Abstract
Daizo, Ushiba (Keio University School of Medicine, Tokyo, Japan), Taiji Nakae, Takehisa Akiyama, and Yoshio Kishimoto. Characterization of “clearance” factor and “cell-bound” antibody in experimental typhoid. J. Bacteriol. 91:1705–1712. 1966.—The “clearance” factor produced in the serum of rabbits immunized with a killed vaccine of an S-type virulent strain of Salmonella enteritidis was identified as O agglutinin and characterized immunochemically as a class of macroglobulin with a sedimentation coefficient of 12.7S. The macroglobulin, which was isolated by means of diethylaminoethyl cellulose column chromatography and sucrose density gradient centrifugation, showed a single protein peak when subjected to cellulose acetate electrophoresis and analytical ultracentrifugation. Its activity as agglutinin and “clearance” factor was inactivated both by mercaptoethanol treatment and absorbtion with the specific O antigen. The H agglutinin was also isolated as a single globulin fraction with relatively low molecular weight, and was characterized as a 7S γ-globulin. This fraction had no “clearance” activity. A macroglobulin, similar to that identified in the rabbit serum, was extracted with urea from the cells of mice immunized with the S-type killed vaccine, but not from mice immunized with a live vaccine (R-type mutant), although this latter vaccine is very effective in producing a solid immunity against typhoid disease. The relation of this “cell-bound” antibody to the mechanism of “cellular” immunity in experimental typhoid is discussed.
