Abstract
Cell therapy is a promising novel option for treatment of cardiovascular disease. Because the role of bone marrow-derived circulating progenitor cells (BM-CPCs) after cell therapy is less clear, we analyzed in this randomized, controlled study the influence of intracoronary autologous freshly isolated bone marrow cell transplantation (BMC-Tx) by using a point-of-care system on cardiac function and on the mobilization of BM-CPCs in patients with ischemic heart disease (IHD). Fifty-six patients with IHD were randomized to either receive freshly isolated BMC-Tx or a control group that did not receive cell therapy. Peripheral blood concentrations of CD34/45+ and CD133/45+ CPCs were measured by flow cytometry pre-, immediately post-, and at 3, 6, and 12 months postprocedure in both groups. Global ejection fraction and the size of infarct area were determined by left ventriculography. We observed in patients with IHD after intracoronary transplantation of autologous freshly isolated BMCs-Tx at 3 and 12 months follow-up a significant reduction of the size of infarct area and increase of global ejection fraction as well as infarct wall movement velocity. The mobilization of CD34/45+ and CD133/45+ BM-CPCs significantly increased at 3, 6, and 12 months after cell therapy when compared with baseline in patients with IHD, although no significant changes were observed between pre- and immediately postintracoronary cell therapy administration. In the control group without cell therapy, there was no significant difference of CD34/45+ and CD133/45+ BM-CPCs mobilization between pre- and at 3, 6, and 12 months postcoronary angiography. Intracoronary transplantation of autologous freshly isolated BMCs by using a point-of-care system in patients with IHD may enhance and prolong the mobilization of CD34/45+ and CD133/45+ BM-CPCs in peripheral blood and this might increase the regenerative potency in IHD.
Introduction
Progenitor cells derived from bone marrow (BM) circulate in the peripheral blood (PB) and have been implicated in neoangiogenesis after tissue ischemia has occurred [1–3]. These BM-derived circulating progenitor cells (BM-CPCs) express unique surface markers, such as CD34+ and the early hematopoietic cell marker CD133+(AC133+) [4,5]. In addition, BM-CPCs are capable of proliferating and differentiating into endothelial cells and are therefore ideal candidates for vascular regeneration [6]. Experiments in animals show that the systemic application or mobilization of stem cells and CPCs beneficially influences the repair of endothelial cells after injury and the progression of atherosclerosis [7–11]. Additionally, clinical trials indicate a beneficial effect of intracoronary infusion of BMCs or CPCs on myocardial function in patients with acute myocardial infarction (AMI) [12–16]. However, the role of BM-CPCs after cell therapy is less clear. It is unknown whether the mobilization of progenitor cells relates to regeneration of infarcted heart muscle after tissue ischemia. In this prospective, randomized, controlled trial, we therefore analyzed the influence of intracoronary freshly isolated cell therapy by using a point-of-care system on cardiac function and on the mobilization of the BM-CPCs in patients with ischemic heart disease (IHD).
Methods
Patient characteristics
In this prospective, randomized, controlled trial, 56 patients between 18 and 80 years of age were eligible for inclusion if they had a documented myocardial infarction at least 3 months and had a clear-cut demarcated region of left ventricular dysfunction with an open infarct-related coronary artery at the time of stem cell therapy (STX). Exclusion criteria were the presence of acutely decompensated heart failure (HF) with a New York Heart Association (NYHA) class of IV, infectious or inflammatory disease, active bleeding, surgery or trauma within 2 months, renal or liver dysfunction, thrombocytopenia, or anemia, a severe comorbidity and alcohol or drug dependency, a history of other severe chronic diseases or cancer, or unwillingness to participate. The local ethics committee approved the study protocol. All IHD patients were discharged with standard medication consisting of acetylsalicylic acid and clopidogrel, an ACE inhibitor, a B-blocker, and a statin.
Study protocol
In this study, 56 patients with IHD were randomly allocated in a 2:1 ratio to either receive intracoronary autologous freshly isolated BMC-Tx or a control group without STX. All patients suffered a transmural myocardial infarction at 28 ± 14 months before STX. All of these patients were treated acutely by percutaneous transluminal coronary angioplasty plus stent implantation. We performed in all patients of both groups at 8 ± 2 months before cell transplantation a coronary angiography as well as a left ventriculography and the patients presented with open infarct-related coronary arteries. These patients were randomized to either receive intracoronary autologous freshly isolated BMC-Tx or a control group without STX. Patients included in the stem cell group underwent a BM puncture and BM aspiration on day 1 after admission. BMCs were separated. Subsequently, after coronary angiography and left ventriculography, the BMCs were freshly transplanted via intracoronary route. Patients in the control group received only coronary angiography and left ventriculography without any cell-based therapy. In both groups, all patients with angiographically relevant coronary restenosis (five patients from the STX group and two patients from the control group) were treated by percutaneous transluminal coronary angioplasty plus stent implantation and have been excluded from the study. We examined all patients in both groups after 3 and 12 months by coronary angiography and left ventriculography. The primary end point of the study was the change in global ejection fraction (EF) as well as the size of infarcted area measured by left ventriculography after 3 and 12 months. Secondary end points were the mobilization of BM-CPCs immediately pre- and post- and at 3, 6, as well as 12 months postprocedure. Functional status was assessed by NYHA classification and brain natriuretic peptide (BNP) level in PB in both groups.
Preparation and administrations of BMCs
A total of approximately 120 mL of BM was aspirated from the iliac crest after local anesthesia and mononuclear cells were isolated and identified including CD34+ and CD133+. The BMC concentrate suspension was isolated using a point-of-care system (Harvest BMAC System; Harvest Technologies GmbH, Munich, Germany) according to the manufacturer's instructions for use to produce 20 mL of concentrated cells. The concentrate consisted of a heterogeneous cell population including hematopoietic, mesenchymal, and other progenitor cells as well as granulocytes and platelets.
