Abstract
Campylobacter concisus infections of the gastrointestinal tract can be accompanied by diarrhea and inflammation, whereas colonization of the human oral cavity might have a commensal nature. We focus on the pathophysiology of C. concisus and the effects of different clinical oral and fecal C. concisus strains on human HT-29/B6 colon cells. Six oral and eight fecal strains of C. concisus were isolated. Mucus-producing HT-29/B6 epithelial monolayers were infected with the C. concisus strains. Transepithelial electrical resistance (Rt) and tracer fluxes of different molecule size were measured in Ussing chambers. Tight junction (TJ) protein expression was determined by Western blotting, and subcellular TJ distribution was analyzed by confocal laser-scanning microscopy. Apoptosis induction was examined by TUNEL-staining and Western blot of caspase-3 activation. All strains invaded confluent HT-29/B6 cells and impaired epithelial barrier function, characterized by a time- and dose-dependent decrease in Rt either after infection from the apical side but even more from the basolateral compartment. TJ protein expression changes were sparse, only in apoptotic areas of infected monolayers TJ proteins were redistributed. Solely the barrier-forming TJ protein claudin-5 showed a reduced expression level to 66±8% (P<0.05), by expression regulation from the gene. Concomitantly, Lactate dehydrogenase release was elevated to 3.1±0.3% versus 0.7±0.1% in control (P<0.001), suggesting cytotoxic effects. Furthermore, oral and fecal C. concisus strains elevated apoptotic events to 5-fold. C. concisus-infected monolayers revealed an increased permeability for 332 Da fluorescein (1.74±0.13 vs. 0.56±0.17 10−6 cm/s in control, P<0.05) but showed no difference in permeability for 4 kDa FITC-dextran (FD-4). The same was true in camptothecin-exposed monolayers, where camptothecin was used for apoptosis induction.
In conclusion, epithelial barrier dysfunction by oral and fecal C. concisus strains could mainly be assigned to apoptotic leaks together with moderate TJ changes, demonstrating a leak-flux mechanism that parallels the clinical manifestation of diarrhea.
Introduction
Campylobacter concisus, first isolated from the human oral cavity, has been proposed as an emerging human enteric pathogen [1]. In contrast to other Campylobacter species no primary animal reservoir has been found for C. concisus, although it has been detected in diarrheic fecal samples from domestic dogs [2]. Recently, Zhang and coworkers found a very high prevalence of C. concisus in saliva from healthy individuals and patients with Crohn's disease suggesting it is an oral commensal rather than a clinical significant oral pathogen [3]. A potential role of emerging C. concisus in human gastrointestinal disease has been proposed, and recent studies describe a high incidence of C. concisus in pediatric diarrheic stool samples as well as in immunocompromised patients with diarrhea in a tertiary hospital setting [1], [4], [5]. However, the overall prevalence of C. concisus in feces is underreported because an easy-to-handle standardized selective culture medium is not available and the time-consuming filter method is not included in standard diagnostics. In a mouse model C. concisus induced weight loss and microabscesses in the liver [6], whereas there is only sparse data on the pathophysiology when the microorganism is introduced to the human intestine. C. concisus possesses virulence factors including secreted and cell-bound hemolytic activities as well as several putative virulence factors e.g. cytolethal distending toxin [7], [8]. Recently, C. concisus genes coding for a presumed Zonula occludens toxin (ZOT) as well as a surface-layer protein belonging to the RTX (repeats in toxin) toxins has been identified [8]. Even though these toxins are recognized as important virulence factors their pathogenic role in C. concisus infection is unknown. In a recent work by Kalischuk and Inglis the ZOT gene was detected more often in C. concisus isolates from healthy individuals than in isolates from diarrheic humans [9]. In consistence with earlier reports [10], the authors identified two main genetically distinct clusters which differed with respect to their pathogenic properties. ALFP cluster 2 isolates were predominantly found in diarrheic patients and showed higher ability in epithelial invasion and translocation. In contrary, C. concisus ALFP cluster 1 were mainly found in fecal isolates from healthy individuals and are present in the oral reference strain ATCC 33237. These strains harbor hemolytic activity and can induce apoptotic DNA fragmentation. Man and coworkers reported recently that C. concisus is able to attach to and invade intestinal epithelial cells, and moreover C. concisus was observed to invade adjacent to the tight junction (TJ) [11]. In the current study we focus on the pathophysiology of C. concisus and describe the effects on epithelial barrier function of different oral and fecal clinical C. concisus strains, using the human colon cell line HT-29/B6.
Results
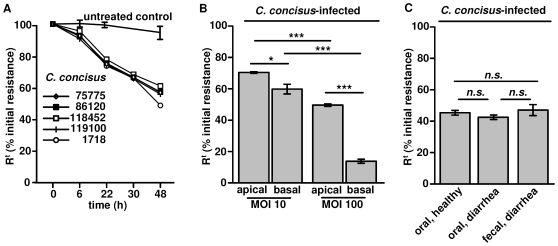
As shown in Figure 1A the incremental decrease in Rt, beginning 22 h after bacterial inoculation (P<0.05, and at 48 h: P<0.001; Student's t-test) showed the same characteristic with all strains used, including ATCC 33237. HT-29/B6 monolayers that were apically or basolaterally infected with C. concisus at MOIs between 10 and 100 showed a dose-dependent decrease in Rt after 48 h, whereas infection from the basal side revealed a stronger response in Rt (Figure 1B ). All oral and fecal strains had similar effects on Rt as shown in Figure 1C .