Full text
PDF

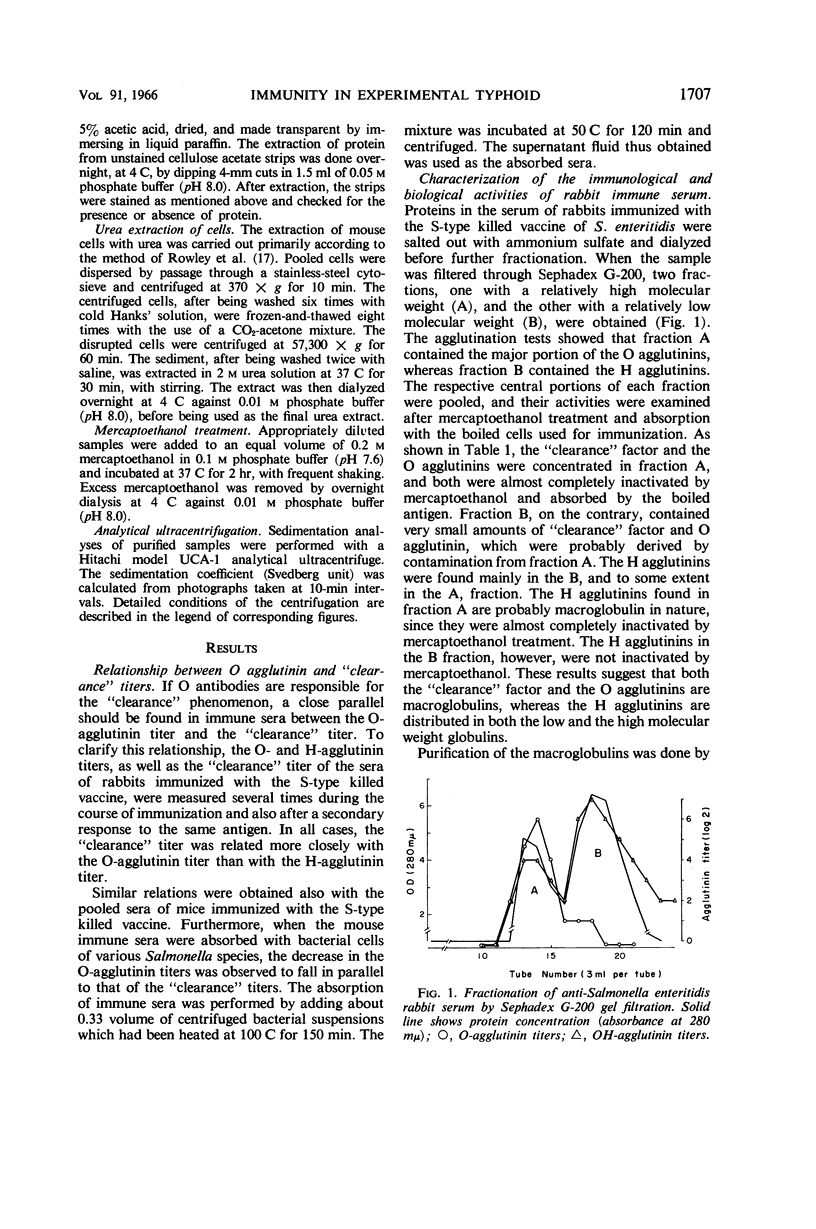
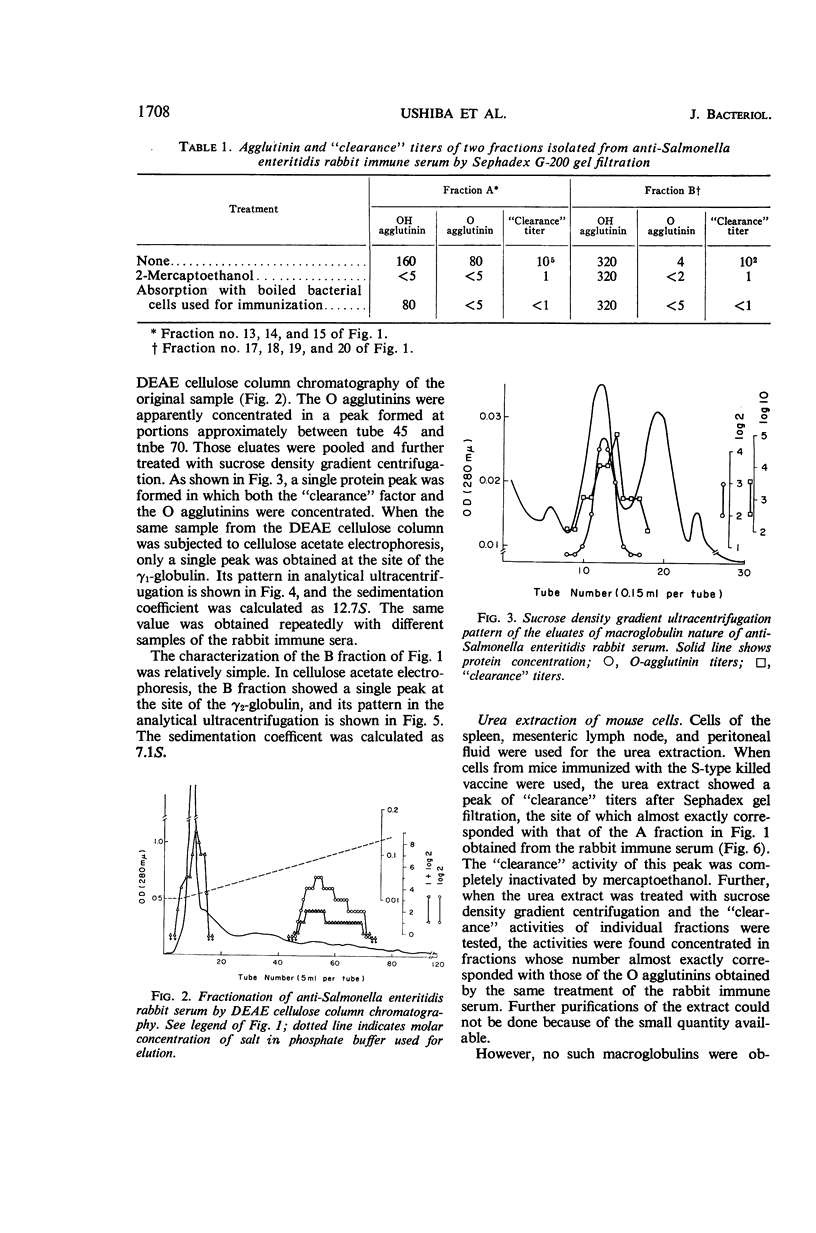

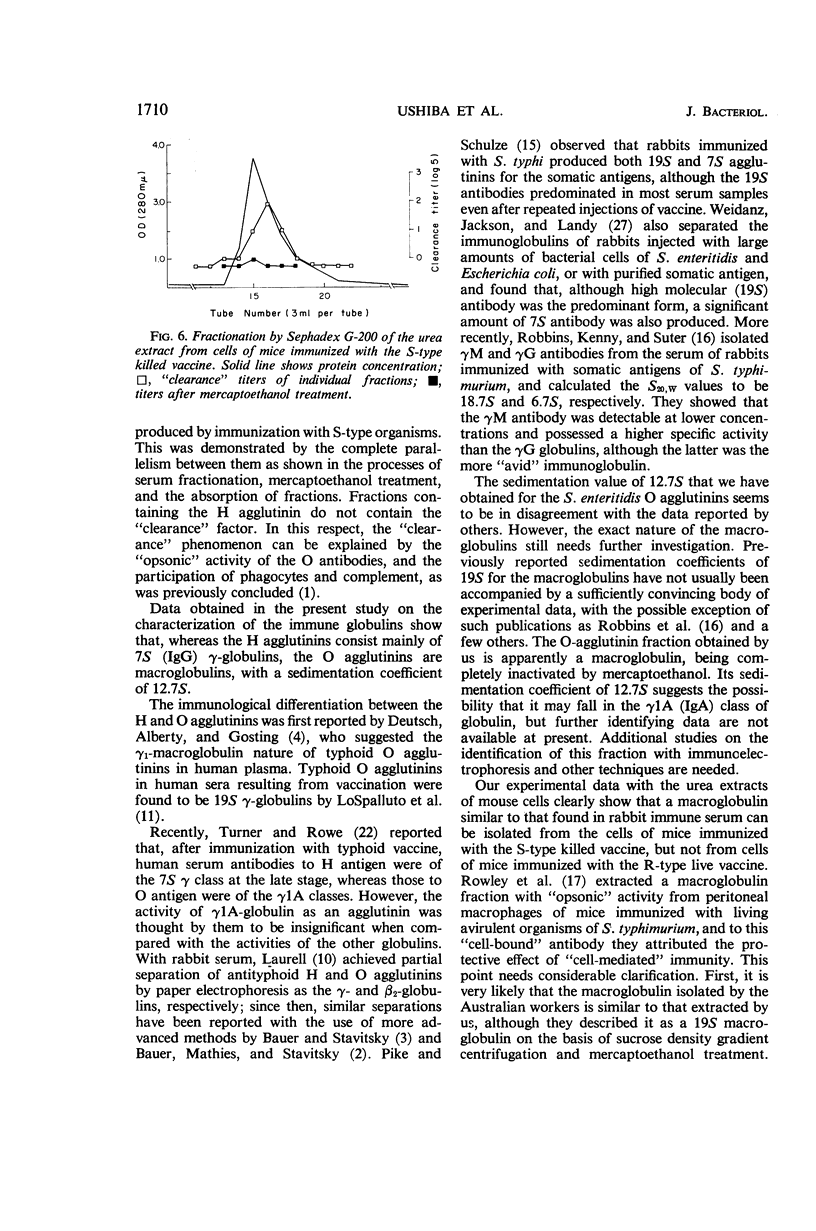


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKIYAMA T., MAEDA K., USHIBA D. [Studies on immunity in experimental typhoid. 2. Mechanism of the clearance of bacteria in the peritoneal cavity of mice passively immunized with antiserum]. Nihon Saikingaku Zasshi. 1962 Nov;17:867–872. doi: 10.3412/jsb.17.867. [DOI] [PubMed] [Google Scholar]
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. C., Stavitsky A. B. ON THE DIFFERENT MOLECULAR FORMS OF ANTIBODY SYNTHESIZED BY RABBITS DURING THE EARLY RESPONSE TO A SINGLE INJECTION OF PROTEIN AND CELLULAR ANTIGENS. Proc Natl Acad Sci U S A. 1961 Oct;47(10):1667–1680. doi: 10.1073/pnas.47.10.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK C. W., MILLER W. E., Jr, DORWARD B., LOSPALLUTO J. The formation of macroglobulin antibodies. II. Studies on neonatal infants and older children. J Clin Invest. 1962 Jul;41:1422–1428. doi: 10.1172/JCI104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLODIN P., KILLANDER J. Fractionation of human-serum proteins by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:402–410. doi: 10.1016/0006-3002(62)90104-x. