Abstract
We demonstrated previously that the blood pressure of patients with IgA nephropathy becomes salt sensitive as renal damage progresses. We also showed that increased urinary angiotensinogen levels in such patients closely correlate with augmented renal tissue angiotensinogen gene expression and angiotensin II levels. Here, we investigated the relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Forty-one patients with IgA nephropathy consumed an ordinary salt diet (12 g/d of NaCl) for 1 week and a low-salt diet (5 g/d of NaCl) for 1 week in random order. The salt-sensitivity index was calculated as the reciprocal of the slope of the pressure-natriuresis curve drawn by linking 2 data points obtained during consumption of each diet. The urinary angiotensinogen:creatinine ratio was significantly higher in patients who consumed the ordinary salt diet compared with the low-salt diet (17.5 μg/g [range: 7.3 to 35.6 μg/g] versus 7.9 μg/g [range: 3.1 to 14.2 μg/g] of creatinine, respectively; P<0.001). The sodium sensitivity index in our patients positively correlated with the glomerulosclerosis score (r=0.43; P=0.008) and changes in logarithmic urinary angiotensinogen:creatinine ratio (r=0.37; P=0.017) but not with changes in urinary protein excretion (r=0.18; P=0.49). In contrast, changes in sodium intake did not alter the urinary angiotensinogen:creatinine ratio in patients with Ménière disease and normal renal function (n=9). These data suggest that the inappropriate augmentation of intrarenal angiotensinogen induced by salt and associated renal damage contribute to the development of salt-sensitive hypertension in patients with IgA nephropathy.
Keywords: angiotensinogen, salt, hypertension, glomerulonephritis, IgA nephropathy, renin-angiotensin system
Salt-sensitive hypertension is often associated with renal damage in patients with chronic kidney disease (CKD).1 Salt-sensitive blood pressure is a risk factor for cardiovascular and renal damage and mortality.2,3 We showed previously that blood pressure becomes salt sensitive as renal damage progresses in patients with IgA nephropathy and normal blood pressure.4 Although the precise mechanisms for the pathogenesis of salt sensitivity of blood pressure in patients with CKD remain unclear, accumulating evidence suggests that the intrarenal renin-angiotensin system (RAS) contributes to its initiation and progression.1
Focus on the role of the RAS in the pathophysiology of hypertension and renal damage has shifted recently to the role of the local RAS in the kidneys.5 Inappropriate increases in levels of angiotensin II (Ang II) in the kidneys lead to enhanced tubular sodium reabsorption and renal tissue damage, both of which might contribute to the development of salt-sensitive hypertension.5,6 That high-salt intake decreases both plasma and kidney Ang II levels by significantly reducing renin release from juxtaglomerular cells under physiological conditions is generally accepted.5 A high-salt intake similarly and significantly decreases both plasma renin activity and Ang II levels in salt-sensitive hypertensive Dahl rats, a model of human salt-sensitive hypertension.7 However, intrarenal Ang II levels increase in these rats during the development of hypertension and renal damage.7–10 Preclinical studies have also shown that increases in intrarenal Ang II levels are associated with inappropriate augmentation of intrarenal angiotensinogen levels.11,12 Nevertheless, no evidence yet supports the notion that the intrarenal RAS is actually activated in salt-dependent hypertensive patients with renal disease. In this regard, our preclinical studies demonstrated that intrarenal angiotensinogen is a key RAS component in regulating local Ang II levels in the kidneys.5 We also recently developed an ELISA system with which to directly measure human angiotensinogen13,14 and showed that increases in urinary angiotensinogen levels correlate with increased angiotensinogen expression and Ang II levels in renal tissues of patients with IgA nephropathy, suggesting that urinary angiotensinogen provides a specific index of intrarenal RAS in such patients.15
Here, we test the hypothesis that the development of salt-sensitive hypertension is associated with increased intrarenal RAS activity in patients with IgA nephropathy by measuring their urinary angiotensinogen excretion rates after consuming diets containing different amounts of salt.
