Abstract
Data on the development of antiretroviral drug resistance in HIV-1-infected children receiving protease inhibitor (PI)-based antiretroviral therapy (ART) are limited. We examined antiretroviral resistance among a cohort of 323 South African HIV-infected children <2 years old exposed to nevirapine for prevention of mother-to-child transmission. Ritonavir (RTV) was used initially for 138 children who were <6 months old or receiving antimycobacterial therapy; otherwise children received lopinavir/ritonavir (LPV/r)-based ART. HIV-1 population sequencing of the pol gene was conducted on all pretreatment samples and on posttreatment samples for children who did not achieve HIV-1 plasma RNA <400 copies/ml by 52 weeks. Among children in the cohort, 38 died, 22 had <24 weeks follow-up, 209 achieved virologic suppression, and 54 did not. Of 41 children without virologic suppression with posttreatment HIV genotype data available, major resistance mutations were found in 32 (78%): 14 (36%) had PI mutations including V82A, M46I, and L90M; 29 (71%) had M184V/I; and three had NNRTI mutations (K103N, Y181C, and G190A). Among the children who did not achieve virologic suppression, none of the seven children treated exclusively with LPV/r developed PI-related mutations, compared with 14 of 32 (44%) who received RTV-based regimens (p=0.036); PI genotypes were unavailable for two children. Seventy-eight percent of children without virologic suppression developed resistance mutations that impact second-line ART options. Only children who received RTV-based ART developed major PI-related resistance mutations, and use of this regimen should be avoided.
Introduction
Despite the dramatic expansion in access to antiretroviral therapy in resource-limited settings over the past decade, HIV-infected children have relatively few antiretroviral treatment (ART) options. Children are one-third as likely to receive ART as adults,1 and treatment selections are limited by a lack of pediatric formulations, the instability of some liquid formulations at room temperature, and drug interactions during treatment for tuberculosis and bacilli Calmette-Guérin (BCG) coinfections.2–5 HIV-infected infants in resource-limited settings are also more likely to have been exposed to short-course or single-dose nevirapine (sdNVP) for prevention of mother-to-child transmission (PMTCT). This exposure may lead to poor virologic outcomes in infants initiating ART with NVP-based regimens, the most common first-line ART regimen in resource-limited settings, as a result of persistent nevirapine-associated resistance mutations.6–8
Initiation of ART prior to 12 weeks of age is shown to significantly reduce early infant mortality.9 NVP-exposed infants are less likely to achieve viral suppression when receiving NVP-based therapy, the most common first-line ART regimen in resource-limited settings, compared with those receiving boosted protease inhibitor (PI)-based ART.10,11 These data have led to recommendations to treat all HIV-infected infants with ART regardless of clinical or immunologic status, and to treat HIV-1-infected infants exposed to NVP during PMTCT with regimens that use a ritonavir (RTV)-boosted PI plus two nucleoside reverse transcriptase inhibitors (NRTIs).12 The combination of these two treatment recommendations leads to widespread use of PI-based treatment regimens, particularly lopinavir/ritonavir (LPV/r), for HIV-infected infants.
Data on response to boosted PI-based ART in children exposed to sdNVP are limited. In adults, despite a high barrier to the development of resistance to LPV/r, the response to LPV/r-based salvage regimens is directly related to the number of preexisting PI-related resistance mutations.13,14 In children, virologic response to LPV/r-based regimens is excellent, with up to 88% of antiretroviral-naive children achieving HIV-1 plasma RNA levels below 400 copies/ml by 48 weeks.15 However, studies from both resource-rich and resource-limited settings suggest that antiretroviral-related resistance mutations develop in 44–94% of children failing PI-based ART, and many children develop multiclass drug resistance.16–20 Antiretroviral resistance mutations that develop while on PI-based ART may lead to decreased susceptibility across many other PIs and limit future treatment options.17,21
Historically, RTV-based regimens have been associated with suboptimal treatment responses, with only 36% of children achieving sustained virologic suppression in an early study.22 Pediatric AIDS Clinical Trials Group investigations have shown that response to ritonavir-based therapy in NTRI-experienced but PI-naive children was robust initially, with 55% achieving virologic suppression by 12 weeks, but only 32% maintained virologic suppression at 48 weeks. The presence of NRTI-associated resistance mutations did not affect rates of virologic failure.23 Recently published data suggest that RTV-based regimens given to children under 6 months of age or receiving concomitant mycobacterial treatment are associated with lack of virologic suppression and increased rates of PI resistance mutations.24,25
This investigation examines this association and the development of antiretroviral-associated resistance mutations in a large cohort of sdNVP-exposed infants and children under 2 years of age initiating PI-based regimens in Johannesburg, South Africa.
Materials and Methods
Study design
We conducted a secondary analysis of response to PI-based ART among NVP-exposed children at a single site in Johannesburg, South Africa. An ART strategies trial enrolled HIV-infected children between 6 and 104 weeks of age who were exposed to NVP for PMTCT and were either eligible for ART based on immunologic or clinical criteria (n=254) or currently receiving a stable PI-based ART regimen (n=69).26–28 The study protocol was approved by the Columbia University Medical Center and University of the Witwatersrand Institutional Review Boards, and each participant's guardian provided signed informed consent. This analysis makes use of data from the observational prerandomization phase of the study only.
Study enrollment and ART regimens
Criteria for study enrollment included: HIV-1 infection, age less than 24 months, exposure to nevirapine for PMTCT (determined by clear and consistent caregiver reports of NVP use as PMTCT interventions are not routinely recorded on health records in South Africa), and eligibility for ART based on South African treatment guidelines in place at the time. Children who met study enrollment criteria were enrolled either prior to ART start or after ART start if they had recently initiated ART, were receiving a first-line PI-based regimen, and had not had any changes to this first-line regimen.