Figure 1. Effect of Campylobacter concisus on epithelial barrier function.
( A ) Different Campylobacter concisus strains on HT-29/B6 cells. Time-dependent decrease in transepithelial electrical resistance (Rt). ( B ) Dose-dependent effects on Rt after infection with C. concisus 1718 from either apical or basal side in HT-29/B6 monolayers 48 h postinfection. ( C ) Comparison of the effect on Rt of C. concisus isolates from oral or fecal source 48 h p.i. Isolates were grouped depending on source in “healthy” (strain no. K2 oral, K4 oral, M2 oral) and “diarrhea” (strain no. 112100 fecal & oral, 113332 fecal & oral, 115605 fecal & oral) as listed in table 1. (n.s. = not significant; *P<0.05; *** P<0.001).
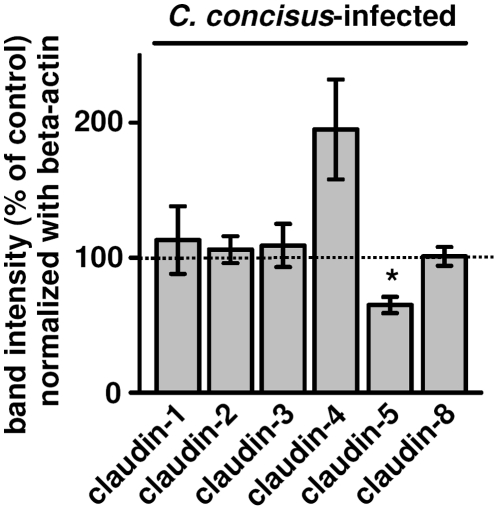
The expression of TJ proteins claudin-1 to -3, -8, occludin and zonula occludens protein-1 (ZO-1) was not changed 48 h postinfection. Only claudin-5 showed a reduced expression level to 66±8% of the control value in whole cell lysate protein preparations (P<0.05, n = 9), whereas claudin-4 showed a tendency towards upregulation which did not reach statistical significance (194±37% of control, n = 9, n.s.) (Figure 2). ZO-1 distribution in C. concisus-infected cells differs only with respect to Triton-X 100 (TX)-soluble and TX-insoluble protein fractions [11], therefore we checked this feature also for the claudins. All claudins showed a slight drift from TX-insoluble to TX-soluble protein preparations. This drift of claudins out of the TJ to intracellular compartments could not be confirmed in confocal laser-scanning microscopy of immunostainings (data not shown). However, 24 h postinfection claudin-5 mRNA level was reduced to 49±16% (P<0.05, n = 4), suggesting expression regulation from the gene, likewise in Arcobacter butzleri infection [12]; a close relative, belonging to the epsilon-proteobacteria.
Figure 2. Effects on tight junction proteins.
Western blot analysis on the expression of tight junction proteins in HT-29/B6 cells 48 h after infection with C. concisus. Densitometry on claudin-1 to -4 and -8 showed no significant difference to control values. Solely claudin-5 expression was reduced with *P<0.05; each n = 9. HT-29/B6 cells were infected with either oral or fecal strains C. concisus K2 oral, K4 oral, M2 oral, 75775 fecal, 86120 fecal, 118452 fecal, 119100 fecal, 1718 fecal or ATCC 33237.
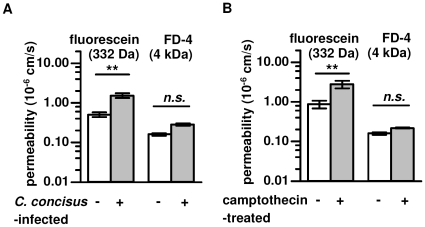
To further characterize epithelial barrier function, the permeability to fluorescein or FD-4 was measured. After 48 h of mucosal exposure to C. concisus, Rt of the HT-29/B6 monolayers had decreased to 60%, whereas the short-circuit current was not induced but rather slightly diminished (2.9±0.2 after C. concisus infection versus 3.2±0.2 µmol h−1 cm−2 in control, n.s., n = 5). Concomitantly, permeability for fluorescein was increased after C. concisus infection (from 0.56±0.17 in control to 1.74±0.13 10−6 cm/s, P<0.01, n = 4) and permeability for FD-4 was unchanged (from 0.14±0.06 in control to 0.29±0.11 10−6 cm/s, n.s., n = 4) (Figure 3A ). To simulate the effects of C. concisus in apoptosis induction we used camptothecin (20 µg/ml) as positive control for 48 h. Camptothecin treatment of HT-29/B6 monolayers revealed similar apoptotic effects, with no changes in TJ integrity [13]. Fluorescein permeability was increased to the same extent as C. concisus-infected cells (from 0.84±0.33 in control to 3.09±0.38 10−6 cm/s, P<0.01, n = 4), whereas permeability to FD-4 was unchanged (from 0.15±0.06 in control to 0.21±0.03 10−6 cm/s, n.s., n = 4, Figure 3B ).