Methods
Patients With IgA Nephropathy
This retrospective exploratory study proceeded in compliance with our protocol and the principles of the Declaration of Helsinki. The ethics committee of Osaka City General Hospital reviewed and approved the protocol, patient information, and informed consent forms. Outpatients diagnosed with renal disease at Osaka City General Hospital between 1998 and 2002 (n=554) provided written informed consent to undergo a renal biopsy. During this period, 150 patients were diagnosed with IgA nephropathy. However, we excluded patients with other renal diseases and those taking any medication. The study was finally composed of 41 patients, including 35 who we had studied previously.16 Table 1 shows the baseline characteristics of the patients.
Table 1.
Baseline Characteristics of Patients With IgA Nephropathy (N=41)
| Clinical Characteristics | Data | Equivalent, mg/dL |
|---|---|---|
| Sex, male/female | 14/27 | |
| Age, y | 45±15 | |
| Serum urea nitrogen, μmol/L | 3.9±1.0 | 11±3 |
| Serum creatinine, μmol/L | 63±19 | 0.71±0.21 |
| Plasma total protein, g/dL | 6.1±0.6 | |
| Glomerulosclerosis score, /400 | 99±76 | |
| Tubulointerstitial damage score, % | 10 (5, 30) |
Values are mean±SD or median (25th and 75th percentiles).
Study Protocols for Patients With IgA Nephropathy
The patients were hospitalized and consumed standard hospital meals containing ≈10 g of NaCl per day for 1 week. They then consumed either a low-salt diet (≈5 g/d of NaCl per day) or an ordinary salt diet (≈12 g/d of NaCl) for 1 week in random order, with no washout period between regimens. The 2 diets contained the same amount of protein (1.2 g/kg of body weight per day) and the same number of calories (35 kcal/kg per day). On the last 3 days of the diets, sodium, protein, and angiotensinogen were assayed in portions of 24-hour urine samples that were obtained daily, frozen, and stored at −80°C. The means of these values were analyzed in the present study. On the last day of each diet, blood pressure during 24 hours was recorded hourly using an automatic oscillometric monitor (Ambulatory Blood Monitoring System, A&D Co, Tokyo, Japan). The mean arterial pressure (MAP) was calculated by adding one third of the pulse pressure to the diastolic pressure, and the results are expressed as means of these 24 values on each final day. Renal plasma flow and creatinine clearance were calculated on the same day using a standard method with para-aminohippurate and endogenous creatinine as markers. Renal clearance was standardized for a body surface area of 1.73 m2. On the last day of each diet, blood samples were obtained from supine patients at 8:00 AM to measure levels of electrolytes, plasma renin activity, and serum aldosterone.
Calculation of Salt-Sensitivity Index
Pressure-natriuretic curves were generated by plotting urinary sodium excretion on the ordinate as a function of the MAP on the abscissa. Assuming a linear relationship between these variables, a curve was drawn for each patient by linking the data points obtained when the sodium balance was in a steady state during each diet. The salt-sensitivity index (SSI) is equal to the reciprocal of the slope (Figure S1, available in the online Data Supplement; please see http://hyper.ahajournals.org).4
Histological Analyses
Sections of biopsy specimens containing ≥10 glomeruli from each patient were stained with periodic acid-Schiff reagent and independently evaluated by 2 investigators who were unaware of the SSI of each patient. The severity of glomerulosclerosis and of tubulointerstitial damage was semiquantified using the described scoring method.4 Severity was graded from 0 to 4 to express the ratio (percentage) of affected glomeruli in biopsy specimens. Damage scores were then calculated by multiplying the grade of individual glomeruli by the ratio (percentage) of glomeruli with the same degree of damage. The severity of glomerulosclerosis for each tissue specimen was determined by adding these damage scores. The severity of tubulointerstitial damage in each specimen was scored as the ratio (percentage) of tubulointerstitial fibrosis, tubular atrophy, and interstitial infiltrates in the cortex.