After undergoing arterial puncture, all patients received 7500–10,000 U of heparin. Cell transplantation was performed via the intracoronary administration route [17] using four fractional infusions parallel to balloon inflation over 2–4 min of 3–5 mL of cell suspension. All cells were infused directly into the infarcted zone through the infarct-related artery via an angioplasty balloon catheter, which was inflated at a low pressure (4 atm) and was located within the previously stented coronary segments. This prevented back flow of cells and produced stop flow beyond the site of balloon inflation to facilitate high-pressure infiltration of cells into the infarcted zone with prolonged contact time for cellular migration. Three and 12 months after catheter-guided cell transplantation, all functional tests were repeated, including coronary angiography and left ventriculography. There were no procedural or cell-induced complications and there were no side effects in any patients.
Coronary angiography and left ventriculography
Patients in both groups underwent left heart catheterization, left ventriculography, and coronary angiography on admission as well as at 3 and 12 months after procedure. Cardiac function and infarct size were determined by left ventriculography. Cardiac function was evaluated by global EF and by auxotonic myocardial contractility index, evaluated by the wall movement velocity of the infarcted area. Global EF was measured with Quantcor software (Siemens, Erlangen, Germany). To quantify the size of infarct area, we used the centerline method according to Sheehan [18] by plotting five axes perpendicular to the long axis of the heart in the main akinetic or dyskinetic segment of ventricular wall. Systolic and diastolic lengths were then measured by two independent observers, and the mean difference was divided by systolic duration in seconds. The follow-up was 3 and 12 months after the treatment. All hemodynamic investigations were obtained by two independent observers.
Mobilization of CD34/45+ and CD133/45+ BM-CPCs
BM-CPCs were collected in PB for CD34/45+ and CD133/45+ in both groups and quantified by flow cytometry (EPICS-XL, Beckmann Coulter). Assessments in patients with BMC-Tx (n = 38) were done immediately pre- and post- as well as at 3, 6, and 12 months postintracoronary cell transplantation. For the control group without BMC-Tx (n = 18), measurements of CD34/45+ and CD133/45+ were performed pre- and immediately post- as well as at 3, 6, and 12 months postcardiac catheterization. PB samples were analyzed within 2 h.
Samples were stained with fluorescein isothiocyanate conjugate of a CD45+ antibody (clone J33; Coulter/Immunotech, Marseille, France) that detects all isoforms and glycoforms of the CD45 family, phycoerythrin (PE) conjugate of a CD34+ antibody (clone 581; Coulter/Immunotech) that detects a class III epitope on all glycoforms of the CD34+ antigen, and PE conjugate of a CD133/1+ (Miltenyi Biotec, Bergisch Gladbach, Germany). Control samples were stained with CD45+ fluorescein isothiacyanate and an IgG1 PE (Coulter/Immunotech) isotype.
Four ethylenediaminetetraacetic acid-added blood samples of each patient were labeled with CD34/45+, CD133/45+, and IgG1/CD45. All tubes were incubated at room temperature in the dark. After incubation, cells were lysed with ammonium chloride and washed with phosphate-buffered saline. Samples were then stored on ice at 4°C in the dark for 20 min and analyzed by flow cytometry [19,20]. Samples were subjected to a 2D side scatter-fluorescence dot plot analysis. After appropriate gating, the concentration of BM-CPCs with low cytoplasmic granularity (low sideward scatter) was quantified and expressed as concentration of cells per million white blood cells.
Safety parameters
To assess any inflammatory response and myocardial reaction after cell therapy, white blood cell count and the serum levels of C-reactive protein, creatine kinase (CK), and troponin were determined immediately before as well as after treatment. Additional analysis was done directly after transplantation and at 3, 6, and 12 months later: BNP level in PB, ECG at rest, 24-h Holter ECG, and echocardiography.
Procedural complications were defined as any ventricular arrythmia, visible thrombus formation, distal embolization, or injury of the coronary artery associated with the cell infusion catheterization procedure.
Statistical analysis
Quantitative data are presented as mean ± SD and qualitative data are tabulated using absolute frequencies and/or percentages. Differences between therapy groups for qualitative variables were tested using Fisher's exact test because of small number of patients in the therapy groups. Within-differences of quantitative variables in each therapy group were compared using the Wilcoxon test for dependent samples, and differences between therapy groups for quantitative variables were compared with the Wilcoxon test for independent samples. Both these nonparametric Wilcoxon tests are preferred because of the more likely expected nonnormal distribution of the data. For all statistical tests, a result will be seen as statistically significant, if the corresponding two-sided P value is smaller or equal to 0.05. If the mean and the median did not differ markedly for a variable, the graphical presentation of the data was done using the mean and SD of this variable. Statistical analysis was performed with SPSS for Windows (Version 15.0).
Results
Baseline characteristics of the patients
We randomized 56 patients with IHD (2:1) in the study. Of them, 38 patients in first group received freshly isolated BMC-Tx into the infarct-related coronary artery, whereas 18 patients in the second group received no intracoronary BMC-Tx. There were no significant differences between the baseline characteristics and demographics of patients in both groups (Table 1).
Table 1.
Baseline Clinical Characteristics of Patients with Ischemic Heart Disease with Bone Marrow Cell Transplantation and Control Group Without Transplantation
| IHD with BMC-Tx (n = 38) | IHD without BMC-Tx (n = l8) | P | |
|---|---|---|---|
| Age | 62 ± 10 | 60 ± 9 | NS |
| M/F | 20/18 | 10/8 | NS |
| Cardiovascular risk factors (%) | |||
| Hypertension | 60 | 65 | NS |
| Hyperlipidemia | 60 | 65 | NS |
| Smoking | 80 | 80 | NS |
| Diabetes | 20 | 25 | NS |
| Positive family history of CAD | 20 | 10 | NS |
| Transmural myocardial infarction, months before Tx | 28 ± 13 | 27 ± 14 | NS |
| No. of diseased vessels | 1.9 ± 0.5 | 2.0 ± 0.6 | NS |
| Infarct-related vessel (LAD/LCX/RCA) | 22/8/8 | 10/5/3 | NS |
| PTCA/stent at the time of AMI | 38/38 | 18/18 | NS |
| Medication (%) | |||
| Aspirin | 100 | 100 | NS |
| Clopidogrel | 100 | 100 | NS |
| ACE inhibitor or AT II blocker | 100 | 100 | NS |
| Beta-blocker | 100 | 100 | NS |
| Aldosterone antagonist | 20 | 20 | NS |
| Statin | 100 | 100 | NS |
| Laboratory parameters | |||
| CK U/L | 2018 ± 560 | 2000 ± 740 | NS |
Quantitative data are presented as mean ± SD.