Figure 3. Epithelial permeability.
Paracellular permeability was characterized by determining 332 Da fluorescein or 4 kDa FITC-dextran (FD-4) fluxes through ( A ) C. concisus-infected 48 h p.i. or ( B ) 48 h camptothecin-treated HT-29/B6 monolayers in Ussing chamber experiments (Student's t-test with *P<0.05, **P<0.01;***P<0.001; each n = 4). Infection with pooled C. concisus strains (oral & fecal) vs. untreated control monolayers were performed.
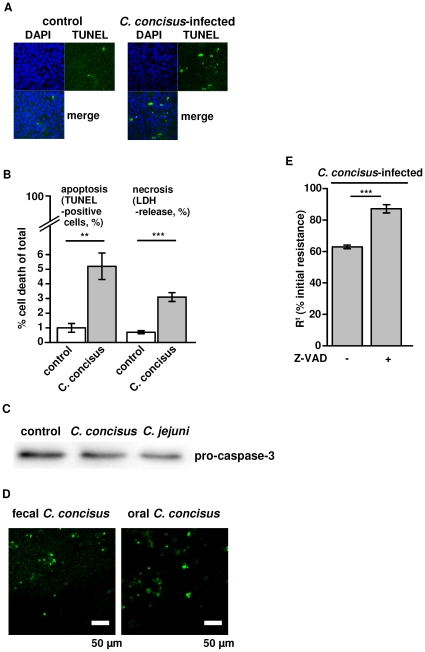
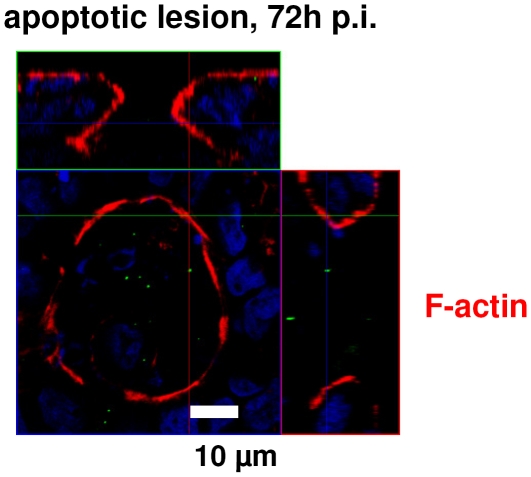
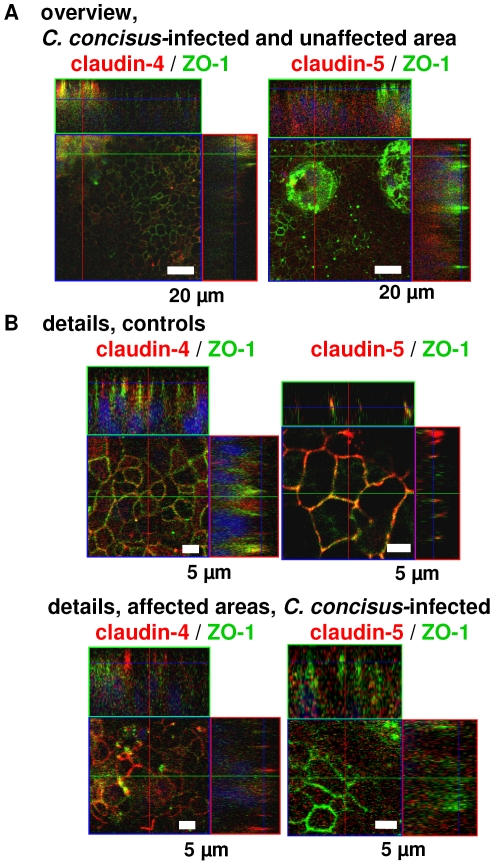
The barrier dysfunction after infection could on the one hand result from changes or disruption of TJ strands but on the other hand also from a loss of epithelial cells by apoptosis or necrosis induction. Apoptosis as examined histologically in TUNEL staining indicated an increased apoptotic ratio in C. concisus-infected cells (Figure 4A ). Furthermore, LDH release was determined as marker for cytotoxicity. LDH release was elevated to 3.1±0.3% versus 0.7±0.1% in control (P<0.001; n = 5) suggesting cytotoxic effects caused by C. concisus (Figure 4B ). After 48 h infected monolayers showed a 5-fold increase in epithelial apoptosis (5.2±0.9% vs. 1.0±0.3% in control; P<0.01; n = 5). In Western blots, pro-caspase-3 band intensity decreased to 70±2% in infected monolayers (P<0.05, n = 7, Figure 4C ), but the caspase-3 activation was weaker when compared to the effects of C. jejuni ATCC 33560, where pro-caspase-3 was decreased to 52±6% of control values (P<0.01, C. concisus vs. C. jejuni, n = 4), while the apoptotic ratio was equal between C. concisus- and C. jejuni-infected cells (4.6±0.3%, n.s. vs. C. concisus, n = 4). Fecal and oral strains did not differ in their ability to induce apoptosis in HT-29/B6 cells with 4.8±1.4% by oral strains vs. 4.7±0.5% apoptosis by fecal strains (n.s., n = 5; Figure 4D ). Pre-treatment with the apoptosis inhibitor Z-VAD showed a reduced loss of Rt in C. concisus-infected cells (at 48 h, C. concisus 112100 fecal & oral; without Z-VAD 63.5±1.5% vs. with Z-VAD 86.7±2.9% of initial resistance, P<0.001; n = 8; Figure 4E ). Moreover, when we prolonged the incubation time to 72 h, we detected C. concisus next to or in epithelial lesions with restitutional purse-string formation in confocal laser-scanning micrographs (Figure 5 and Figure S1). Also, patchy distributed apoptotic areas with TJ changes were found beside largely unaffected areas in each monolayer (Figure 6). As an example it is shown in Figure 6 that claudins of infected monolayers were not clearly redistributed towards intracellular compartments.