Urinary Sampling From Patients With Ménière Disease
A low-salt diet has become a popular therapy recently for Ménière disease.17,18 Because Ménière disease is an inner ear disease that is not systemic, urinary samples were collected as controls from outpatients with Ménière disease and normal renal function. Between December 2007 and December 2008, we recruited 16 patients who were diagnosed at Kagawa University Medical School with Ménière disease based on the clinical course and diagnostic categories according to the 1995 guidelines from the American Academy of Otolaryngology-Head and Neck Surgery Committee of Hearing and Equilibrium.19 We excluded patients who were pregnant, hypertensive (systolic blood pressure/diastolic blood pressure >140/90 mm Hg), diabetic, or who had kidney disease, hepatic diseases, infections, or malignancies. Finally, a dietitian provided nutritional guidance to 9 enrolled patients with Ménière disease to consume a low-salt diet (6 g/d of NaCl per day). Twenty-four–hour urine was collected and assayed for sodium, protein, and angiotensinogen before and 4 weeks after consuming the low-salt diet. Blood pressure was measured between 9:00 and 10:00 AM after 30 minutes of bed rest. This study proceeded in compliance with our protocol and the principles of the Declaration of Helsinki. The local institutional review board at Kagawa University Medical School reviewed and approved the protocol, the patient information, and informed consent forms.
Other Measurements
Urinary angiotensinogen levels were measured using a sandwich ELISA as described13,14 and urinary angiotensinogen:creatinine ratios were calculated. Serum urea nitrogen, serum and urinary creatinine, serum and urinary protein, and urinary sodium concentrations were determined in the clinical laboratory at Osaka City General Hospital. Plasma renin activity was measured using an angiotensin I radioimmunoassay. Serum aldosterone was also measured using a radioimmunoassay.
Statistical Analysis
Values are expressed as mean±SD, except for scores for tubulointerstitial damage, as well as values for urinary excretion of protein, urinary angiotensinogen, plasma renin activity, and SSI, which are expressed as medians (25th and 75th percentiles), because these values were not normally distributed. Significant differences among creatinine clearance, renal plasma flow, filtration fraction, and serum aldosterone in the 2 diets were evaluated using Student t test for paired samples. Significant differences among urinary excretion of protein, urinary angiotensinogen:creatinine ratio, and plasma renin activity in the 2 diets were evaluated using the Wilcoxon test. Correlations among SSI, glomerulosclerosis score, and changes in urinary excretion of protein and in logarithmic transformed urinary angiotensinogen were evaluated using a Pearson correlation. Scores for tubulointerstitial damage and other parameters were evaluated using a Spearman correlation. These parameters were assessed by multiple linear regression analysis. Data were statistically analyzed using JMP version 8 (SAS Institute Inc, Cary, NC). P<0.05 was considered statistically significant.
Results
Systemic and Renal Function in Patients With IgA Nephropathy
Table 2 shows urinary excretion rates of sodium and protein, MAP renal function, plasma renin activity, and serum aldosterone levels in hospitalized patients with IgA nephropathy. The MAP and the urinary excretion rate of sodium were significantly higher among patients who consumed an ordinary salt diet compared with a low-salt diet. Urinary excretion rates of protein, creatinine clearance, and effective renal plasma flow were also significantly higher among patients who consumed the ordinary salt diet. The filtration fraction did not significantly differ between the 2 diets. Plasma renin activity and serum aldosterone levels were significantly lower among patients who consumed the ordinary salt diet. The SSI in all of the patients with IgA nephropathy was 0.040 (range: 0.013 to 0.092).
Table 2.