IHD, ischemic heart disease; BMC-Tx, bone marrow cell transplantation; CAD, coronary artery disease; PTCA, percutaneous transluminal coronary angioplasty; CK, creatine kinase; LAD, left anterior descending coronary artery; LCX, left circumflex artery; RCA, right coronary artery; NS, not significant.
Effect of BMCs-Tx
The mobilization of BM-CPCs
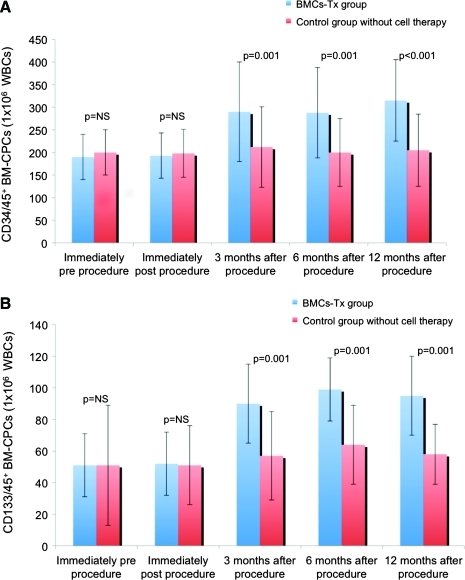
The mobilization of BM-CPCs was analyzed in the first group immediately pre- and post- and at 3, 6, and 12 months postintracoronary cell transplantation as well as in the second group without cell therapy pre-, immediately post-, and at 3, 6, and 12 months postcardiac catheterization. There was a significant increase of CD34/45+ mobilization at 3, 6, and 12 months after intracoronary cell transplantation compared with the control group, whereas there were no significant changes between immediately pre- and postintervention within both groups (Fig. 1A). The mobilization of CD133/45+ showed the same pattern at 3, 6, and 12 months after intervention with a significant increase in the cell therapy group compared with the control group without cell transplantation (Fig. 1B). In contrast to intracoronary cell therapy group with a significant increase of CD34/45+ and CD133/45+ mobilization between baseline and after 3, 6, and 12 months follow-up, there was no difference in the control group between baseline and after 3, 6, and 12 months follow-up (Table 2).
FIG. 1.
(A, B) The mobilization of BM-CPCs was analyzed immediately pre- and post- and at 3, 6, and 12 months postprocedure in both groups. CD34/45+ and CD133/45+ BM-CPC mobilization significantly increased at 3, 6, and 12 months after BMCs-Tx compared with baseline, whereas no significant differences were observed between immediately pre- and postprocedure in both groups. There were no significant changes in the mobilization of BM-CPCs at 3, 6, and 12 months after coronary angiography compared with baseline in the control group without cell therapy. Moreover, there were significant differences of BM-CPC mobilization at 3, 6, and 12 months after procedure between both groups. BM-CPCs, bone marrow-derived circulating progenitor cells; BMC-Tx, bone marrow cell transplantation; WBCs, white blood cells. Color images available online at www.liebertonline.com/scd
Table 2.
Cardiac Function, Clinical Parameters, and Mobilization of BM-CPCs Immediately Pre- and Post- and at 3, 6, and 12 Months Postcoronary Angiography in the Control Group Without Bone Marrow Cell Transplantation
| Immediately precoronary angiography | Immediately postcoronary angiography | 3 months after coronary angiography | 6 months after coronary angiography | 12 months after coronary angiography | |
|---|---|---|---|---|---|
| Global EF (%) | 46 ± 10 | 47 ± 7a | 46 ± 9a | ||
| The size of infarct area (%) | 29 ± 9 | 28 ± 9a | 26 ± 8a | ||
| Infarct wall movement velocity (cm/s) | 1.86 ± 0.96 | 1.93 ± 0.76a | 1.99 ± 0.9a | ||
| LVEDV (mL) | 141 ± 28 | 142 ± 31a | 140 ± 30a | ||
| LVESV (mL) | 76 ± 17 | 75 ± 15a | 76 ± 16a | ||
| SVI (mL/m) | 36 ± 10 | 35 ± 8a | 34 ± 9a | ||
| CD34/45+ BM-CPCs | 200 ± 50 | 198 ± 53a | 212 ± 89a | 200 ± 75a | 205 ± 80a |
| CD133/45+ BM-CPCs | 51 ± 38 | 51 ± 25a | 57 ± 28a | 64 ± 25a | 58 ± 19a |
| BNP (pg/mL) | 169 ± 95 | 128 ± 97a | 138 ± 59a | 125 ± 70a | |
| NYHA classification | 2.50 ± 0.9 | 2.30 ± 0.7a | 2.40 ± 0.7a | 2.29 ± 0.9a |
Values are mean ± SD.
There was no significant difference in baseline cardiac function, clinical function status parameters, as well as mobilization of BM-CPCs between both groups at baseline.
P = not significant.
BM-CPCs, bone marrow-derived circulating progenitor cells; NYHA, New York Heart Association; BNP, B-type natriuretic peptide; EF, ejection fraction; LVEDV, end-diastolic volume; LVESV, end-systolic volume; SVI, stroke volume index.
