk2, but not Gprk1, downregulates Smo in Hh-responding cells
Although signaling was normal in gprk2 mutants at 25°C, Smo regulation was not. In wild-type wing discs, smo is ubiquitously expressed, but Smo protein levels are high in posterior compartment cells where Ptc is thought to be absent, highest in the first two rows of anterior cells adjacent to the A/P compartment boundary (where Ptc is strongly inhibited by high levels of Hh), and low in more anterior Ptc-expressing cells (Fig. 2A,E) (Capdevila and Guerrero, 1994; Denef et al., 2000; Phillips et al., 1990). In gprk2 mutants, Smo upregulation in the two cell rows abutting the A/P boundary appeared normal (Fig. 2B,F). However, Smo levels in more anterior cells were abnormally high and comparable to those of cells in the posterior compartment, as previously observed upon Gprk2 knockdown (Molnar et al., 2007). Ectopic Smo accumulation appeared to be limited to Hh-responding cells, identified by expression of dpp-LacZ. Similar observations were made at 29°C (Fig. 2C,G). In this case the domain of Smo accumulation was wider, likely reflecting farther diffusion of Hh as a result of reduced ptc induction (Chen and Struhl, 1996). Smo accumulation in gprk1 mutant discs was normal, even at 29°C (see Fig. S4B in supplementary material), indicating again that Gprk2 plays the predominant role in regulating Smo. To see if ectopic Smo accumulation was Hh-dependent, we tested the effect of removing hh in gprk2 mutants using a temperature sensitive allele (hhts2). In hhts2, gprk2 double mutant discs at the restrictive temperature, no ectopic anterior Smo accumulation was observed (Fig. 2H). We conclude that Gprk2 normally functions to downregulate Smo protein in Hh-responding cells.
Gprk2 and β-Arrestin can downregulate Smo without affecting signaling
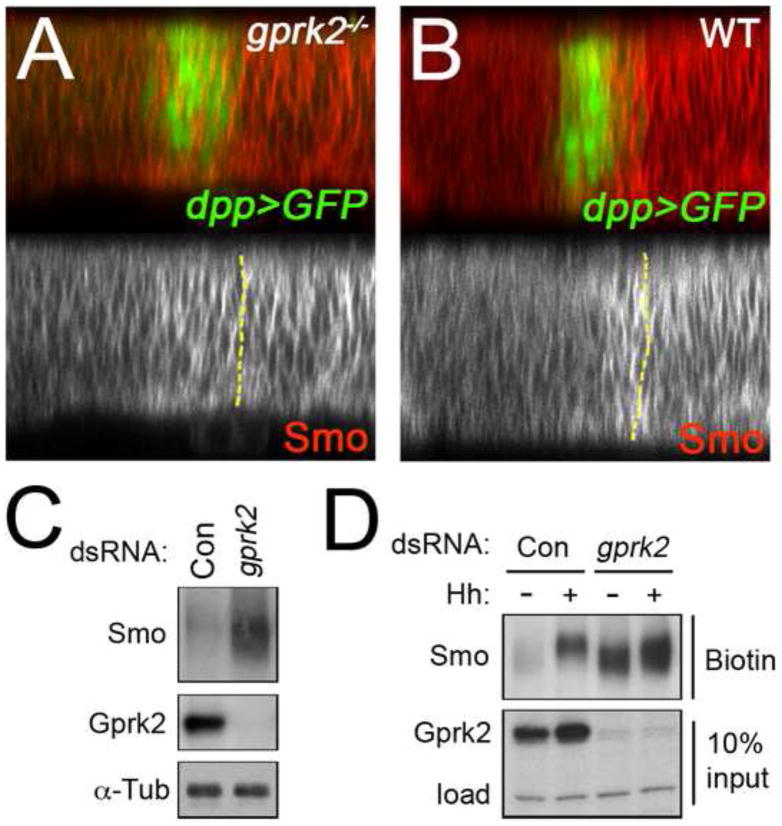
Given the role of GRKs in regulating GPCR trafficking, the simplest explanation for the observed accumulation of Smo is that Gprk2 promotes Smo internalization from the cell surface. There is some evidence for this from mammalian cell culture, where siRNA-mediated knockdown of GRK2 reduced intracellular accumulation of Smo-YFP in response to agonist treatment in transfected HEK293 cells (Chen et al., 2004). In vivo, we noted that endogenous Smo protein accumulated at the lateral surface of Hh-stimulated gprk2-mutant cells (Fig. 4A). This distribution was indistinguishable from that seen in wild-type posterior compartment cells, where Smo is constitutively active and accumulates primarily at the lateral plasma membrane (Fig. 4B) (Denef et al., 2000; Nakano et al., 2004). This suggested that Smo accumulates at the cell surface in the absence of Gprk2. To test this directly, we turned to a Drosophila S2 cell culture model. We confirmed that S2 cells express Gprk2. A protein of the expected size was detected in S2 cell lysates by Western blotting, and disappeared upon treatment with a grpk2-specific dsRNA (Fig. 4C). Endogenous Smo levels were 3.2-fold higher in gprk2 dsRNA-treated cells than in control dsRNA-treated cells (Fig. 4C). This fits with our observations in vivo, and indicates that Gprk2 functions similarly in S2 cells. To see if the ectopically accumulated Smo protein is at the plasma membrane, we biotinylated cell surface proteins on control and gprk2 dsRNA-treated S2 cells, with or without HhN stimulation. In control conditions, a minimal amount of Smo biotinylation was observed (Fig. 4D), suggesting that a small amount of basally active Smo was present on the surface of unstimulated cells (as previously observed in cl8 cells) (Denef et al., 2000). Surface levels of Smo were 3.6–times higher in cells treated with gprk2 dsRNA (Fig. 