Changes in Urinary Excretion of Sodium, Blood Pressure, Renal Function, Plasma Renin Activity, and Serum Aldosterone Between Diets in Patients With IgA Nephropathy
| Renal and Other Results | Ordinary Salt Diet | Low-Salt Diet | P |
|---|---|---|---|
| Urinary excretion of sodium, mmol/d | 166±37 | 48±14 | <0.0001 |
| Mean blood pressure, mm Hg | 96±9 | 89±9 | <0.0001 |
| Urinary excretion of protein, mg/d | 509 (207, 1916) | 372 (142, 1134) | 0.004 |
| Creatinine clearance, mL/min | 114±25 | 108±23 | <0.0001 |
| Effective renal plasma flow, mL/min | 549±192 | 516±199 | 0.0012 |
| Filtration fraction, % | 22±5 | 23±5 | 0.22 |
| Plasma renin activity, ng/mL per h | 0.6±0.9 | 1.7±1.8 | <0.0001 |
| Serum aldosterone, ng/dL | 7.1±3.1 | 13.9±8.3 | <0.0001 |
Values are mean±SD or median (25th and 75th percentiles).
Urinary Angiotensinogen and Correlation in Patients With IgA Nephropathy
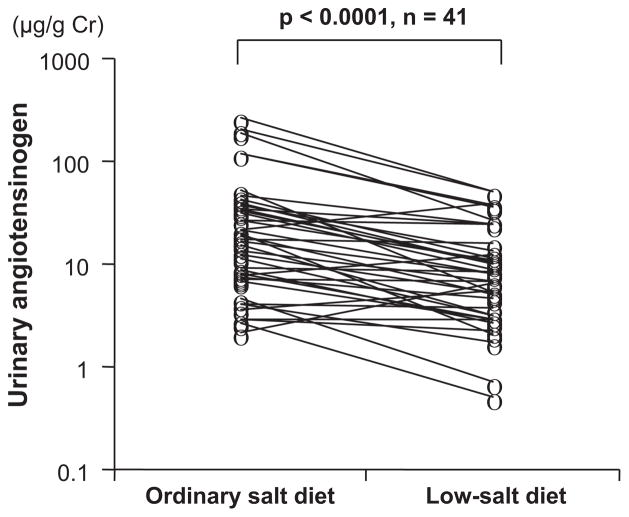
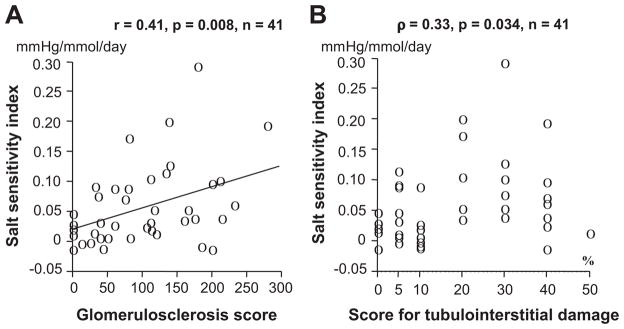
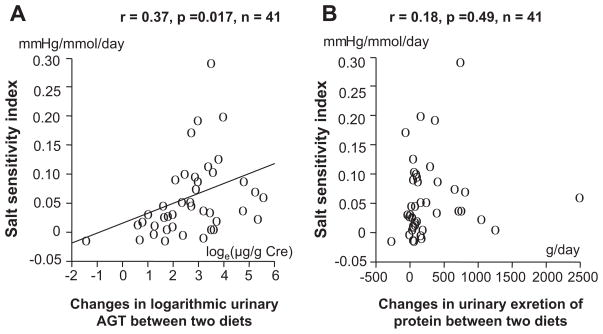
The urinary angiotensinogen:creatinine ratio was significantly higher among patients with IgA nephropathy on the ordinary salt diet compared with the low-salt diet (17.5 μg/g [range: 7.3 to 35.6 μg/g] versus 7.9 μg/g [range: 3.1 to 14.2 μg/g] of creatinine, respectively; P<0.001; Figure 1). The SSI significantly correlated with scores for glomerulosclerosis or tubulointerstitial damage (Figure 2A and 2B). The SSI also significantly correlated with changes in the logarithmic urinary angiotensinogen:creatinine ratio (Figure 3A) but not with changes in the urinary excretion rate of protein (Figure 3B). Multiple regression analysis of these 4 explanatory variables showed that SSI correlated with changes in the logarithmic urinary angiotensinogen:creatinine ratio but not with glomerulosclerosis scores, scores for tubulointerstitial damage, or changes in urinary protein excretion (Table 3). Using 1 of the 2 histological parameters and the remaining 2 variables, multiple regression analysis was re-evaluated, whereas the 2 histological damage scores correlated with each other. Multiple regression analysis of 3 explanatory variables showed that SSI correlated with glomerulosclerosis scores and changes in the logarithmic urinary angiotensinogen:creatinine ratio but not with scores for tubulointerstitial damage or changes in urinary protein excretion (Table 3). Changes in the logarithmic urinary angiotensinogen:creatinine ratio significantly correlated with scores for glomerulosclerosis (r=0.38; P=0.014) or tubulointerstitial damage (ρ=0.36; P=0.021), changes in urinary excretion of protein (r=0.63; P<0.0001), and SSI (r=0.37; P=0.017). Multiple regression analysis of these 3 or 4 explanatory variables revealed that changes in logarithmic urinary angiotensinogen: creatinine ratio correlated with changes in urinary protein excretion rates and SSI but not with the scores for glomerulosclerosis or tubulointerstitial damage (Table 4).