Figure 4. Effects of C. concisus on epithelial apoptosis.
( A ) Apoptotic effects of C. concisus on confluent HT-29/B6 cells visualized with TUNEL staining and fluorescence microscopy 48 h after infection, the number of cell rosette formations was increased, condensed or fragmentized nuclei were visible, and a more generalized loss of cells was observed. ( B ) After 48 h of incubation with C.concisus TUNEL staining of confluent HT-29/B6 monolayers revealed an increase in the number of apoptotic cells and LDH release was measured as a sign of necrosis induction (Student's t-test with **P<0.01; ***P<0.001; each n = 5). ( C ) Increased apoptosis was indicated by caspase-3 activation 48 h after infection. Band intensity of pro-caspase-3 (35 kDa) was significantly diminished (P<0.05, n = 7). C. jejuni ATCC 33560 served as positive control. ( D ) After 48 h of incubation with C. concisus from oral or fecal source apoptotic cell number was equal in TUNEL staining of confluent HT-29/B6 monolayers (n.s., n = 5). ( E ) For verification of C. concisus-induced apoptosis effects on barrier function, HT-29/B6 monoalyers were pre-treated with 50 µM Z-VAD (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl-ketone), a caspase inhibitor that completely blocks caspase-mediated cell death. Transepithelial resistance was partially although not completely reconstituted by Z-VAD. Isolates were grouped depending on source in “oral” and “fecal” as listed in table 1.
Figure 5. Apoptotic leaks.
Confocal laser-scanning microscopy (Z-axis scans, XY plane) of an apoptotic lesion in HT-29/B6 monolayers infected with C. concisus. Cytoskeletal F-actin is marked red by Phalloidin-Alexa-Fluor594, the blue staining with DAPI ( = 4′-6-diamidino-2-phenylindole dihydrochloride) marks the nuclei of epithelial cells and C. concisus is marked green by anti-Campylobacter antibody. Details are presented in Figure S1. In controls, superficial single cell lesions were closed within 16 min, accompanied by formation of an actin ring (“purse-string”) that is essential for restitution. Recovery of apoptotic lesions, induced by C. concisus, seems unaffected as the purse-string actin formation is clearly visible.
Figure 6. Apoptosis and tight junctions.
Confocal laser-scanning microscopy of HT-29/B6 monolayers revealed patchy distributed areas with tight junction changes and apoptotic events as well as largely unaffected areas in ( A ) overviews with low magnification and ( B ) details with higher magnifcation. Immunostaining with green signal for ZO-1; and claudin-4 and -5 marked red; merge appears yellow. Nuclei are stained blue with DAPI.