4D). This is comparable to the change in total Smo levels (Fig. 4C), suggesting that most of the Smo that accumulates is at the cell surface. Hh stimulation of control cells also increased levels of cell surface Smo (by 3.0–fold), as previously reported (Denef et al., 2000). This effect was enhanced by reducing Gprk2 levels, resulting in a 1.6 –fold increase compared to Hh-stimulated control cells (Fig. 4D). Knockdown of Gprk2 also affected Smo phosphorylation in response to Hh (see below). We conclude that Gprk2 activity is important for internalization and downregulation of Smo, both in vivo and in cultured cells. In the absence of Gprk2, Smo accumulates at the plasma membrane.
Fig. 4. Loss of Gprk2 leads to accumulation of Smo at the plasma membrane.
(A,B) Confocal optical Z-sections of wing discs from a gprk2del1/gprk2del2 mutant animal (A) or wild-type control (B). Red channel is Smo immunostaining, green channel is GFP expressed using dpp-GAL4. The anterior/posterior compartment boundary, determined by the boundary of GFP expression, is indicated in each panel by a yellow dotted line. (C) S2 cells were treated with dsRNAs targetting gfp (Control) or gprk2. Lysates were separated by SDS-PAGE and immunoblotted with antibodies against Smo (top) and Gprk2 (middle). Samples were run in parallel on a higher percentage gel and reprobed with antibody against α-Tubulin to ensure equal loading (bottom). (D) S2 cells were pretreated with dsRNA targetting gfp (Control) or gprk2, and then incubated for 2 h with control (−) or HhN (+) conditioned medium. Cell surface proteins were then labeled by surface biotinylation. After lysis, biotinylated proteins were recovered by avidin-mediated affinity purification, and separated by SDS-PAGE. Smo was detected in the biotin-labeled surface protein fraction by immunoblotting (top). 1/10 of the input was run separately and probed with an antibody against Gprk2 (middle). A background band served as a loading control (load) to ensure that the starting samples had equivalent amounts of protein (bottom).
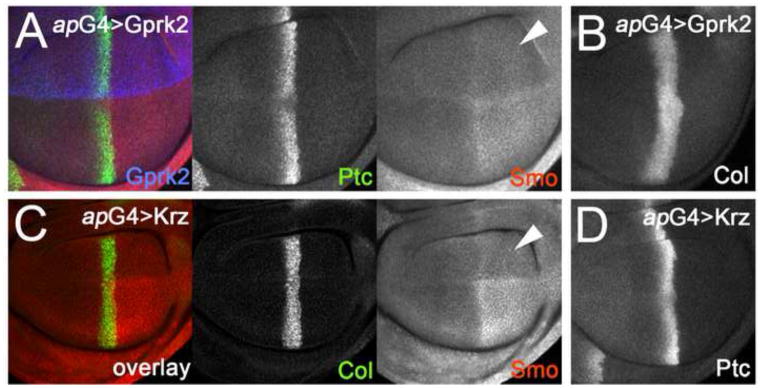
To determine whether Gprk2 is sufficient to promote Smo downregulation, we used ap-GAL4 to drive transgenic expression of gprk2 in the dorsal compartment (Fig. 5A, blue channel). Smo levels in Gprk2-overexpressing dorsal-posterior cells were lower than in wild-type ventral-posterior cells, and were more similar to those seen in the anterior compartment, confirming that Gprk2 is capable of downregulating Smo (Fig. 5A). A similar effect on Smo has been reported upon overexpression of several proteins affecting Hh signaling, including Ptc, Cos-2, dominant-negative Fu, or a mutant form of the PKA regulatory subunit which blocks PKA activity. These proteins inhibit Smo accumulation by preventing its activation. Accordingly, expression of each also caused a loss of Hh-induced Smo stabilization in anterior compartment boundary cells and of high-threshold target gene activation (Denef et al., 2000; Jia et al., 2004; Johnson et al., 1995; Liu et al., 2007). The effect of Gprk2 was different, as it did not impair signaling in Hh-stimulated cells. Anterior En, Col, and Ptc expression were normal (Fig. 5A,B; see also Fig. S5A in supplementary material). Smo stabilization was still observed in Gprk2-overexpressing cells abutting the A/P boundary, although the levels were reduced (Fig. 5A). This difference between Gprk2 and other proteins that inhibit Smo accumulation is consistent with Gprk2 acting on Smo after it has been activated and signalled. This fits well with the canonical function of GRKs - because they regulate only activated GPCRs, they often limit rather than prevent GPCR activity (Lohse, 1993).