During the prerandomization observational phase used for this analysis, children over 6 months of age were treated with LPV/r (230 mg/m2), lamivudine (3TC, 4 mg/kg), and stavudine (d4T, 1 mg/kg). Children younger than 6 months of age and those receiving rifampin-based regimens for tuberculosis or BCG disease received antiretroviral therapy with ritonavir (RTV 400–450 mg/m2) instead of LPV/r plus the NRTIs above. Children who started therapy below the age of 6 months were switched to LPV/r once they aged past 6 months, as were children who completed TB therapy. At the time of the study, recommendations regarding doubling of LPV/r doses or super-RTV boosted LPV for children receiving rifampin were not yet widely practiced in South Africa.29
Study measurements and outcome determination
Routine study visits occurred at enrollment, 2, 4, 8, 16, 24, 36, and 52 weeks post-ART initiation. At each study visit, a history and physical examination determined the child's height, weight, and antiretroviral and antimycobacterial regimens. All caregivers were given education about HIV treatment and adherence counseling at each visit. Adherence was assessed using a questionnaire to the caregivers, and pharmacists weighed the medication bottles to reconcile the amount returned with expected quantities used since the prior visit. For the purposes of this analysis, nonadherence was defined as returning 20% more than expected of any antiretroviral medication at any study visit. A second nonadherence parameter based on the adherence assessment only at the time of the virologic endpoint was also examined and found to be similar to the adherence measure used. Weight- and height-for-age z-scores were calculated using software from the World Health Organization.30
The virologic outcome of antiretroviral therapy was determined only for those children with at least 24 weeks of follow-up; those not known to have died who were lost to follow-up prior to the 24 week study visit were excluded from the analysis. Children were classified as achieving virologic suppression if they had achieved a plasma HIV-1 RNA level <400 copies/ml by 52 weeks of follow-up. A cut-off of <400 copies/ml for virologic suppression was selected for this analysis as this was one of the criteria used for randomization into the subsequent phase of the ART strategies trial.28 Children who never achieved plasma HIV-1 RNA levels <400 copies/ml or had levels below 400 copies/ml that then rose to over 400 copies/ml by 52 weeks of follow-up were classified as not achieving virologic suppression.
Laboratory methods
Blood samples were drawn at the enrollment visit to determine baseline CD4+ cell counts, CD4+ percentages, and HIV-1 plasma RNA levels in those children initiating ART under supervision of the study team, but were not routinely available for those children who enrolled in the study already receiving first-line ART. Further blood samples were taken from all children at each study visit to determine CD4+ cell count and percentage and HIV-1 plasma RNA level while on therapy. CD4+ cell count and percentage were determined using panleukogating. HIV-1 plasma RNA levels were determined using the Roche Amplicor assay (standard assay, version 1.5, quantification range 400–750,000 copies/ml or ultrasensitive test quantification range 50–150,000 copies/ml, Branchburg, NJ).
HIV-1 genotype testing for antiretroviral resistance-associated mutations was conducted on samples collected pretreatment for 244 children who enrolled prior to ART initiation. Pretreatment samples were not available for the children who enrolled in the study already receiving ART. Posttreatment samples for genotyping were sought for all participants if and when they met criteria for the endpoint of lack of virologic suppression on ART. HIV-1 genotype results were unlinked from personal identifiers and were tested for mutations in the reverse transcriptase and protease genes using population sequencing. Plasma samples were used to isolate viral RNA using MagNa Pure LC Total Nucleic Acid Isolation kits on the MagNa Pure Automated System (Roche Diagnostics, Indianapolis, IN). An in-house nested polymerase chain reaction (PCR) to amplify the pol region was performed as described previously.31,32 For samples where no product was obtained, two separate PCRs for reverse transcriptase and protease were performed.33,34 All PCR products were sequenced using BigDye Terminators v3.1 on an ABI3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). All sequences were submitted to the Stanford University HIV drug resistance database,35 and the 2009 International AIDS Society-USA (IAS-USA) guidelines were used to define antiretroviral resistance-associated mutations for NRTIs, nonnucleoside reverse transcriptase inhibitors (NNRTIs), and PIs.36
Statistical methods
All analyses presented are limited to the prerandomization phase of the larger ART strategies trial. For all analyses, means are reported for normally distributed continuous variables and medians for those not normally distributed. Statistical significance of continuous variables was tested using two-sided t-tests for normally distributed variables and Wilcoxon rank-sum tests for nonnormally distributed variables. Categorical variables were examined using two-sided chi-squared or Fisher's exact tests. p-values less than or equal to 0.05 were considered significant.
The first analysis was limited to infants and children who enrolled in the study prior to ART initiation, and compared the presence of pretreatment major resistance mutations by three outcome groups: virologically suppressed, not virologically suppressed, and death. Associations found to be statistically significant in this univariate analysis (p<0.05) were included in multivariate logistic regression models to allow adjustment for possible confounding. Secondary univariate analyses were also conducted on demographic and clinical parameters of the cohort at enrollment by the same outcome groups.
For children who did not achieve virologic suppression, the profile of drug resistance mutations in posttreatment samples collected at the time of endpoint was determined. Among children who met criteria for lack of virologic suppression and had HIV genotype results, those with and without posttreatment PI-associated resistance mutations were compared. Odds ratios and 95% confidence intervals (CI) were calculated using logistic regression for the significant bivariate associations. STATA 11.0 (College Station, TX) was used to conduct 2×3 two-sided Fisher's exact tests; all other analyses were performed using PASW Statistics 10.9.2 (Chicago, IL).
Results
Study population
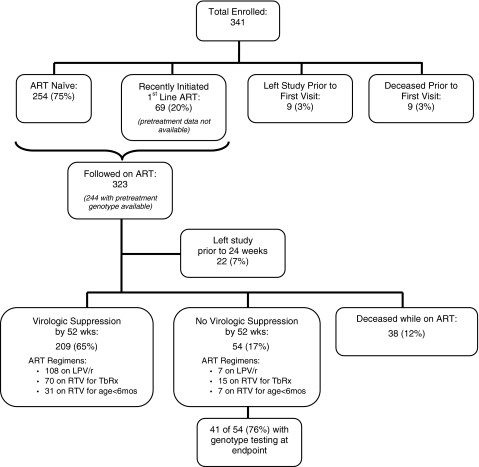
Of 341 children who were either treatment naive (80%) or on a stable PI-based regimen (20%) enrolled in the study, 323 initiated ART, nine discontinued the study prior to their first visit, and nine died prior to their first visit. Of those who initiated study visits, 244/323 (76%) had a pretreatment HIV-1 genotype prior to treatment initiation; 69 (21%) enrolled in the study while on ART and thus did not have a pretreatment sample collected, and 10 (3%) who had a pretreatment sample collected did not have sufficient volume stored for genotype testing. Of the children initiating study visits, 54 (17%) did not achieve HIV-1 plasma RNA levels below 400 copies/ml by their final study visit, 209 (65%) achieved levels below 400 copies/ml by 52 weeks of follow-up, 38 (12%) died while on ART, and 22 (7%) left the study prior to 24 weeks of follow up (Fig. 1).