Discussion
As a main finding, we demonstrated that C. concisus was able to induce barrier dysfunction in confluent HT-29/B6 cells from apical but even more from the basolateral compartment. Although controversially discussed [9], [11], also oral C. concisus strains, even from healthy carriers, had the ability to impair epithelial barrier function. Man and coworkers demonstrated the polar flagellum of C. concisus to be involved in attachment and invasion of Caco-2-cells [11]. We found similar invasion abilities for our C. concisus strains in HT-29/B6 as well as Caco-2-cells (data not shown). Recently, it was also reported that C. concisus translocated across the Caco-2 monolayers and suggested that the concomitant increase in permeability for 44 kDa horseradish peroxidase (HRP) 6 h postinfection was through a paracellular pathway [11]. In contrast to this, Söderholm and coworkers reported that HRP fluxes through the epithelium were due to a transcytotic pathway via HRP-containing endosomes [14], at least in intact epithelial layers. Therefore it might be that Campylobacter invasion is rather correlated with the transcytosis ratio (macropinocytosis) and not with a paracellular disruption, but perhaps a spatial TJ redistribution is accelerated by this process. However, the causal link between the barrier dysfunction of C. concisus-infected cells and a pathomechanism via the paracellular pathway by apoptosis induction and/or TJ affection ( = leak-flux dysfunction) is supported by our data. We investigated the protein expression of barrier-forming claudins. The claudin protein family is most important for epithelial barrier function even though not all claudins have sealing properties. We did only find a weak influence on claudin-4 and -5 and no effect on claudin-1 to -3, -8, occludin or ZO-1. This is contrary to other enteric pathogens with a stronger impact on TJs, e.g. enteropathogenic Escherichia coli or Arcobacter butzleri [12]. Our results did not display an overall alteration of the TJ. The only reduction in the sealing TJ protein claudin-5 was rather weak, thus it seems likely that the TJ is functionally only moderately affected. However, infected epithelial cells were destroyed, as demonstrated by a release of LDH and an increase in apoptotic events. The most important result of our study was that both, oral and fecal strains induced apoptotic leaks leading to an increase in epithelial permeability to fluorescein. Pre-treatment with the apoptosis inhibitor Z-VAD showed a reduced loss of Rt over 48 h and confirms the impact of C. concisus-induced apoptosis on barrier function. Interestingly, a similar experiment with A. butzleri, which shows more impact on the TJ and also an effect on apoptosis, results in a partial reconstitution of A. butzleri–induced barrier dysfunction by inhibition of apoptosis with Z-VAD. This enabled us to distinguish the barrier impairment caused by TJ disruption from apoptotic influences [12]. The comparison of tracer fluxes in C. concisus- or camptothecin-exposed monolayers revealed an increase in 332 Da fluorescein permeability but no difference for 4 kDa dextran in the same time period with both treatments, suggesting that apoptosis induction could be the main cause of C. concisus-induced barrier dysfunction. This result correlates to previous results of our group that revealed increased permeability in apoptotic monolayers to the same characteristic molecule size cut-off at 4 kDa. In these experiments camptothecin-induced apoptosis in HT-29/B6 monolayers showed also an increased permeability towards intermediate molecule size of 344 Da (H3-lactulose) and no change for 4 kDa tracer molecules (H3-polyethylene glycol) [13]. Thus, the apoptosis induction by C. concisus seems to be the prominent feature of infection. The induction of epithelial cell death could be caspase-dependent but also caspase-independent as already shown for C. jejuni [15]. As shown for the hemolytic toxin aerolysin from Aeromonas hydrophila, the pore-forming activity of aerolysin interferes with restitutional purse-string formation [16] of small epithelial lesions via MLCK-dependent actomyosin constriction [17]. However, this rapid inhibitory consequence on epithelial recovery was not the case with C. concisus. Here, purse-string formation was still visible even 72 h p.i. The hemolytic activity of C. concisus is low, compared to typical hemolytic bacteria, but all C. concisus strains used in our study showed some hemolytic activity (data not shown), measured by the contact hemolysin assay [7].
Our findings suggest C. concisus as an enteric pathogen, characterized by defined spatial interactions with human epithelial cells. We demonstrate that both oral and fecal C. concisus strains induce impairment of the intestinal barrier function. The epithelial necrosis and apoptosis induction are mechanisms that can contribute to a leak-flux type of diarrhea. Also, this leads to a higher amount of antigens passing the mucosa that can accelerate inflammatory processes. Campylobacteriosis is a well-known zoonosis, mostly caused by C. jejuni through contaminated food. In our study eight patients with diarrhea had C. concisus in their feces, whereas three healthy individuals had C. concisus only in saliva samples. Investigations on biofilm formation in the oral cavity and on the ecological niche of C. concisus would be worthwhile also for research on the route of transmission (oral-oral and/or fecal-oral). Humans might be the main reservoir for C. concisus infection, as known from Helicobacter pylori infection. There is a need for extensive investigation of veterinary samples in search for possible additional reservoirs of C. concisus. Furthermore, there is an urgent need for clinical data with a proper follow-up period to ascertain, if this emerging pathogen is an etiologic factor in the development of chronic diarrhea or inflammatory bowel diseases as recently proposed for Salmonella or Campylobacter enteritis [18].
Materials and Methods
Isolation and growth condition of C. concisus
Six oral and eight fecal strains of C. concisus were selected for the study. Fecal strains were sampled from eight patients with diarrhea (age 2–63 years). Three of the patients were also asked to deliver saliva samples from which C. concisus was cultivated. The remaining three oral strains were cultivated from saliva samples of three healthy individuals (age 25–31 years) who had no C. concisus in their fecal samples and no history of gastrointestinal disease (Table 1). This study adhered to the Declaration of Helsinki, and ethics approval for research was obtained from The Ethics Committee for Region Nordjylland, Denmark (Den Videnskabsetiske Komité for Region Nordjylland; N-20080056). All patients who participated in the investigation signed written informed consent forms. For children written informed consent forms were signed by their parents. All strains were analyzed at the Department of Clinical Microbiology, Aalborg Hospital, Aarhus University Hospital, Denmark. C. concisus was isolated by the filter technique on 5% horse blood agar plates, containing 1% yeast extract (SSI Diagnostica, Hillerød, Denmark), and incubated at 37°C in a microaerobic atmosphere with 3% hydrogen [19]. Final confirmation was obtained through a species-specific real-time PCR based on the cpn60 gene, as described elsewhere [20]. No other pathogen was isolated in the patients' fecal samples. All strains were stored in nutrient beef broth with 10% glycerin (SSI Diagnostica) at −80°C until use. Prior to experimental infection of epithelial cell cultures, bacteria were grown on 5% horse blood agar plates, containing 1% yeast extract (SSI Diagnostica) at 37°C in microaerobic atmosphere with 3% hydrogen for 48 h. Then bacteria cells were transfered to liquid culture medium RPMI 1640 (PAA Laboratories, Pasching, Austria) and cultured again for 48 h to log-phase. Bacterial density was counted microscopically and adjusted to a choosen multiplicity of infection (MOI). The C. concisus reference strain ATCC 33237 from human gingival sulcus was cultured in the same way.