Fig. 5. Overexpression of Gprk2 or the β-arrestin Krz decreases Smo accumulation.
(A,B) Wing discs with Gprk2 (blue channel in A) expressed throughout the dorsal compartment under the control of ap-GAL4. Smo (red channel in A) accumulation is reduced in dorsal-posterior cells (arrowhead). Target gene expression in Hh-responding dorsal-anterior cells, reflected by levels of Ptc (green channel in A) and Col (B), was unaffected. (C,D) Wing discs with HA-tagged Krz expressed throughout the dorsal compartment under the control of ap-GAL4. Smo (red channel in C) accumulation is reduced in dorsal-posterior cells (arrowhead). Target gene expression in Hh-responding dorsal-anterior cells, reflected by levels of Col (green channel in C) and Ptc (D), was unaffected.
GRKs typically regulate GPCRs by promoting their association with arrestins. β-arrestins have been implicated in Hh signaling in both mammalian cells and zebrafish embryos (Chen et al., 2004; Meloni et al., 2006; Wilbanks et al., 2004). To test whether arrestins can regulate Smo in vivo, we overexpressed an HA-tagged form of the Drosophila β-arrestin orthologue Kurtz (Krz) (Mukherjee et al., 2005). Arrestin overexpression is often sufficient to promote internalization of agonist-activated GPCRs (Ferguson et al., 1996; Goodman et al., 1996). Consistent with this, overexpression of HA-Krz with ap-GAL4 had very similar effects as Gprk2. The levels of activated Smo were reduced in dorsal-posterior Krz-expressing cells compared to wild-type ventral posterior cells (Fig. 5C). Krz overexpression had no effect on the expression of Hh target genes (Fig. 5C,D and Fig. S5B). Hh still induced Smo stabilization at the A/P boundary, although the levels were reduced (Fig. 5C). These observations demonstrate that arrestins can also control cell surface Smo accumulation in vivo. Together with the Gprk2 gain- and loss-of-function studies, these results support a model in which Gprk2 and Krz/β-arrestin control activity-dependent Smo internalization in flies.
Gprk2 promotes Smo phosphorylation in vivo
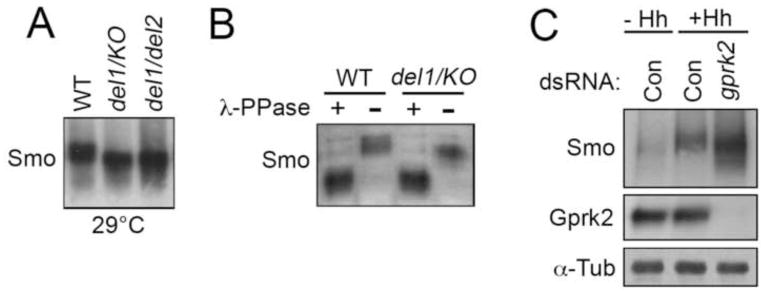
GRKs regulate GPCR internalization by directly phosphorylating the receptors (Reiter and Lefkowitz, 2006). Mammalian GRK2 has been shown to promote phosphorylation of Smo in metabolic labeling studies (Chen et al., 2004). In Drosophila, Smo is a substrate for phosphorylation by PKA and CKI, both of which are required for Smo activation. Given that Hh signaling is impaired in gprk2 mutants, we examined the phosphorylation status of Smo to see if the absence of Gprk2 affects PKA and CKI-dependent regulation. Most of the Smo protein in wild-type wing discs exists in a slow-migrating, PKA- and CKI-phosphorylated active form (Denef et al., 2000). In immunoblots of wing disc lysates from wild-type and gprk2 mutant animals cultured at 29°C, we found that endogenous Smo existed in a faster migrating form in the mutants (Fig. 6A). λ-phosphatase treatment of the samples prior to fractionation eliminated the Smo mobility difference between wild-type and gprk2 mutants, confirming that it was due to altered phosphorylation (Fig. 6B). Based on the small difference in Smo mobility in the mutants versus wild-type, compared to the large shift induced by lambda phosphatase treatment, it appears that loss of Gprk2 caused a small reduction in Smo phosphorylation.