FIG. 1.
Participant disposition tree for all children enrolled in the NEVEREST cohort. Antiretroviral therapy (ART) regimens include “LPV/r”: lopinavir-ritonavir-based therapy throughout the observation period; “RTV for TbRx”: RTV-based therapy at some point during the observation period due to cotreatment for mycobacterial infection; “RTV for age <6 months”: RTV-based therapy at some point during the observation period due to age <6 months. Percentages in the second tier are of the total 341 enrolled. Percentages below 323 “Followed on ART” are of that total.
Pretreatment profile of resistance mutations and treatment outcomes
Overall, 226 children who met criteria for the three treatment outcomes of interest, virologic suppression, lack of suppression, or death, had a pretreatment HIV-1 genotype to assess for antiretroviral-associated resistance mutations (Table 1a). Sixty-eight children (30%) carried virus with at least one major resistance mutation: 39/155 (25%) of children who suppressed, 13/38 (34%) of those who did not achieve virologic suppression, and 16/33 (49%) of children who died. Eleven children had major resistance mutations in more than one of the three drug classes tested. The specific major mutations present at baseline were distributed as follows: three had PI-related mutations (one I47V and two M46L), seven had NRTI-related mutations (one D67N, three K70E, seven L74V, one L210W, and eight K219E/Q), and 58 had NNRTI-related mutations (10 K103N, 45 Y181C, two Y188C, and two G190A). One child had both K103N and Y188C at baseline.
Table 1.
Pretreatment Characteristics of the Cohort by Treatment Outcome: Virologic Suppression, No Virologic Suppression, or Death by 52 Weeks
| Virologic suppression | No virologic suppression | p value (suppressed vs. no) | Death | p value (death vs. others) | |
|---|---|---|---|---|---|
| a. Baseline major resistance mutations (pretreatment HIV-1 genoype tests only, n=226) | |||||
| n (%) | 155 (68%) | 38 (17%) | 33 (15%) | ||
| Any | 39 (25%) | 13 (34%) | 0.26a | 16 (49%) | 0.013a |
| PI (I47V, M46L) | 2 (1%) | 0 (0%) | 1.000b | 1 (3%) | 0.379b |
| NRTI (D67N, K70E, L74V, L210W, K219EQ) | 11 (7%) | 4 (11%) | 0.501b | 3 (9%) | 0.732b |
| NNRTI (K103N, Y181C, Y188C, G190A) | 33 (21%) | 12 (32%) | 0.179a | 13 (39%) | 0.051a |
| b. Clinical characteristics (limited to 236 children enrolled pretreatment and not lost to follow-up <24 weeks) | |||||
| n (%) | 164 (70%) | 39 (16%) | 33 (14%) | ||
| Median age at enrollment in months (IQR) | 8 (5, 14) | 7 (5, 15) | 0.755c | 7 (4, 9.5) | 0.074c |
| Female sex | 79 (48%) | 18 (46%) | 0.821a | 15 (46%) | 0.804a |
| Mean weight for age z-score (IQR) | −2.07 (−3.11, −1.08) | −2.74 (−3.77, −1.44) | 0.026d | −3.43 (−4.41, −2.01) | <0.001d |
| Median height for age z-score (IQR) | −2.98 (−3.86, −2.02) | −3.96 (−4.84, −2.86) | 0.001c | −3.84 (−4.68, −2.89) | 0.014c |
| Median CD4% (IQR) | 19.6% (13, 26%) | 16.7% (13, 24%) | 0.283c | 14.6% (10, 21%) | 0.015c |
| Median CD4 count, cells/μl (IQR) | 929 (626, 1472) | 852 (384, 1583) | 0.272c | 449 (294, 851) | <0.001c |
| CDC Immunologic stage [CD4 cells/μl] | |||||
| 1 [≥500] | 131 (80%) | 27 (69%) | 0.211b | 14 (42%) | <0.001b |
| 2 [200–499] | 23 (14%) | 7 (18%) | 14 (42%) | ||
| 3 [<200] | 10 (6%) | 5 (13%) | 5 (15%) | ||
| Pretreatment HIV-1 plasma RNA (copies/ml) ≥750K | 95 (56%) | 28 (72%) | 0.223b | 31 (94%) | 0.001a |
| WHO stage | |||||
| I | 19 (12%) | 2 (5%) | 0.866b | 1 (3%) | 0.153b |
| II | 6 (4%) | 1 (3%) | 1 (3%) | ||
| III | 73 (44%) | 19 (49%) | 10 (30%) | ||
| IV | 42 (26%) | 11 (28%) | 13 (39%) | ||
| not staged | 24 (15%) | 6 (15%) | 8 (24%) | ||
| Nonadherent | 36 (22%) | 22 (56%) | <0.001a | 11 (33%) | 0.195a |
| TB treatment | 57 (35%) | 23 (59%) | 0.005a | 17 (52%) | 0.190a |
Predictors significant at p≤0.05 are in italic.
Chi-squared test (two-sided).
Fisher's exact test.
Wilcoxon rank-sum test (two-tailed).
Student's t-test (two-tailed).
PI, protease inhibitor; NRTI, nucleoside reverse transcription inhibitor; NNRTI, nonnucleoside reverse transcription inhibitor; IQR, interquartile range; TB, tuberculosis.
We were surprised to observe that the presence of a major drug-associated resistance mutation pretreatment was a statistically significant predictor of death within the cohort (p=0.013). This association was strongest, though not statistically significant, in children with pretreatment NNRTI mutations (p=0.051). Pretreatment resistance mutations did not predict lack of virologic suppression, likely because all children were treated with PI-based ART and the majority of baseline resistance was to NNRTIs (Table 1a). Resistance mutations were more common among younger children, likely due to the resurgence of wild-type virus over time after sdNVP exposure,37,38 and after adjustment for both age and pretreatment HIV-1 plasma RNA level the association was no longer significant. A final model incorporating baseline major drug-associated mutations, age, pretreatment HIV-1 plasma RNA level, pretreatment CD4+ cell count, and pretreatment weight-for-age z-score showed that only HIV-1 plasma RNA level, CD4+ cell count, and the weight-for-age z-score were significantly associated with death in the cohort. Results of this multivariate analysis are shown in Table 2.