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. BASIS FOR IMMUNITY TO TYPHOID IN MICE AND THE QUESTION OF "CELLULAR IMMUNITY". Bacteriol Rev. 1963 Dec;27:391–404. doi: 10.1128/br.27.4.391-404.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., SATO I., TANAKA T. Experimental salmonellosis. Intracellular growth of Salmonella enteritidis ingested in mononuclear phagocytes of mice, and cellular basis of immunity. J Bacteriol. 1961 Jun;81:863–868. doi: 10.1128/jb.81.6.863-868.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi S., Saito K. IN VITRO TRANSFER OF CELLULAR IMMUNITY OF MOUSE PHAGOCYTES IN EXPERIMENTAL SALMONELLOSIS. J Bacteriol. 1962 Sep;84(3):592–593. doi: 10.1128/jb.84.3.592-593.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIKE R. M., SCHULZE M. L. PRODUCTION OF 7S AND 19S ANTIBODIES TO THE SOMATIC ANTIGENS OF SALMONELLA TYPHOSA IN RABBITS. Proc Soc Exp Biol Med. 1964 Mar;115:829–833. doi: 10.3181/00379727-115-29050. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- SAITO K., AKIYAMA T., NAKANO M., USHBA D. Interaction between Salmonella enteritidis and tissue cultured macrophages derived from immunized animals. Jpn J Microbiol. 1960 Oct;4:395–407. doi: 10.1111/j.1348-0421.1960.tb00188.x. [DOI] [PubMed] [Google Scholar]