Fig. 6. Gprk2 promotes Smo phosphorylation.
(A) Immunoblot analysis of wing disc lysates probed with antibody against Smo. Lysates were prepared from wild-type (w1118), gprk2del1/grpk2KO, and gprk2del1/grpk2del2 larvae cultured at 29°C (B) Wing disc lysates prepared from wild-type or gprk2del1/grpk2KO larvae were treated with (+) or without (−) λ-phosphatase and immunoblotted with antibody against Smo. (C) S2 cells were pretreated with dsRNA targetting gfp (Control) or gprk2, and then incubated for 2 h with control (− Hh) or HhN (+ Hh) conditioned medium. Lysates were separated by SDS-PAGE and immunoblotted with antibodies against Smo (top) and Gprk2 (middle). Samples were run in parallel on a higher percentage gel and reprobed with antibody against α-Tubulin to ensure equal loading (bottom).
We made similar observations in S2 cells. In control cells, Smo was divided between faster and slower migrating forms representing unphosphorylated and partially phosphorylated Smo (Fig. 6C). Treatment with HhN induced stabilization of Smo (which increased 1.9-fold) as well as its phosphorylation (Fig. 6C). Prior treatment of cells with gprk2 dsRNA altered the response to HhN stimulation. In addition to a 2.6-fold higher level of accumulation of Smo relative to control Hh-treated cells, the Hh-induced decrease in Smo mobility was slightly reduced, indicating that Smo was less phosphorylated (Fig. 6C; see also Fig. 4D). As in wing discs, however, Smo was still phosphorylated in response to Hh. This suggests that Hh-dependent regulation by PKA and CKI still occurs in the absence of Gprk2. Together with the effects of Gprk2 on Smo membrane accumulation, our results suggest that Gprk2 promotes Smo internalization after it is activated and re-localized to the plasma membrane, possibly by direct phosphorylation, consistent with the mechanism of regulation of other GPCRs by GRKs.
Distinct Ptc-dependent and Gprk2-dependent mechanisms of Smo regulation
As both Ptc and Gprk2 affect Smo accumulation, we were interested in exploring the relationship between the two. In both wild-type and gprk2 mutant wing discs, we consistently noticed a region with lower levels of Ptc situated between the A/P boundary (where Ptc is upregulated) and the far anterior compartment (where Ptc is expressed at “baseline” levels) (Fig. 2A–C, quantified in Fig. 2E–G). dpp-LacZ was expressed throughout the “low-Ptc” region, indicating that it was Hh-responding cells that had reduced Ptc levels.
One explanation for the very low levels of Ptc in Hh-responding cells away from the A/P boundary is that Hh binding promotes Ptc internalization and degradation, as previously observed in cultured cells (Denef et al., 2000; Incardona et al., 2002). If so, we predicted that the width of the “low-Ptc” domain would change if the Hh gradient were expanded or contracted. To test the effects of expanding the gradient, we overexpressed Hh within its endogenous domain using hh-GAL4. This induced typical gain-of-function phenotypes including overgrowth of the anterior compartment (Fig. 7A) (Ingham and Fietz, 1995) Under these conditions, the anterior “low-Ptc” domain was also expanded, extending throughout most of the anterior wing pouch (Fig. 7A). Ptc levels returned to baseline only in the anterior-most region. Also in agreement with our prediction, elimination of a functional Hh gradient by culturing hhts2 animals at restrictive temperature led to the disappearance of the anterior “low-Ptc” domain (Fig. 7B). Because ptc is a Hh target gene, it is possible that these changes were due to altered ptc transcription. To test this, we examined Ptc levels in clones of cells in which Hh signaling was abolished by expression of a dsRNA transgene targeting Smo. Smo dsRNA-expressing cells adjacent to the A/P compartment boundary failed to induce Hh target genes, confirming that the transgene was functional (see Fig. S6 in supplementary material). Expression of Smo dsRNA in clones within the “low-Ptc” domain had no effect on Ptc levels (Fig. 7C), indicating that the reduction in Ptc levels in this region is independent of Hh pathway activity. Together, these observations are consistent with the downregulation of Ptc levels being attributable to Hh-induced turnover. The fact that Ptc levels are low in Hh-responding cells away from the A/P boundary suggests that Ptc activity may also be low in these cells.