Table 2.
Multivariate Analysis of the Associations Between the Presence of Pretreatment Major Resistance Mutations and Death Within the Cohort
| OR Model 1 (95% CI) | p value | ORs Model 2 (95% CI) | p value | ORs Model 3 (95% CI) | p value | ORs Model 4 (95% CI) | p value | |
|---|---|---|---|---|---|---|---|---|
| Pretreatment major resistance mutations | 2.55 (1.20, 5.42) | 0.015 | 2.16 (0.98, 4.76) | 0.055 | 1.95 (0.86, 4.42) | 0.107 | 1.29 (0.54, 3.12) | 0.566 |
| Age in months at treatment initiation | — | — | 0.95 (0.88, 1.03) | 0.198 | 0.98 (0.90, 1.05) | 0.504 | 0.94 (0.87, 1.02) | 0.136 |
| Pretreatment HIV-1 plasma RNA (copies/ml) ≥750K | — | — | — | — | 8.46 (1.94, 36.9) | 0.004 | 5.75 (1.26, 26.2) | 0.024 |
| Pretreatment CD4+ cell count (cells/μl) <500 | 2.99 (1.28, 6.99) | 0.011 | ||||||
| Pretreatment weight For age z-score | 0.716 (0.55, 0.93) | 0.012 |
Model 1 is unadjusted; Model 2 is adjusted for age at time of treatment initiation; Model 3 is adjusted for age and pretreatment plasma HIV-1 RNA level; and Model 4 is adjusted for age, pretreatment plasma HIV-1 RNA level, pretreatment CD4+ cell count, and pretreatment weight-for-age z-score. OR, odds ratio; K=1000.
Other predictors of treatment outcome before 52 weeks
Additional characteristics of the cohort stratified into the three outcome groups are shown in Table 1b. This analysis was limited to children who enrolled prior to ART initiation for whom pretreatment data were available. Significant predictors for lack of virologic suppression were lower pretreatment weight and height-for-age z-scores, nonadherence, and concomitant treatment for mycobacterial disease. Predictors of mortality included lower pretreatment weight and height-for-age z-scores, lower pretreatment CD4% and CD4 cell count, CDC immunologic stage,39 and higher pretreatment plasma HIV-1 RNA level.
Posttreatment genotype results in children with lack of virologic suppression by 52 weeks
Of the 54 children meeting criteria for lack of virologic suppression, 41 had HIV genotype testing and 13 did not, either because samples were unavailable or did not amplify. Two of the 41 genotypes gave results only for the reverse transcriptase gene, so protease resistance mutations after treatment were available for 39 children. Comparing those children without genotype testing at time of lack of virologic suppression to those for whom genotype testing was available, children without genotype testing at the endpoint had lower HIV-1 plasma RNA levels at time of failure, but otherwise did not significantly differ from children with genotype data available (data not shown).
Of the 41 children with lack of virologic suppression and posttreatment genotype results at the time of virologic endpoint, 37 (90%) had at least one antiretroviral resistance-associated mutation, and 32 (78%) had a major resistance mutation. Over a third of those with resistance mutations at virologic endpoint also had major drug resistance mutations pretreatment (nine of 24 with baseline tests). Fourteen (36%) had major PI-related resistance mutations posttreatment at the time of virologic endpoint. These included V82A (n=14), M46I (n=4), and L90M (n=1) and 29 (71%) children had M184V/I. None of the major PI or NRTI mutations found posttreatment was present pretreatment. Of the five children with NNRTI mutations posttreatment, two had major NNRTI mutations persisting from baseline, two had new mutations, one had a minor mutation persisting from baseline, and two did not have pretreatment results. The antiretroviral-associated resistance mutations found in each sample are listed in Table 3.
Table 3.
Genotypic Resistance Profiles, Pretreatment and Posttreatment by Antiretroviral Regimen, Lopinavir/Ritonavir-Based (LPV/r) Compared with Ritonavir-Based (RTV-Based), of 41 Children Who Were Not Virologically Suppressed
| Number | Pretreatment PI | Pretreatment NNRTI | Pretreatment NRTI | Endpoint PI | Endpoint NNRTI | Endpoint NRTI |
|---|---|---|---|---|---|---|
| Maintained on LPV/r-based regimen throughout study period | ||||||
| 1 | M184V | |||||
| 2 | K103N | K103N | M184V | |||
| 3 | M184V | |||||
| 4 | M184V | |||||
| 5 | K70E | |||||
| 6 | ||||||
| 7 | ||||||
| 8 | (protease did not amplify) | |||||
| Received RTV-based regimen during study period | ||||||
| 9 | K103N, Y188C | I54V, V82A | M184V | |||
| 10 | Y181C | K219E | V82A, L90M | M184V | ||
| 11 | Y181C | V82A | M184I | |||
| 12 | V82A | M184V | ||||
| 13 | V82A | M184V | ||||
| 14 | V82A | M184V | ||||
| 15 | Y181C | L10F, M46I, I54V, V82A | M184V | |||
| 16 | Y181C | K219E | L10F, M46I, I54V, V82A | M184V | ||
| 17 | V179D | M46I, F53L, I54V, V82A | D67N, M184V | |||
| 18 | M46I, I54V, V82A | A62V, M184V | ||||
| 19 | Y181C | L33F, I54V, V82A | M184V | |||
| 20 | L10R, I54V, V82A | A62V, M184V | ||||
| 21 | L10I | L10I, V82A | M184V | |||
| 22 | L10F, I54V, Q58E, V82A | D67N, M184V | ||||
| 23 | E138A | I54V | E138A | M184V | ||
| 24 | L24I, I54V | E138A | M184V | |||
| 25 | L10V | M184V | ||||
| 26 | A71T | A71T | ||||
| 27 | Y181C | |||||
| 28 | G190A | G190A | ||||
| 29 | Y181C | M184V | ||||
| 30 | M184V | |||||
| 31 | M184V | |||||
| 32 | M184V | |||||
| 33 | M184V | |||||
| 34 | (protease did not amplify) | M184V | ||||
| 35 | M184V | |||||
| 36 | M184V | |||||
| 37 | ||||||
| 38 | ||||||
| 39 | ||||||
| 40 | ||||||
| 41 | ||||||
Gray shading, data not available; italics, major mutations maintained from baseline; bold, major mutations defined by 2009 IAS-USA list.