Figure 1.
Changes in urinary angiotensinogen:creatinine ratios in patients with IgA nephropathy who consumed diets containing different amounts of salt.
Figure 2.
Relationship between salt-sensitivity index and scores for glomerulosclerosis (A) and tubulointerstitial damage (B) in patients with IgA nephropathy.
Figure 3.
Relationship between salt-sensitivity index and changes in logarithmic angiotensinogen:creatinine ratio (A) and urinary excretion of protein (B) in patients with IgA nephropathy.
Table 3.
Multiple Regression Analysis of Salt-Sensitivity Index in Patients With Nephropathy
| Parameters | Estimate | SD | t | P |
|---|---|---|---|---|
| Four parameters | ||||
| Intercept | −0.0085 | 0.022 | −0.38 | 0.70 |
| Glomerulosclerosis score | 0.00029 | 0.00018 | 1.58 | 0.12 |
| Score for TID | 3.95e−6 | 0.00094 | 0.00 | 0.997 |
| Changes in U-P | −0.000034 | 2.53e−5 | −1.34 | 0.19 |
| Changes in Loge, U-AGT | 0.019 | 0.0087 | 2.13 | 0.044* |
| Three parameters 1 | ||||
| Intercept | −0.0085 | 0.022 | −0.39 | 0.70 |
| Glomerulosclerosis score | 0.00029 | 0.00013 | 2.15 | 0.0038* |
| Changes in U-P | −0.000034 | 2.53e−5 | −1.34 | 0.19 |
| Changes in Loge, U-AGT | 0.019 | 0.0087 | 2.13 | 0.040* |
| Three parameters 2 | ||||
| Intercept | −0.0021 | 0.022 | −0.09 | 0.92 |
| Score for TID | 0.0010 | 0.00072 | 1.39 | 0.17 |
| Changes in U-P | −3.47e−6 | 2.65e−5 | −1.31 | 0.20 |
| Changes in Loge, U-AGT | 0.020 | 0.0090 | 2.25 | 0.031* |
TID indicates tubulointerstitial damage; U-P, urinary excretion of protein; U-AGT, urinary angiotensinogen.
P<0.05.
Table 4.