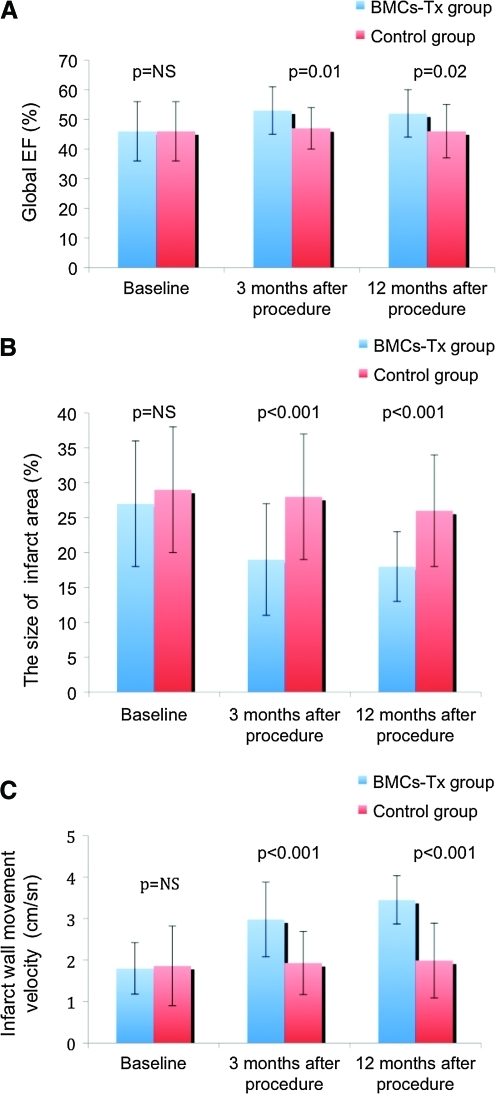
Left ventricular function, infarct size, and infarct wall movement velocity
Patients in both groups underwent left heart catheterization, left ventriculography, and coronary angiography on admission as well as at 3 and 12 months after procedure. Global EF, left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), stroke volume index (SVI), infarct size, and the wall movement velocity of the infarcted area were measured by left ventriculography in the first group immediately pre- and at 3 and 12 months post-BMC-Tx as well as in the second group without BMC-Tx pre- and at 3 and 12 months postcardiac catheterization. There were no significant baseline differences in global EF, infarct size, and infarct wall movement velocity between the two groups (Tables 2 and 3 and Fig. 2A–C). At 3 and 12 months after cell therapy, we observed a significant increase of global EF and infarct wall movement velocity, whereas there was no significant difference in the control group. Further, we found a significant decrease of the size of infarct area after 3 and 12 months. Moreover, we found a significant increase of SVI and decrease of LVESV, whereas no significant change was observed in LVEDV at 3 and 12 months after cell therapy (Table 3). In the control group, there were no significant changes in global EF, LVEDV, LVESV, SVI, infarct size, and the wall movement velocity of the infarcted area at 3 and 12 months after coronary angiography (Table 2). Moreover, we observed that the global EF and the wall movement velocity of the infracted area significantly increased at 3 and 12 months after cell therapy compared with the control group. The size of infarct area was significantly decreased at 3 and 12 months after BMCs-Tx when compared with the control group without cell therapy (Fig. 2A–C).
Table 3.
Cardiac Function, Clinical Parameters, and Mobilization of BM-CPCs Immediately Pre- and Post- and at 3, 6, and 12 Months Postbone Marrow Cell Transplantation in the Intervention Group
| Immediately pre-BMC-Tx | Immediately post-BMC-Tx | 3 months after BMC-Tx | 6 months after BMC-Tx | 12 months after BMC-Tx | |
|---|---|---|---|---|---|
| Global EF (%) | 46 ± 10 | 53 ± 8a | 52 ± 8a | ||
| The size of infarct area (%) | 27 ± 9 | 19 ± 8b | 18 ± 5b | ||
| Infarct wall movement velocity (cm/s) | 1.80 ± 0.74 | 2.98 ± 0.89b | 3.45 ± 0.5b | ||
| LVEDV (mL) | 138 ± 34 | 139 ± 32c | 138 ± 32c | ||
| LVESV (mL) | 75 ± 20 | 65 ± 10a | 66 ± 10a | ||
| SVI (mL/m) | 34 ± 10 | 42 ± 9a | 41 ± 8a | ||
| CD34/45+ BM-CPCs | 190 ± 50 | 193 ± 50c | 290 ± 110a | 288 ± 100a | 315 ± 90b |
| CD133/45+ BM-CPCs | 51 ± 20 | 52 ± 20c | 90 ± 25a | 99 ± 20b | 95 ± 25a |
| BNP (pg/mL) | 178 ± 89 | 65 ± 28b | 61 ± 19b | 60 ± 15b | |
| NYHA classification | 2.40 ± 0.8 | 1.80 ± 0.8b | 1.7 ± 0.8b | 1.5 ± 0.8b |
P = 0.01–0.001 (compared with baseline).
P < 0.001 (compared with baseline).
P = not significant.
FIG. 2.
(A–C) Global EF, infarct size, and the wall movement velocity of the infarcted area were measured by left ventriculography immediately pre-and at 3 and 12 months postprocedure in both groups. There were no significant baseline differences in global EF, infarct size, and infarct wall movement velocity between the two groups. Global EF and infarct wall movement velocity significantly increased at 3 and 12 months after cell therapy when compared with the control group. Further, there was a significant decrease of infarct size at 3 and 12 months after cell transplantation when compared with the control group. No significant changes were observed in global EF, infarct size, and infarct wall movement velocity at 3 and 12 months after coronary angiography compared with baseline in the control group without cell therapy. Further, there were significant changes in global EF, infarct size, and infarct wall movement velocity at 3 and 12 months after procedure between both groups. EF, ejection fraction. Color images available online at www.liebertonline.com/scd
Functional status and clinical safety parameters
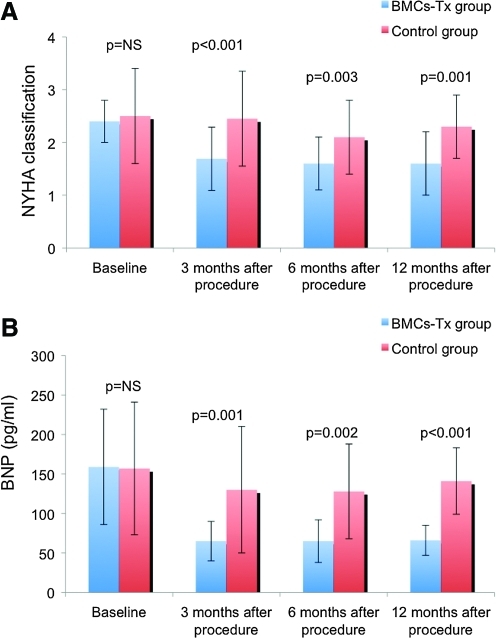
To determine the functional status, we assessed NYHA classification in both groups by two independent and blinded physicians. We observed a significant improvement in NYHA classification at 3, 6, and 12 months after intracoronary cell therapy, whereas there was no significant difference in the control group at 3, 6, and 12 months after coronary angiography. Further, we found a significant decrease of BNP level in PB at 3, 6, and 12 months after BMC-Tx, whereas no significant difference was observed in the control group at 3, 6, and 12 months after coronary angiography (Tables 2 and 3). There were no significant differences of baseline NYHA classification and BNP levels between both groups. The NYHA classification and BNP levels significantly decreased at 3, 6, and 12 months after cell therapy compared with the control group (Fig. 3A, B).