There was a striking complementarity in the patterns of Smo and Ptc accumulation in gprk2 mutants. Cells within the “low-Ptc” region showed ectopic Smo accumulation, whereas far anterior compartment cells with basal levels of Ptc did not (Fig. 2A-C and E-G). This suggested to us that Smo protein levels may be controlled by two distinct mechanisms, one Ptc-dependent (in the absence of Hh) and the other Gprk2-dependent (in Hh-responding cells). To see if Ptc and Gprk2 function independently, we tested whether one could downregulate Smo in the absence of the other. The observation that Gprk2 overexpression is sufficient to reduce Smo levels in posterior compartment cells (Fig. 5A), which are not thought to express Ptc (Capdevila and Guerrero, 1994; Phillips et al., 1990), suggests that Gprk2 functions independently of Ptc. To rule out any requirement for Ptc, we used the MARCM system (Lee and Luo, 2001) to express Gprk2 in ptc mutant clones. In ptcS2 homozygous clones situated in the posterior compartment, Smo levels were unchanged (data not shown). However, when Gprk2 was overexpressed in posterior ptcS2 clones there was a reduction in Smo levels (Fig. 7D), confirming that Gprk2 downregulates Smo independent of Ptc. The converse was also true. Overexpression of Ptc throughout the dorsal compartment of gprk2 mutant wing discs specifically reduced dorsal Smo levels, including in the region where Smo ectopically accumulates (Fig. 7E, arrowhead). Thus Ptc is capable of downregulating Smo in the absence of Gprk2. We conclude that Smo undergoes at least two distinct modes of downregulation in flies, one Ptc-dependent and the other Gprk2-dependent.
DISCUSSION
Studies in several systems have highlighted the strong correlation between Smo activity and cell surface accumulation. Activation of Smo by a number of different means is consistently associated with its accumulation at the cell surface (Corbit et al., 2005; Denef et al., 2000; Jia et al., 2004; Zhang et al., 2004, Nakano, 2004 #480; Zhu et al., 2003). In normal tissues, it appears the situation is more complex. In the wing disc, posterior compartment cells lacking Ptc or anterior cells exposed to the very highest levels of Hh do indeed show significant stabilization of endogenous Smo. However, Hh-responding anterior cells farther from the source of Hh have only low levels of Smo, despite the fact that Ptc levels are reduced and the downstream signaling pathway is active. Our results indicate that this is due to the activity of Gprk2, which promotes internalization and downregulation of Smo in Hh-responding cells.
For many GPCRs, internalization is controlled by two distinct mechanisms. In the absence of ligand these receptors undergo constitutive or tonic endocytosis, which contributes to receptor homeostasis (Moore et al., 2007). Once activated by ligand, GPCR trafficking changes to an activity-dependent mode of internalization, typically mediated by GRKs and arrestins. Our results suggest that Smo undergoes two distinct modes of internalization leading to its downregulation in flies, reminiscent of constitutive and activity-dependent regulation of GPCRs. Constitutive turnover is controlled by Ptc. In wing discs, Ptc-dependent regulation of Smo was evident in far anterior cells, beyond the range of Hh diffusion. These cells produce baseline levels of Ptc sufficient to prevent Smo accumulation and keep the signaling pathway inactive (Capdevila et al., 1994a; Denef et al., 2000). Smo downregulation in far anterior cells was normal in gprk2 mutants, suggesting that Ptc-dependent constitutive regulation of Smo does not require Gprk2. This was confirmed by the observation that overexpressed Ptc was capable of downregulating Smo in gprk2 mutants. In contrast, downregulation of Smo in Hh-responding cells is controlled by Gprk2. In the absence of Gprk2, Smo ectopically accumulated in response to Hh stimulation. Ectopic Smo accumulation was confined to cells exposed to very high levels of Hh or with below-baseline levels of Ptc. This suggests that Gprk2 independently controls Smo levels in cells in which Ptc activity is reduced. This was confirmed by the demonstration that Gprk2 overexpression can downregulate Smo in ptc mutant posterior cells.