Multiple Regression Analysis of Changes in Logarithmic Urinary Angiotensinogen:Creatinine Ratios in Patients With Nephropathy
| Parameters | Estimate | SD | t | P |
|---|---|---|---|---|
| Four parameters | ||||
| Intercept | 1.64 | 0.29 | 5.67 | <0.001* |
| Glomerulosclerosis score | 0.00048 | 0.0033 | 0.14 | 0.89 |
| Score for TID | 0.0093 | 0.017 | 0.56 | 0.58 |
| Changes in U-P | 0.0017 | 0.00038 | 4.34 | 0.0001* |
| SSI | 5.83 | 2.79 | 2.09 | 0.044* |
| Three parameters 1 | ||||
| Intercept | 1.67 | 0.28 | 5.93 | <0.001* |
| Glomerulosclerosis score | 0.0017 | 0.0025 | 0.67 | 0.51 |
| Changes in U-P | 0.0017 | 0.00037 | 4.65 | <0.0001* |
| SSI | 5.88 | 2.77 | 2.13 | 0.040* |
| Three parameters 2 | ||||
| Intercept | 1.65 | 0.27 | 6.04 | <0.001* |
| Score for TID | 0.011 | 0.012 | 0.87 | 0.39 |
| Changes in U-P | 0.0017 | 0.00038 | 4.42 | <0.0001* |
| SSI | 5.95 | 2.64 | 2.25 | 0.031* |
TID indicates tubulointerstitial damage; U-P, urinary excretion of protein; SSI, salt-sensitivity index.
P<0.05.
Urinary Angiotensinogen in Patients With Ménière Disease
None of the outpatients with Ménière disease developed proteinuria or hypertension during the study (<140/90 mm Hg; data not shown). After 4 weeks of consuming a low-salt diet, the urinary excretion rate of sodium significantly decreased from 108±37 to 67±21 mEq/d (P<0.05), and plasma renin activity significantly increased from 0.97±0.41 to 1.36±0.73 ng/mL per hour (P<0.05). However, consuming the low-salt diet for 4 weeks did not significantly change either mean blood pressure (data not shown) or the urinary angiotensinogen:creatinine ratio (11.6±6.7 to 11.1±4.2 μg/g of creatinine).
Discussion
Both preclinical11,20,21 and clinical studies15,22–24 have indicated that urinary angiotensinogen can serve as a specific index of intrarenal RAS status. We showed previously that blood pressure becomes salt sensitive as renal damages progress in patients with normotensive IgA nephropathy.4 We also found recently that patients with IgA nephropathy have increased urinary angiotensinogen levels, suggesting activation of the intrarenal RAS.15 We, therefore, investigated the relationship between increased intrarenal RAS activity and the development of salt-sensitive hypertension in patients with IgA nephropathy. The present study is the first to determine that the salt sensitivity of blood pressure significantly correlates with urinary angiotensinogen excretion and renal damage in patients with IgA nephropathy. These data support the hypothesis based on previous preclinical findings7–12 that salt-induced inappropriate activation of the intrarenal RAS and associated renal damage contribute to the development of salt-sensitive hypertension in patients with CKD.
The salt sensitivity of blood pressure is induced by an increase in tubular sodium reabsorption and/or a reduction in the glomerular ultrafiltration coefficient.1,25 The present study found that the salt sensitivity of blood pressure significantly correlated with increased levels of urinary angiotensinogen, a marker of intrarenal RAS activity. Therefore, enhanced intrarenal Ang II levels might result in an increase in tubular sodium reabsorption and worsen the salt sensitivity of blood pressure. This notion is supported by recent clinical data showing that an Ang II receptor blocker can enhance natriuresis in patients with CKD.26 In agreement with previous clinical findings in patients with IgA nephropathy,15 the present study found that the filtration fraction was not significantly changed by a short-term high-salt diet in patients with IgA nephropathy. However, we cannot explain why the high-salt diet increased urinary angiotensinogen levels but did not significantly alter the filtration fraction. A high-salt–induced decrease in circulating Ang II might partially compensate for the effect of the activated intrarenal RAS in patients with IgA nephropathy who have diminished but relatively maintained renal function. This notion is supported by the clinical findings that a high-salt diet significantly decreases the filtration fraction in normal individuals but increases it in patients with advanced renal disease.27 One limitation of the present study is that we could not administer a high-salt diet over the long term to patients with IgA nephropathy because of ethical issues.