Predictors of drug resistance-associated mutations at time of lack of virologic suppression
None of the pretreatment characteristics found to be associated with lack of virologic suppression or death was associated with resistance mutations posttreatment at time of lack of virologic suppression (data not shown). However, treatment regimen, adherence, and pretreatment major mutations were found to be associated with PI resistance-associated mutations in children who did not achieve virologic suppression (Table 4). As all children with major PI resistance-associated mutations had a V82A, restricting the analysis to LPV/r- or RTV-associated resistance mutations did not change the outcome.
Table 4.
Characteristics of Children on Antiretroviral Therapy by Treatment Outcome at ≤52 Weeks
| |
No virologic suppression |
Virologic suppression |
|||
|---|---|---|---|---|---|
| Characteristic | With major PI mutations (% or IQR) | Without major PI mutations (% or IQR) | p value (with vs. without PI mutations) | p value (suppressed vs. not suppressed) | |
| n (%) | 14 (5.6%) | 25 (10%) | 209 (84.3%) | ||
| Median age at ART start, months | 7.66 (2.40, 11.5) | 12.9 (4.94, 17.7) | 0.181a | 9.1 (5.36, 15.0) | 0.972a |
| Median age at endpoint, monthsb | 17.5 (14.7, 21.1) | 18.7 (14.1, 27.0) | 0.489a | 17.7 (14.5, 23.5) | 0.532a |
| Median ART days at endpoint | 363 (179, 369) | 329 (226, 371) | 0.970a | 365 (248, 365) | 0.867a |
| Median WFA Z at endpoint | −1.59 (−2.74, −1.12) | −1.22 (−2.26, −0.36) | 0.303a | −0.78 (−1.46, 0.18) | 0.002a |
| Median WFA Z ≥−0.845 at endpoint | 2 (15%) | 12 (48%) | 0.077c | 107 (53%) | 0.038d |
| Median HFA Z at endpoint | −3.67 (−4.66, −2.51) | −3.75 (−4.96, −1.90) | 0.619a | −3.14 (−4.02, −2.13) | 0.106a |
| Median HFA Z ≥−3.2 at endpoint | 3 (27%) | 11 (46%) | 0.461c | 100 (53%) | 0.100d |
| Median CD4% at endpoint (IQR) | 24.2 (16.0, 34.0) | 26.6 (16.0, 34.2) | 0.785a | 29.5 (23.1, 36.4) | 0.015a |
| Median HIV-1 plasma RNA, copies/ml, at resistance test | 39,300 (12,400, 74,400) | 16,180 (4,135, 100,000) | 0.468a | n/a | |
| ART regimen at endpointe | |||||
| LPV/r throughout | 0 | 7 (6%) | 0.041c | 108 (94%) | <0.001d |
| on RTV for TB/BCG Rx | 12 (13%) | 13 (14%) | 70 (74%)) | ||
| on RTV for <6 months age | 2 (5%) | 5 (13%) | 31 (82%) | ||
| Nonadherent | 4 (29%) | 16 (64%) | 0.034c | 46 (22%) | <0.001d |
| TB/BCG Rx | 13 (93%) | 15 (60%) | 0.060c | 30 (73%) | <0.001d |
| Baseline major mutationse | |||||
| Any | 6 (13%) | 3 (6%) | 0.046c | 39 (81%) | 0.37d |
| PI | None | None | — | 2 (100%) | 1.00c |
| NRTI | 2 (15%) | 0 (0%) | 0.132c | 11 (85%) | 1.00c |
| NNRTI | 6 (14%) | 3 (7%) | 0.046c | 33 (79%) | 0.17d |
Predictors significant at p<0.05 are in italics.
Wilcoxon rank-sum test (two-tailed).
The endpoint described for those who did not achieve virologic suppression is the point at which lack of virologic suppression was determined: first HIV-1 plasma RNA level ≥400 copies/ml for those who achieved levels <400 copies/ml on therapy, or after 24 weeks of continuous antiretroviral therapy for those children who never achieved HIV-1 plasma RNA levels <400 copies/ml. The endpoint for those who achieved virologic suppression is the last complete study visit (closest to 52 weeks).
Fisher's exact test.
Chi-squared test (two-sided).
Percentages given are by row (not column).
IQR, intraquartile range; ART, antiretroviral therapy; WFA Z, weight-for-age z-score; HFA Z, height-for-age z-score; LPV/r, lopinavir/ritonavir; RTV, ritonavir; TB/BCG Rx, cotreatment for mycobacterial disease; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
Ritonavir-based therapy and antimycobacterial treatment
Of the children included in this analysis, 38 (15%) received RTV-based therapy during follow up because they were <6 months of age, 95 (39%) received RTV-based therapy because of cotreatment for mycobacterial disease, and 115 (46%) were maintained on LPV/r-based therapy throughout the observation period (Fig. 1). All 14 of the children who developed major PI mutations received RTV-based ART at some point during the study period, compared with none of the seven children who were maintained exclusively on LPV/r-based regimens (p=0.036) (Table 4, Row: “ART regimen at endpoint”).
Overall, examining the 257 children with 52-week outcome data, children who received RTV-based regimens were more likely to have an unsuppressed plasma HIV-1 RNA level at endpoint (28%) than those maintained on LPV/r (10%, p<0.001). Relatively few children (38) received RTV-based ART because they were <6 months of age, making it difficult to determine if RTV alone, without concomitant treatment for mycobacterial disease, was associated with PI mutations. The association of treatment for mycobacterial disease (which required RTV-based regimens) with the development of PI mutations did not reach statistical significance (p=0.06) (Table 4, Row: “TB/BCG Rx”).
HIV-1 plasma RNA level at endpoint
Median HIV-1 plasma RNA at resistance testing was higher, though not statistically significantly so, in children who had lack of virologic suppression with PI mutations compared to those without. When stratified by quartiles of HIV-1 plasma RNA levels at the time of resistance testing, the presence of PI mutations was highest in those children in the second and third quartiles. No children with levels <6331 copies/ml (in the first quartile) had PI mutations, and the difference, by HIV-1 plasma RNA level quartile, for prevalence of PI mutations was statistically significant (p=0.007).