Table 1. Proband data at time of Campylobacter concisus isolation.
| Age | Sex | Clinical characteristic | Source | Isolate no. |
| 25 | Female | Healthy | oral | K2 |
| 31 | Female | Healthy | oral | K4 |
| 26 | Male | Healthy | oral | M2 |
| 29 | Male | Crohn's disease | fecal | 75775 |
| 60 | Male | Diarrhea | fecal | 86120 |
| 19 | Female | Crohn's disease | fecal | 118452 |
| 63 | Female | Collagenous colitis | fecal | 119100 |
| 2 | Female | Bloody diarrhea | fecal | 1718 |
| 36 | Female | Crohn's disease | fecal & oral | 112100 |
| 57 | Female | Diarrhea | fecal & oral | 113332 |
| 42 | Male | Diarrhea | fecal & oral | 115605 |
Epithelial cell culture
HT-29/B6 cells represent an established cell model for studies on bacterial infection [12], [17], [21]. HT-29/B6 cells were established and described by Kreusel et al. in 1991, as a stable and highly differentiated subclone derived from wild-type HT-29 cells by glucose deprivation [22]. The cells were cultured in RPMI cell culture medium (RPMI 1640, PAA Laboratories, Pasching, Austria) as described previously [22]. Cells were grown to confluence on polycarbonate filter supports (Millicell-PCF™, effective membrane area 0.6 cm2, pore size 3 µm, Millipore, Billerica, MA., USA), and formed high-ohmic epithelial monolayers with a transepithelial electrical resistance (Rt) between 500 and 800 Ohm⋅cm2 eight days after seeding. In parallel to the infection with all C. concisus strains, three types of controls were performed: (i) without adding bacteria, (ii) with addition of heat-inactivated C. concisus or C. concisus lysates (60 min at 75°C) and (iii) with Escherichia coli K12.
Electrophysiological studies
In order to investigate the effect of the oral and fecal C. concisus strains on epithelial integrity, Rt of polarized HT-29/B6 monolayers was measured after infection with chopstick electrodes (Word Precision Instruments, Sarasota, FL., USA) combined with an ohmmeter (manufactured by D. Sorgenfrei at the Institute of Clinical Physiology, Charité, Berlin) in the cell culture dish under sterile conditions at 37°C. Bacteria were added to the apical compartment of confluent HT-29/B6 cell monolayers to yield an initial concentration of 2×107 cfu/ml equaling a MOI of 20. Infected HT-29/B6 cell monolayers were mounted into modified Ussing-type chambers (manufactured by the Institute of Clinical Physiology, Charité, Berlin) [22] after 48 h. Short circuit current (Isc) and Rt were determined by using a computerized automatic clamp device (Fiebig Hard & Software, Berlin, Germany). Infections were done in an atmosphere with 5% CO2 in ambient air in favor for the HT-29/B6 cell line. For an additional assay concerning dose-dependent effects, monolayers were apically or basolaterally infected with C. concisus at MOIs between 10 and 100.
Epithelial permeability
Unidirectional tracer flux studies were performed from the apical to the basolateral compartment under short-circuit conditions in Ussing chambers with fluorescein (332 Da) and fluorescein isothiocyanate-labeled 4 kDa dextran (FD-4) (Sigma, St. Louis, MO., USA) according to previous described protocols [23], [24]. Medium in the basolateral compartment was initially free of dyes. At specific intervals, medium was withdrawn from the basolateral chamber and fluorescence was measured in a spectrophotometer (Tecan Infinite M200, Durham, NC., USA). Permeability was calculated from flux over concentration difference. Camptothecin-treated monolayers were also investigated by tracer flux measurements, in order to quantify barrier disturbance by apoptosis induction.
Western blot analysis
TJs play a key role in epithelial integrity and may be affected during infection. We investigated the TJ protein expression of barrier-forming claudins after infection with C. concisus. Western blot analysis TJ proteins were quantified by densitometry. Immunoblots were performed and analyzed as described elsewhere [25]. Detergent-soluble protein fractions were prepared from infected monolayers. The following antibodies were used: anti-ZO-1, anti-occludin (1∶2000; Zymed, San Francisco, CA., USA), anti–claudin-1–5 and -8 (1∶1000; Zymed), anti–β-actin (1∶5000; Sigma), and anti–caspase-3 (1∶1000; Cell Signaling Technology, Beverly, MA., USA).