Knockdown of specific GRKs can increase cell surface levels of GRK-regulated receptors after agonist stimulation. Conversely, GRK or arrestin overexpression can promote GPCR internalization (Claing et al., 2002; Kim et al., 2005). In this respect, Smo behaves like a typical GPCR. Knockdown of Gprk2 in S2 cells led to increased accumulation of Smo at the plasma membrane, and the same appeared to be true in imaginal discs, whereas overexpression of Gprk2 or the β-arrestin orthologue Krz led to a reduction of Smo levels. This is the first evidence that arrestins participate in Smo regulation in flies, as they do in mammalian cells and zebrafish (Kovacs et al., 2008; Wilbanks et al., 2004). However, Smo levels were unchanged in krz mutant wing disc clones (data not shown), suggesting that one of the other Drosophila arrestins may be involved in Smo regulation, either alone or redundantly with Krz.
Several observations suggest that Gprk2 regulates Smo in an activity-dependent manner. First, the ectopic Smo accumulation observed in gprk2 mutants was restricted to Hh-responding cells and was Hh-dependent. Second, overexpression of Gprk2 or Krz downregulated Smo levels in posterior compartment cells, where it is constitutively activated. Third, unlike other proteins that downregulate Smo by preventing its activation (Denef et al., 2000; Jia et al., 2004; Johnson et al., 1995; Liu et al., 2007), overexpression of Gprk2 or Krz had no effect on Hh signaling. This fits well with the canonical function of GRKs, which act on the receptors only after they have been activated and often limit rather than prevent GPCR signaling (Lohse, 1993). Activity-dependent regulation of Smo by Gprk2 may provide an explanation for two somewhat surprising observations – first, that Smo levels are highest in A/P boundary cells, which also have the highest levels of Gprk2; and second, that Gprk2 overexpression reduced but did not eliminate Smo “hyper-accumulation” in these cells. The A/P boundary cells are exposed to the highest levels of Hh. The accumulation of Smo in these cells despite the presence of high levels of Gprk2 could be because the rate of Smo activation and plasma membrane translocation is greater than the rate at which activated Smo is phosphorlyated by Gprk2 and subsequently internalized. It is also possible that Smo is protected from Gprk2-dependent downregulation in strongly-stimulated cells.
Our analysis of Smo phosphorylation further supports the idea that Smo is regulated like other GPCRs in vivo. We found that Smo is hypophosphorylated in gprk2 mutant wing discs and in Hh-treated S2 cells after gprk2 knockdown. Smo is still heavily phosphorylated in both systems. We showed that this phosphorylation is Hh-dependent in S2 cells, suggesting that activating phosphorylation by PKA and CKI still occurs in the absence of Gprk2. The effects on Smo phosphorylation are most consistent with a model in which Gprk2 directly phosphorylates activated Smo, to promote its internalization.
GRKs and Hh signaling
We found that the effects of gprk2 deletion are temperature-sensitive. Whereas most mutant animals survived when cultured at 25°C, the majority died before reaching adulthood when raised at 29°C. Those that survived showed moderate Hh loss-of-function wing phenotypes and reduced Hh target gene activation in discs. These observation clearly indicate that Gprk2 is involved in Hh signaling, as previously observed upon dsRNA-mediated knockdown of gprk2 expression (Molnar et al., 2007). We note that the Hh signaling defects we observed in gprk2 null mutants were less severe than those obtained using the gprk2 dsRNA, although in both cases experiments were conducted at 29°C. This suggests that the dsRNA construct that was used may have off-target effects on other transcripts, possibly including gprk1. By analyzing animals mutant for both Drosophila GRKs, we found that Smo signaling in gprk2 mutants is partially rescued by Gprk1. gprk1/gprk2 double homozygous mutants were not viable. However, a single gene copy of gprk2 was sufficient to support all developmental GRK functions in the absence of Gprk1. In contrast, gprk2 homozygous mutants with only a single gene copy of gprk1 showed reduced Hh signaling, even at 25°C. This is the first demonstration that Gprk1 and Gprk2 share some essential redundant functions. Our results suggest that Gprk2 is the principal GRK required for Hh signaling, but Gprk1 can substitute, albeit less efficiently, at 25°C. Inefficient rescue by Gprk1 could explain the temperature-dependent effects of gprk2 deletion alleles on Hh signaling, for example if Gprk1 is unable to stably interact with Smo (or other targets) at higher temperatures.