Adherence to ART
Overall, the children who did not achieve virologic suppression were more likely to have poor adherence, defined as returning 20% more than expected of any antiretroviral medication at any study visit, than those who suppressed (OR 4.10, 95% CI: 2.05, 8.24). In contrast, restricting to those children who did not achieve virologic suppression, major PI-related mutations were less common if they also had poor adherence (OR 0.22, 95% CI: 0.05, 0.93).
Pretreatment major resistance mutations
The presence of PI mutations after treatment was more likely in children with pretreatment major resistance mutations, an association primarily driven by the presence of pretreatment NNRTI mutations (Table 4, Row “Baseline major mutations.”) The odds for the presence of pretreatment NNRTI mutations in children with PI mutations at the endpoint were 6.5 (95% CI: 1.09, 38.6) higher than those without PI mutations at the endpoint. Only two children who lacked virologic suppression had pretreatment major NRTI-related resistance mutations, and none had pretreatment PI-related resistance mutations, so the statistical analysis was inconclusive for these antiretroviral classes.
Of interest, the T74S protease polymorphism/mutation, which is not included on the IAS-USA mutation list, but has been shown to resensitize subtype B and C virus to ritonavir in vitro,40 was found in 22% of the children who did not to achieve virologic suppression (data not shown). The presence of T74S in the endpoint specimen was significantly associated with the presence of major PI mutations (OR 5.5, 95% CI: 1.10, 27.4). The odds ratio for the presence of T74S in those children who were always maintained on a RTV-based regimen was 5.0 (95% CI: 1.02, 24.5) compared with those who were on a LPV-r-based regimen at least part of the time.
There were no statistically significant predictors for NRTI- or NNRTI-related resistance mutations posttreatment among those who did not suppress. The presence of an M184V mutation was statistically associated with weight-for-age z-score only at time of lack of virologic suppression; 25% of children with M184V and 58% without M184V had median weight-for-age z-scores higher than the group median of −0.845 (p=0.043).
Discussion
The data presented are unique in describing treatment outcomes and patterns of antiretroviral resistance in a large cohort within a relatively understudied population: HIV-infected infants and young children on ART in a resource-limited setting. The results are concerning in terms of the percentage of children who developed major antiretroviral resistance mutations within 52 weeks (78%) when not virologically suppressed and in the limitations that this resistance places on future ART options for these children.
In this cohort, 17% of children on PI-based ART did not achieve virologic suppression by 52 weeks, and 78% of these had major antiretroviral-associated resistance mutations at the time of virologic endpoint posttreatment. The majority of children with resistance mutations had the lamivudine-associated resistance mutation M184V, which does not significantly limit future ARV regimen options. However, 36% developed major PI-associated resistance mutations, all of whom had a V82A, a mutation known to be associated with RTV.41 The high proportion of children with major PI-associated resistance mutations differs from findings in adults and children treated with RTV-boosted PI-based ART, where the development of major PI-associated resistance mutations is uncommon.13,19,42–44 The prevalence of major PI-associated resistance mutations in this cohort is likely a result of RTV-based ART, as seen in earlier adult cohorts prior to the advent of RTV-boosted PI-based ART.45,46
Supporting this assertion, the children who received RTV-based regimens at any point were less likely to achieve virologic suppression and more likely to develop major PI-associated resistance mutations, in contrast to those exclusively treated with a LPV/r-based regimen. None of the children treated solely with LPV/r developed major PI mutations, confirming the findings in other cohorts that virologic failure on LPV/r or other ritonavir-boosted PI-based regimens does not usually lead to the development of major PI-associated resistance mutations.13,14,19,44 As only a small number of children treated exclusively with LPV/r-based therapy did not achieve virologic suppression in our cohort, further investigation is needed to determine the profile of resistance mutations that are selected in a group unsuccessfully treated with a LPV/r-based regimen.
Our findings strongly support avoidance of RTV-only based ART for children who are being treated for mycobacterial infections or who are less than 6 months of age. Known drug–drug interactions, particularly with antimycobacterial therapy, the poor palatability of ritonavir, and the inferior efficacy of RTV-only regimens to RTV-boosted PI-based regimens may all have contributed to these findings.5,22,47,48 Fortunately, appropriate dosing of LPV/r for younger children is now available.48 This was not available at the time the study was conducted. Superboosting of LPV/r is also now recommended in South Africa for children requiring mycobacterial infection cotreatment.47,49
Other findings that merit attention include the effect of adherence to ART on treatment outcome and the development of resistance. Poor adherence was strongly associated with lack of virologic suppression in the cohort. In contrast, among the subgroup of children without virologic suppression, rates of nonadherence were highest (64%) in the children who did not develop major PI mutations compared with 29% in those who did develop major PI mutations. This is likely caused by a lack of drug-selective pressure on the virus in children with poor adherence. The link between higher levels of adherence and increased development of resistance mutations is described in adult studies of RTV-boosted and unboosted PI-based ART. Several investigations have shown that adherence levels over 75–85% were associated with development of resistance mutations, and lower levels of adherence led to ART failure without resistance.45,46,50 The adherence measure used in this study likely distinguished between children with less than or equal to 75% true adherence and those with more than 75% adherence, but was not likely to distinguish children with 75–85% adherence and those with >85% adherence. As such, adherence was correlated with virologic suppression, but nonadherence did not predict resistance. However, this is a speculation, and further studies with more rigorous adherence monitoring would be necessary in this population to determine the true cause of this parodoxical finding.
The data presented here also demonstrate an association between pretreatment major resistance mutations, most of which are mutations affecting the viral reverse transcriptase, and the development of PI-associated resistance mutations on treatment. Interaction between resistance-associated mutations has been seen for NRTI-related mutations, particularly in the sequence of thymidine analogue resistance-associated mutations.51,52 In viral protease, minor mutations frequently emerge after major mutations arise, and may have variable effects on antiretroviral efficacy and viral fitness.36 To our knowledge, an interaction between NNRTI- or NRTI-related resistance mutations and the development of subsequent PI-related resistance mutations has not been described. The possibility that the presence of resistance mutations in one enzyme would predispose HIV to develop resistance mutations in another enzyme is a finding that merits further exploration. However, small numbers precluded valid multivariate investigation in our study and we cannot rule out that the association may be due to confounding. For example, maternal viral load is related both to the presence of NNRTI-related resistance mutations at baseline and with poor treatment outcomes in children, so it is also plausible that high maternal viral load may explain the association.53
The limitations to this analysis include that the fact that it was conducted at a single site and findings may not be applicable to other settings. The size of the cohort is relatively small, although for pediatric cohorts it represents one of the largest in which the development of resistance has been monitored over time using HIV-1 genotypes. Resistance mutation profiles were not available for 24% of the children at baseline and at the study endpoint for 26% of the children who did not achieve virologic suppression, but the results were not significantly affected by stratification of the analysis by availability of HIV-1 genotype testing. The analyses involving plasma HIV-1 RNA level were limited due to the inability of the assay to detect levels over 750,000 copies/ml, which led to the need for categorization of this variable.