FIG. 3.
(A, B) To determine the functional status of patients, NYHA classification and BNP levels were measured at 3, 6, and 12 months after procedure in both groups. There were no significant differences of baseline NYHA classification and BNP level in both groups. At 3, 6, and 12 months after cell therapy, there was a significant decrease of NYHA classification and BNP level compared with baseline. In the control group without cell therapy, there were no significant changes in NYHA classification and BNP level at 3, 6, and 12 months after coronary angiography compared with baseline. Moreover, significant differences in NYHA classification and BNP level were observed at 3, 6, and 12 months after procedure between both groups. NYHA, New York Heart Association; BNP, brain natriuretic peptide. Color images available online at www.liebertonline.com/scd
ECG at rest and on exercise and 24-h Holter ECG revealed no rhythm disturbances at any time point. There was no inflammatory response or myocardial reaction (white blood cell count, C-reactive protein, CK, troponin) after cell therapy. No immediate pre- as well as postprocedure adverse complications, no new electrocardiographic changes or significant elevations in CK or troponin, as well as no inflammatory response were observed in patients with BMC-Tx.
Discussion
In this study, we examined the influence of autologous intracoronary freshly isolated BMC-Tx on the mobilization of BM-CPCs and left ventricular function in patients with IHD after 3, 6, and 12 months.
Cardiac ischemia leading to postinfarction HF, particularly in patients with large myocardial infarction, is associated with a high mortality and morbidity. The use of stem cell-based therapy is becoming increasingly recognized as having the potential to salvage damaged myocardium and to promote endogenous repair of cardiac tissue, thus having the potential for the treatment of HF. Experimental data show that infusion or injection of stem/progenitor cells derived from various sources may enhance blood flow and neovascularization and may improve heart function after myocardial infarction [21,22]. Pilot and randomized clinical trials suggested that the intracoronary infusion of autologous BMCs is safe and feasible as well as beneficially affects postinfarction remodeling or perfusion in patients with AMI [12–16]. Likewise, it was reported that intracoronary infusion of BMCs in patients with IHD improves the function of myocardium [23,24]. Our findings showed that the infarct size reduced, whereas the global EF and regional infarct wall movement velocity increased at 3 and 12 months after freshly isolated intracoronary cell therapy in patients with IHD, similar to the data of Strauer et al. [23] and Assmus et al. [24]. Additionally, we found improvement of the functional status (NYHA classification) and BNP level at 3, 6, and 12 months after cell therapy. Cell isolation procedures are crucial for the functional activity of the administered cellular product. In our trial, we chose to use a point-of-care system for the preparation of the treating cell composition. We demonstrated the same results for the first time with intracoronary freshly isolated BMC-Tx by using a point-of-care system with Harvest BMAC system for the preparation of the treating cell composition, not Ficoll gradient separation as in other studies. Many previously conducted trials employed Ficoll gradient separation as the method of cell collection, which produces a very limited cell linage spectrum. The cellular composition of the concentrate, which was prepared using a point-of-care system, differs from that prepared using the Ficoll method. The Ficoll composition contains predominantly mononuclear cells (lymphocytes, erythroblasts, and monocytes) and very few granulocytes. The point-of-care system concentrates contain nucleated cell population with mononuclear cells and specific stem cell population (CD34+ and CD133+) as well as the platelets from the marrow aspirate (Table 4). Importantly, however, the point-of-care device provided advantage of significantly higher yield of isolated BMCs compared with the Ficoll protocol. Thus, if the number of infused cells in in vivo neovascularization model was adjusted for this higher yield of BMCs, the treatment effect was significantly greater compared with Ficoll BMCs, as assessed by limb perfusion measurement [25]. One obvious difference in the two compositions is the presence of significant numbers of granulocytes and platelets in the point-of-care system concentrate composition. Platelets and granulocytes have been shown to have a positive effect on the neovascular potential of the resulting concentrate. The presence of platelets could be important because it has been shown that these platelet-derived mediators also potently enhance postnatal angiogenesis. Iba and colleagues demonstrated that implantation of mononuclear cells together with platelets into ischemic limbs more effectively augments collateral vessel formation by supplying various angiogenic factors, in which vascular endothelial growth factor (VEGF) played a key role [26]. Indeed, Massberg and colleagues provided compelling evidence that platelets generate the critical signal that recruits CD34+ BMCs and c-Kit+ Sca-1+ Lin− BM-derived progenitor cells to sites of injury [27]. Therefore, these findings strongly support the notion that implanted platelets play a pivotal role in stem and progenitor recruitment and provide a rationale for the fact that the point-of-care system produced functional in vivo results similar to or better than Ficoll. In our study, despite higher number of platelets, we observed no immediate pre- as well as postprocedure adverse complications. In addition, unlike Ficoll isolation, where cells are resuspended in a serum-free medium, the point-of-care system is always resuspended in the patient's own plasma. Thus, the isolated cells are not removed from their natural plasma microenvironment, which may help to sustain the functionality of the cells. This has been further supported by an experimental study of Hermann et al., who showed the point-of-care system composition to be significantly more bioactive than the Ficoll composition. Intriguingly, however, because of the greater yield of cells generated using a point-of-care system, the cellular product isolated from a given BM aspirate by using a point-of-care device may actually translate into even greater therapeutic effects. Additionally, practical aspects may also deserve consideration. Importantly, a major limitation of the Ficoll isolation procedure for clinical applications is that it is strongly investigator dependant, is immensely time consuming, and requires a good manufacturing practice facility. In this study, we were able to demonstrate that such complex methods are not necessary to achieve established results. As the concentration process uses a point-of-care system, everything can be accomplished in one session without adding excessive time to the overall procedure circumventing the previously mentioned disadvantages of the Ficoll isolation process. The point-of-care device provides a much shorter turnaround time. Therefore, this device represents a cost-effective and time-efficient stand-alone technique for the isolation of autologous BMCs suitable for cell therapy regimens in the rapidly growing field of regenerative medicine.