Studies in several systems have now shown that reduction of GRKs by a variety of means impairs Hh signaling. This is somewhat surprising, for two reasons. First, because GRKs function to reduce GPCR signaling, their loss typically leads to receptor hypersensitivity and excessive rather than reduced signaling in vivo (Premont and Gainetdinov, 2007). Second, given the strong correlation between Smo accumulation at the plasma membrane and activity, one would expect ectopic Smo accumulation to result in too much rather than too little signaling. One possible explanation for this apparent contradiction is that Gprk2 phosphorylation contributes to the process of Smo activation, along with PKA and CKI. According to this model, Smo would be unable to reach its most active state in the absence of phosphorylation by Gprk2. It is also conceivable that activity-dependent internalization of Smo promotes its signaling activity. For many GPCRs, GRK and arrestin-dependent internalization leads to recycling of the receptor to the cell surface, which could potentiate signaling (Moore et al., 2007; Reiter and Lefkowitz, 2006). In other cases, internalization is required for assembling G-protein-independent GPCR-associated signaling complexes (Reiter and Lefkowitz, 2006). β-arrestins were required for association of mammalian Smo with Kif3a, a subunit of the kinesin II motor complex involved in transport of proteins within cilia. Kif3a plays a role in the mammalian pathway that is in some ways similar to Cos2 (Huangfu and Anderson, 2005; Kovacs et al., 2008). It may be that association of Cos2 with Smo likewise depends on Gprk2/arrestins.
Finally, it is important to note that the effects of mutating GRKs are almost certainly not restricted to misregulation of Smo. Indeed, at 29°C, most gprk2 mutants fail to survive to adulthood, but those that do show only moderate Hh signaling defects. Misregulation of other GPCRs may well contribute to the lethality. If so, the effects of GRK deletion on GPCR signaling and second messenger production could be complex, depending on which receptors are active and whether they couple to stimulatory or inhibitory G-proteins. Reduction of Gprk2 levels in female fly ovaries does affect bulk cAMP levels, reducing them to one-third the normal level in this tissue (Lannutti and Schneider, 2001). G-protein-dependent regulation of cAMP levels downstream of Smo is important for Hh signaling, likely through effects on PKA activity (Ogden et al., 2008). Misregulation of cAMP metabolism in GRK mutants, either as a direct consequence of altered G-protein-mediated signaling by Smo or more global changes in GPCR activity, could have profound effects on Hh signaling output. Additional work will be required to define the precise mechanism by which Gprk2 affects Hh signaling.
Supplementary Material
Acknowledgments
We thank Stephen Cohen, Spyros Artavanis-Tsakonas, Tom Kornberg, Michèle Crozatier, and Dan Kiehart for generously providing reagents, Stephen Cohen, William Brook, and Marko Horb for helpful comments on the manuscript, and Karen Oh for expert technical assistance. This work was supported by Canadian Institutes of Health Research grant #81187. D.R.H. is supported by a Junior II Research Scholarship from Fonds de la recherche en santé Québec.
References
- Apionishev S, Katanayeva NM, Marks SA, Kalderon D, Tomlinson A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nat Cell Biol. 2005;7:86–92. doi: 10.1038/ncb1210. [DOI] [PubMed] [Google Scholar]
- Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Estrada MP, Sanchez-Herrero E, Guerrero I. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. Embo J. 1994a;13:71–82. doi: 10.1002/j.1460-2075.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. Embo J. 1994;13:4459–68. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, Pariente F, Sampedro J, Alonso JL, Guerrero I. Subcellular localization of the segment polarity protein patched suggests an interaction with the wingless reception complex in Drosophila embryos. Development. 1994b;120:987–98. doi: 10.1242/dev.120.4.987. [DOI] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–60. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–31. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, 3rd, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–6. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Gallet A, Therond PP. Temporal modulation of the Hedgehog morphogen gradient by a patched-dependent targeting to lysosomal compartment. Dev Biol. 2005;277:51–62. doi: 10.1016/j.ydbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Glise B, Jones DL, Ingham PW. Notch and Wingless modulate the response of cells to Hedgehog signalling in the Drosophila wing. Dev Biol. 2002;248:93–106. doi: 10.1006/dbio.2002.0720. [DOI] [PubMed] [Google Scholar]
- Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2556–61. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–50. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–12. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Ingham P. Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- Hipfner DR, Keller N, Cohen SM. Slik Sterile-20 kinase regulates Moesin activity to promote epithelial integrity during tissue growth. Genes Dev. 2004;18:2243–8. doi: 10.1101/gad.303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Gruenberg J, Roelink H. Sonic hedgehog induces the segregation of patched and smoothened in endosomes. Curr Biol. 2002;12:983–95. doi: 10.1016/s0960-9822(02)00895-3. [DOI] [PubMed] [Google Scholar]