Immunofluorescence microscopy
To test for the integrity of the TJ meshwork, TJ proteins ZO-1, occludin, claudin-1 to 5 and claudin-8 were visualized using confocal laser-scanning microscopy (Zeiss LSM510, Jena, Germany), analyzed by Carl Zeiss LSM Image Examiner software according to prior descriptions [25]. For detection, antibodies raised against TJ proteins (1∶50; Zymed) or Campylobacter (Santa Cruz Biotechnology Inc, Santa Cruz, CA. USA) were used. For cytoskeletal F-actin, staining with Phalloidin-Alexa-Fluor594 (Sigma) was performed.
Cytotoxicity. LDH release assay
Lactate dehydrogenase (LDH) release from HT-29/B6 cells was measured according to the method of Madara and Stafford [26]. TUNEL staining was performed according to manufacturer's protocol (In-situ Cell Death Detection Kit, Roche, Mannheim, Germany). Stained apoptoses were counted microsopically. We investigated the properties of oral and fecal C. concisus isolates in apoptosis induction by TUNEL staining and Western blotting on caspase-3 activation.
Invasion assay
Bacteria were harvested from agarplates (5% horse blood agar +1% yeast extract; SSI Diagnostica) after 48 h and equally diluted in 3 ml RPMI-media, vortexed, and incubated another 48 h in a 37°C microaerobic atmosphere with 3% hydrogen. Monolayers were infected from the apical side with a MOI of 10 and treated (i) with gentamicin (100 µg/ml) prior to infection, (ii) with gentamicin after 2 hours of incubation, or (iii) without gentamicin. Bacterial supernatants were proven for bacterial count. The monolayers were then washed three times with PBS and flooded with 300 µl 0.5% Triton-X 100. C. jejuni ATCC 33560 was used as positive control.
Quantitative Realtime-PCR
Total RNA was obtained from HT-29/B6 cells by phenol-chloroform extraction and complementary DNA (cDNA) was synthesized by reverse-transcription PCR with High-Capacity cDNA Archive Kit (Applied Biosystems, Mannheim, Germany) with random primers. Real-time PCR was performed according to manufacturer's instructions with an ABI-7900HT PCR device using the TaqMan Gene Expression Assay (no. Hs00533949_s1; claudin-5) with FAM dye-labeled primers. Glyceraldehyde 3-phosphate dehydrogenase cDNA was quantified using VIC reporter dyes as endogenous control (all Applied Biosystems). Differential expression was calculated according to the 2−ΔΔCT method [27].
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical analysis was performed using the Student t-test. P<0.05 was considered significant.
Supporting Information
Apoptotic leaks. 360° view of the confocal laser-scanning micrograph of an apoptotic lesion in HT-29/B6 monolayers infected with C. concisus, based on Figure 5 (Z-axis scans, XY plane). Cytoskeletal F-actin is marked red by Phalloidin-Alexa-Fluor594, the blue staining with DAPI ( = 4′-6-diamidino-2-phenylindole dihydrochloride) marks the nuclei of epithelial cells and C. concisus is marked green by anti-Campylobacter antibody. In controls, superficial single cell lesions were closed within 16 min, accompanied by formation of an actin ring (“purse-string”) that is essential for restitution. Recovery of apoptotic lesions, induced by C. concisus, seems unaffected as the purse-string actin formation is clearly visible.