Table 4.
The Cellular Composition of Bone Marrow Aspirate and Bone Marrow Concentrate by Using a Point-of-Care System in the Group with BMC-Tx
| Bone marrow aspirate (preseparation, 120 cc) | Bone marrow concentrate (postseparation, 20 cc) | |
|---|---|---|
| Total nucleated cells (× 106 mL) | 25 ± 9 | 99 ± 25 |
| CD34+ cells (× 106 mL) | 0.21 ± 0.07 | 0.95 ± 0.1 |
| CD133+ cells (× 106 mL) | 0.07 ± 0.004 | 0.35 ± 0.02 |
| Platelet count (× 103 per μL) | 155 ± 19 | 705 ± 174 |
| Viability of cells (%) | 98 ± 1.5 | |
Several hypotheses have been proposed about how intracoronary cell therapy improves myocardial function. Experimental studies addressing the capacity of transplanted BM-derived stem cells to differentiate into the cardiomyogenic lineage yielded conflicting results [28,29]. Recent well-conducted studies suggest that the BMCs do not transdifferentiate into cardiomyocytes but adopt mature hematopoietic characteristics [30]. In contrast to embryonic stem cells, most adult stem or progenitor cells do not spontaneously differentiate into cardiomyocytes but rather require an adequate stimulus to do so. Another proposed mechanism is that cell therapy may increase angiogenesis and improve blood supply to ischemic regions, potentially aiding in the revascularization of hibernating myocardium and preventing cardiomyocyte apoptosis. Additionally or alternatively, the local microenvironment plays an important role to induce cell fate changes by physical cell-to-cell interaction or by providing paracrine factors promoting tissue repair [31,32].
Cell-based therapy is a promising option for treatment of ischemic disease. However, cell therapy is in its early stages, and various questions remain. For example, the mechanisms of action by which cells exhibit beneficial effects [33]. BMCs are best characterized and have been used in the majority of clinical trials performed to date. BMCs contain a complex assortment of progenitor cells, including hematopoietic stem cells, mesenchymal stem cells, or stromal cells and multipotential adult progenitor cells. BM-CPCs are another population of progenitor cells that has also been shown to have therapeutic potential. These cells were characterized by the expression of at least two hematopoietic stem cell markers (CD133+ or CD34+) [34]. Tissue ischemia was found to mobilize BM-CPCs into the PB and contribute to neovascularization in an animal model [35]. In humans, it was demonstrated that the mobilization of CD34/45+ and CD133/45+ BM-CPCs significantly increased with a peak on day 7 and decreased on day 8 following myocardial infarction. On the basis of these findings, it is plausible to correlate this spontaneous mobilization of CD34/45+ and CD133/45+ BM-CPCs response to myocardial repair after AMI. Moreover, this response is mostly inadequate because of reduced mobilization of BM-CPCs by increased cardiovascular risk factors in patients with large myocardial infarction [36]. An important point in our results is the significant increase of CD34/45+ and CD133/45+ BM-CPCs mobilization in the PB at 3, 6, and 12 months after intracoronary cell transplantation, with no significant change in the control group. The presence of immature circulating cells in the PB has been advocated as a marker of an organism's regenerative capacity [37]. The potential to form collateral vessels in ischemic tissue is increased with a combination of both platelets and mononuclear cells when compared with only mononuclear cells. This effect has been attributed to the increased presence of “angiogenic factors (mainly VEGF) and cytokines” [26]. In fact, Massberg et al. [27] offer strong evidence that platelets play an important role in recruiting CD34+ BM-derived progenitor cells to sites of injury. Specific inhibition of platelet adhesion essentially abrogated the accumulation of CD34+ and c-Kit+ Sca-1+ Lin− BM progenitor cells into the site of injury. Walter et al. [38] have shown that activated platelets secrete the potent chemokine SDF-1, thereby supporting further primary adhesion and migration of circulating stem and progenitor cells. This has been further supported by Stellos and Gawaz, who concluded “platelet interaction with progenitor cells seems to play a decisive role in vascular and tissue regeneration” [39]. The point-of-care system concentrates, which was used in our study, also contain this important combination—both platelets and mononuclear cell populations. In our study, we showed that the intracoronary BMC-Tx in IHD patients may enhance and prolong the mobilization of BM-CPCs. Experimental and clinical studies suggest that there is an evolving role for CPCs in neoangiogenesis and rejuvenation of the endothelial monolayer [1,7,10]. Indeed, the mobilization of BM-CPCs is inversely correlated with endothelial function [40], which explains that the BM-CPCs may play an important role in endogenous repair mechanisms of the injured endothelial monolayer and thereby reduce atherosclerotic lesion formation [41]. The occurrence of a first major cardiovascular event (AMI, hospitalization, revascularization, or death from cardiovascular causes) was associated with reduced BM-CPC levels in patients with coronary artery disease [42]. Moreover, intracoronary administration of BMCs is associated with a significant reduction of major adverse cardiovascular events after AMI [43]. Previous studies demonstrate that patients with HF show endothelial dysfunction, and in HF, nitric oxide production is diminished, whereas rate of endothelial apoptosis is increased [44,45]. The impaired neovascularization in mice lacking expression of endotheliale sticksoffmonoxid-synthase (eNOS) is related to a defect in progenitor cell mobilization from BM. The enhanced expression of endotheliale stickstoffmonoxid-synthase (eNOS) and vascular endothelial growth factor (VEGF) might improve the mobilization of BM-CPCs into the PB and enhance the process of vasculogenesis [46,47]. Moreover, the transient increase in BM-CPCs after regular symptom-limited (ischemic and/or subischemic) exercise training reached a maximum after the regular exercise training for 3 weeks, but did not persist up until 3 months after the regular training [48]. Laufs and colleagues described a significant increase in BM-CPCs after a 4-week, noncontrolled rehabilitation training program in patients with stable coronary artery disease without exercise-induced ischemia [49]. In that trial, it was speculated that asymptomatic tissue ischemia leads to an increase in vasculogenic cytokines, in the same way as symptomatic tissue ischemia does. The improved perfusion capacity, which was demonstrated in the TOPECARE- and REPAIR-AMI trial in patients after cell therapy, increases epicardial artery shear stress and stimulates the endothelium to release NO, which may enhance the mobilization of BM-CPCs and exerts antiatherosclerotic functions [50–52].