- Incardona JP, Lee JH, Robertson CP, Enga K, Kapur RP, Roelink H. Receptor-mediated endocytosis of soluble and membrane-tethered Sonic hedgehog by Patched-1. Proc Natl Acad Sci U S A. 2000;97:12044–9. doi: 10.1073/pnas.220251997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, Fietz MJ. Quantitative effects of hedgehog and decapentaplegic activity on the patterning of the Drosophila wing. Curr Biol. 1995;5:432–40. doi: 10.1016/s0960-9822(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr Biol. 2000;10:1315–8. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- Jia J, Tong C, Wang B, Luo L, Jiang J. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature. 2004;432:1045–50. doi: 10.1038/nature03179. [DOI] [PubMed] [Google Scholar]
- Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Grenier JK, Scott MP. patched overexpression alters wing disc size and pattern: transcriptional and post-transcriptional effects on hedgehog targets. Development. 1995;121:4161–70. doi: 10.1242/dev.121.12.4161. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–7. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–81. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannutti BJ, Schneider LE. Gprk2 controls cAMP levels in Drosophila development. Dev Biol. 2001;233:174–85. doi: 10.1006/dbio.2001.0219. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Montell C. Rhodopsin kinase activity modulates the amplitude of the visual response in Drosophila. Proc Natl Acad Sci U S A. 2004;101:11874–9. doi: 10.1073/pnas.0402205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–4. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao X, Jiang J, Jia J. Fused-Costal2 protein complex regulates Hedgehog-induced Smo phosphorylation and cell-surface accumulation. Genes Dev. 2007;21:1949–63. doi: 10.1101/gad.1557407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ. Molecular mechanisms of membrane receptor desensitization. Biochim Biophys Acta. 1993;1179:171–88. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- Martin V, Carrillo G, Torroja C, Guerrero I. The sterol-sensing domain of Patched protein seems to control Smoothened activity through Patched vesicular trafficking. Curr Biol. 2001;11:601–7. doi: 10.1016/s0960-9822(01)00178-6. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- Meloni AR, Fralish GB, Kelly P, Salahpour A, Chen JK, Wechsler-Reya RJ, Lefkowitz RJ, Caron MG. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26:7550–60. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar C, Holguin H, Mayor F, Jr, Ruiz-Gomez A, de Celis JF. The G protein-coupled receptor regulatory kinase GPRK2 participates in Hedgehog signaling in Drosophila. Proc Natl Acad Sci U S A. 2007;104:7963–8. doi: 10.1073/pnas.0702374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol. 2005;7:1191–201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Nystedt S, Shivdasani AA, Strutt H, Thomas C, Ingham PW. Functional domains and sub-cellular distribution of the Hedgehog transducing protein Smoothened in Drosophila. Mech Dev. 2004;121:507–18. doi: 10.1016/j.mod.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis E, Hui CC. Hedgehog signaling and congenital malformations. Clin Genet. 2005;67:193–208. doi: 10.1111/j.1399-0004.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456:967–70. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–92. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Philipp M, Fralish GB, Meloni AR, Chen W, MacInnes AW, Barak LS, Caron MG. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol Biol Cell. 2008;19:5478–89. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Roberts IJ, Ingham PW, Whittle JR. The Drosophila segment polarity gene patched is involved in a position-signalling mechanism in imaginal discs. Development. 1990;110:105–14. doi: 10.1242/dev.110.1.105. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–34. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Schneider LE, Spradling AC. The Drosophila G-protein-coupled receptor kinase homologue Gprk2 is required for egg morphogenesis. Development. 1997;124:2591–602. doi: 10.1242/dev.124.13.2591. [DOI] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. A Hedgehog activity gradient contributes to AP axial patterning of the Drosophila wing. Development. 1997;124:4697–705. doi: 10.1242/dev.124.22.4697. [DOI] [PubMed] [Google Scholar]
- Strutt H, Thomas C, Nakano Y, Stark D, Neave B, Taylor AM, Ingham PW. Mutations in the sterol-sensing domain of Patched suggest a role for vesicular trafficking in Smoothened regulation. Curr Biol. 2001;11:608–13. doi: 10.1016/s0960-9822(01)00179-8. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, Ryner L, Cheung LM, Chong A, Erickson C, Fisher WW, Greer K, Hartouni SR, Howie E, Jakkula L, Joo D, Killpack K, Laufer A, Mazzotta J, Smith RD, Stevens LM, Stuber C, Tan LR, Ventura R, Woo A, Zakrajsek I, Zhao L, Chen F, Swimmer C, Kopczynski C, Duyk G, Winberg ML, Margolis J. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–7. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–7. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- Zhang C, Williams EH, Guo Y, Lum L, Beachy PA. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc Natl Acad Sci U S A. 2004;101:17900–7. doi: 10.1073/pnas.0408093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–8. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Zheng L, Suyama K, Scott MP. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 2003;17:1240–52. doi: 10.1101/gad.1080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.