Despite these limitations, the clinical implications of the patterns of resistance observed in this cohort are of particular concern. The children participating in this study were well-monitored at regular study visits; the study team provided adherence counseling to caregivers at each encounter, and all children had access to HIV-1 plasma RNA level monitoring that enabled researchers to rapidly identify poor outcomes. These conditions are not currently replicated in most resource-limited settings.
The international community has begun to recognize the importance of virologic monitoring and resistance surveillance in adults as a tool to prevent the emergence of drug resistance and achieve success on first- and second-line therapy.54 The data presented here suggest that even in the setting of frequent virologic monitoring and adherence counseling, resistance can develop rapidly on RTV-based regimens. This is of concern for HIV-infected children, for whom second-line treatment options are more limited, and issues of exposure to nevirapine and cotreatment for mycobacterial disease are more prevalent than in HIV-infected adults.4 There is an urgent need for further research to determine the outcomes of second-line ART in children, to provide expanded options for second-line ART regimens, and to better understand the development of resistance to LPV/r-based regimens.
Acknowledgments
The study was supported in part by grants from the National Institutes of Child Health and Human Development (NICHD) HD 47177, Secure the Future Foundation, and the National Institute for Allergy and Infectious Diseases (NIAID) K23AI081538 [B.S.T.]. These data were presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI), Montreal, February 8–11, 2009. Poster Presentation #957.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Prendergast A. Tudor-Williams G. Jeena P. Burchett S. Goulder P. International perspectives, progress, and future challenges of paediatric HIV infection. The Lancet. 2007;370(9581):68–80. doi: 10.1016/S0140-6736(07)61051-4. [DOI] [PubMed] [Google Scholar]
- 2.Meyers T. Moultrie H. Naidoo K. Cotton M. Eley B. Sherman G. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis. 2007;196(Suppl 3):S474–481. doi: 10.1086/521116. [DOI] [PubMed] [Google Scholar]
- 3.Renaud-Thery F. Nguimfack BD. Vitoria M, et al. Use of antiretroviral therapy in resource-limited countries in 2006: Distribution and uptake of first- and second-line regimens. AIDS. 2007;21(Suppl 4):S89–95. doi: 10.1097/01.aids.0000279711.54922.f0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO; 2007. A research agenda for childhood tuberculosis: Improving the management of childhood tuberculosis within national tuberculosis programmes, research priorities based on a literature review. [Google Scholar]
- 5.Ren Y. Nuttall JJ. Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50(5):439–443. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 6.Arrive E. Newell ML. Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: A meta-analysis. Int J Epidemiol. 2007;36(5):1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 7.Martinson NA. Morris L. Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44(2):148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 8.Jourdain G. Ngo-Giang-Huong N. Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351(3):229–240. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 9.Violari A. Cotton MF. Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo P. Violari A. Lindsey J, et al. Nevirapine (NVP) vs lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) in single dose nevirapine (sdNVP)-exposed HIV-infected infants: Preliminary results from the IMPAACT P1060 trial. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Cape Town, South Africa. 2009. [Google Scholar]
- 11.Jaspan HB. Berrisford AE. Boulle AM. Two-year outcomes of children on non-nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008;27(11):993–998. doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Revised Treatment Recommendations for Infants. Geneva: WHO; 2008. [Google Scholar]
- 13.Kempf DJ. King MS. Bernstein B, et al. Incidence of resistance in a double-blind study comparing lopinavir/ritonavir plus stavudine and lamivudine to nelfinavir plus stavudine and lamivudine. J Infect Dis. 2004;189(1):51–60. doi: 10.1086/380509. [DOI] [PubMed] [Google Scholar]
- 14.Bongiovanni M. Virological success of lopinavir/ritonavir salvage regimen is affected by an increasing number of lopinavir/ritonavir-related mutations. Antivir Ther. 2003;8(3):209–214. [PubMed] [Google Scholar]
- 15.Saez-Llorens X. Violari A. Deetz CO, et al. Forty-eight-week evaluation of lopinavir/ritonavir, a new protease inhibitor, in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22(3):216–224. doi: 10.1097/01.inf.0000055061.97567.34. [DOI] [PubMed] [Google Scholar]
- 16.Badolato R. Schumacher RF. Rodella E, et al. Genotyping for guiding drug choice in human immunodeficiency virus-infected children failing multiple antiretroviral treatment regimens. Pediatr Infect Dis J. 2005;24(8):747–749. doi: 10.1097/01.inf.0000172910.79381.64. [DOI] [PubMed] [Google Scholar]
- 17.Delaugerre C. Warszawski J. Chaix ML. Veber F. Macassa E. Prevalence and risk factors associated with antiretroviral resistance in HIV-1-infected children. J Med Virol. 2007;79(9):1261–1269. doi: 10.1002/jmv.20940. [DOI] [PubMed] [Google Scholar]
- 18.Page TN. Archary M. Gordon ML, et al. Drug resistance patterns, genotypic analysis of viral tropism in HIV-1 subtype C-infected children failing antiretroviral therapy in South Africa. Paper presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Jul 19–22;2009 ; Cape Town, South Africa. [Google Scholar]
- 19.Delaugerre C. Teglas JP. Treluyer JM. Vaz P. Jullien V. Predictive factors of virologic success in HIV-1-infected children treated with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2004;37(2):1269–1275. doi: 10.1097/01.qai.0000137408.78031.37. [DOI] [PubMed] [Google Scholar]
- 20.Wallis CL. Erasmus L. Varughese S. Ndiweni D. Stevens WS. Emergence of drug resistance in HIV-1 subtype C infected children failing the South African national antiretroviral roll-out program. Pediatr Infect Dis J. 2009;28(12):1123–1125. doi: 10.1097/INF.0b013e3181af5a00. [DOI] [PubMed] [Google Scholar]
- 21.Luis Jimenez J. Resino S. Martinez-Colom A. Bellon JM. Angeles Munoz-Fernandez M. Mutations at codons 54 and 82 of HIV protease predict virological response of HIV-infected children on salvage lopinavir/ritonavir therapy. J Antimicrob Chemother. 2005;56(6):1081–1086. doi: 10.1093/jac/dki356. [DOI] [PubMed] [Google Scholar]