A high-salt diet significantly increased urinary angiotensinogen excretion in patients with IgA nephropathy but not in those with Ménière disease. Studies in vitro have shown that proximal tubular Ang II production is enhanced by conditioned culture medium from mesangial cells activated by IgA.28 Furthermore, in mice29 and in patients30 with IgA nephropathy, the augmentation of intrarenal angiotensinogen expression is associated with increases in reactive oxygen species. Therefore, we speculate that increases in reactive oxygen species play an important role in mediating the intrarenal augmentation of angiotensinogen as IgA nephropathy progresses, whereas these changes do not occur in patients with Ménière disease who have normal renal function. However, the present study did not investigate reactive oxygen species in the kidney. Further clinical studies are needed to investigate the role of reactive oxygen species in the regulation of intrarenal angiotensinogen and Ang II in patients with IgA nephropathy.
We31 and others24 have recently found significantly increased urinary angiotensinogen excretion in patients with CKD. Intrarenal and urinary angiotensinogen levels are also increased in salt-sensitive hypertensive Dahl rats, which are models of human salt-sensitive hypertension.11,32 Angiotensinogen is the only known substrate for renin, which is the rate-limiting enzyme of the RAS. Because the level of angiotensinogen is close to the Michaelis-Menten constant for renin, levels not only of renin, but also of angiotensinogen, can dictate the activity of the RAS, and upregulated angiotensinogen levels might lead to increased Ang II formation and consequent sodium reabsorption.33,34 This concept is very important for the development of salt-sensitive hypertension in which plasma renin activities are significantly decreased by a high-salt intake.7 Oxidative stress might participate in intrarenal/urinary enhanced angiotensinogen levels induced by a high-salt intake, because superoxide dismutase mimetics offset such augmentation in Dahl salt-sensitive rats.12 Ohashi et al35 also showed recently that augmented intrarenal angiotensinogen levels are associated with increased oxidative stress in the kidneys of mice with IgA nephropathy. Furthermore, a recent study in vitro has generated firm evidence that oxidative stress induces Ang II generation via a conformational change of angiotensinogen.36 However, separate interventional studies are required to address this issue in a clinical setting.
We showed recently that baseline urinary angiotensinogen levels are not correlated with proteinuria in patients with IgA nephropathy and minor glomerular abnormalities.15 We also showed previously that baseline levels of urinary angiotensinogen and albumin or protein are not always significantly related in hypertensive patients.23 A high-salt diet increased proteinuria and urinary angiotensinogen levels among patients with IgA nephropathy in the present study. Changes in the excretion rates of urinary protein in these patients positively correlated with changes in urinary angiotensinogen: creatinine ratio. However, SSI positively correlated only with changes in urinary angiotensinogen excretion rate. These data support the concept based on clinical15,22,23,37 and preclinical11,20,21 evidence indicating that urinary angiotensinogen excretion is not a simple consequence of proteinuria.
Perspectives
The present retrospective exploratory study supports the hypothesis that salt-induced inappropriate augmentation of intrarenal RAS activity and associated renal damage play important roles in the development of salt-sensitive blood pressure in patients with IgA nephropathy. We showed previously that normotensive patients with IgA nephropathy develop salt-sensitive blood pressure,15 which, together with the present findings, indicates that increases in urinary angiotensinogen levels can predict the progression of salt-dependent hypertension. We have already started prospective clinical studies to determine whether increases in urinary angiotensinogen levels can predict the later progression of renal damage and hypertension in patients with metabolic syndrome and type 2 diabetes mellitus who have normal kidney function.
Supplementary Material
Acknowledgments
We acknowledge the excellent technical assistance provided by Akemi Katsurada and Dr Toshie Saito (Tulane University Health Sciences Center).
Sources of Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to A.N.) and by grants from Mitsui Life Social Welfare Foundation (to A.N.), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408 to H.K.), and the Medical Research Fund to Osaka City General Hospital (to Y.K. and M.I.).
Footnotes
This study was presented in part at the symposium of the International Society of Nephrology, Nexus Kyoto, Kyoto, Japan, April 4, 2010, and at the 63rd High Blood Pressure Research Conference, Chicago, IL, September 24, 2009.
Disclosures
None.