On the basis of our findings, mechanistically, that transplantation of freshly isolated BMCs via inflated angioplasty balloon catheter, which increases epicardial artery shear stress and stimulates paracrine factors as well as the endothelium to release NO, with an additional effect of platelets by interaction with cytokines/VEGF may enhance the mobilization of BM-CPCs in the PB. Enhanced NO production by improved perfusion capacity as well as enhanced mobilization of BM-CPCs may improve cardiac contractile function and reduce infarct size after cell therapy. Finally, increase in BM-CPCs by enhanced NO production in the PB may improve neoangiogenesis as well as rejuvenation of the injured endothelial monolayer and thereby reduce atherosclerotic lesion formation. Therefore, patients receiving STX may tend to adopt a healthier behavior in everyday life. This may lead to improved clinical outcome after intracoronary cell therapy in patients with IHD.
In the present study, we could demonstrate that intracoronary transplantation of autologous freshly isolated BMCs improved global EF and reduced infarct size significantly in patients with IHD after 3 and 12 months. Moreover, we observed a significant mobilization of BM-CPCs even at 3, 6, and 12 months after cell transplantation. This interesting observation could be implemented in future large-scale randomized studies, where the BM-CPC mobilization after transplantation may serve as a predictor for identifying IHD patients with greater benefit after cell therapy.
Author Disclosure Statement
No conflicts of interest exist.
References
- 1.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T. Witzenbichler B. Schatteman G. Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Kalka C. Masuda H. Takahashi T. Kalka-Moll WM. Silver M. Kearney M. Li T. Isner JM. Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafii S. Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 4.Peichev M. Naiyer AJ. Pereira D. Zhu Z. Lane WJ. Williams M. Oz MC. Hicklin DJ. Witte L. Moore MA. Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 5.Yin AH. Miraglia S. Zanjani ED. Almeida-Porada G. Ogawa M. Leary AG. Olweus J. Kearney J. Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 6.Gunsilius E. Duba HC. Petzer AL. Kahler CM. Gasti GA. Contribution of endothelial cells of hematopoietic origin to blood vessel formation. Circ Res. 2001;88:E1. doi: 10.1161/01.res.88.1.e1. [DOI] [PubMed] [Google Scholar]
- 7.Kong D. Melo LG. Gnecchi M. Zhang L. Mostosiavsky G. Liew CC. Pratt RE. Dzau VJ. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation. 2004;110:2039–2046. doi: 10.1161/01.CIR.0000143161.01901.BD. [DOI] [PubMed] [Google Scholar]
- 8.Walter DH. Rittig K. Bahlmann FH. Kirchmair R. Silver M. Murayama T. Nishimura H. Losordo DW. Asahara T. Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 9.Werner N. Junk S. Laufs U. Link A. Walenta K. Bohm M. Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 10.Werner N. Priller J. Laufs U. Endres M. Böhm M. Dirnagl U. Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 11.Sondergaard CS. Bonde J. Dagnaes-Hansen F. Nielsen JM. Zachar V. Holm M. Hokland P. Pedersen L. Minimal engraftment of human CD34+ cells mobilized from healthy donors in the infarcted heart of athymic nude rats. Stem Cells Dev. 2009;18:845–856. doi: 10.1089/scd.2008.0006. [DOI] [PubMed] [Google Scholar]
- 12.Strauer BE. Brehm M. Zeus T. Köstering M. Hernandez A. Sorg RV. Kögler G. Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 13.Assmus B. Schachinger V. Teupe C. Britten M. Lehmann R. Döbert N. Griinwald F. Aicher A. Urbich C. Martin H. Hoelzer D. Dimmeler S. Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 14.Wollert KC. Meyer GP. Lotz J. Ringes-Lichtenberg S. Lippolt P. Breidenbach C. Fichtner S. Korte T. Hornig B. Messinger D. Arseniev L. Hertenstein B. Ganser A. Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 15.Janssens S. Dubois C. Bogaert J. Theunissen K. Deroose C. Desmet W. Kalantzi M. Herbots L. Sinnaeve P. Dens J. Maertens J. Rademakers F. Dymarkowski S. Gheysens O. Van Cleemput J. Bormans G. Nuyts J. Belmans A. Mortelmans L. Boogaerts M. Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 16.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Hölschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Süselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 17.Strauer BE. Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–934. doi: 10.1161/01.cir.0000057525.13182.24. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan FH. Bolson EL. Dodge HT. Mathey DG. Schofer J. Woo HW. Advantages and applications of the centreline method for characterizing regional ventricular function. Circulation. 1986;74:293–305. doi: 10.1161/01.cir.74.2.293. [DOI] [PubMed] [Google Scholar]
- 19.Keeney M. Chin-Yee I. Weir K. Popma J. Nayar R. Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based o the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;15:61–70. [PubMed] [Google Scholar]
- 20.Sutherland DR. Anderson L. Keeney M. Nayar R. Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S. Zeiher AM. Cell therapy of acute myocardial infarction: open questions. Cardiology. 2009;113:155–160. doi: 10.1159/000187652. [DOI] [PubMed] [Google Scholar]
- 22.Dimmeler S. Zeiher AM. Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assmus B. Honold J. Schächinger V. Britten MB. Fischer-Rasokat U. Lehmann R. Teupe C. Pistorius K. Martin H. Abolmaali ND. Tonn T. Dimmeler S. Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 24.Strauer BE. Brehm M. Zeus T. Bartsch T. Schannwell C. Antke C. Sorg RV. Kögler G. Wernet P. Muller HW. Köstering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease (The IACT Study) J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 25.Hermann PC. Huber SL. Herrler T. von Hesler C. Andrassy J. Kevy SV. Jacobson MS. Heeschen C. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–1069. [PubMed] [Google Scholar]