(MPG)
Acknowledgments
The excellent technical assistance of In-Fah Lee, Claudia May, Detlef Sorgenfrei and Anja Fromm is gratefully acknowledged.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Deutsche Forschungsgemeinschaft [DFG Schu 559/11-1 to MZ, JDS, RB]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Newell DG. Campylobacter concisus: an emerging pathogen? Eur J Gastroenterol Hepatol. 2005;17(10):1013–1014. doi: 10.1097/00042737-200510000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Chaban B, Ngeleka M, Hill JE. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010;10:73. doi: 10.1186/1471-2180-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Budiman V, Day AS, Mitchell H, Lemberg DA, et al. Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J Clin Microbiol. 2010;48(8):2965–2967. doi: 10.1128/JCM.02391-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aabenhus R, Permin H, On SL, Andersen LP. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis. 2002;34(4):248–252. doi: 10.1080/00365540110080566. [DOI] [PubMed] [Google Scholar]
- 5.Lastovica AJ. Clinical relevance of Campylobacter concisus isolated from pediatric patients. J Clin Microbiol. 2009;47(7):2360. doi: 10.1128/JCM.00568-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aabenhus R, Stenram U, Andersen LP, Permin H, Ljungh A. First attempt to produce experimental Campylobacter concisus infection in mice. World J Gastroenterol. 2008;14(45):6954–6959. doi: 10.3748/wjg.14.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Istivan TS, Coloe PJ, Fry BN, Ward P, Smith SC. Characterization of a haemolytic phospholipase A(2) activity in clinical isolates of Campylobacter concisus. J Med Microbiol. 2004;53(Pt 6):483–493. doi: 10.1099/jmm.0.45554-0. [DOI] [PubMed] [Google Scholar]
- 8.Kaakoush NO, Man SM, Lamb S, Raftery MJ, Wilkins MR, et al. The secretome of Campylobacter concisus. FEBS J. 2010;277(7):1606–1617. doi: 10.1111/j.1742-4658.2010.07587.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalischuk LD, Inglis GD. Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiol. 2011;11:53. doi: 10.1186/1471-2180-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engberg J, Bang DD, Aabenhus R, Aarestrup FM, Fussing V, et al. Campylobacter concisus: an evaluation of certain phenotypic and genotypic characteristics. Clin Microbiol Infect. 2005;11(4):288–295. doi: 10.1111/j.1469-0691.2005.01111.x. [DOI] [PubMed] [Google Scholar]
- 11.Man SM, Kaakoush NO, Leach ST, Nahidi L, Lu HK, et al. Host Attachment, Invasion, and Stimulation of Proinflammatory Cytokines by Campylobacter concisus and Other Non-Campylobacter jejuni Campylobacter Species. J Infect Dis. 2010;202(12):1855–1865. doi: 10.1086/657316. [DOI] [PubMed] [Google Scholar]
- 12.Bücker R, Troeger H, Kleer J, Fromm M, Schulzke JD. Arcobacter butzleri induces barrier dysfunction in intestinal HT-29/B6 cells. J Infect Dis. 2009;200(5):756–764. doi: 10.1086/600868. [DOI] [PubMed] [Google Scholar]
- 13.Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, et al. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J Physiol. 2001;535(Pt 2):541–552. doi: 10.1111/j.1469-7793.2001.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soderholm JD, Streutker C, Yang PC, Paterson C, Singh PK, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut. 2004;53(12):1817–1824. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalischuk LD, Inglis GD, Buret AG. Strain-dependent induction of epithelial cell oncosis by Campylobacter jejuni is correlated with invasion ability and is independent of cytolethal distending toxin. Microbiology. 2007;153(Pt 9):2952–2963. doi: 10.1099/mic.0.2006/003962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Florian P, Schöneberg T, Schulzke JD, Fromm M, Gitter AH. Single-cell epithelial de-fects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J Physiol. 2002;545(Pt 2):485–499. doi: 10.1113/jphysiol.2002.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, et al. Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis. 2011 doi: 10.1093/infdis/jir504. In Press. [DOI] [PubMed] [Google Scholar]
- 18.Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, et al. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Engberg J, On SL, Harrington CS, Gerner-Smidt P. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for Campylobacters. J Clin Microbiol. 2000;38(1):286–291. doi: 10.1128/jcm.38.1.286-291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaban B, Musil KM, Himsworth CG, Hill JE. Development of cpn60-based real-time quantitative PCR assays for the detection of 14 Campylobacter species and application to screening of canine fecal samples. Appl Environ Microbiol. 2009;75(10):3055–3061. doi: 10.1128/AEM.00101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troeger H, Richter JF, Beutin L, Günzel D, Dobrindt U, et al. Escherichia coli alpha-haemolysin induces focal leaks in colonic epithelium: a novel mechanism of bacterial translocation. Cell Microbiol. 2007;9(10):2530–2540. doi: 10.1111/j.1462-5822.2007.00978.x. [DOI] [PubMed] [Google Scholar]
- 22.Kreusel KM, Fromm M, Schulzke JD, Hegel U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol. 1991;261(4 Pt 1):C574–C582. doi: 10.1152/ajpcell.1991.261.4.C574. [DOI] [PubMed] [Google Scholar]
- 23.Epple HJ, Kreusel KM, Hanski C, Schulzke JD, Riecken EO, et al. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflugers Arch. 1997;433(5):638–647. doi: 10.1007/s004240050325. [DOI] [PubMed] [Google Scholar]
- 24.Schulzke JD, Fromm M, Bentzel CJ, Zeitz M, Menge H, et al. Ion transport in the experimental short bowel syndrome of the rat. Gastroenterology. 1992;102(2):497–504. doi: 10.1016/0016-5085(92)90096-h. [DOI] [PubMed] [Google Scholar]
- 25.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321(1):89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 26.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression datausing real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apoptotic leaks. 360° view of the confocal laser-scanning micrograph of an apoptotic lesion in HT-29/B6 monolayers infected with C. concisus, based on Figure 5 (Z-axis scans, XY plane). Cytoskeletal F-actin is marked red by Phalloidin-Alexa-Fluor594, the blue staining with DAPI ( = 4′-6-diamidino-2-phenylindole dihydrochloride) marks the nuclei of epithelial cells and C. concisus is marked green by anti-Campylobacter antibody. In controls, superficial single cell lesions were closed within 16 min, accompanied by formation of an actin ring (“purse-string”) that is essential for restitution. Recovery of apoptotic lesions, induced by C. concisus, seems unaffected as the purse-string actin formation is clearly visible.
(MPG)