References
- 1.Kimura G, Frem GJ, Brenner BM. Renal mechanisms of salt sensitivity in hypertension. Curr Opin Nephrol Hypertens. 1994;3:1–12. [PubMed] [Google Scholar]
- 2.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Konishi Y, Okada N, Okamura M, Morikawa T, Okumura M, Yoshioka K, Imanishi M. Sodium sensitivity of blood pressure appearing before hypertension and related to histological damage in immunoglobulin A nephropathy. Hypertension. 2001;38:81–85. doi: 10.1161/01.hyp.38.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 6.Silva GB, Garvin JL. Angiotensin II-dependent hypertension increases na transport-related oxygen consumption by the thick ascending limb. Hypertension. 2008;52:1091–1098. doi: 10.1161/HYPERTENSIONAHA.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Antioxidative effect of p38 mitogen-activated protein kinase inhibitor in the kidney of hypertensive rat. J Hypertens. 2005;23:165–174. doi: 10.1097/00004872-200501000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens. 2005;27:355–367. [PubMed] [Google Scholar]
- 10.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 11.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzaki Y, Ozawa Y, Kobori H. Quantification of human angiotensinogen by a novel sandwich ELISA. Peptides. 2006;27:3000–3002. doi: 10.1016/j.peptides.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konishi Y, Morikawa T, Yasu T, Teramoto K, Okada N, Yoshioka K, Okumura M, Imanishi M. Blunted response of the renin-angiotensin system and nitric oxide synthesis related to sodium sensitivity in immunoglobulin A nephropathy. Hypertens Res. 2004;27:7–13. doi: 10.1291/hypres.27.7. [DOI] [PubMed] [Google Scholar]
- 17.Devaiah AK, Ator GA. Clinical indicators useful in predicting response to the medical management of meniere’s disease. Laryngoscope. 2000;110:1861–1865. doi: 10.1097/00005537-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Santos PM, Hall RA, Snyder JM, Hughes LF, Dobie RA. Diuretic and diet effect on Meniere’s disease evaluated by the 1985 committee on hearing and equilibrium guidelines. Otolaryngol Head Neck Surg. 1993;109:680–689. doi: 10.1177/019459989310900408. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Otolaryngology-Head and Neck Foundation, Inc. Committee on Hearing and Equilibrium Guidelines for the Diagnosis and Evaluation of Therapy in Meniere’s Disease. Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 20.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan N. Primary hypertension: pathogenesis. In: Kaplan N, editor. Kaplan’s Clinical Hypertension. Philadelphia, PA: Lippincott Williams and Wilkins; 2006. pp. 50–121. [Google Scholar]
- 26.Fukuda M, Yamanaka T, Mizuno M, Motokawa M, Shirasawa Y, Miyagi S, Nishio T, Yoshida A, Kimura G. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J Hypertens. 2008;26:583–588. doi: 10.1097/HJH.0b013e3282f2fded. [DOI] [PubMed] [Google Scholar]
- 27.Cianciaruso B, Bellizzi V, Minutolo R, Colucci G, Bisesti V, Russo D, Conte G, De Nicola L. Renal adaptation to dietary sodium restriction in moderate renal failure resulting from chronic glomerular disease. J Am Soc Nephrol. 1996;7:306–313. doi: 10.1681/ASN.V72306. [DOI] [PubMed] [Google Scholar]
- 28.Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J Am Soc Nephrol. 2005;16:2306–2317. doi: 10.1681/ASN.2004121117. [DOI] [PubMed] [Google Scholar]
- 29.Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:750–755. doi: 10.1111/j.1440-1681.2009.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–163. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal renin-angiotensin system activity. Am J Nephrol. 31:318–325. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296:F771–F779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–89. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 34.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–475. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Activation of reactive oxygen species and the renin-angiotensin system in iga nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:509–515. doi: 10.1111/j.1440-1681.2008.05107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, Stein PE, Broughton Pipkin F, Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338:478–480. doi: 10.1097/MAJ.0b013e3181b90c25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.