- 22.Chadwick EG. Rodman JH. Britto P, et al. Ritonavir-based highly active antiretroviral therapy in human immunodeficiency virus type 1-infected infants younger than 24 months of age. Pediatr Infect Dis J. 2005;24(9):793–800. doi: 10.1097/01.inf.0000177281.93658.df. [DOI] [PubMed] [Google Scholar]
- 23.Fiscus SA. Kovacs A. Petch LA, et al. Baseline resistance to nucleoside reverse transcriptase inhibitors fails to predict virologic response to combination therapy in children (PACTG 338) AIDS Res Ther. 2007;4:2. doi: 10.1186/1742-6405-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zyl G. van der Merwe L. Claassen M, et al. Protease inhibitor resistance in South African children with virologic failure. Pediatr Infect Dis J. 2010;28(12):1125–1127. doi: 10.1097/INF.0b013e3181af829d. [DOI] [PubMed] [Google Scholar]
- 25.Reitz C. Coovadia A. Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: Effect of cotreatment for tuberculosis. J Infect Dis. 2010;201(8):1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith K. Kuhn L. Coovadia A, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23(9):1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coovadia A. Abrams E. Strehlau R, et al. Randomized clinical trial of switching to nevirapine-based therapy for infected children exposed to nevirapine prophylaxis. Paper presented at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Jul 19–22;2009 ; Cape Town, South Africa. [Google Scholar]
- 28.Coovadia A. Abrams EJ. Stehlau R, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: A randomized controlled trial. JAMA. 2010;304(10):1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.South African Government. South African HIV Treatment Guidelines, factsheet section 10–Antiretroviral. 2007.
- 30.World Health Organization. WHO Child Growth Standards and WHO Anthro Software and Macros, 2005. www.who.int/childgrowth/software/en/2005. [Sep;2009 ]. www.who.int/childgrowth/software/en/2005
- 31.Loubser S. Balfe P. Sherman G. Hammer S. Kuhn L. Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20(7):995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillay V. Ledwaba J. Hunt G, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1-infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antivir Ther. 2008;13(Suppl 2):101–107. [PubMed] [Google Scholar]
- 33.Coovadia A. Hunt G. Abrams EJ. Sherman G. Meyers T. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48(4):462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt G. Coovadia A. Abrams E, et al. Drug resistance at treatment initiation in HIV-infected infants exposed to single-dose nevirapine for prevention of transmission in South Africa. Johannesburg: AIDS Research Unit, National Institute for Communicable Diseases; 2010. Submitted. [Google Scholar]
- 35.Stanford HIVDB Team. Stanford University HIV Drug Resistance Database. http://hivdb.stanford.edu/pages/algs/HIVdb.html. [Aug;2009 ]. http://hivdb.stanford.edu/pages/algs/HIVdb.html
- 36.Johnson VA. Brun-Vezinet F. Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17(5):138–145. [PubMed] [Google Scholar]
- 37.Eshleman SH. Mracna M. Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15(15):1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kamya MR. Mayanja-Kizza H. Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46(2):187–193. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Revised surveillance case definitions for hiv infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years–United States, 2008. MMWR. 2008;57(No. RR-10):1–12. [PubMed] [Google Scholar]
- 40.Soares EA. Santos AF. Gonzalez LM, et al. Mutation T74S in HIV-1 subtype B and C proteases resensitizes them to ritonavir and indinavir and confers fitness advantage. J Antimicrob Chemother. 2009;64(5):938–944. doi: 10.1093/jac/dkp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eastman PS. Mittler J. Kelso R, et al. Genotypic changes in human immunodeficiency virus type 1 associated with loss of suppression of plasma viral RNA levels in subjects treated with ritonavir (Norvir) monotherapy. J Virol. 1998;72(6):5154–5164. doi: 10.1128/jvi.72.6.5154-5164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eron J., Jr. Yeni P. Gathe J, Jr, et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: A randomised non-inferiority trial. Lancet. 2006;368(9534):476–482. doi: 10.1016/S0140-6736(06)69155-1. [DOI] [PubMed] [Google Scholar]
- 43.Riddler SA. Haubrich R. DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima VD. Gill VS. Yip B. Hogg RS. Montaner JS. Harrigan PR. Increased resilience to the development of drug resistance with modern boosted protease inhibitor-based highly active antiretroviral therapy. J Infect Dis. 2008;198(1):51–58. doi: 10.1086/588675. [DOI] [PubMed] [Google Scholar]
- 45.King MS. Brun SC. Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naive, HIV-1-infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191(12):2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 46.Bangsberg DR. Acosta EP. Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20(2):223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 47.CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online] http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm. 2007. http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm
- 48.Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children: Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2009. [Apr 16;2010 ]. pp. 1–139.
- 49.McIlleron H. Ren Y. Nuttall J, et al. Double-dose lopinavir/ritonavir provides insufficient lopinavir exposure in children receiving rifampicin-based anti-TB treatment. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada. 2009. [Google Scholar]
- 50.Maggiolo F. Airoldi M. Kleinloog HD, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clin Trials. 2007;8(5):282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 51.Kellam P. Boucher CA. Larder BA. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992;89(5):1934–1938. doi: 10.1073/pnas.89.5.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitcomb JM. Parkin NT. Chappey C. Hellmann NS. Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188(7):992–1000. doi: 10.1086/378281. [DOI] [PubMed] [Google Scholar]
- 53.Prendergast A. Mphatswe W. Tudor-Williams G, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22(11):1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. HIVResNet: The global HIV drug resistance network. http://www.who.int/hiv/topics/drugresistance/hivresnet/en/index.html. [Feb 10;2010 ]. http://www.who.int/hiv/topics/drugresistance/hivresnet/en/index.html