- SATO I., TANAKA T., SAITO K., MITSUHASHI S. Inhibition of Salmonella enteritidis ingested in mononuclear phagocytes from liver and subcutaneous tissue of mice immunized with live vaccine. J Bacteriol. 1962 Jun;83:1306–1312. doi: 10.1128/jb.83.6.1306-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato I., Mitsuhashi S. Experimental salmonellosis. VII. In vitro transfer of cellular immunity by ribosomal fraction of mouse mononuclear phagocytes. J Bacteriol. 1965 Nov;90(5):1194–1199. doi: 10.1128/jb.90.5.1194-1199.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER K. J., JENKIN C. R., ROWLEY D. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 2. ANTIBODY FORMATION DURING THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:229–236. doi: 10.1038/icb.1964.24. [DOI] [PubMed] [Google Scholar]
- TURNER M. W., ROWE D. S. CHARACTERIZATION OF HUMAN ANTIBODIES TO SALMONELLA TYPHI BY GEL-FILTRATION AND ANTIGENIC ANALYSIS. Immunology. 1964 Nov;7:639–656. [PMC free article] [PubMed] [Google Scholar]
- USHIBA D. Phagocytosis of bacteria and their intracellular multiplication in experimental typhoid. Tohoku J Exp Med. 1962 Mar 25;76:133–143. doi: 10.1620/tjem.76.133. [DOI] [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- WEIDANZ W. P., JACKSON A. L., LANDY M. SOME ASPECTS OF THE ANTIBODY RESPONSE OF RABBITS TO IMMUNIZATION WITH ENTEROBACTERIAL SOMATIC ANTIGENS. Proc Soc Exp Biol Med. 1964 Jul;116:832–837. doi: 10.3181/00379727-116-29386. [DOI] [PubMed] [Google Scholar]