- 26.Iba O. Matsubara H. Nozawa Y. Fujiyama S. Amano K. Mori Y. Kojima H. Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106:2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 27.Massberg S. Konrad I. Schürzinger K. Lorenz M. Schneider S. Zohlnhoefer D. Hoppe K. Schiemann M. Kennerknecht E. Sauer S. Schulz C. Kerstan SH. Peluso M. Goyal P. Vestweber D. Emambokus NR. Busch DH. Frampton J. Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B. Bodine DM. Leri A. Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Murry CE. Soonpaa MH. Reinecke H. Nakajima H. Nakajima HO. Rubart M. Pasumarthi KB. Virag JI. Bartelmez SH. Poppa V. Bradford G. Dowell JD. Williams DA. Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 30.Balsam LB. Wagers AJ. Christensen JL. Kofidis T. Weissman IL. Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 31.Dimmeler S. Burchfield J. Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 32.Lipinski MJ. Biondi-Zoccai GGL. Abbate A. Khianey R. Scheiban I. Bartunek J. Vanderheyden M. Kim HS. Kang HJ. Strauer BE. Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 33.Sharma R. Raghubir R. Stem cell therapy: a hope for dying hearts. Stem Cells Dev. 2007;16:517–536. doi: 10.1089/scd.2006.0070. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Y. Jahagirdar BN. Reinhardt RL. Schwartz CD. Keene CD. Ortiz-Gonzalez XR. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low WC. Largaespada DA. Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2007;447:879–880. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T. Masuda H. Takahashi T. Kalka C. Pastore C. Silver M. Kearne M. Magner M. Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 36.Turan RG. Brehm M. Koestering M. Zeus T. Bartsch T. Steiner S. Picard F. Ebner P. Schannwell CM. Strauer BE. Factors influencing spontaneous mobilization of CD34+ and CD133+ progenitor cells after myocardial infarction. Eur J Clin Invest. 2007;37:842–851. doi: 10.1111/j.1365-2362.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- 37.Blau HM. Brazelton TR. Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 38.Walter DH. Haendeler J. Reinbold J. Rochwalsky U. Seeger F. Honold J. Hoffmann J. Urbich C. Lehmann R. Arenzana-Seisdesdos F. Aicher A. Heeschen C. Fichtlscherer S. Zeiher AM. Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 39.Stellos K. Gawaz M. Platelet interaction with progenitor cells: potential implications for regenerative medicine. Thromb Haemost. 2007;98:922–931. doi: 10.1160/th07-02-0147. [DOI] [PubMed] [Google Scholar]
- 40.Hill JM. Zalos G. Halcox JP. Schenke WH. Waclawiw MA. Quyyumi AA. Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 41.Urbich C. Dimmeler S. Endothelial progenitor cell: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 42.Werner N. Kosiol S. Schiegl T. Ahlers P. Walenta K. Link A. Bohm M. Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 43.Schächinger V. Erbs S. Elsässer A. Haberbosch W. Hambrecht R. Hölschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Süselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 44.Agnoletti L. Curello S. Bachetti T. Malacarne F. Gaia G. Comini L. Volterrani M. Bonetti P. Parrinello G. Cadei M. Grigolato PG. Ferrari R. Serum from patients with severe heart failure downregulates eNOS and is proapoptotic: role of tumor necrosis factor-alpha. Circulation. 1999;100:1983–1991. doi: 10.1161/01.cir.100.19.1983. [DOI] [PubMed] [Google Scholar]
- 45.Katz SD. Khan T. Zeballos GA. Mathew L. Potharlanka P. Knecht M. Whelan J. Decreased activity of the L-arginine-nitric-oxide metabolic pathway in patients with congestive heart failure. Circulation. 1999;99:2113–2117. doi: 10.1161/01.cir.99.16.2113. [DOI] [PubMed] [Google Scholar]
- 46.Aicher A. Heeschen C. Mildner-Rihm C. Urbich C. Ihling C. Technau-Ihling K. Zeiher AM. Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi T. Kalka C. Masuda H. Chen D. Silver M. Kearney M. Magner M. Isner JM. Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 48.Brehm M. Picard F. Ebner P. Turan G. Bölke E. Köstering M. Schüller P. Fleissner T. Ilousis D. Augusta K. Peiper M. Schannwell CH. Strauer BE. Effects of exercise training on mobilization and functional activity of blood-derived progenitor cells in patients with acute myocardial infarction. Eur J Med Res. 2009;14:393–405. doi: 10.1186/2047-783X-14-9-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laufs U. Werner N. Link A. Endres M. Wassmann S. Jurgens K. Miche E. Böhm M. Nickenig G. Physical training increases endothelial progenitor cell, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 50.Candipan RC. Wang BY. Bultrago R. Tsao PS. Cooke JP. Regression or progression. Dependency on vascular nitric oxide. Arterioscler Thromb Vasc Biol. 1996;16:44–50. doi: 10.1161/01.atv.16.1.44. [DOI] [PubMed] [Google Scholar]
- 51.Coke JP. Singer AH. Tsao P. Zera P. Rowan RA. Billingham ME. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J Clin Invest. 1992;90:1168–1172. doi: 10.1172/JCI115937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bassaneze V. Barauna VG. Lavini-Ramos C. Kalil J. Schettert IT. Miyakawa AA. Krieger JE. Shear stress induces nitric oxide-mediated vascular endothelial growth factor production in human adipose tissue mesenchymal stem cells. Stem Cells and Development. 2010;19:371–378. doi: 10.1089/scd.2009.0195. [DOI] [PubMed] [Google Scholar]